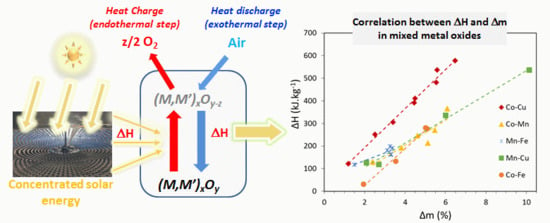
Mixed Metal Oxide Systems Applied to Thermochemical Storage of Solar Energy: Benefits of Secondary Metal Addition in Co and Mn Oxides and Contribution of Thermodynamics
Abstract
1. Introduction
2. Materials and Methods
3. Experimental Assessment of Mixed Metal Oxide Systems for TCES
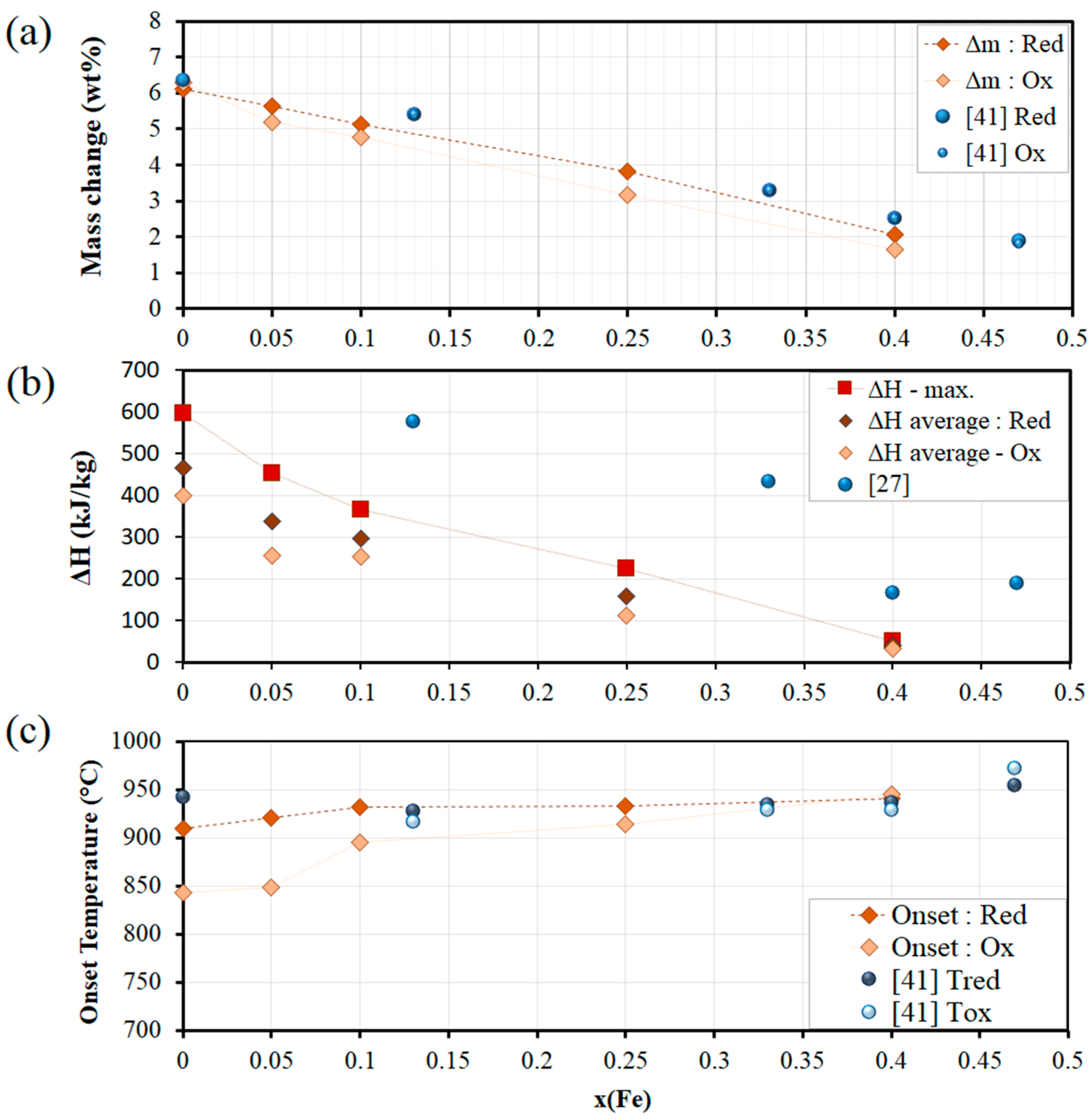
3.1. Co-Fe-O Mixed Oxide System
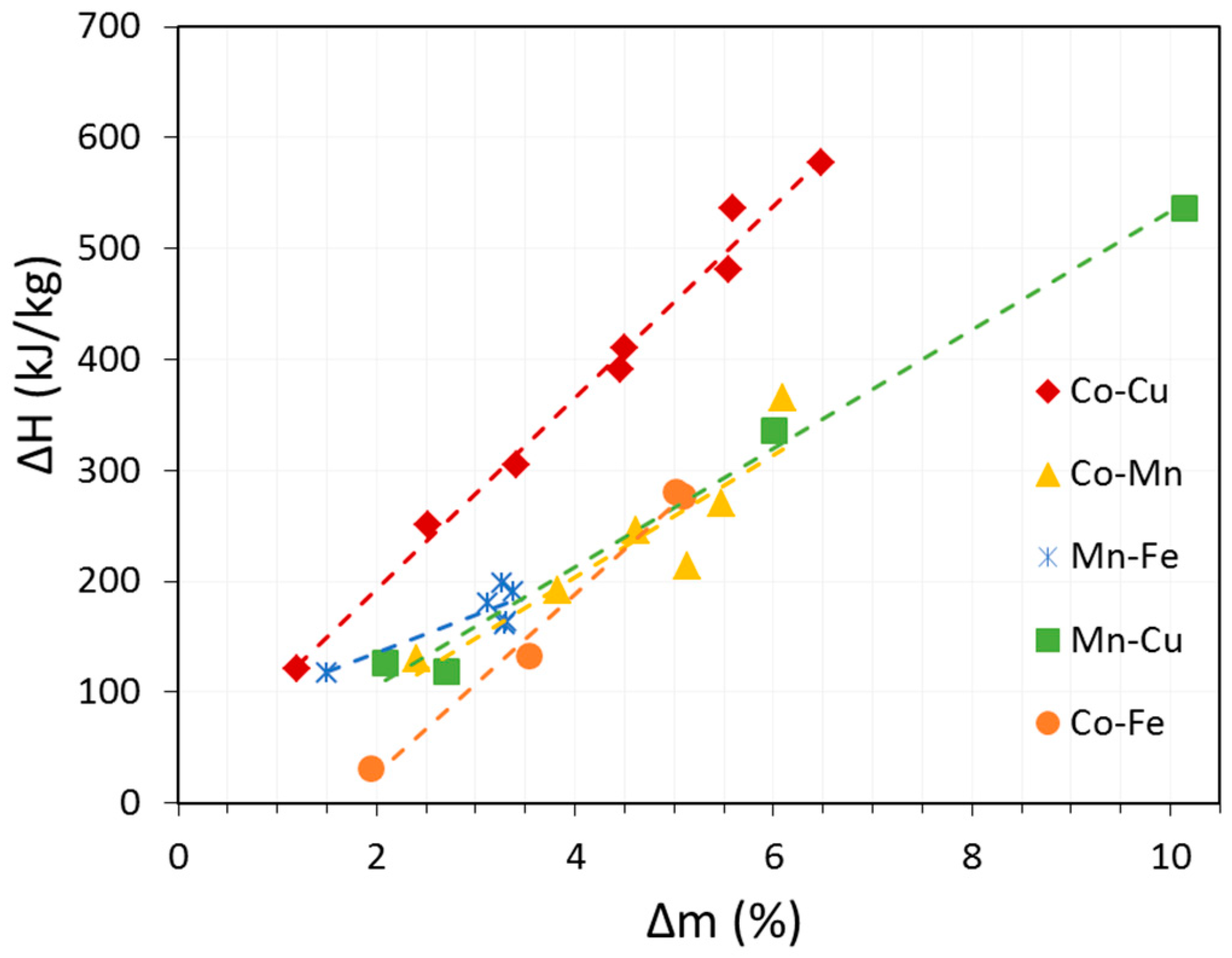
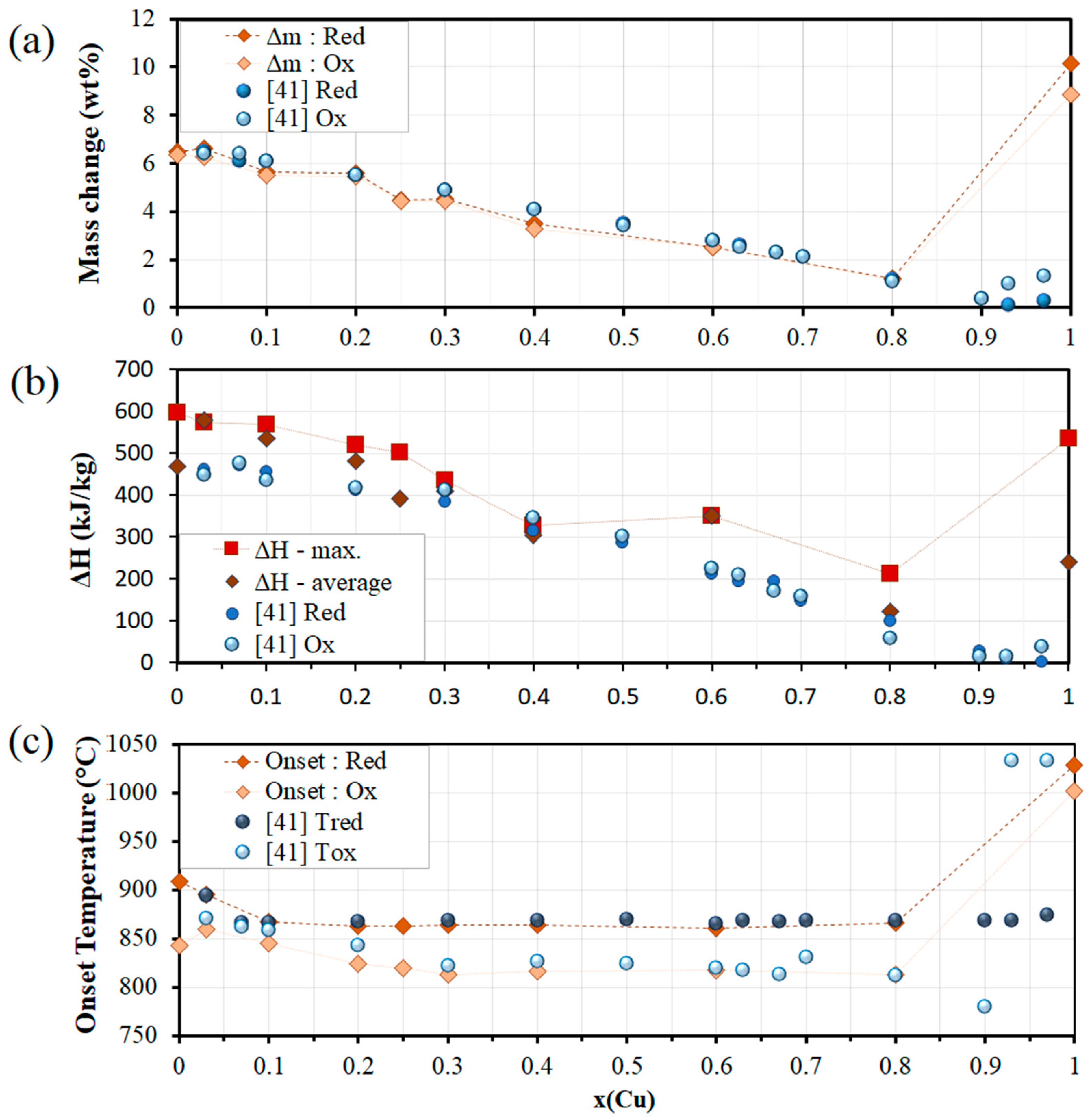
3.2. Co-Cu-O Mixed Oxide System
3.3. Mn-Fe-O Mixed Oxide System
3.4. Mn-Cu-O Mixed Oxide System
3.5. Mn-Co-O Mixed Oxide System
4. Contribution of Thermodynamic Calculations
- Existence of an accurate phase diagram for the pseudo-binary system,
- Existence of thermochemical data such as enthalpy of mixing between phases or heat capacity functions (cp(T)) for all phases of the system,
- Existence of a model established with the Calphad method, allowing equilibrium computations with a dedicated software.
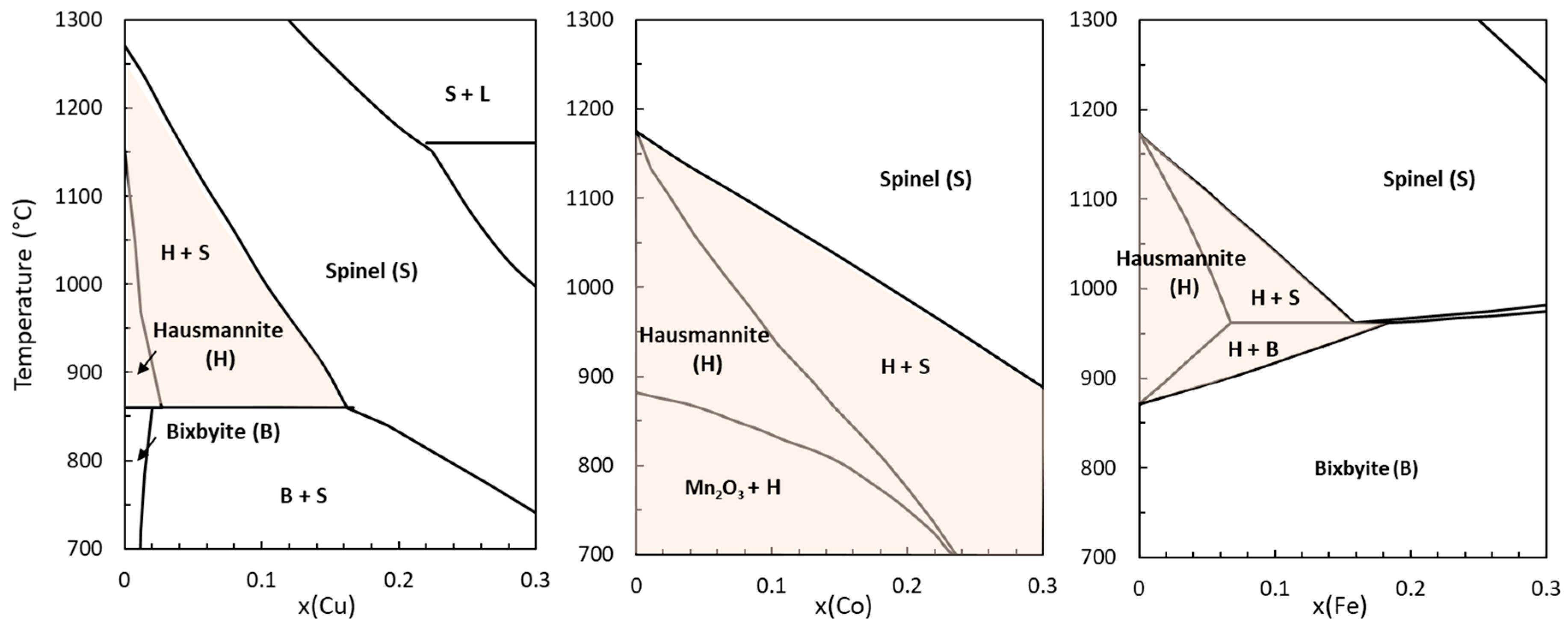
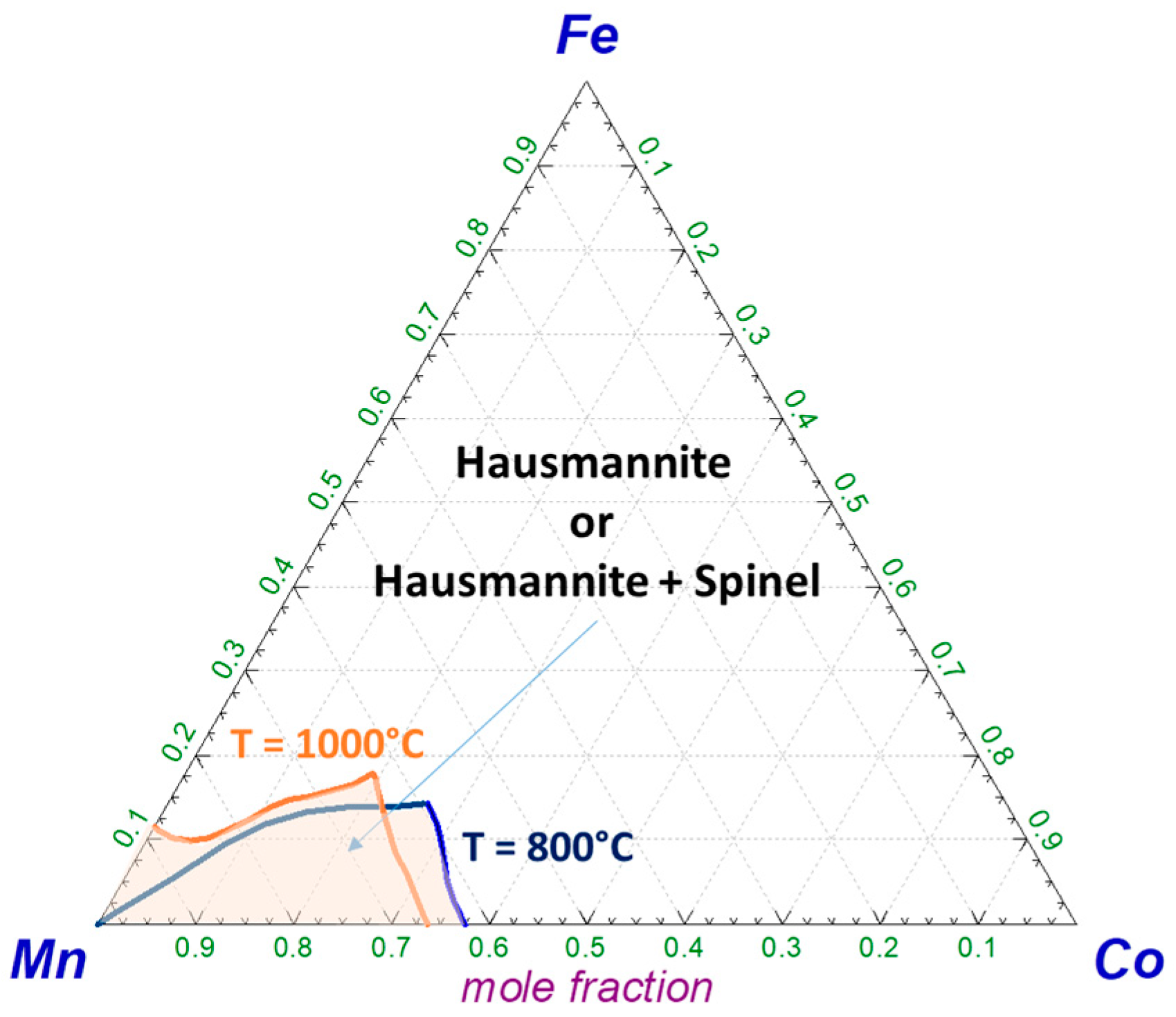
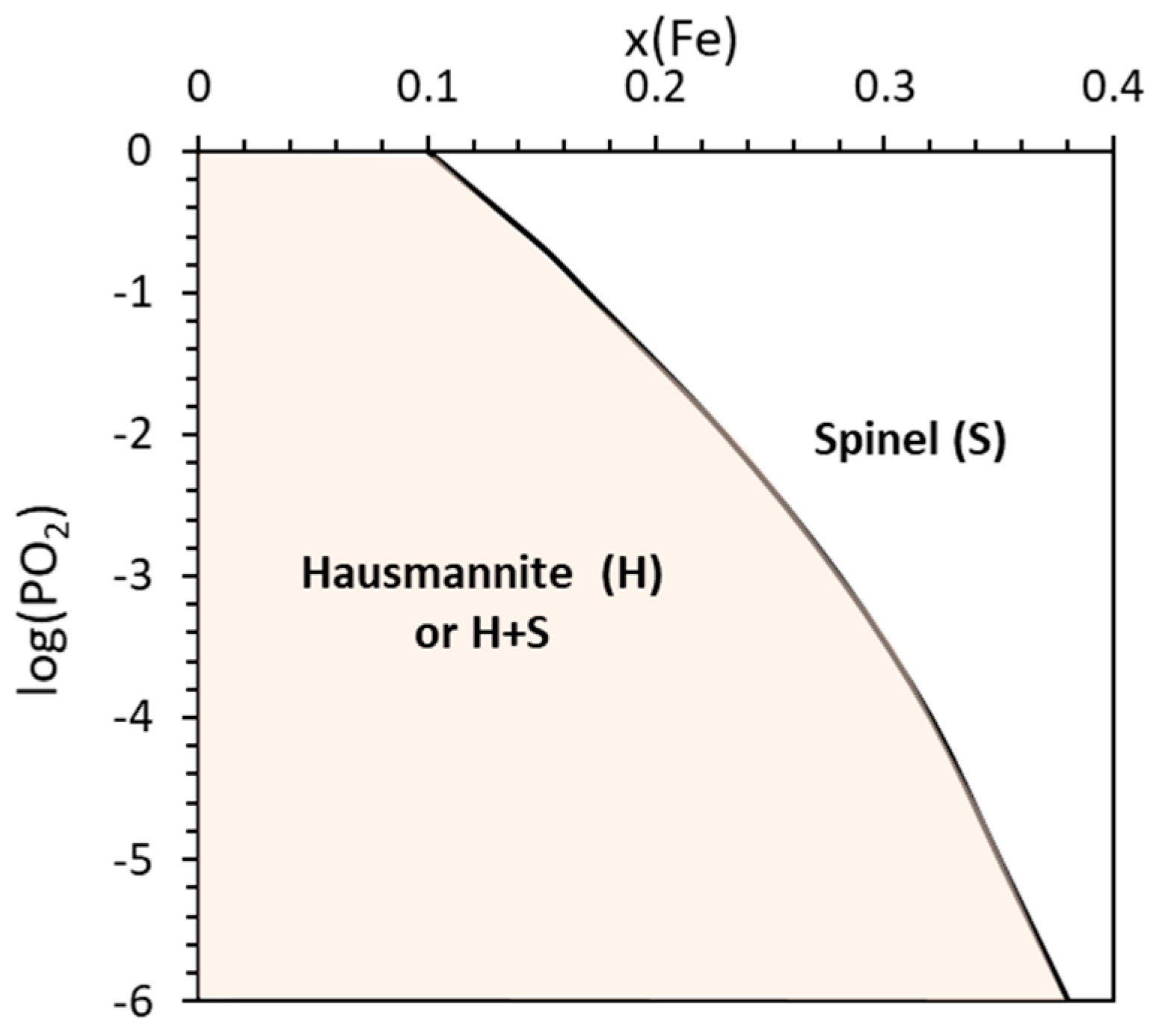
4.1. Hausmannite Phase Boundaries
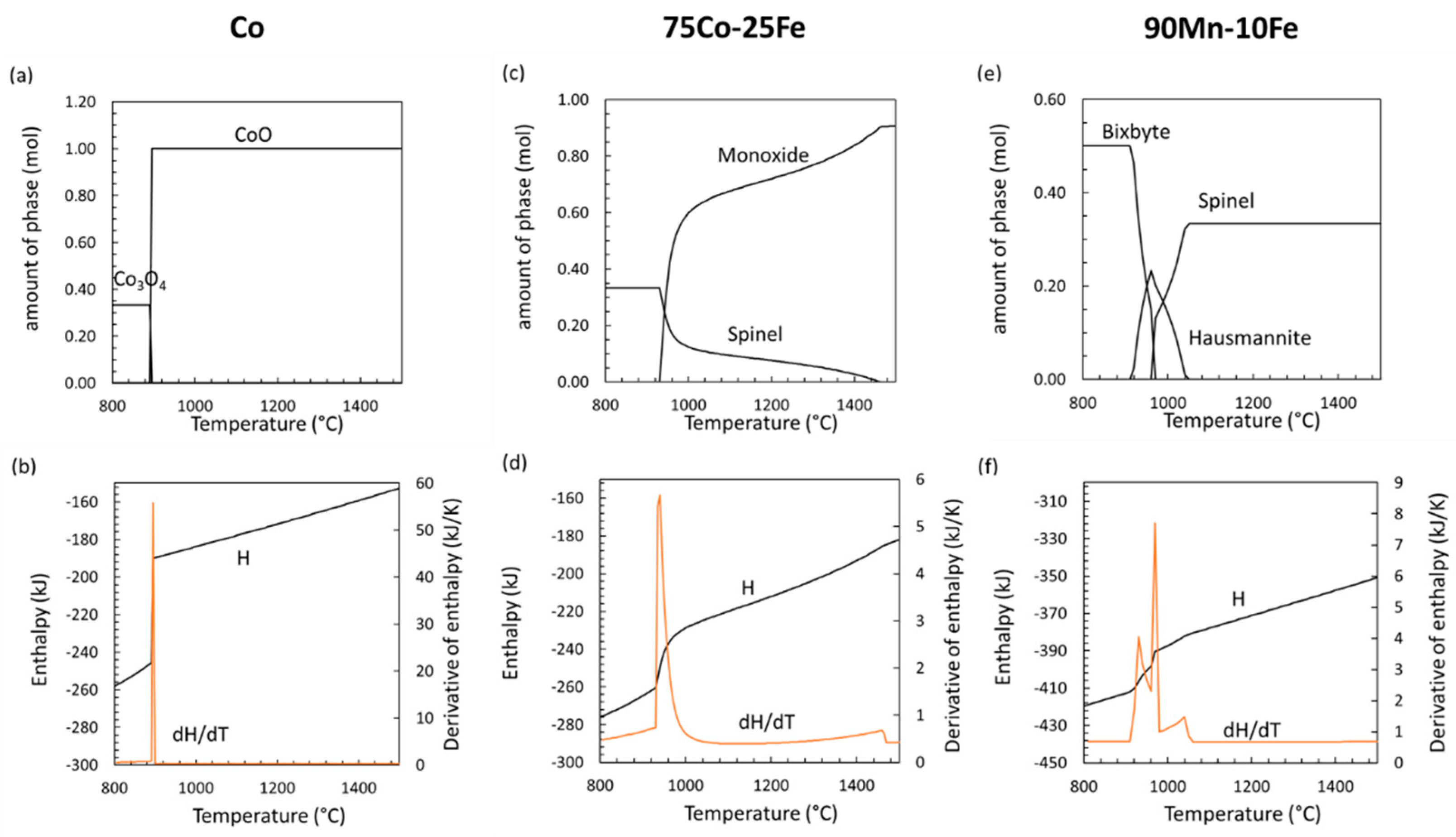
4.2. Phase Transitions in Mixed Oxide Systems
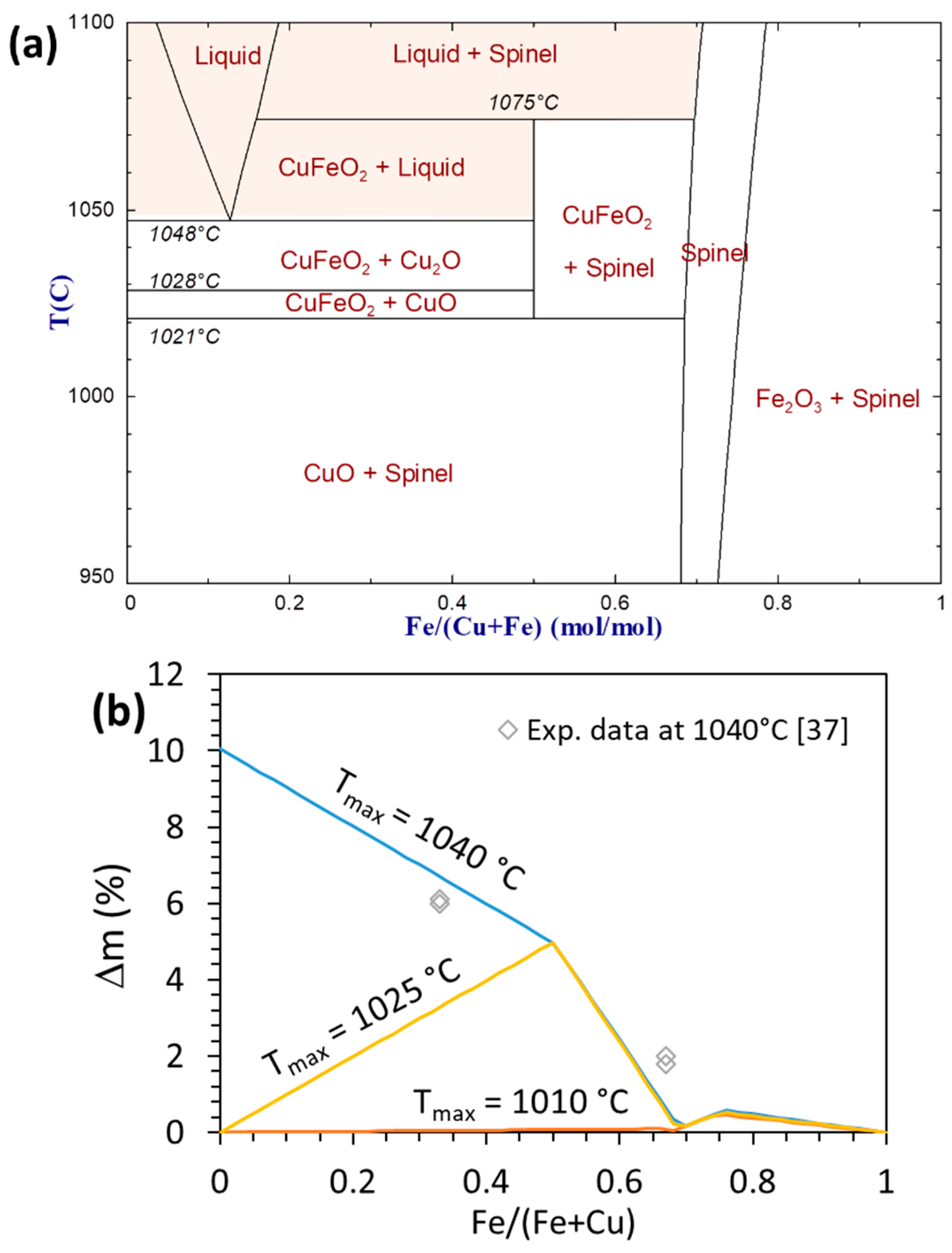
4.3. Calculations in the Cu-Fe-O System
5. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pardo, P.; Deydier, A.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cabassud, M.; Cognet, P. A review on high temperature thermochemical heat energy storage. Renew. Sustain. Energy Rev. 2014, 32, 591–610. [Google Scholar] [CrossRef]
- Yan, T.; Wang, R.Z.; Li, T.X.; Wang, L.W.; Fred, I.T. A review of promising candidate reactions for chemical heat storage. Renew. Sustain. Energy Rev. 2015, 43, 13–31. [Google Scholar] [CrossRef]
- Abedin, A.H. A Critical Review of Thermochemical Energy Storage Systems. Open Renew. Energy J. 2011, 4, 42–46. [Google Scholar] [CrossRef]
- Pelay, U.; Luo, L.; Fan, Y.; Stitou, D.; Rood, M. Thermal energy storage systems for concentrated solar power plants. Renew. Sustain. Energy Rev. 2017, 79, 82–100. [Google Scholar] [CrossRef]
- Ströhle, S.; Haselbacher, A.; Jovanovic, Z.R.; Steinfeld, A. The effect of the gas-solid contacting pattern in a high-temperature thermochemical energy storage on the performance of a concentrated solar power plant. Energy Environ. Sci. 2016, 9, 1375–1389. [Google Scholar] [CrossRef]
