Abstract
The enzymatic system of vegetables is well known as an efficient biocatalyst in the stereoselective reduction of ketones. Therefore, we decided to use the comminuted material of several plants including five vegetables (Apium graveolens L., Beta vulgaris L., Daucus carota L., Petroselinum crispum L., and Solanum tuberosum L.) and three fruits (Malus pumila L. “Golden” and “Kortland” as well as Pyrus communis L. “Konferencja”) to obtain enantiomerically pure carveol, which is commercially unavailable. Unexpectedly, all of the used biocatalysts not only reduced the carbonyl group of (4R)-(–)-carvone and (4S)-(+)-carvone, but also reduced the double bond in the cyclohexene ring. The results revealed that (4R)-(–)-carvone was transformed into (1R, 4R)- and (1S, 4R)-dihydrocarvones, and (1R,2R,4R)-dihydrocarveol. Although the enzymatic system of the potato transformed the substrate almost completely, the %de was not the highest. Potato yielded 92%; however, when carrot was used as the biocatalyst, it was possible to obtain 17% of (1R, 4R)-(+)-dihydrocarvone with 100% diastereomeric excess. In turn, the (4S)-(+)-carvone was transformed, using the biocatalysts, into (1R, 4S)- and (1S, 4S)-dihydrocarvones and dihydrocarveols. Complete substrate conversion was observed in biotransformation when potato was used. In the experiments using apple, (1R, 4S)-dihydrocarvone with 100% diastereomeric excess was obtained. Using NMR spectroscopy, we confirmed both diastereoisomers of 4(R)-1,2-dihydrocarveols, which were unseparated in the GC condition. Finally, we proved the high usefulness of vegetables for the biotransformation of both enantiomers of carvone as well as dihydrocarvone.
1. Introduction
Carvone (p-mentha-6,8-dien-2-one) is one of the most frequently cited examples of odoriferous compounds where both enantiomers differ in smell. The (4R)-(–)-carvone (1) with a mint odor is a component of the oil of Mentha viridis and Mentha spicata. Next to (1), dihydrocarveol, cis-dihydrocarvone (2) are also present in the latter essential oil [1]. In turn, (4S)-(+)-carvone (6) has a caraway fragrance and is found in essential oil obtained from Carum carvi L. and Anethum graveolens L. [2]. As a scented compound, carvone is used in the production of cosmetics, toothpastes, and chewing gum [3]. It also has a number of interesting biological activities such as anticancer [4,5] and free radical scavenging activities [6]. It may also be a potential inhibitor of acetylcholinesterase (AChE) [7].
Both carvone isomers are used as the starting material in the synthesis of many other biologically active compounds such as dihydroagarofuran and eudesmane sesquiterpenoids [8], guiaiane sesquiterpenes [9], polyoxygenated atisane-type and spongiane-type diterpenoids [10], 8,14-secosteroids [3], nemorensic acid [11], and cryptophycin A [12]. Often, the first step in their synthesis is a stereoselective reduction of the double bond.
In order to obtain other odoriferous compounds, carvone has also been biotransformed using a variety of biocatalysts. During microbiological and plant transformations, the formation of reaction products where the carbonyl group or double bond is reduced in addition to hydroxylation on the inactivated carbon atom is observed [13,14].
To transform carvone, the following plant fragments were used: roots of Manihot esculenta Crantz and Manihot dulcis Crantz [15], sugar cane juice from Saccharum officinarum L. [16], and coconut water from Cocos nucifera L. [17]. Only the reduction of carvone to carveol was observed, and the degree of substrate conversion did not exceed 15%. Therefore, we decided to choose new vegetables and fruits as biocatalysts for obtaining enantiomerically pure carveol, which is commercially unavailable.
Plant biocatalysts are adept at carrying out a number of reactions, including the reduction of aldehydes, ketones [18,19], and ketoesters [20] as well as the oxidation of alcohols or the hydrolysis of acetates. Besides carrots, the active reagents used in the abovementioned reactions were celeriac, potatoes, Jerusalem artichoke [21], apples, onions, and others. It is not known what types of enzymes are involved in these reactions. Considering the wide range of obtainable, taxonomically different plants, in the future, it would be feasible to use biocatalysts for practically every reaction. The general benefits of plants as reagents are that they are easily disposed because they are biodegradable, they have mild reaction conditions, and are available at low cost.
Thus far, little information about the transformation of carvone using fragmented vegetables is available in the literature; to address this research gap, the biotransformation of both carvone isomers via the comminuted plant material of five vegetables and three fruits is presented in this paper.
2. Materials and Methods
2.1. Biotransformation
2.1.1. Biocatalyst
For the biotransformation of carvone, five vegetables and three fruits were selected: Daucus carota L. (carrot), Petroselinum sativum Hoffm. (parsley), Apium graveolens L. var. rapaceum (celeriac), Solanum tuberosum L. (potato), Beta vulgaris L. (beetroot), Malus pumila L. cv. “Golden”, Malus pumila L. cv. “Kortland” (apple), and Pyrus communis L. cv. “Konferencja” (pear). The biocatalysts were purchased at a local store and were identified by our botanist. Vouchers were deposited in the herbarium of Wroclaw University of Environmental and Life Sciences.
In order to ensure optimal conditions for the enzymes of the intracellular biocatalyst to carry out the reaction, a phosphate buffer with a pH close to the natural juice of the vegetable or fruit was used (Table 1).

Table 1.
