Design, Synthesis and Antifungal Activity of Novel Benzoylcarbamates Bearing a Pyridine Moiety
Abstract
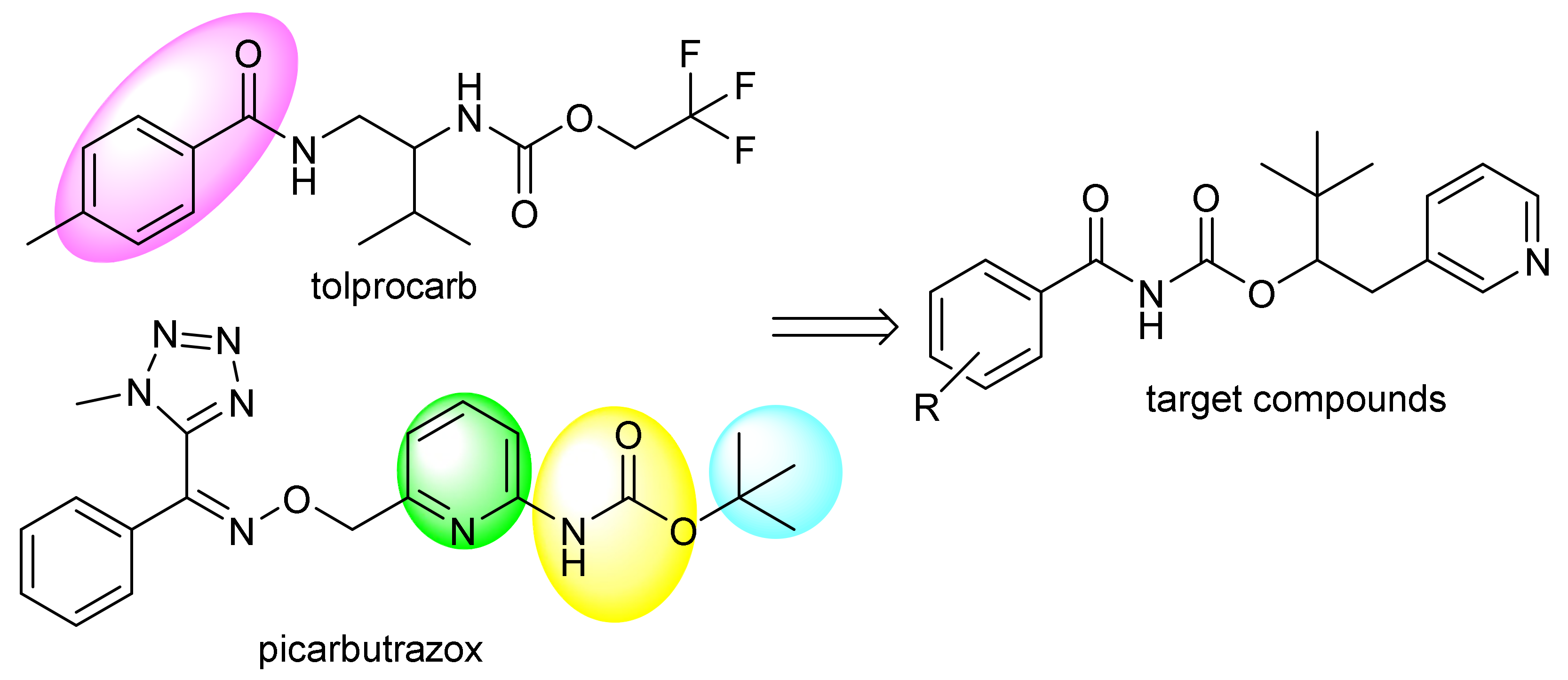
1. Introduction
2. Materials and Methods
2.1. Synthesis
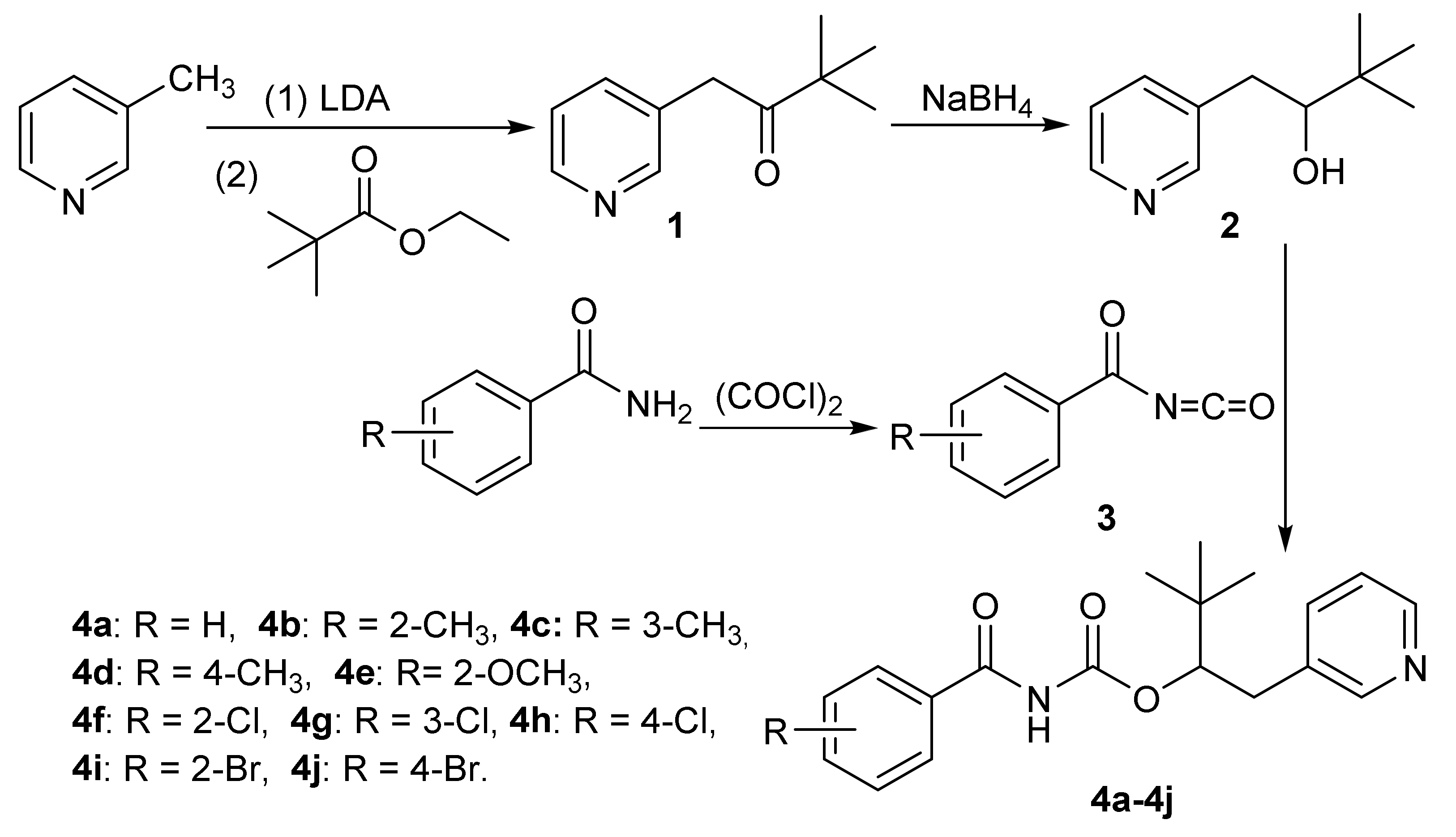
2.1.1. Synthesis of 3,3-Dimethyl-1-(pyridin-3-yl)butan-2-one (1)
2.1.2. Synthesis of 3,3-Dimethyl-1-(pyridin-3-yl)butan-2-ol (2)
2.1.3. General Procedure for the Synthesis of Substituted Benzoyl Isocyanate (3)
2.1.4. General Procedure for the Synthesis of Substituted Benzoylcarbamates (4a–4j)
2.2. Biological Assay
3. Results and Discussion
3.1. Chemistry
3.2. Antifungal Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bardas, G.A.; Veloukas, T.; Koutita, O.; George, S.K. Multiple resistance of Botrytis cinereal from kiwifruit to SDHIs, Qo Is and fungicides of other chemical groups. Pest Manag. Sci. 2010, 66, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Hua, N.Z. Formulation and Application of Fungicide Tebuconazole. Agrochemicals 2013, 52, 781–786. [Google Scholar] [CrossRef]
- Min, L.J.; Shi, Y.X.; Yang, M.Y.; Zhai, Z.W.; Weng, J.Q.; Tan, C.X.; Liu, X.H.; Li, B.J.; Zhang, Y.G. Microwave assisted synthesis and antifungal activity of some novel hydrazones containing pyridine moiety. Lett. Drug Des. Discov. 2016, 13, 324–328. [Google Scholar] [CrossRef]
- Gomha, S.M.; Muhammad, Z.A.; Abdel-aziz, M.R.; Abdel-aziz, H.M.; Gaber, H.M.; Elaasser, M.M. One-pot synthesis of new thiadiazolyl-pyridines as anticancer and antioxidant agents. J. Heterocycl. Chem. 2018, 55, 530–536. [Google Scholar] [CrossRef]