- Bayon, A.; Bader, R.; Jafarian, M.; Fedunik-Hofman, L.; Sun, Y.; Hinkley, J.; Miller, S.; Lipiński, W. Techno-economic assessment of solid-gas thermochemical energy storage systems for solar thermal power applications. Energy 2018, 149, 473–484. [Google Scholar] [CrossRef]
- André, L.; Abanades, S.; Flamant, G. Screening of thermochemical systems based on solid-gas reversible reactions for high temperature solar thermal energy storage. Renew. Sustain. Energy Rev. 2016, 64, 703–715. [Google Scholar] [CrossRef]
- Fujii, I.; Tsuchiya, K.; Higano, M.; Yamada, J. Studies of an energy storage system by use of the reversible chemical reaction: CaO + H2O ⇌ Ca(OH)2. Sol. Energy 1985, 34, 367–377. [Google Scholar] [CrossRef]
- Pardo, P.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cognet, P.; Cabassud, M. Ca(OH)2/CaO reversible reaction in a fluidized bed reactor for thermochemical heat storage. Sol. Energy 2014, 107, 605–616. [Google Scholar] [CrossRef]
- Linder, M.; Roßkopf, C.; Schmidt, M.; Wörner, A. Thermochemical energy storage in kW-scale based on CaO/Ca(OH)2. Energy Procedia 2013, 49, 888–897. [Google Scholar] [CrossRef]
- Dai, L.; Long, X.F.; Lou, B.; Wu, J. Thermal cycling stability of thermochemical energy storage system Ca(OH)2/CaO. Appl. Therm. Eng. 2018, 133, 261–268. [Google Scholar] [CrossRef]
- Criado, Y.A.; Huille, A.; Rougé, S.; Abanades, J.C. Experimental investigation and model validation of a CaO/Ca(OH)2 fluidized bed reactor for thermochemical energy storage applications. Chem. Eng. J. 2017, 313, 1194–1205. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, C.Y. First-principle study of CaO/Ca(OH)2 thermochemical energy storage system by Li or Mg cation doping. Chem. Eng. Sci. 2014, 117, 293–300. [Google Scholar] [CrossRef]
- Criado, Y.A.; Alonso, M.; Abanades, J.C. Enhancement of a CaO/Ca(OH)2 based material for thermochemical energy storage. Sol. Energy 2016, 135, 800–809. [Google Scholar] [CrossRef]
- Ortiz, C.; Romano, M.C.; Valverde, J.M.; Binotti, M.; Chacartegui, R. Process integration of Calcium-Looping thermochemical energy storage system in concentrating solar power plants. Energy 2018, 155, 535–551. [Google Scholar] [CrossRef]