The pH values of natural cell juice for the selected bioreagents.
2.1.2. Procedure for Screening Biotransformation
A total of 50 mL of phosphate buffer at the appropriate pH comparable to the pH of cell juice (Table 1) was placed in a 300-mL Erlenmeyer flask, where 20 mL of the comminuted biocatalyst was suspended. Then, 20 μL of the substrate dissolved in 200 μL of acetone was added to each flask and shaken (23 °C, 130 rpm). After 48 h of biotransformation, the samples were extracted with 50 mL of chloroform. The post-reaction mixture was centrifuged for 10 min at 6000 rpm. The collected chloroform layer was dried with anhydrous MgSO4. After the evaporation of the solvent, the composition of the reaction mixture was identified by gas chromatography and the structures of the obtained products were confirmed by spectroscopic methods.
Each biotransformation was carried out in duplicate. In addition, control samples (buffer + biocatalyst) were performed to eliminate the influence of plant-derived metabolites on the interpretation of the results obtained.
2.1.3. Procedure for Preparative Biotransformation
Preparative biotransformations were executed in the same way as the screening. Fresh, healthy, and undamaged vegetables and fruits were ground after washing. For each of the 10 300-mL Erlenmeyer flasks, 20 mL of the ground catalyst in 50 mL of phosphate buffer was added and 20 μL of the substrate dissolved in 200 μL of acetone was included in each of the samples. After 48 h, the transformation mixture was extracted with 50 mL of chloroform. The chloroform layer was then carefully collected and dried using anhydrous MgSO4. The resulting solution was filtered and concentrated in a vacuum evaporator.
The structures of the products obtained were determined on the basis of the physicochemical data:
(1R, 4R)-(+)-dihydrocarvone (2) (Figures S1–S3) 1H NMR (CDCl3): δ 1.04 (d, JHH = 6 Hz, 3H, 7-CH3), 1.38 (qd, JHH = 3.5 and 13 Hz, 1H, 6a-CH2), 1.63 (qd, JHH = 4 and 11 Hz, 1H, 5a-CH2), 1.75 (s, 3H, 9-CH3), 1.94 (m, 1H, 5b-CH2), 2.13 (m, 1H, 6b-CH2), 2.27 (m, 1H, 3a-CH2), 2.33 (m, 1H, 4-CH), 2.35 (m, 1H, 1-CH), 2.45 (m, 1H, 3b-CH2), 4.74 (brs, 1H one proton of 10->C=CH2), 4.76 (brs, 1H, one proton of =CH2) 13C NMR (CDCl3): 14.35 (C-7), 20.45 (C-9), 30.78 (C-5), 34.92 (C-6), 44.75 (C-4), 46.88 (C-1), 47.03 (C-3), 109.62 (C-10), 147.63 (C-8), 212.64 (C-2).
(1R,2S,4R)-neodihydrocarveol (5) (Figures S4–S6) 1H NMR (CDCl3): 0.98 (d, JHH = 6.8 Hz, 3H, 7-CH3), 1.23 (dddd, JHH = 12.8, 12.4, 4.5 and 3.2 Hz,1H, 5a-CH2), 1.42 (m, 1H, 3a-CH2), 1.44 (m, 2H, 6-CH2), 1.53 (m, 1H, 1-CH), 1.74 (t, JHH = 1.0 Hz, 3H, 9-CH3), 1.77 (dddd, JHH = 12.8, 3.5, 3.3 and 3.2 Hz, 1H, 5b-CH2), 1.93 (ddd, JHH = 13.6, 3.3 and 3.2 Hz, 1H, 3b-CH2), 2.28 (tt, JHH = 12.4 and 3.2 Hz, 1H, 4-CH), 3.91 (m, 1H, 2-CH), 4.71 (brs, 2H, 10->C=CH2) 13C NMR (CDCl3): 18.32 (C-9), 20.97 (C-7), 28.14 (C-6), 31.40 (C-5), 36.07 (C-1), 37.82 (C-4), 38.67 (C-3), 71.01 (C-2), 108.4 (C-10), 150.3 (C-8).
(1R,2R,4R)-dihydrocarveol (4) (Figure S7)1HNMR (CDCl3): 1.05 (d, JHH = 6.8 Hz, 3H, 7-CH3), 1.24–1.62 (m, 7H, 1-CH, 3-CH2, 5-CH2, 6-CH2) 1.74 (brs, 3H, 9-CH3), 2.15 (m, 1H, 4-CH), 3.20 (ddd, JHH = 10.0, 10.0 and 4.0 Hz, 1H, 2-CH), 4.72 (brs, 2H, 10->C=CH2).
2.1.4. Investigation of Biotransformation over Time
In order to verify the course of the biotransformation over time, a suitable number of flasks with biocatalysts were prepared as described above in the screening procedure. The reactions were terminated after 1, 2, 3, 5, 22, 23, 26, 28, 45, and 48 h in the case of (4R)-(–)-carvone (1) and after 2, 4, 6, 8, 10, 12, 24, 28, and 48 h for (4S)-(+)-carvone (6).