- Li, T.X.; Zhang, J.; Pan, J.K.; Wu, Z.X.; Hu, D.Y.; Song, B.A. Design, synthesis, and antiviral activities of 1,5-benzothiazepine derivatives containing pyridine moiety. Eur. J. Med. Chem. 2017, 125, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Renard, F.; Lecomte, F.; Hubert, P.; Leval, X.D.; Piootte, B. N-(3-Arylaminopyridin-4-yl)alkanesulfon amides as pyridine analogs of nimesulide: Cyclooxygenases inhibition, anti-inflammatory studies and insight on metabolism. Eur. J. Med. Chem. 2014, 74, 12–22. [Google Scholar] [CrossRef]
- Zaheer, Z.; Shaikh, S.I.; Mokale, S.N.; Lokwani, D.K. Synthesis, biological evaluation and computational study of new quinoline hybrids as antitubercular agent. Lett. Drug Des. Discov. 2018, 15, 914–922. [Google Scholar] [CrossRef]
- Zhang, X.L.; Wang, B.L.; Mao, M.Z.; Xiong, L.X.; Yu, S.J.; Li, Z.M. Synthesis and insecticidal activity of 5-chloro-N-(4-chloro-2-(substitutedcarbamoyl) -6-methylphenyl)-1-aryl-3-(trifluoromethyl) -1H- pyrazole-4-carboxamide. Chem. J. Chin. Univ. 2013, 34, 96–102. [Google Scholar] [CrossRef]
- Sun, G.X.; Shi, Y.X.; Sun, Z.H.; Yang, M.Y.; Wu, H.K.; Weng, J.Q.; Tan, C.X.; Liu, X.H.; Li, B.J.; Zhang, Y.G. Synthesis and bioactivities of novel 1,3,4-oxadiazole derivatives containing pyridine moiety. Lett. Drug Des. Discov. 2014, 9, 1119–1123. [Google Scholar] [CrossRef]
- Guan, A.Y.; Liu, C.L.; Sun, X.F.; Xie, Y.; Wang, M.A. Discovery of pyridine-based agrochemicals by using Intermediate Derivatization Methods. Bioorg. Med. Chem. 2016, 24, 342–353. [Google Scholar] [CrossRef]
- Ye, X. A novel fungicide picarbutrazox. World Pestic. 2018, 40, 63–64. [Google Scholar] [CrossRef]