- Chen, X.; Jin, X.; Liu, Z.; Ling, X.; Wang, Y. Experimental investigation on the CaO/CaCO3 thermochemical energy storage with SiO2 doping. Energy 2018, 155, 128–138. [Google Scholar] [CrossRef]
- Benitez-Guerrero, M.; Valverde, J.M.; Sanchez-Jimenez, P.E.; Perejon, A.; Perez-Maqueda, L.A. Multicycle activity of natural CaCO3 minerals for thermochemical energy storage in Concentrated Solar Power plants. Sol. Energy 2017, 153, 188–199. [Google Scholar] [CrossRef]
- André, L.; Abanades, S. Evaluation and performances comparison of calcium, strontium and barium carbonates during calcination/carbonation reactions for solar thermochemical energy storage. J. Energy Storage 2017, 13, 193–205. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, C.; Doroodchi, E.; Nellore, R.; Moghtaderi, B. A review on high-temperature thermochemical energy storage based on metal oxides redox cycle. Energy Convers. Manag. 2018, 168, 421–453. [Google Scholar] [CrossRef]
- Bulfin, B.; Vieten, J.; Agrafiotis, C.; Roeb, M.; Sattler, C. Applications and limitations of two step metal oxide thermochemical redox cycles; A review. J. Mater. Chem. A 2017, 5, 18951–18966. [Google Scholar] [CrossRef]
- Alonso, E.; Pérez-Rábago, C.; Licurgo, J.; Fuentealba, E.; Estrada, C.A. First experimental studies of solar redox reactions of copper oxides for thermochemical energy storage. Sol. Energy 2015, 115, 297–305. [Google Scholar] [CrossRef]
- Haseli, P.; Jafarian, M.; Nathan, G.J. High temperature solar thermochemical process for production of stored energy and oxygen based on CuO/Cu2O redox reactions. Sol. Energy 2017, 153, 1–10. [Google Scholar] [CrossRef]
- Hänchen, M.; Stiel, A.; Jovanovic, Z.R.; Steinfeld, A. Thermally driven copper oxide redox cycle for the separation of oxygen from gases. Ind. Eng. Chem. Res. 2012, 51, 7013–7021. [Google Scholar] [CrossRef]
- Clayton, C.K.; Whitty, K.J. Measurement and modeling of decomposition kinetics for copper oxide-based chemical looping with oxygen uncoupling. Appl. Energy 2014, 116, 416–423. [Google Scholar] [CrossRef]
- Alonso, E.; Pérez-Rábago, C.; Licurgo, J.; Gallo, A.; Fuentealba, E.; Estrada, C.A. Experimental aspects of CuO reduction in solar-driven reactors: Comparative performance of a rotary kiln and a packed-bed. Renew. Energy 2017, 105, 665–673. [Google Scholar] [CrossRef]