2.2. Analysis
Analytical thin-layer chromatography (TLC) was performed on silica gel-coated aluminum plates (DC-Alufolien Kieselgel 60 F254, Merck, Darmstadt, Germany) with a mixture of hexane and acetone in various ratios. Compounds were detected by spraying the plates with 20% ethanolic H2SO4 containing 0.1% of anisaldehyde, followed by heating to 120 °C. Preparative column chromatography was performed on silica gel (Kieselgel 60, 230–400 mesh ASTM, Merck) with a mixture of hexane and acetone (20:1) as the eluent. Gas chromatography (GC) analysis was carried out on an Agilent Technologies 6890N instrument (H2 as the gas carrier) using a DB-17 column (cross-linked methyl silicone gum, 30 m × 0.32 mm × 0.25 µm) and the following temperature program: 110 °C for 7 min, 30 °C/min to 280 °C (hold for 1 min); injector temperature 200 °C; detector temperature 280 °C, or on an Varian CP3380 instrument (H2 as gas carrier) using the capillary columns of Thermo TR-5 (30 m × 0.32 mm × 1.0 µm) and the following temperature program: 115 °C for 7 min, 3 °C/min to 120 °C, 30 °C/min to 300 °C (hold for 1 min); injector temperature 200 °C; and detector temperature 300 °C. The structures of the obtained compounds were also confirmed by gas chromatography-mass spectrometry (GC-MS) analysis using a Varian SATURN 2000 instrument (electron ionization (EI), He as the carrier gas) with an HP-1 column (cross-linked methyl silicone gum, 25 m × 0.32 mm × 0.52 µm) under the following conditions: injector 200 °C, detector 300 °C, column temperature: 120 °C (hold for 2 min), 120–300 °C (rate 20 °C/min), 300 °C (hold for 3 min).
Optical rotations were determined on a P-2000 polarimeter (Jasco) in chloroform solutions, and concentrations are denoted in g/100 mL.
NMR spectra were recorded in a CDCl3 solution on a Bruker AvanceTM 600 MHz spectrometer. IR spectra were recorded on a Thermo-Nicolet IR300 FT-IR spectrometer.
3. Results
3.1. Biotransformation of (4R)-(–)-Carvone (1)
The main purpose of our work was to verify and determine the ability of comminuted vegetable bioreagents to reduce α,β-unsaturated ketones. Consequently, both enantiomers of carvone were selected for biotransformation.
At the beginning, (4R)-(–)-carvone (1) was subjected to biotransformation by means of five vegetables and three fruits. Based on the literature [15,16,17], we expected carveol as a biotransformation product, but we observed a mixture of products in each sample. Another course of biotransformation could lead to the formation of dihydrocarvone after the reduction of the double bond in the carvone ring. Commercial (+)-dihydrocarvone is available in the form of a mixture of two isomers: (1R, 4R)-(+)-dihydrocarvone (2) and (1S, 4R)-(+)-dihydrocarvone (3). Hence, to assign a given signal to a particular isomer, they were separated by column chromatography. GC analysis (Figure 1) and NMR data allowed us to determine that the transformation products were (1R, 4R)-(+)-dihydrocarvone, (1S, 4R)-(+)-dihydrocarvone, and dihydrocarveol.
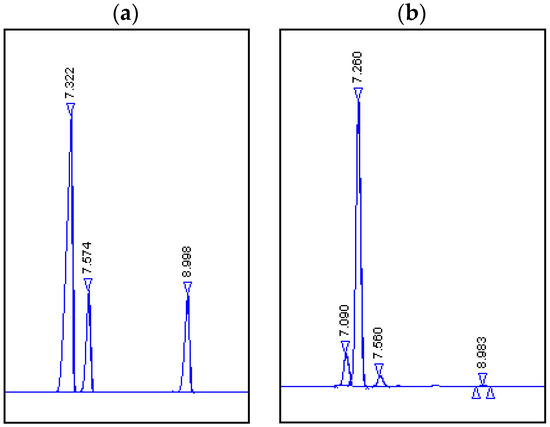
Figure 1.
Chromatograms. (a) Products obtained by biotransformation using potato: Rf = 7.090, (1R,2R,4R)-dihydrocarveol (4); Rf = 7.260, (1R, 4R)-(+)-dihydrocarvone (2); Rf = 7.560, (1S, 4R)-(+)-dihydrocarvone (3); unreacted substrate Rf = 8.983, (4R)-(–)-carvone (1). (b) Standards: Rf = 7.322, (1R, 4R)-(+)-dihydrocarvone (2); Rf = 7.574 (1S, 4R)-(+)-dihydrocarvone (3); Rf = 8.998, (4R)-(–)-carvone (1).
Based on these results, it was found that (4R)-(–)-carvone (1) was primarily reduced to (1R, 4R)-(+)-dihydrocarvone (2), which was slightly reduced to alcohol 4 (Scheme 1). A comparison of the post-reaction chromatograms and standards indicated that during the reduction of the double bond in (4R)-(–)-carvone, more (1R, 4R)-(+)-dihydrocarvone (2) than (1S, 4R)-(+)-dihydrocarvone (3) was formed.
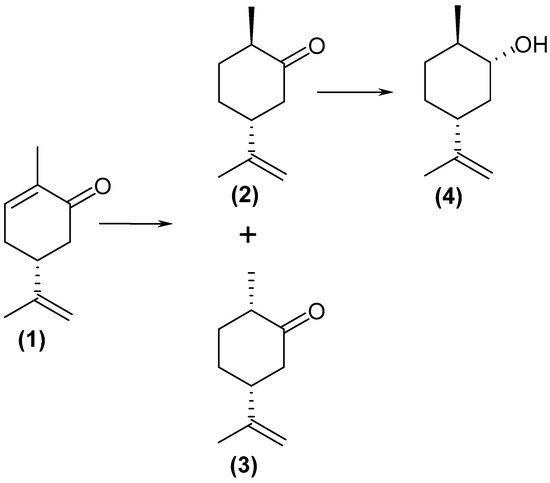
Scheme 1.
