1. Introduction
Three dimensional (3D) printing enables fabrication of complex structures [
1,
2,
3]. Among the established 3D printing techniques, material extrusion is a prevailing technique due to facile process implementation under relatively moderate ambient conditions and a diverse material palette [
4]. As a result of these materials processing characteristics, microextrusion-based bioprinting (micro-EBB) of cell-laden biopolymer materials has been advanced to fabricate analog tissue constructs towards targeted cell-based models and therapeutic applications [
5,
6,
7,
8,
9]. The micro-EBB materials fabrication process extrudes suspensions of live cells within hydrogels, enabling tissue scale constructs with high flexural and mechanical strength and cell proliferation rates [
10]. The operating principle behind the micro-EBB techniques is one that combines a fluid-dispensing system with an automated robotic system for precision printing. During printing, computer-controlled deposition of cells within continuous filament structures results in rapid fabrication of the desired 3D customized structures with adequate mechanical stiffness [
11,
12,
13]. Various bioprinting process innovation strategies have recently been developed, including single axis printing and co-axial printing. Moreover, beyond the traditional implementation of bioprinting in an air medium, emerging techniques have implemented printing in a liquid bath or hydrogel support bath medium. Among the various bioprinting techniques, extrusion within a support bath medium is a burgeoning technique that enables complex 3D structured materials processing of low viscosity liquid hydrogel precursors [
14,
15,
16,
17]. By depositing structural filaments within a support bath, the limitations of conventional printing in air processing configurations can be mitigated, including clogging [
18,
19], gravity-induced structural collapse with weak interfacial strength [
20], and the absence of a support structure [
21,
22]. Specifically, bioprinting within a support bath enables low viscosity materials processing to minimize the extent of the material occlusion of the extruding nozzle. Also, the layer-to-layer interfacial strength can be maintained due to the characteristically low surface tension of the bio-ink within a liquid medium [
15]. Also, the bath material serves as a provisional frame for the in-process support of the printed bio-ink towards an integrated 3D structure with high shape fidelity upon post-process material gelation.
To better understand bioprinting performance outcomes within a support bath material, the key consideration is how to confer and maintain the complex structural properties, including construct shape fidelity and mechanical stiffness, in the context of low viscosity prints. Related work includes studies in which the printability of gelatin-alginate hydrogel blends within a yield stress bath has been demonstrated to yield complex 3D structures [
10]. Moreover, print feasibility studies with various materials, including PDMS and agarose hydrogels, have been reported for hydrophilic support baths such as Carbopol gel and perfluorotributylamine [
17,
23]. Supermolecular hydrogels have also been printed in self-healing hydrogels to achieve fine, multi-material structures [
24]. Developing a support bath material is critical for the printing of liquid hydrogel precursors. Currently, gelatin microparticles, Carbopol microgels, nanoclay, and pluronic F127 represent some of the most promising candidate support bath materials. Gelatin is a thermosensitive material that can serve as a support bath in gel form at a relatively low working temperature (<25 °C), which can be removed by increasing the temperature. In this paper, gelatin is selected as a component of the bio-ink, thereby limiting the use of gelatin as the prescribed support bath material. The ion-sensitive Carbopol can be used to print hydrogels without an ionic cross-linking post-processing step. It is reported that the printed outcome exhibits poor surface quality when using Carbopol as a support bath [
15]. The pluronic F127 is stable at a temperature range of 25 °C–40 °C, which is typically used as mold and sacrificial material for bioprinting [
5,
25]. Pluronic F127 has poor mechanical strength and tends to dissolve in aqueous environments, which may affect printed structure shape properties while using liquid hydrogel precursors [
26]. The nanoclay is selected here as the support structure due to its superior properties such as the ionic insensitivity, thermal stability, and unltraviolet transparency. As a yield stress bath material, the nanoclay formulation begins in the gel state before processing. When the bioprinting nozzle translates in the nanoclay support bath, nanosilicates around the nozzle experience shear stress higher than the yield stress of the nanoclay, causing a material phase transition from the gel to sol state. After the nozzle travels away, the region rapidly recovers to its native gel state when the shear stress falls below the yield stress.
Although bioprinting within a support bath has been widely investigated by using a different fluid-dispensing system and hydrogel materials with different rheological properties, there are fundamental questions about how the relationships can be mapped between the printing process parameters and the printing performance outcomes. To systematically evaluate the related outcomes for bioprinting within a support bath, this paper advances an experimental parametric study to optimize the 3D structural shape fidelity by tuning the process parameters including the layer height, extrusion flowrate, printing temperature, and print head speed. The
Table 1 lists the key parameters and their values that will be investigated in this study.
The tubular structure, which is widely studied as a mimic of human vessel tissue, is selected as an example to be investigated [
13,
23]. Next, optimal printing conditions are then explored to achieve 3D tissue constructs with a smooth surface, shape integrity, and adequate stiffness. Based on the experimentally optimized printing condition, this paper aims to provide needed insight into how cell-laden hydrogel materials can be systematically processed under the emerging bioprinting support bath paradigm to produce tissue constructs with precise control of structural properties.