- Schrader, A.J.; Muroyama, A.P.; Loutzenhiser, P.G. Solar electricity via an Air Brayton cycle with an integrated two-step thermochemical cycle for heat storage based on Co3O4/CoO redox reactions: Thermodynamic analysis. Sol. Energy 2015, 118, 485–495. [Google Scholar] [CrossRef]
- Block, T.; Knoblauch, N.; Schmücker, M. The cobalt-oxide/iron-oxide binary system for use as high temperature thermochemical energy storage material. Thermochim. Acta 2014, 577, 25–32. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Roeb, M.; Schmücker, M.; Sattler, C. Exploitation of thermochemical cycles based on solid oxide redox systems for thermochemical storage of solar heat. Part 2: Redox oxide-coated porous ceramic structures as integrated thermochemical reactors/heat exchangers. Sol. Energy 2015, 114, 440–458. [Google Scholar] [CrossRef]
- Neises, M.; Tescari, S.; de Oliveira, L.; Roeb, M.; Sattler, C.; Wong, B. Solar-heated rotary kiln for thermochemical energy storage. Sol. Energy 2012, 86, 3040–3048. [Google Scholar] [CrossRef]
- Tescari, S.; Agrafiotis, C.; Breuer, S.; De Oliveira, L.; Neises-Von Puttkamer, M.; Roeb, M.; Sattler, C. Thermochemical solar energy storage via redox oxides: Materials and reactor/heat exchanger concepts. Energy Procedia 2013, 49, 1034–1043. [Google Scholar] [CrossRef]
- Singh, A.; Tescari, S.; Lantin, G.; Agrafiotis, C.; Roeb, M.; Sattler, C. Solar thermochemical heat storage via the Co3O4/CoO looping cycle: Storage reactor modelling and experimental validation. Sol. Energy 2017, 144, 453–465. [Google Scholar] [CrossRef]
- Karagiannakis, G.; Pagkoura, C.; Halevas, E.; Baltzopoulou, P.; Konstandopoulos, A.G. Cobalt/cobaltous oxide based honeycombs for thermochemical heat storage in future concentrated solar power installations: Multi-cyclic assessment and semi-quantitative heat effects estimations. Sol. Energy 2016, 133, 394–407. [Google Scholar] [CrossRef]
- Pagkoura, C.; Karagiannakis, G.; Zygogianni, A.; Lorentzou, S.; Kostoglou, M.; Konstandopoulos, A.G.; Rattenburry, M.; Woodhead, J.W. Cobalt oxide based structured bodies as redox thermochemical heat storage medium for future CSP plants. Sol. Energy 2014, 108, 146–163. [Google Scholar] [CrossRef]