Scheme of the reduction reactions of (4R)-(–)-carvone (1).
On the basis of the GC chromatograms, we determined the percentage composition of the reaction mixtures obtained after the biotransformation of (4R)-(–)-carvone (1). The results are recorded in Table 2.

Table 2.
Results of the biotransformation of (4R)-(–)-carvone (1).
The acquired results indicated that the degree of substrate conversion depended on the type of bioreagent used. It can be seen that the enzymatic system of vegetables was more effective in the transformations of (4R)-(–)-carvone (1) than the fruits used in our experiments. The comminuted potato pulp proved to be the most effective biocatalyst, since an almost complete conversion of the substrate was observed at 99.7%. A relatively high degree of conversion was also noted using celeriac for transformation, which proved to be the most effective biocatalyst from the plants belonging to the Umbelliferous family (carrots and parsley) used for biotransformation. Among the fruits used, the best biocatalyst was pear, with 50.7% substrate conversion. The apple enzymatic system was proven to be essentially incapable of bioreduction (1). It is worth noting that although potato transformed (4R)-(–)-carvone (1) in the most effective way, the %de was not the highest. Potato yielded 92% while pear yielded 97%, and even 100% diastereomeric excess was observed with carrots.
Verifying the Biotransformation of (4R)-(–)-Carvone (1) over Time Using Potato as a Biocatalyst
Based on the results obtained during the screening biotransformations, the shredded potato tubers were selected as a biocatalyst to determine the order of reactions occurring during biotransformation. The biotransformation process was carried out under conditions analogous to those employed in the screening procedure. The samples were collected after 1, 2, 3, 5, 22, 23, 26, 28, 45, and 48 h. The results obtained are shown in Figure 2.
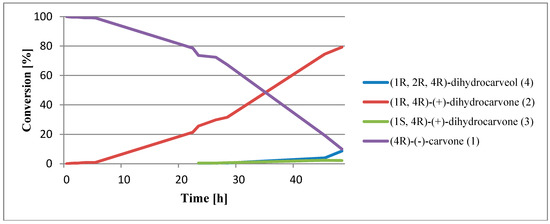
Figure 2.
Biotransformation of 4R)-(–)-carvone (1) via a comminuted potato.
Analyzing the results, it was found that by far the largest loss of substrate occurred on the second day of biotransformation. A steady decrease in the content of (4R)-(–)-carvone (1) may indicate a stable level of oxidoreductases during biotransformation.
Due to the fact that the yield of produced dihydrocarveol was low and insufficient for detailed NMR analysis after compound purification by column chromatography, we decided to biotransform trans-(+)-dihydrocarvone (2), which was one of the products formed.
3.2. Biotransformation of (1R, 4R)-(+)-Dihydrocarvone (2)
As mentioned before, (+)-dihydrocarvone is not available as a pure diastereoisomer, but in the form of two cis and trans isomers. Using the Aldrich catalog description, it is known that the ratio between trans and cis isomers is 70:30, which can additionally be confirmed by the following chromatogram made from a commercially available mixture of (+)-dihydrocarvone (Figure 3).
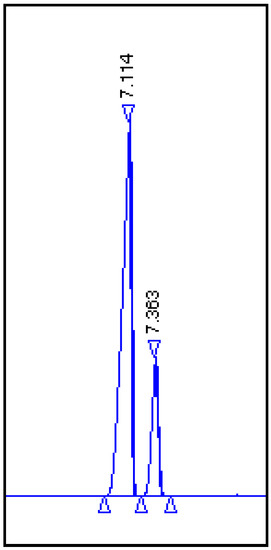
Figure 3.
Chromatogram of the (+)-dihydrocarvone mixture of two diasteoisomers: trans (1R, 4R)-(+)-dihydrocarvone (2) with Rf = 7.114 and cis (1S, 4R)-(+)-dihydrocarvone (3) with Rf = 7.363.
The diastereomers were separated by column chromatography using a ratio of hexane:acetone (20:1) as an eluent. After purification, the specific rotation of (1R, 4R)-(+)-dihydrocarvone (2) was measured and compared to the literature ( = +14.6 CHCl3, c = 1.0; lit [22]; = 14.2, CHCl3, c = 0.8).
The obtained pure (1R, 4R)-(+)-dihydrocarvone (2) was biotransformed using previously selected plant biocatalysts. The composition of the reaction mixture was determined by comparing the chromatograms of the standards and the reaction mixtures.
The results obtained during biotransformation are summarized in Table 3. This evidence supports the conclusion that the substrate was transformed by each of the catalysts used. In contrast to the biotransformation of (4R)-(–)-carvone (1) described above, the enzymatic system of potato turned out to be a poor biocatalyst in the reduction of (1R, 4R)-(+)-dihydrocarvone (2). The largest loss of substrate was observed using the carrot enzymatic system as a bioreagent.

Table 3.
Results of the (1R, 4R)-(+)-dihydrocarvone (2) biotransformation.