2. Materials and Methods
2.1. Cell Culture and Viability Assay
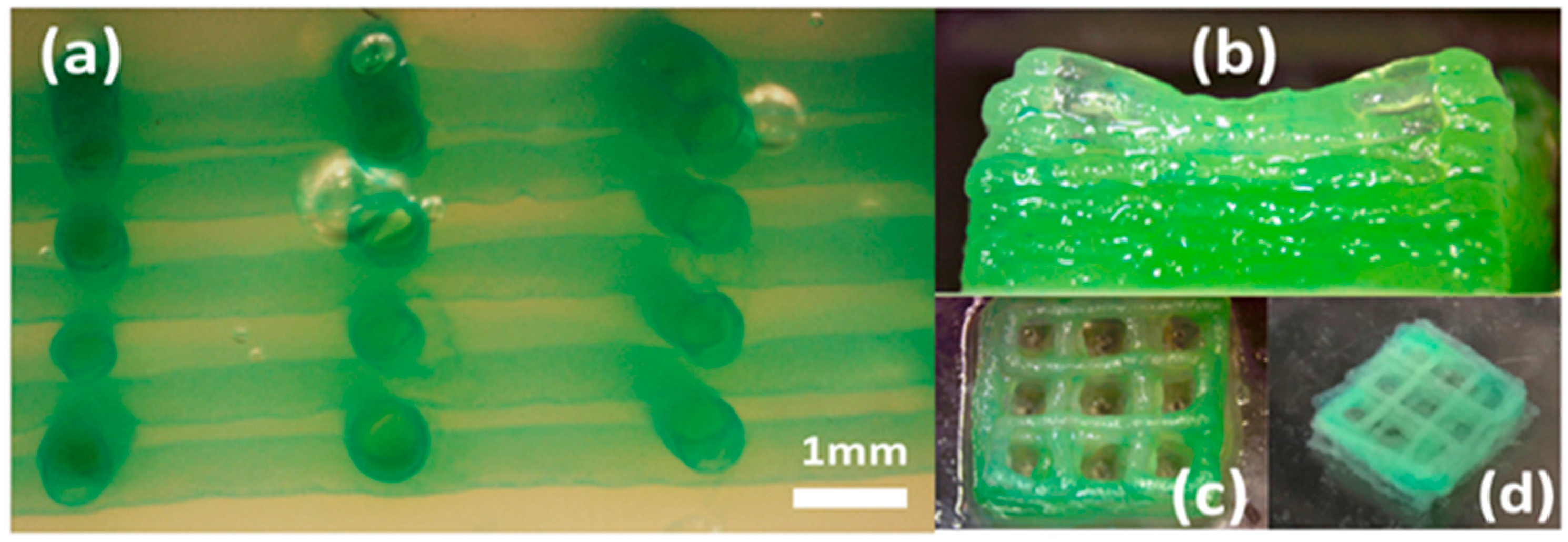
Normal human dermal fibroblast (NHDF) cells are cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 15% fetal bovine serum (FBS), 1% penicillin-streptomycin (P/S) solution. The NHDFs are cultured in an incubator at 37°C and with 5% CO2. The growth medium is changed every 2 days. Dissociation of NHDFs with 0.25% trypsin is applied at approximately 90% confluence to passage the cells. Each live cell suspension is fluorescently stained with Calcein-AM (2 μmol/L, Enzo), centrifuged, re-suspended in the liquid hydrogel precursor, and visualized post-print with a wide-field fluorescent microscope.
2.2. Material Preparation
2.2.1. Alginate-Gelatin Blend Preparation
Gelatin powder (type A, 300 bloom, with average molecular weight of 100,000, Sigma-Aldrich, St. Louis, MO, USA), and sodium alginate powder ((C6H7NaO6)n, with molecular weight of 216.121 g/mol, (Sigma-Aldrich, St. Louis, MO, USA) are dissolved in 1 × Dulbecco’s phosphate-buffered saline (DPBS, without calcium and magnesium) at 95 °C to make gelatin-alginate solution with various concentrations. A warm gelatin-alginate solution is sterile-filtered with 0.45 μm and 0.20 μm filters and then stored at 4 °C for future use. A calcium chloride (CaCl2) stock solution is prepared by dissolving CaCl2 in DI water at a concentration of 2% w/v. A sodium chloride (NaCl) stock solution is prepared by dissolving NaCl in DI water at a concentration of 0.9% w/v.
2.2.2. Nanoclay Support Bath Preparation
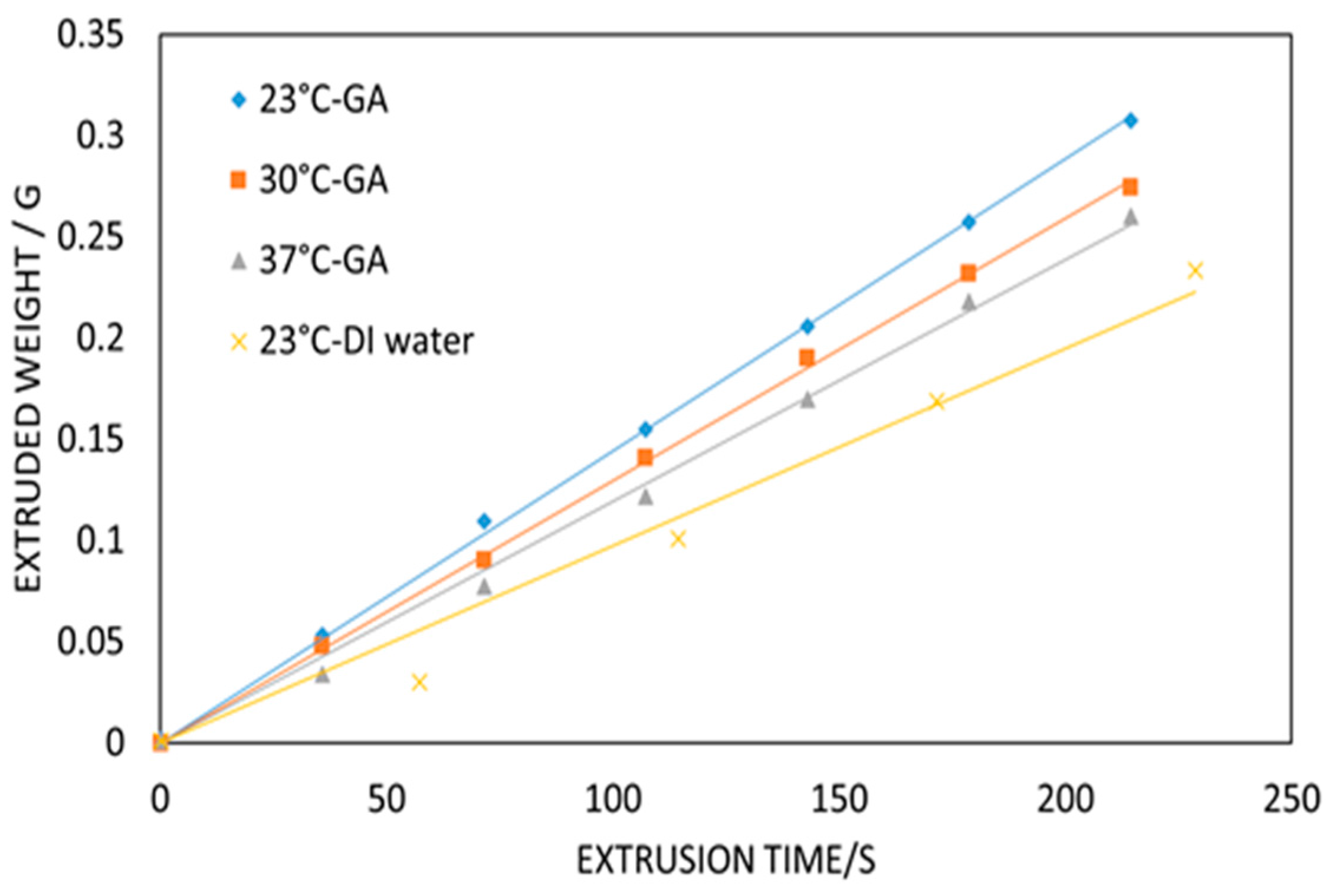
The Laponite nanoclay (Na0.7Si8Mg5.5Li0.3O20(OH)4) is in the form of disk shaped particles with thickness 1 nm and diameter 25 nm. Laponite EP nanoclay (BYK Additives Inc., Gonzales, TX, USA) is used as a yield stress bath to support hydrogel printing. Laponite EP nanoclay powder is dispersed in DI water under vigorous stirring for 24 h to ensure uniform dissolution. A range of nanoclay concentrations has been tested. The 4% nanoclay with 0.5% CaCl2 is finalized as the optimal for both rheological properties (moderate viscosity). The lower concentration of nanoclay fails to support the printing of complex structures, causing material discontinuities during printing. The higher concentration of nanoclay is more viscous, exerting more pressure on the printed cell-laden filament within the bath during printing. A 4% nanoclay formulation is prepared to investigate the effects of the support bath properties on the filament formation. To improve the hydrogel cross-linking properties of the 3D structure, the 4% nanoclay with 0.5% CaCl2 is prepared as the optimal bath concentration for printing.