- André, L.; Abanades, S. Investigation of metal oxides, mixed oxides, perovskites and alkaline earth carbonates/hydroxides as suitable candidate materials for high-temperature thermochemical energy storage using reversible solid-gas reactions. Mater. Today Energy 2018, 10, 48–61. [Google Scholar] [CrossRef]
- André, L.; Abanades, S.; Cassayre, L. High-temperature thermochemical energy storage based on redox reactions using Co-Fe and Mn-Fe mixed metal oxides. J. Solid State Chem. 2017, 253, 6–14. [Google Scholar] [CrossRef]
- André, L.; Abanades, S.; Cassayre, L. Experimental Investigation of Co–Cu, Mn–Co, and Mn–Cu Redox Materials Applied to Solar Thermochemical Energy Storage. ACS Appl. Energy Mater. 2018, 1, 3385–3395. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Improving the Thermochemical Energy Storage Performance of the Mn2O3/Mn3O4 Redox Couple by the Incorporation of Iron. ChemSusChem 2015, 8, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Karagiannakis, G.; Pagkoura, C.; Zygogianni, A.; Lorentzou, S.; Konstandopoulos, A.G. Monolithic ceramic redox materials for thermochemical heat storage applications in CSP plants. Energy Procedia 2013, 49, 820–829. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Thermochemical heat storage based on the Mn2O3/Mn3O4 redox couple: Influence of the initial particle size on the morphological evolution. J. Mater. Chem. A 2014, 2, 19435–19443. [Google Scholar] [CrossRef]
- Gillot, B.; El Guendouzi, M.; Laarj, M. Particle size effects on the oxidation-reduction behavior of Mn3O4 hausmannite. Mater. Chem. Phys. 2001, 70, 54–60. [Google Scholar] [CrossRef]
- Block, T.; Schmücker, M. Metal oxides for thermochemical energy storage: A comparison of several metal oxide systems. Sol. Energy 2016, 126, 195–207. [Google Scholar] [CrossRef]
- Monazam, E.R.; Breault, R.W.; Siriwardane, R. Kinetics of magnetite (Fe3O4) oxidation to hematite (Fe2O3) in air for chemical looping combustion. Ind. Eng. Chem. Res. 2014, 53, 13320–13328. [Google Scholar] [CrossRef]
- Kozuka, H. Handbook of Sol-Gel Science and Technology; Volume 1: Sol-gel Processing; Sakka, S., Ed.; Springer Science & Business Media: Berlin, Germany, 2005; 680 p. [Google Scholar]