As Daucus carota emerged as the best biocatalyst, a preparative biotransformation was carried out, which would allow us to attain a greater amount of product for spectroscopic investigation. The chromatograms (Figure 4) demonstrate that (1R, 4R)-(+)-dihydrocarvone (2) was converted to alcohol. After the separation of the reaction mixture into its components by column chromatography, however, it appeared that two diastereomeric alcohols, (4) and (5), were the reaction products. These alcohols were visible in the GC analysis as one signal and, despite numerous attempts, we were not able to separate them.
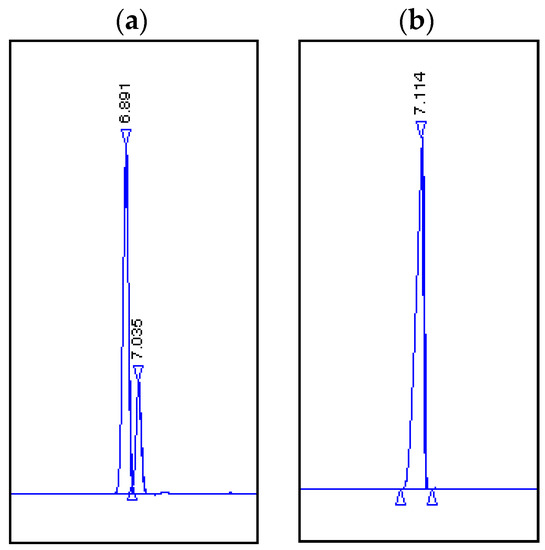
Figure 4.
Chromatograms. (a) Products obtained by biotransformation using carrots: Rf = 6.891, (1R,2R,4R)-dihydrocarveol (4) and (1R,2S,4R)-neodihydrocarveol (5); unreacted substrate: Rf = 7.035, (1R, 4R)-(+)-dihydrocarvone (2). (b) Standard: Rf = 7.114 (1R, 4R)-(+)-dihydrocarvone (2).
3.3. Biotransformation of (4S)-(+)-Carvone (6)
(4S)-(+)-carvone (6) was converted to (1R, 4S)-dihydrocarvone (7), (1S, 4S)-dihydrocarvone (8), and dihydrocarveols (9) and (10) (Scheme 2). Thus, as with the reduction of (4R)-(–)-carvone (1) described above, the reduction of the double bond followed by the carbonyl group was detected.
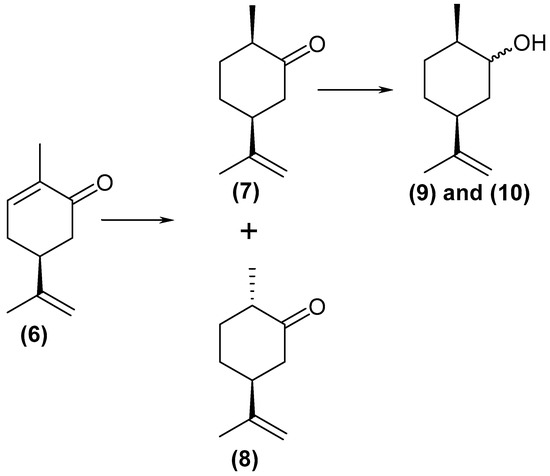
Scheme 2.
Scheme of the (4S)-(+)-carvone (6) reduction reaction.
Chromatograms of the standards and products obtained by biotransformation using a ground potato are shown in Figure 5. Products (7) and (8) were identified as (1R, 4S)-dihydrocarvone (7) and (1S, 4S)-dihydrocarvone (8) by means of gas chromatography by comparing the Rf values with the standards.
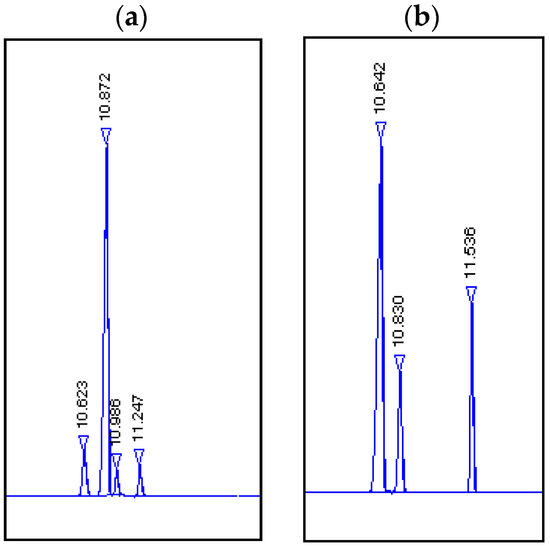
Figure 5.
Chromatograms. (a) Products obtained by biotransformation using potato: Rf =10.623, (1S,4S)-dihydrocarvone (8); Rf =10.872 (1R,4S)-dihydrocarvone (7); Rf =10.986 dihydrocarveol (9); Rf =11.247 dihydrocarveol (10). (b) Standards: Rf =10.642, (1S,4S)-dihydrocarvone, Rf =10.830, (1R,4S)-dihydrocarvone; Rf =11.536 (4S)-(+)-carvone (6).
Moreover, to confirm their structure, we attempted to separate them using column chromatography. By using eluents with a different composition, it was not possible to separate the carvone from the products (7) and (8) being formed. In addition to dihydrocarvons, alcohols (9) and (10) were also obtained by the stereospecific reduction of the carbonyl group. Unfortunately, due to the fact that they formed in minute amounts, they could not be isolated, thus their structures were indeterminable.
The results detected via GC chromatograms are displayed in Table 4.

Table 4.
Results of the biotransformation of (4S)-(+)-carvone (6).