2.3. Bio-Ink Formulation
The initial cell-free bio-ink is constituted at 20% w/v gelatin and 6% w/v alginate. When bubbles are observed upon loading into a syringe material reservoir, the syringe is placed at an ambient temperature of 37 °C to accelerate bubble removal. The bio-ink formulation is prepared by mixing gelatin-alginate hydrogels and the NHDF cells. The bio-ink consisting of alginate, gelatin, and cells is prepared and processed for the final fabricated 3D bifurcation structure. As an example, 3% w/v alginate, 10% w/v gelatin with a prescribed bio-ink cell density is prepared by first collecting the NHDFs by centrifugation at 1000 rpm for 5 min. The NHDF cells are dispersed into a 0.5 mL solution of DPBS by gently pipetting the cell pellet so as to avoid bubble formation that may confound the cell density measurements. The mixed solution of 0.5 mL 6% w/v alginate and 20% w/v gelatin at 37 °C is then added to the cell suspension to yield a final bio-ink formulation with 3% w/v alginate and 10% w/v gelatin with 2 × 106 cells/mL. For complete mixing, the bio-ink is maintained at 37 °C and transferred to 4 °C for 5 min to induce rapid gelation with minimal time-dependent cell settling. Prior to printing, the bio-ink is warmed at a prescribed processing temperature for 10 mins.
2.4. 3D Bioprinter System Configuration
All cell-laden constructs are created by implementing an in-house 3D bioprinter based on a customized 3-axis CNC machine mounted with an independently addressable printing head. The toolpath is designed and transformed into G-code using Matlab (MathWorks, Inc., Natick, MA, USA), and then imported to the stand-alone open source software grblController to control the three-axis translational platform. A temperature-controlled heating pad (New Era Pump Systems, Inc., Wantagh, NY, USA) is wrapped around the bio-ink syringe to maintain the bio-ink material at 37 °C. The bio-ink material formulation (both cell-laden experimental and cell-free control groups) is loaded into a 3ml syringe-based material reservoir equipped with a 22 gauge nozzle (EFD, Inc., Madison Heights, MI, USA). A bio-ink deposition flowrate of 1–10 mL/h is controlled by modulating the motor rotation speed.