- Carrillo, A.J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Understanding Redox Kinetics of Iron-Doped Manganese Oxides for High Temperature Thermochemical Energy Storage. J. Phys. Chem. C 2016, 120, 27800–27812. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Moya, J.; Bayón, A.; Jana, P.; De La Peña O’Shea, V.A.; Romero, M.; Gonzalez-Aguilar, J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Thermochemical energy storage at high temperature via redox cycles of Mn and Co oxides: Pure oxides versus mixed ones. Sol. Energy Mater. Sol. Cells 2014, 123, 47–57. [Google Scholar] [CrossRef]
- Motuzas, J.; Diniz Da Costa, J.C. Copper aided exchange in high performance oxygen production by CuCo binary oxides for clean energy delivery. J. Mater. Chem. A 2015, 3, 17344–17350. [Google Scholar] [CrossRef]
- Kang, Y.B.; Jung, I.H. Thermodynamic modeling of oxide phases in the Fe–Mn–O system. J. Phys. Chem. Solids 2016, 98, 237–246. [Google Scholar] [CrossRef]
- Adánez-Rubio, I.; Izquierdo, M.T.; Abad, A.; Gayán, P.; de Diego, L.F.; Adánez, J. Spray granulated Cu-Mn oxygen carrier for chemical looping with oxygen uncoupling (CLOU) process. Int. J. Greenh. Gas Control 2017, 65, 76–85. [Google Scholar] [CrossRef]
- Golikov, Y.V.; Tubin, S.Y.; Barkhatov, V.P.; Balakirev, V.F. Phase Diagrams of the Co-Mn-O System in Air. J. Phys. Chem. Solids 1985, 46, 539–544. [Google Scholar] [CrossRef]
- Driessens, F.C.M.; Rieck, G.D. Phase Equilibria in the System Cu-Mn-O. Z. Anorg. Allg. Chem. 1967, 351, 48–62. [Google Scholar] [CrossRef]
- Wei, P.; Bieringer, M.; Cranswck, L.M.D.; Petric, A. In-situ High Temperature X-ray and Neutron Diffraction of Cu-Mn Oxide Phases. J. Mater. Sci. 2010, 45, 1056–1064. [Google Scholar] [CrossRef]
- Shishin, D.; Hidayat, T.; Jak, E.; Decterov, S.A. Critical assessment and thermodynamic modeling of the Cu–Fe–O system. Calphad 2013, 41, 160–179. [Google Scholar] [CrossRef]
- Jacob, K.T.; Fitzner, K.; Alcock, C.B. Activities in the spinel solid solution, phase equilibria and thermodynamic properties of ternary phases in the system Cu-Fe-O. Met. Trans. B 1977, 8, 451–460. [Google Scholar] [CrossRef]
- Zhang, W.-W.; Chen, M. Thermodynamic Modeling of the Co-Fe-O System. Calphad 2013, 41, 76–88. [Google Scholar] [CrossRef]
- Jung, I.-H.; Decterov, S.A.; Pelton, A.D.; Kim, H.-M.; Kang, Y.-B. Thermodynamic Evaluation and Modeling of the Fe-Co-O System. Acta Mater. 2004, 52, 507–519. [Google Scholar] [CrossRef]