Similar to (4R)-(–)-carvone (1), the biotransformation of (4S)-(+)-carvone (6) took place with each of the applied biocatalysts and a higher degree of conversion was observed when the enzymatic system from vegetables was used for the reaction. Furthermore, another common feature of the bioreduction process for both carvone enantiomers is that the comminuted potato resulted in a complete reaction of the substrate, and a high diasteroisomeric purity of 82%. Attractive biocatalysts turned out to be an apple of the “Kortland” variety and a pear, at which a 100% diastereomeric excess was seen with a relatively high degree of conversion. Celeriac was not as effective as a bioreagent as in the transformation of (4R)-(–)-carvone (1).
Verifying the Biotransformation of (4S)-(+)-Carvone (6) over Time Using Potato as a Biocatalyst
To determine the course of the biotransformation of (4S)-(+)-carvone (6) catalyzed by the enzymatic system of higher plants, shredded potato tubers were used as a biocatalyst. The samples were collected at 2, 4, 6, 8, 10, 12, 24, 28, and 48 h.
The obtained results (Figure 6) prove that the reaction progressed in the same way as in the case of (4R)-(–)-carvone (1).
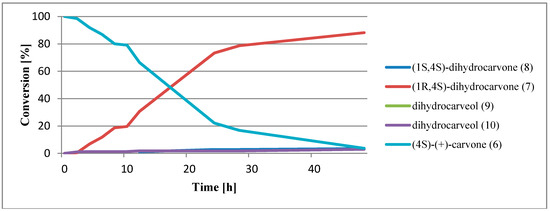
Figure 6.
Biotransformation of (4S)-(+)-carvone (6) via a comminuted potato.
In contrast to the biotransformation of (4R)-(–)-carvone (1), the reduction of the double bond in (4S)-(+)-carvone (6) ensued predominantly during the first day of transformation. The principal product formed was (1R, 4S)-dihydrocarvone (7). The process had high diastereoselectivity because almost no formation of a second dihydrocarvone stereoisomer was observed.
4. Discussion
The biocatalysts used in this study performed the reduction of the double bond in the carvone ring as the first step, followed by the reduction of the carbonyl group. This course of transformation is well known in biotransformations observed in plant cell cultures, e.g., Nicotiana tabacum [14], Mentha piperita [23], Medigo sativa, and Astasia longa [24]. In the paper by Yadav et al. [25], the transformation of (4R)-(–)-carvone (1) via comminuted carrot was described. The acquired results differed from those presented by us in this paper, where the main difference was related to the stereospecificity of the reduction of the double bond in the carvone ring. While the dihydrocarvone obtained by Yadav et al. had the configuration (1S, 4R), in our results, the configuration was (1R, 4R)-dihydrocarvone (3). The degree of substrate conversion was also different; we established 18%, while Yadav et al. achieved 60%. Another difference is that in the work of Yadav et al., the principal product of biotransformation was (1S, 2R, 4R)-neoisodihydrocarveol, which was obtained after 8 h with very high enantioselectivity (95%). The reduction of the carbonyl group in both studies advanced in the same way—R-alcohol was formed, contrary to Prelog’s rule. Such a sizeable discrepancy in the results can be explained by the use of another carrot variety and the prevailing climatic conditions in our latitudes. The results from Yadev et al. were more compatible with biotransformations carried out with yeast [25] than with the use of plant cultures [23].
In conclusion, the biotransformations executed in our study demonstrated that the first step of the transformation was the regioselective reduction of the double bond in the ring of both carvone stereoisomers (4R)-(–)-(1) and (4S)-(+)-carvone (6), which delivered the appropriate dihydrocarvones. The identical stereochemistry of reduction was observed in both carvone stereoisomers. The hydrogen atom attack at C-1 was from the Si side, while it was at C-6 from the re side (Figure 7).
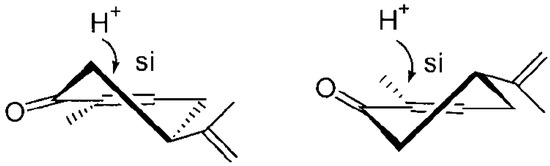
Figure 7.
Hydrogen atom attack to C-1 atom during the bioreduction of (4R)-(–)-carvone (1) and (4S)-(+)-carvone (6), respectively.
The enzymatic hydrogenation reactions of the double bond are catalyzed primarily by enoate reductases (ERs, E.C. 1.3.1.x), which are dependent on flavin mononucleotide. Most of them belong to the “Old Yellow Enzyme” family (OYE) and are involved in the metabolism of fatty acids or in the detoxification process [26]. Although a number of enzymes in this group have been isolated and tested even with the use of microorganisms; in practice, entire cultures are utilized. This is caused by enzyme instability as their special sensitivity even to trace amounts of oxygen can effectively deactivate the enzyme [27]. Thus, the use of an isolated enzyme requires a system of cofactor regeneration.
During enzymatic hydrogenation, usually the trans addition of hydrogen to the double bond is observed. This reaction occurs more readily when the double bond is coupled to a second double bond or when it is activated by the presence of electron withdrawing substituents. It is worth noting that, unlike enzymes isolated from microorganisms, known plant reductases catalyze cis-additions [23].
In this study, the trans or cis addition was examined depending on the carvone enantiomer. In the case of (4R)-(–)-carvone (1), compound (2) was formed (trans product), while from (4S)-(+)-carvone (6), cis-dihydrocarvone (7) was formed.