2.5. Temperature Control System for Support Bath
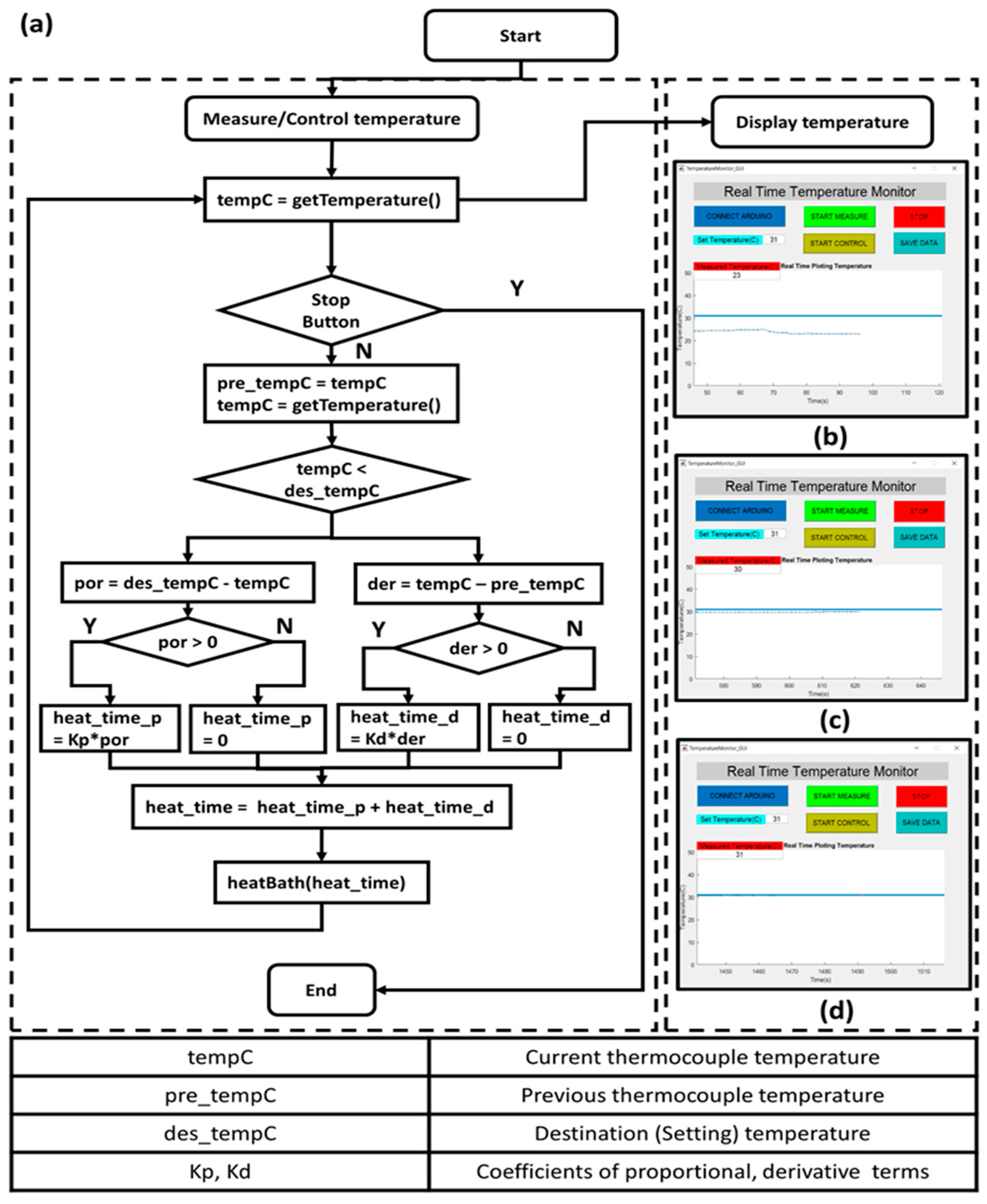
The support bath temperature is controlled by a customized temperature control system. The control system contains an Arduino Uno board, a solid state relay, a heating unit, a thermocouple amplifier (MAX31855), and a thermocouple. The system configuration is shown in
Figure 1. The control box is connected to a host computer. A software “Real Time Temperature Monitor” with GUI is programmed in Matlab to communicate with the Arduino board. The software functions to measure the current temperature, and control the object temperature by using a proportional-integral-derivative (PID) controller. The control algorithm details are shown in
Figure 2. The support bath is pre-heated to the desired temperature to save the temperature control time. The printing platform is surrounded by a latex sheet to mitigate heat losses.
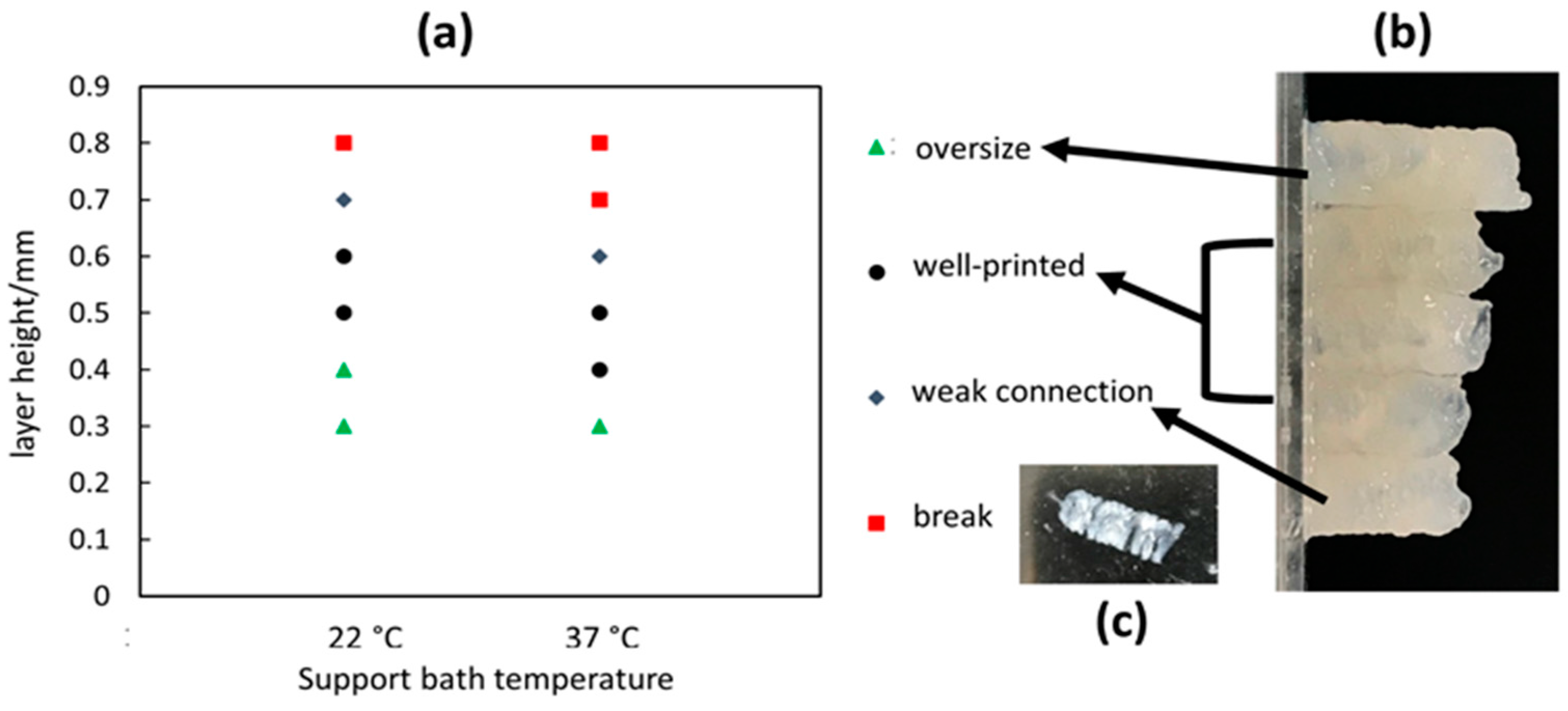
Figure 2b–d shows the temperature control procedure. The set temperature is 31 °C, and the bath temperature is gradually increased and stabilized at 31 °C within 10 min. The customized temperature control system aims to maintain the support bath conditions. This is of particular importance when ambient temperatures fall below 22 °C, resulting in fast gelation of the gelatin material with accompanying nozzle occlusion.