- Zabdyr, L.A.; Fabrichnaya, O.B. Phase Equilibria in the Cobalt Oxide-Copper Oxide System. J. Phase Equilib. 2002, 23, 149–155. [Google Scholar] [CrossRef]
- Crum, J.V.; Riley, B.J.; Vienna, J.D. Binary Phase Diagram of the Manganese Oxide-Iron Oxide System. J. Am. Ceram. Soc. 2009, 92, 2378–2384. [Google Scholar] [CrossRef]
- Kjellqvist, L.; Selleby, M. Thermodynamic assessment of the Cr-Mn-O system. J. Alloys Coump. 2010, 507, 84–92. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. FactSage Thermochemical Software and Databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Manganese oxide-based thermochemical energy storage: Modulating temperatures of redox cycles by Fe-Cu co-doping. J. Energy Storage 2016, 5, 169–176. [Google Scholar] [CrossRef]
- Pashkova, E.V.; Novosadvoa, E.B.; Chalyi, V.P.; Antishko, A.N. Phase Diagram of the Manganese, Iron, and Cobalt oxides. Ukr. Chem. J. 1987, 53, 26–29. [Google Scholar]
- Lalanne, M.; Barnabé, A.; Mathieu, F.; Tailhades, P. Synthesis and Thermostructural Studies of a CuFe1−xCrxO2 Delafossite Solid Solution with 0 ≤ x ≤ 1. Inorg. Chem. 2009, 48, 6065–6071. [Google Scholar] [CrossRef] [PubMed]
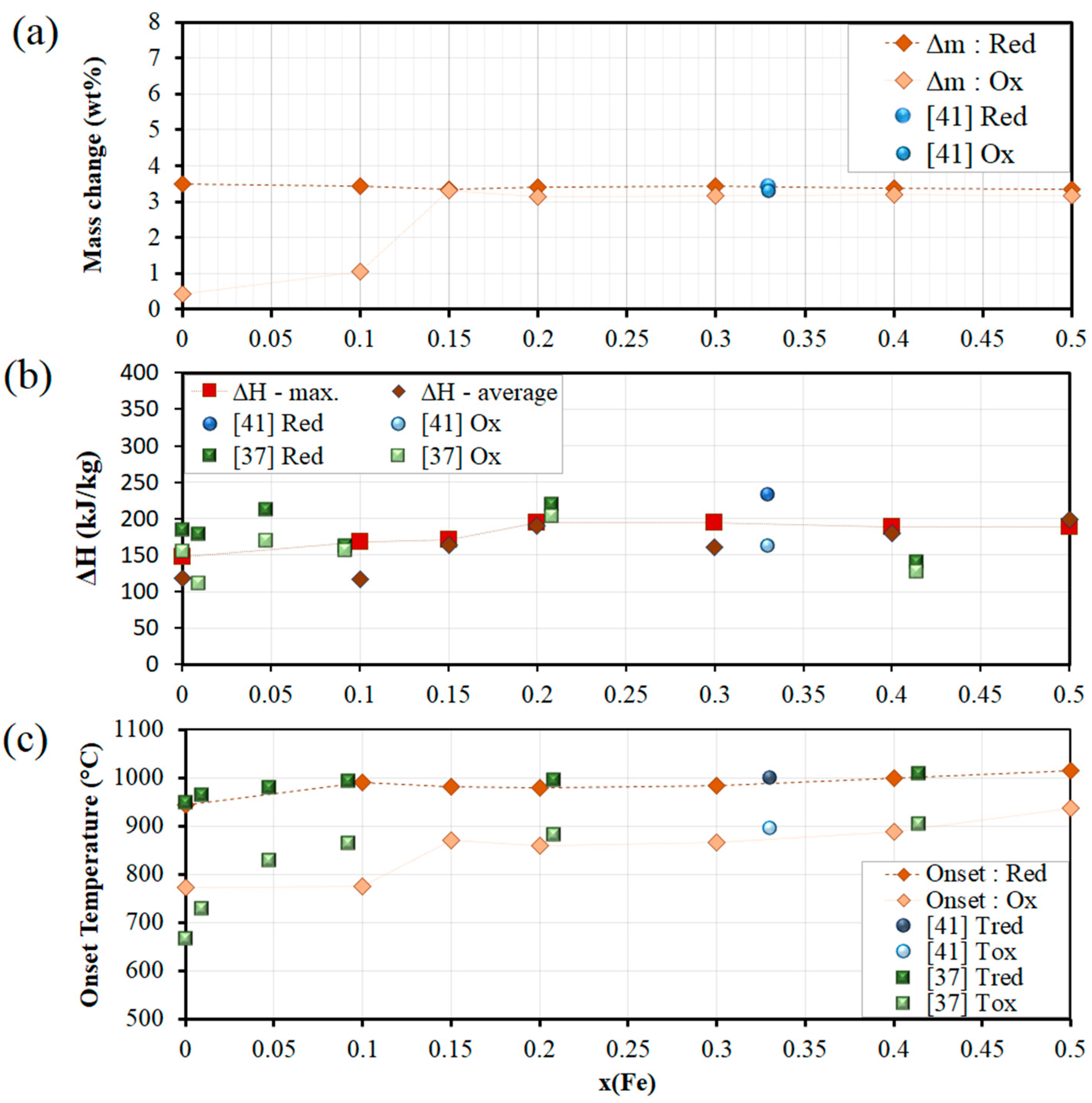
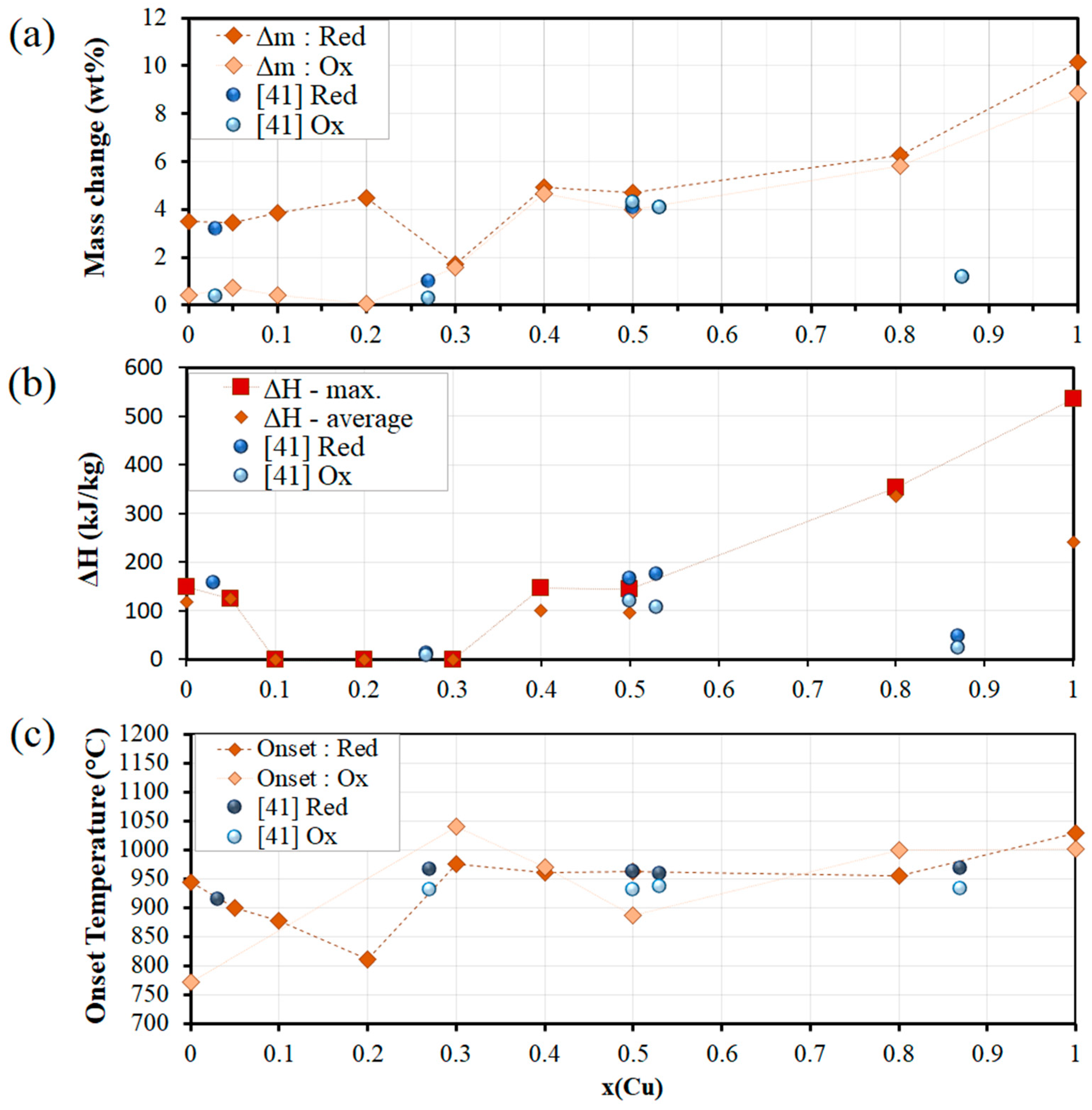
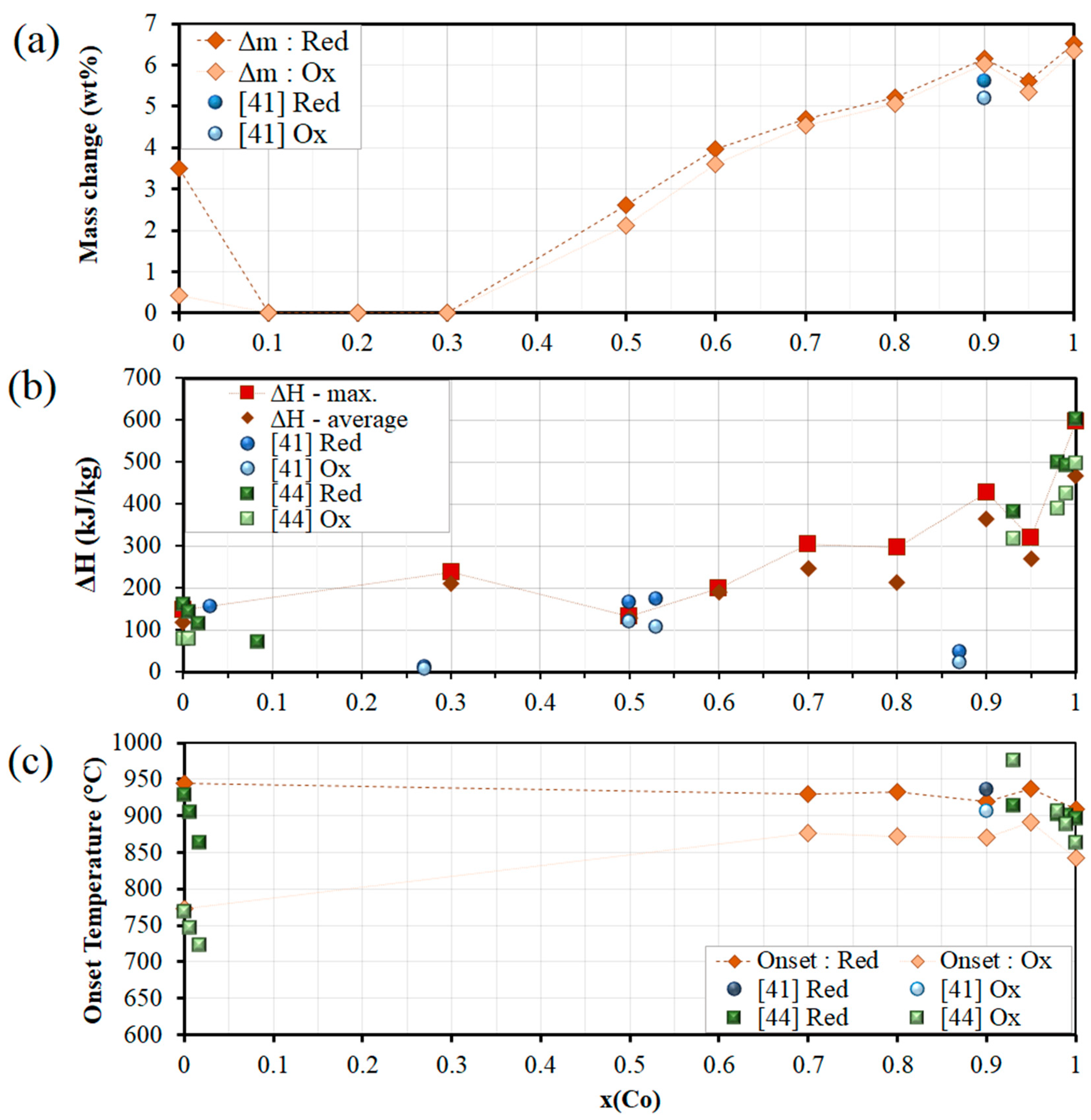
| TCES System | Onset Temperature (°C) | Temperature Gap (°C) | Conversion (%) | Exp. Reaction Enthalpy (kJ/kg) | Phase Transition & Redox Couple | |||
|---|---|---|---|---|---|---|---|---|
| Reduction | Oxidation | |||||||
| Co3O4/CoO | 909 | 843 | 66 | 95.6 | 597 | |||
| Mn2O3/Mn3O4 | 944 | 772 | 172 | 19.2 | 148 | |||
| CuO/Cu2O | 1028 | 1002 | 26 | 87.1 | 536 | |||
| Fe2O3/Fe3O4 | 1391 | 1350 | 41 | 86 | 183 | |||
| Co-Cu-O | x(Cu) | 0.03 | 896 | 860 | 36 | 94.3 | 574 | |
| 0.1 | 867 | 845 | 22 | 97.4 | 570 | |||
| 0.2 | 864 | 824 | 40 | 97.3 | 520 | |||
| 0.25 | 863 | 820 | 43 | 99.7 | 503 | |||
| 0.3 | 864 | 813 | 51 | 97.5 | 436 | |||
| 0.4 | 864 | 816 | 48 | 93 | 327 | |||
| 0.6 | 861 | 818 | 43 | 98.8 | 351 | |||
| 0.8 | 867 | 813 | 54 | 93.4 | 212 | |||
| Co-Fe-O | x(Fe) | 0.05 | 921 | 848 | 73 | 84.4 | 454 | |
| 0.1 | 931 | 896 | 35 | 83.9 | 365 | |||
| 0.25 | 933 | 914 | 19 | 82.4 | 224 | |||
| 0.4 | 941 | 945 | 4 | 82.5 | 51 | |||
| Mn-Co-O | x(Co) | 0.5 | 989 (Ar) | - | - | 64.2 | 133 | |
| 0.6 | 942 (Ar) | - | - | 84.7 | 200 | |||
| 0.7 | 930 | 877 | 53 | 96.7 | 306 | |||