In the biotransformation of (4R)-(–)-carvone (1) and (4S)-(+)-carvone (6), apart from the hydrogenation products, the corresponding alcohols were also obtained. The reduction of substrate (1) provided alcohol R; consequently, it ran against Prelog’s rule. The reaction of the carbonyl group reduction was catalyzed by dehydrogenases or corresponding reductases. As a result of the desymmetrization of the substrate molecule, optically pure products are often obtained.
In almost all of the reactions of enzymatic reduction of the carbonyl group, the hydrogen ion is transferred from the reducing cofactor. The enzyme distinguishes substituents around the carbonyl group, hence enzymatic reductions are characterized by enantioselectivity when the resulting products are chiral.
5. Conclusions
In the biotransformation of both enantiomers of carvone (1) and (6) by enzymatic systems of selected plants, dihydrocarvones were achieved, which can be used as chirones in the chemical synthesis of compounds with interesting biological activities such as eudesmane sesquiterpenoids [8] with diastolic activity, or isolated from blue-green algae depsipeptide cryptophycin A [12] with high antitumor activity, or dihydrotachysteroles [28] analogs of vitamin D3. When using the comminuted plant material in the transformation of (4S)-(+)-carvone (6), cis-dihydrocarvone was created mainly while in transformation by microbial reductase, and the trans addition of hydrogen to the double bond of carvone was observed. The enzymatic systems of vegetables transformed the substrates more efficiently than the fruit enzymatic systems. The highest degree of substrate conversion was observed during biotransformation using the comminuted potato.
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/2076-3417/8/12/2605/s1. Figure S1: 1H NMR (600 MHz, CDCl3) spectrum of (1R, 4R)-(+)-dihydrocarvone (2), Figure S2: 13C NMR (500 MHz, CDCl3) spectrum of (1R, 4R)-(+)-dihydrocarvone (2), Figure S3: COSY NMR spectrum of (1R, 4R)-(+)-dihydrocarvone (2), Figure S4: 1H NMR (600 MHz, CDCl3) spectrum of (1R, 2S, 4R)-neodihydrocarveol (5), Figure S5: 13C NMR (500 MHz, CDCl3) spectrum of (1R, 2S, 4R)-neodihydrocarveol (5), Figure S6: COSY NMR spectrum of (1R, 2S, 4R)-neodihydrocarveol (5), Figure S7: 1H NMR (600 MHz, CDCl3) spectrum of (1R, 2R, 4R)-dihydrocarveol (4).
Author Contributions
W.M. conceived, designed the experiments, and analyzed the data; W.M., D.S., and K.W. performed the experiments; W.M., K.W., and M.G. wrote the paper; and A.S. and M.G. analyzed the NMR spectrum of the products.
Funding
This research received no external funding.
Acknowledgments
This publication was supported by the Wroclaw Centre of Biotechnology, programme The Leading National Research Centre (KNOW) for the years 2014–2018.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sivropoulou, A.; Kokkini, S.; Lanaras, T.; Arsenakis, M. Antimicrobial activity of mint essential oils. J. Agric. Food. Chem. 1995, 43, 2384–2388. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.; Konings, M.C.J.M.; Gershenzon, J.; Karp, F.; Croteau, R. Cytochrome P-450 dependent (+)-limonene-6-hydroxylation in fruits of caraway (Carum carvi). Phytochemistry 1999, 50, 243–248. [Google Scholar] [CrossRef]
- De Carvalho, C.C.C.R.; da Fonseca, M.M.R. Carvone: Why and how should one bother to produce this terpene. Food Chem. 2006, 95, 413–422. [Google Scholar] [CrossRef]
- Aydın, E.; Turkez, H.; Keles, M.S. Potential anticancer activity of carvone in N2a neuroblastoma cell line. Toxicol. Ind. Health 2015, 31, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Chen, H. Anticancer effects of carvone in myeloma cells is mediated through the inhibition of p38 MAPK signalling pathway, apoptosis induction and inhibition of cell invasion. JBUON 2018, 23, 747–751. [Google Scholar] [PubMed]
- Sabir, S.M.; Singh, D.; Rocha, J.B.T. In vitro antioxidant activity of S-carvone isolated from Zanthoxylum alatum. Pharm. Chem. J. 2015, 49, 187–191. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A.; Targowska-Duda, K.; Klimek, K.; Ginalska, G.; Joźwiak, K.; Waksmundzka-Hajnos, M.; Cieśla, Ł. Volatile terpenoids as potential drug leeds in Alzheimer’s disease. Open Chem. 2017, 15, 332–343. [Google Scholar] [CrossRef]
- Kumaran, R.S.; Mehta, G. A versatile, RCM based approach to eudesmane and dihydroagarofuran sesquiterpenoids from (−)-carvone: A formal synthesis of (−)-isocelorbicol. Tetrahedron 2015, 71, 1718–1731. [Google Scholar] [CrossRef]