2.6. Post-Processing of Printed Constructs
After printing, the constructs are first immersed in a 0.9% NaCl2 to rinse the support bath, followed by the addition of 2% CaCl2 solution to physically cross-link the sodium alginate material for 10 mins. Any residual nanoclay support bath material is removed by gently pipetting the CaCl2 solution on the surface of the constructs. After cross-linking, the CaCl2 solution is gently removed, and DPBS is applied to wash the construct. The structures become smooth due to the post-processing under solution with hydrogel cross-linking to maintain the shape fidelity at ambient temperatures.
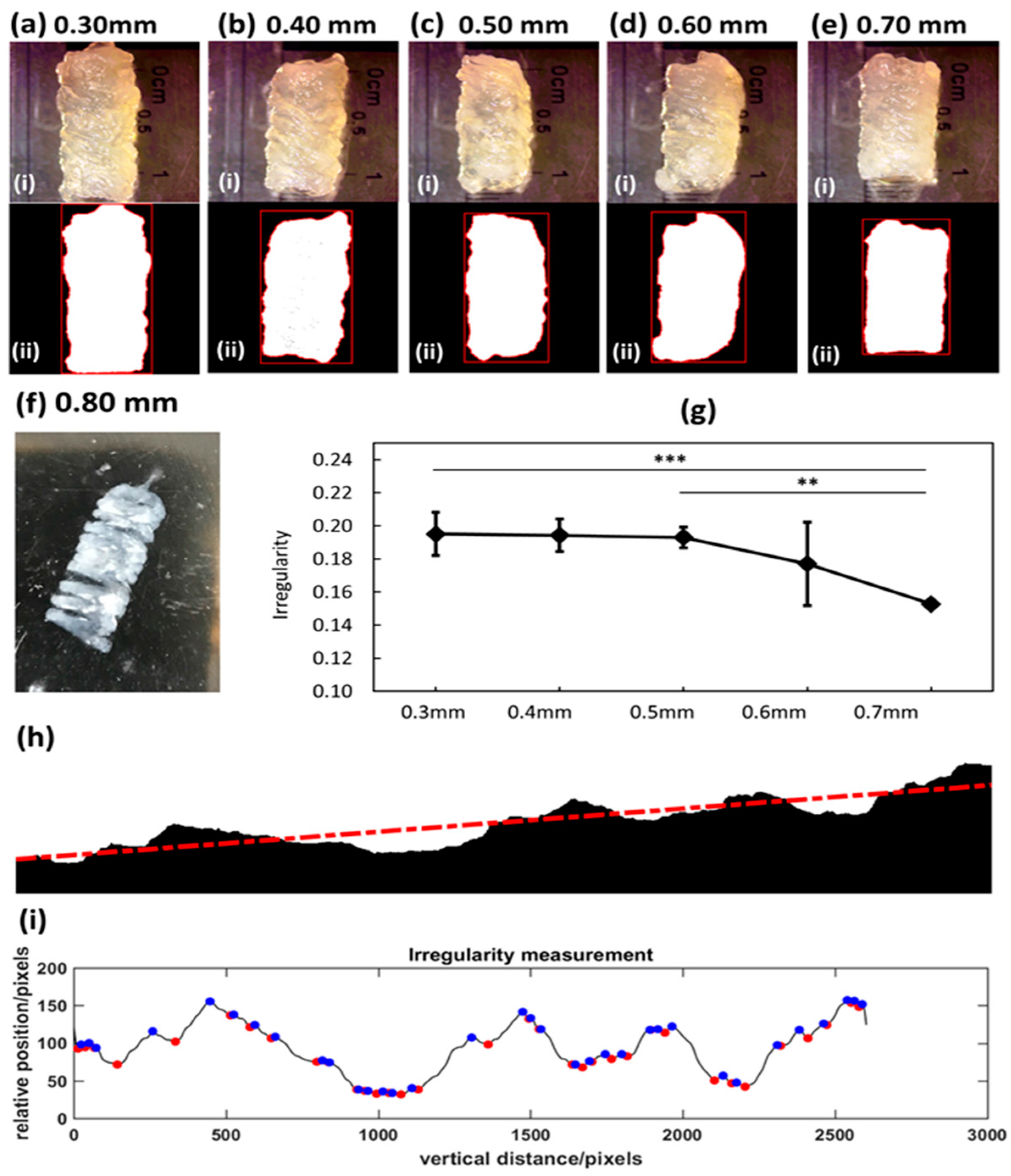
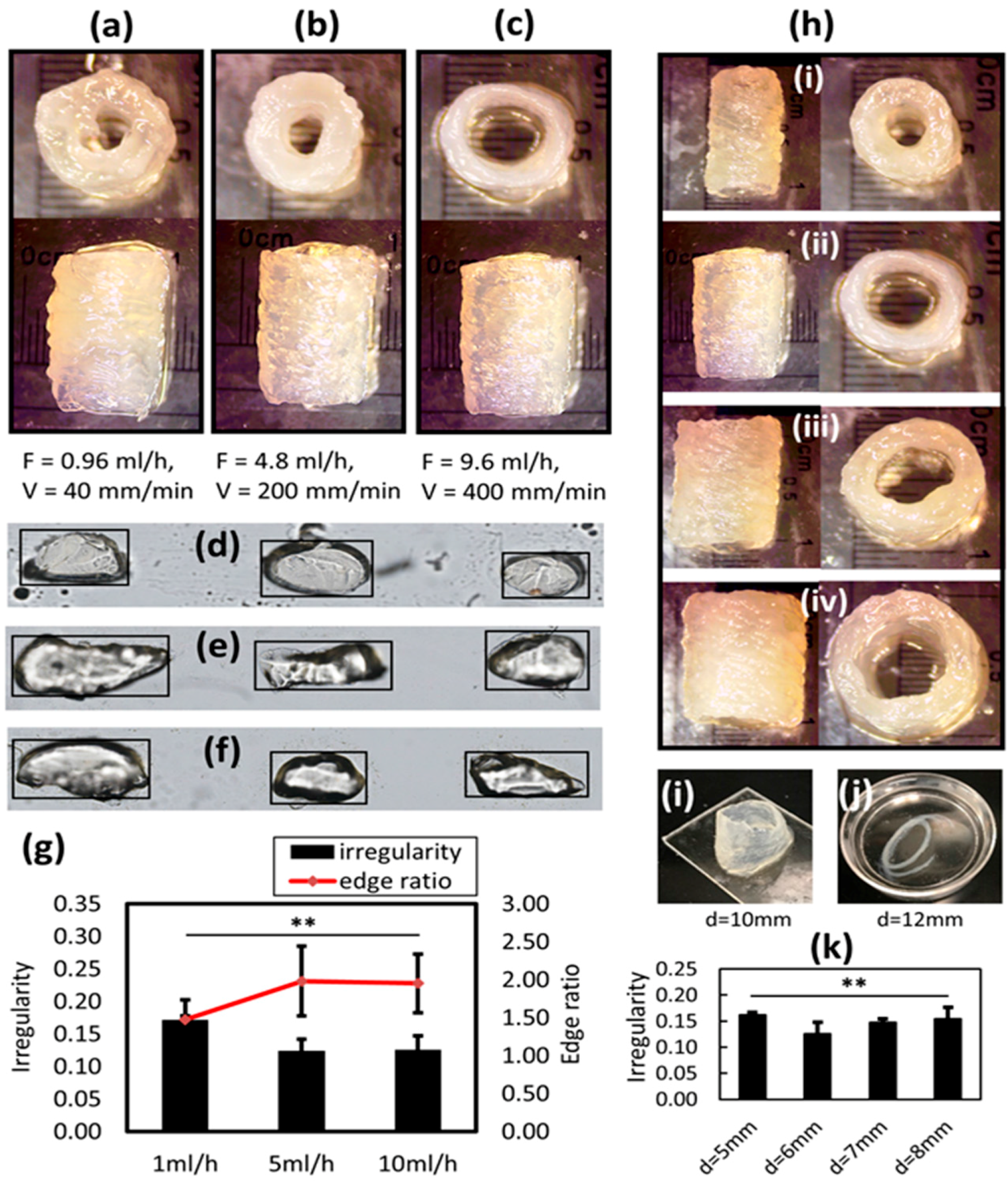
2.7. Irregularity Measuremen
The irregularity is defined as a metric to characterize the outer surface quality of the printed tubular structure. The irregularity (or roughness) is defined as the average vertical distance between the successive local maximum and minimum points of a curve that represents a surface. To measure the irregularity of a surface (or a projected curve of a surface), a binary image of the object (e.g., Figure 5h, in which the binary image represents the arbitrary projected outline of a surface) is used. The irregularity is measured by the following algorithm: (1) By computing a fitted linear model of the outline, for example, the outline can be represented as:
(2) Project the outline to the fitted linear model (rotate the whole outline to eliminate the effect of global incline. Thus, the projected data can be represented as:
(3) The projected data will be further filtered to remove noise by using Fast Fourier Transform (FFT), and inverse-FFT algorithm, which is implemented by Matlab. A threshold is selected to filter the signals with higher frequency than the threshold, which is noise signal. After denoising, the filtered outline is represented as:
(4) A set of local maximum and local minimum points are detected from the final smooth outline yfft. Successive local maximum and local minimum are set as a pair, and their absolute y direction difference is summed up and then normalized (divided by the length of measured area L). The irregularity is defined as:
in which
yfft (
max,
i),
yfft (
min,
i) are
ith successive local max and min points.
2.8. Imaging and Data Analysis
An inverted bright field-fluorescence (IX83, Olympus) microscope is used to image the printed samples. Fluorescent images of printed cell-laden samples are processed using Cellsens, ImageJ, and Matlab to yield quantitative measurements of printed surface irregularity through fluorescent image line profile analysis. Statistical significance of measurements with a student’s t-test and one-way ANOVA is computed using GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA). Differences are considered significant as p < 0.05 (*), p < 0.01 (**), p < 0.005(***).