| 0.8 | 933 | 873 | 60 | 96.6 | 296 | |||
| 0.9 | 920 | 871 | 49 | 97.9 | 427 | |||
| 0.95 | 937 | 991 | 54 | 94.9 | 319 | |||
| Mn-Cu-O | x(Cu) | 0.05 | 900 | no re-ox. | - | 21.2 | 126 | |
| 0.1 | 877 | no re-ox. | - | 10.7 | - | |||
| 0.2 | 811 | no re-ox. | - | 1.6 | - | |||
| 0.3 | 976 | 1040 | 64 | 90.8 | - | - | ||
| 0.4 | 960 | 969 | 9 | 96.9 | - | |||
| 0.5 | 962 | 886 | 76 | 88.2 | - | |||
| 0.8 | 954 | 1000 | 46 | 89.7 | 354 | |||
| Mn-Fe-O | x(Fe) | 0.1 | 990 | 774 | 216 | 31 | 168 | |
| 0.15 | 981 | 870 | 111 | 99.1 | 171 | |||
| 0.2 | 980 | 860 | 120 | 98.2 | 194 | |||
| 0.3 | 984 | 866 | 118 | 94.1 | 195 | |||
| 0.4 | 998 | 903 | 95 | 95.3 | 189 | |||
| 0.5 | 1014 | 937 | 77 | 94.7 | 189 | |||
| Oxide System | Phase Diagram | Model |
|---|---|---|
| Cu-Fe-O | Jacob, 1977 [53] | Shishin, 2013 [52] |
| Co-Fe-O | Zhang, 2013 [54] | Jung, 2004 [55] Zhang, 2013 [54] |
| Co-Cu-O | Zabdyr, 2002 [56] | Zabdyr, 2002 [56] |
| Mn-Fe-O | Crum, 2009 [57] | Kjellqvist, 2010 [58] Kang, 2016 [47] |
| Mn-Co-O | Golikov, 1985 [49] | None |
| Mn-Cu-O | Driessens, 1967 [50] Wei, 2009 [51] | None |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
André, L.; Abanades, S.; Cassayre, L. Mixed Metal Oxide Systems Applied to Thermochemical Storage of Solar Energy: Benefits of Secondary Metal Addition in Co and Mn Oxides and Contribution of Thermodynamics. Appl. Sci. 2018, 8, 2618. https://doi.org/10.3390/app8122618
André L, Abanades S, Cassayre L. Mixed Metal Oxide Systems Applied to Thermochemical Storage of Solar Energy: Benefits of Secondary Metal Addition in Co and Mn Oxides and Contribution of Thermodynamics. Applied Sciences. 2018; 8(12):2618. https://doi.org/10.3390/app8122618
Chicago/Turabian StyleAndré, Laurie, Stéphane Abanades, and Laurent Cassayre. 2018. "Mixed Metal Oxide Systems Applied to Thermochemical Storage of Solar Energy: Benefits of Secondary Metal Addition in Co and Mn Oxides and Contribution of Thermodynamics" Applied Sciences 8, no. 12: 2618. https://doi.org/10.3390/app8122618
APA StyleAndré, L., Abanades, S., & Cassayre, L. (2018). Mixed Metal Oxide Systems Applied to Thermochemical Storage of Solar Energy: Benefits of Secondary Metal Addition in Co and Mn Oxides and Contribution of Thermodynamics. Applied Sciences, 8(12), 2618. https://doi.org/10.3390/app8122618