- De Faria, M.L.; Magalhaes, R.D.A.; Silva, F.C.; Luiz, G.D.O.; Ceschi, M.A.; Brocksom, U.; Brocksom, T.J. Enantiodivergent syntheses of cycloheptenone intermediates for guaiane sesquiterpenes. Tetrahedron 2000, 11, 4093–4103. [Google Scholar] [CrossRef]
- Abad, A.; Agullo, C.; Cunat, A.C.; Garcıa, A.B.; Gimenez-Saiz, C. Synthetic studies on the preparation of oxygenated spongiane diterpenes from carvone. Tetrahedron 2003, 59, 9523–9536. [Google Scholar] [CrossRef]
- Honda, T.; Ishikawa, F. Enantiospecific synthesis of (+)-nemorensic acid, a necic acid component of the macropyrrolizidine alkaloid, nemorensine. J. Org. Chem. 1999, 64, 5542–5546. [Google Scholar] [CrossRef] [PubMed]
- Eggen, M.J.; Georg, G.I. The cryptophycins: their synthesis and anticancer activity. Med. Res. Rev. 2002, 71, 1718–1731. [Google Scholar] [CrossRef] [PubMed]
- Hamada, H.; Yasumune, H.; Fuchikami, Y.; Hirata, T.; Sattler, I.; Williams, H.J.; Scott, J. Biotransformation of geraniol, nerol and (+)- and (−)-carvone by suspension cultured cells of Catharanthus roseus. Phytochemistry 1997, 44, 615–621. [Google Scholar] [CrossRef]
- Hirata, T.; Hamada, H.; Aoki, T.; Suga, T. Stereoselectivity of the reduction of carvone and dihydrocarvone by suspension cells of Nicotiana tabacum. Phytochemistry 1982, 21, 2209–2212. [Google Scholar] [CrossRef]
- Machado, L.L.; Souza, J.S.N.; de Mattos, M.C.; Sakata, S.K.; Cordell, G.A.; Lemos, T.L.G. Bioreduction of aldehydes and ketones using Manihot species. Phytochemistry 2006, 67, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Assuncao, J.C.C.; Machado, L.L.; Lemos, T.L.G.; Cordell, G.A.; Monte, F.J.Q. Sugar cane juice for the bioreduction of carbonyl compounds. J. Mol. Catal. B Enzym. 2008, 52–53, 194–198. [Google Scholar] [CrossRef]
- Fonseca, A.M.; Monte, F.J.Q.; de Oliveira, M.C.F.; de Mattos, M.C.; Cordell, G.A.; Braz-Filho, R.; Lemos, T.L.G. Coconut water (Cocos nucifera L.)—A new biocatalyst system for organic synthesis. J. Mol. Catal. B Enzym. 2009, 57, 78–82. [Google Scholar] [CrossRef]
- Mączka, W.K.; Mironowicz, A. Enantioselective reduction of bromo- and methoxy-acetophenone derivatives using carrot and celeriac enzymatic system. Tetrahedron 2004, 15, 1965–1967. [Google Scholar] [CrossRef]
- Liu, X.; Pan, Z.G.; Xu, J.H.; Li, H.X. Enantioselective reduction of acetophenone analogues using carrot and celeriac enzymes system. Chin. Chem. Lett. 2010, 21, 305–308. [Google Scholar] [CrossRef]
- Mączka, W.K.; Grabarczyk, M.; Wińska, K.; Anioł, M. Plant-mediated stereoselective biotransformation of phenylglyoxylic acid esters. Z. Naturforsch. 2014, 69, 309–316. [Google Scholar] [CrossRef]
- Mironowicz, A. Biotransformations of racemic acetates by potato and topinambur tubers. Phytochemistry 1998, 47, 1531–1534. [Google Scholar] [CrossRef]
- Iqbal, N.; Rudroff, F.; Brig, A.; van Beeumen, J.; Mihovilovic, M.D. Asymmetric bioreduction of activated carbonecarbon double bonds using Shewanella yellow enzyme (SYE-4) as novel enoate reductase. Tetrahedron 2012, 68, 7619–7623. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-S.; Park, S.-H.; Chang, Y.-J.; Lim, Y.-H.; Kim, S.-U. Transformation of menthane monoterpenes by Mentha piperita cell culture. Biotechnol. Lett. 2002, 24, 1553–1556. [Google Scholar] [CrossRef]
- Shimoda, K.; Hirata, T. Biotransformation of enones with biocatalysts—Two enone reductases from Astasia longa. J. Mol. Catal. B Enzym. 2000, 8, 255–264. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, G.S.K.K.; Sabitha, G.; Krishna, A.D.; Prasad, A.R.; Rahaman, H.; Rao, K.V.; Rao, A.B. Daucus carota and baker’s yeast mediated bio-reduction of prochiral ketones. Tetrahedron 2007, 18, 717–723. [Google Scholar] [CrossRef]
- Magallanes-Noguera, C.; Cecati, F.M.; Mascotti, M.L.; Reta, G.F.; Agostini, E.; Orden, A.A.; Kurina-Sanz, M. Plant tissue cultures as sources of new ene- and ketoreductase activities. J. Biotechnol. 2017, 251, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Winkler, C.K.; Clay, D.; van Heerden, E.; Faber, K. Overcoming co-product inhibition in the nicotinamide independent asymmetric bioreduction of activated C=C- bonds using flavin-dependent ene-reductases, Biotechnol. Bioeng. 2013, 110, 3085–3092. [Google Scholar] [CrossRef]
- Maestro, M.A.; Casledo, L.; Mourino, A. A convergent approach to the dihydrotachysteroldiene system. Application to the synthesis of dihydrotachysterol (DHT2), 25-hydroxydihydrotachysterol (25-OH-DHT), 10(R),19-dihydro-(5E)-3-epivitamin D2, and 25-hydroxy-10(R),19-dihydro-(5E)-3-epivitamin D2. J. Org. Chem. 1992, 57, 5208–5213. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).