4. Discussion
As a current state-of-art bioprinting technique, microextrusion-based printing liquid hydrogel precursors within a supporting bath medium enable the formation of complex 3D structures. Although the essential role of a support bath in bioprinting is well-recognized, underlying structural phenomena and mechanisms are still largely unknown. In the context of emergent phenomena distinct from traditional bioprinting in air medium, control of the structural outcomes with quality design attributes requires optimization of key printing process parameters. By optimizing the bioprinting parameters for filament and tubular structures within a nanoclay support bath, the optimal printing parameters are identified and reported. First, the design and implementation of a temperature control system to the bioprinter enables the support bath temperature to be stably maintained. This temperature control system is controlled by PID controller and operated with customized software with GUI. Compared to other studies that implement nanoclay as a support bath material, the findings from this paper show that the support bath temperature has a significant effect on the formation of the printed hydrogel structures. Upon lowering the support bath temperature below room temperature (22 °C), the issue of clogging arises, which has not been reported in previous studies [
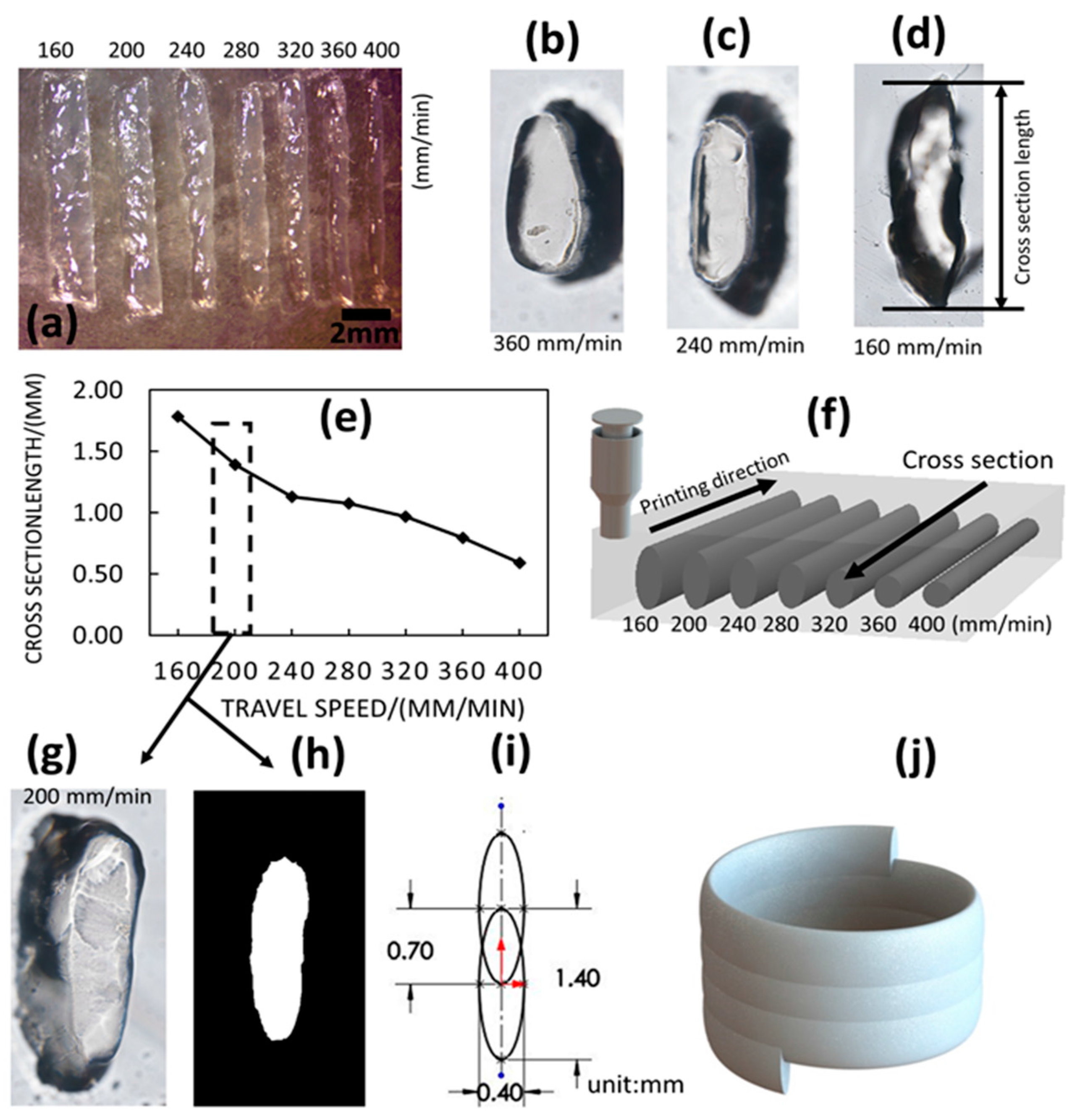
14]. Furthermore, by analyzing the single filament dimension, a relationship between the filament cross-sectional long axis with extrusion flowrate and travel speed is revealed. Based on this, the optimal layer height is determined to enable solid material fusion between successively printed layers. At first, the optimal layer height for printing tubular structure is identified as 0.5 mm when the flowrate is 4.8 mL/h and travel speed is 200 mm/min. The temperature of the support bath is set near room temperature (22 °C). However, to improve printing time efficiency, different travel speeds are tested, resulting in the identification of optimal parameters for printing tubular structures with uniform surface characteristics at shorter print times. Although other state-of-art studies do consider the rheological properties as major parameters, the interplay with key process parameters has not been systematically studied, along with the effect on printed 3D structure. However, this paper highlights the key process parameters that are critical for bioprinting complex structural outcomes within a support bath medium.
To characterize the printed structure, the irregularity is proposed to measure the surface quality of the tubular structure. The irregularity serves as a quantitative metric that reveals the relationship between the investigated parameters and printed outcomes. Another property that is critical to the bioprinting structure is the resolution. It is reported herein that the single printed filament is on a millimeter scale, which is a relatively low resolution for micro-EBB technique. However, the print resolution can be significant improved by using smaller diameter nozzles. The resolution of the demonstrated bioprinted 3D tubular structures is still within the range of current state-of-art bioprinted structures. The typical printed tube has a 5~10 mm diameter when using pluronic F127 and GelMA blend [
26]. The “Y” shape bifurcation structure printed using alginate within CaCl
2 bath has an average diameter of 10 mm [
13]. The filament diameter printed by FRESH method ranges between 160 and 260 µm; however, the variation of filament diameter is large, which means the single printed filament is non-uniform [
16]. In general, although the current state-of-art bioprinted tubular structure is still at the millimeter scale, the materials, methodologies, and optimized parameters identified by each approach lay a solid foundation for the optimization of the final structural resolution in future studies.
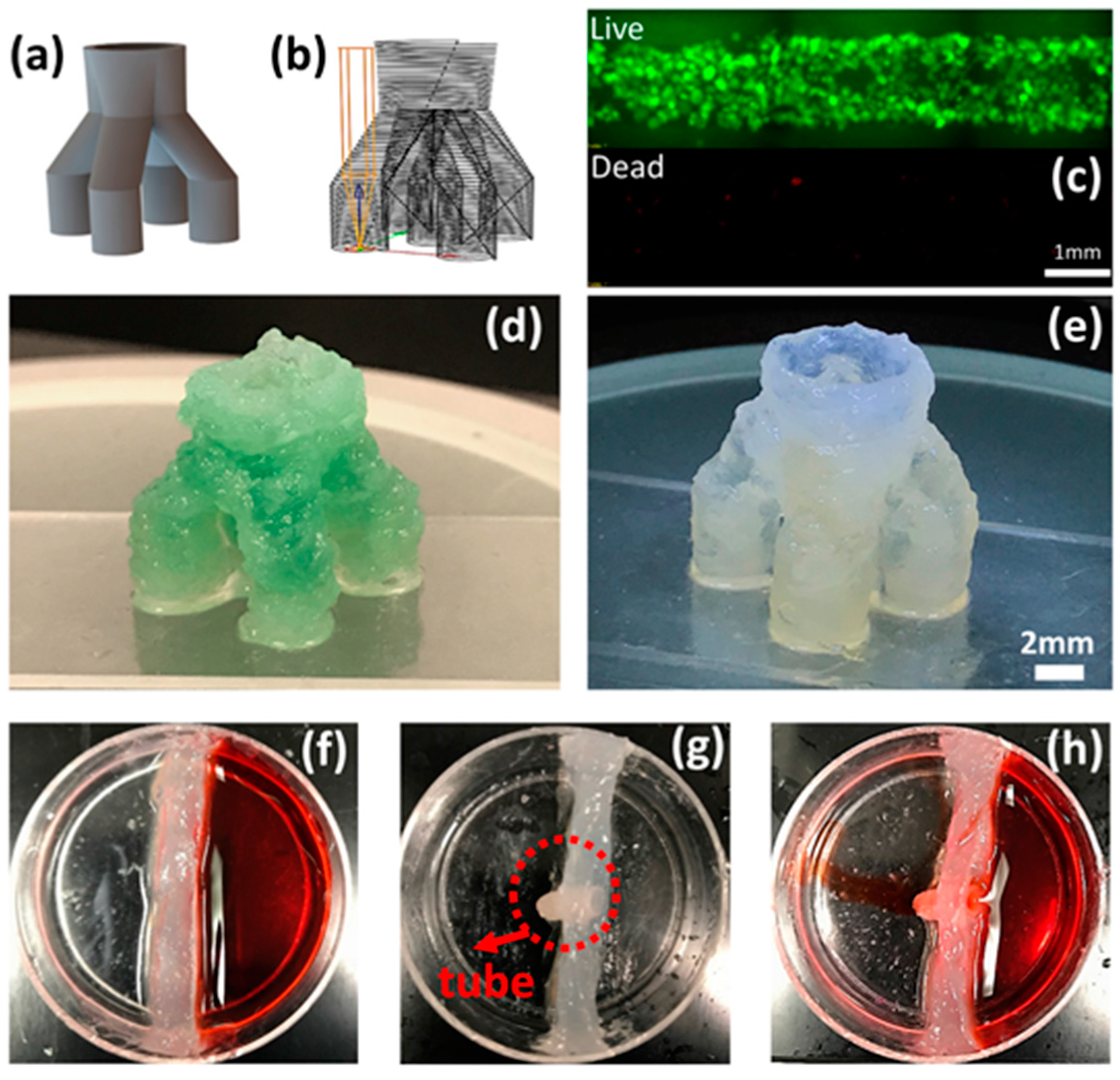
In this paper, the experimntal observations and analyses demonstrate that although printing within support bath enables fabrication of complex structure, the results indicate that the printed structure should be designed with structural stability. Otherwise, the structure will collapse after extracting the support bath. In addition, the design and printing of a bifurcating tubular structure that mimics the human vascular structures validates the parameters derived from the previous experimental results. Typically, based on the bioprinting in a support bath paradigm, a “Y” shape bifurcating print geometry is enabled, which is a planar structure [
13]. However, to demonstrate increased 3D structural complexity, in this study, a customized one-to-four bifurcation toolpath is designed and printed. The printed one-to-four tubular structure can stand on the cover slide with adequate stiffness and shape fidelity. This study also investigates the shape fidelity for another prevailing bioprinting tissue structure in the mesh scaffold. The results indicate that when printing in a support bath, it is more challenging to maintain whole mesh structural integrity. The weak interfacial strength between layers is insufficient for preserving the overall shape fidelity in the context of outside disturbances. In order to further investigate some of these fundamental printability issues, ongoing research is suggested to better understand the rheological property and behavior of the support bath and bio-ink materials.
5. Conclusions
In this paper, experimental studies are conducted to investigate the printability of bifurcating tubular structures during the bioprinting process along with post-print structural outcomes. Optimal printing parameters are investigated and identified for a fast, high-quality complex bifurcating structure prints. Typically, the key parameters of bioprinting of gelatin-alginate hydrogel blend include the rheological properties of hydrogel material (tuned by material concentration and printing temperature). For bioprinting of gelatin-alginate hydrogel within a support bath medium, the material concentration of both the hydrogel and support bath are prescribed, which is optimal, based on phenomenological observations. The printability of a single filament is investigated. Therefore, in this paper, the further parametric study about other key parameters includes layer height that determines how the successive layers are fused, the printing travel speed of the shape outer surface irregularity, and the structure design that enables adequate structural stability. After all of those parameters are well understood, the final print of a bifurcation structure demonstrates the well-controlled printability of the liquid hydrogel precursor within a support bath. For future studies, the cell density within the hydrogel, as another key parameter for the bioprinted cell-laden constructs, will be systematically investigated. Also, the optimization of those process parameters has set a fundamental basis upon which the resolution of printed single filament or tubular structure can be further improved. One promising future study is to extend the printed 3D complex bifurcation structure to the micrometer feature sizes (e.g., 100–200 µm diameter), where the cells can experience an in vivo microenvironment with physiologically relevant dimensional scales.