- Zhang, Y.B. A novel fungicide-pyribencarb. World Pestic. 2013, 35, 61–62. [Google Scholar]
- Hamada, T.; Asanagi, M.; Satozawa, T.; Araki, N.; Banba, S.; Higashimura, N.; Akase, T.; Hirase, K. Action mechanism of the novel rice blast fungicide tolprocarb distinct from that of conventional melanin biosynthesis inhibitors. J. Pestic. Sci. 2014, 39, 152–158. [Google Scholar] [CrossRef]
- Liu, Y.P.; Yang, J.C.; Cai, B.S.; Wu, Q.; Liu, C.L. A novel fungicide benthiavalicarb-isopropyl. Agrochemicals 2011, 50, 756–758. [Google Scholar] [CrossRef]
- Xu, S.F.; Sun, L.; Fei, X.L.; Sun, M.D.; Wang, H.G.; Wang, S.; Li, Y.C.; Wang, W.; Yang, Q.B.; Li, Y.X. Study on synthesis of fungicide diethofencar. Appl. Chem. Ind. 2010, 39, 30–32. [Google Scholar] [CrossRef]
- Li, Y.; Li, B.J.; Ling, Y.; Miao, H.J.; Shi, Y.X.; Yang, X.L. Synthesis and fungicidal activity of aryl carbamic acid-5-aryl-2-furanmethyl ester. J. Agric. Food Chem. 2010, 58, 3037–3042. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Wan, F.X.; Liu, S.; Zhang, J.H.; Jiang, L. Synthesis and Antifungal Activity Evaluation of Novel Substituted Pyrimidine-5-Carboxamides Bearing the Pyridine Moiety. J. Chin. Chem. Soc. 2018, 65, 445–451. [Google Scholar] [CrossRef]
- Zhang, J.H.; Niu, L.Z.; Li, Y.; Liu, S.; Jiang, L. Facile Synthesis of 2-(pyridin-3-yl)-2-benzoyloxy acetamides via Passerini reaction and evaluation of their biological activity. Chin. J. Org. Chem. 2018, 38, 1842–1848. [Google Scholar] [CrossRef]
- Yang, Y.P.; Cui, M.C.; Zhang, X.Y.; Dai, J.P.; Zhang, Z.Y.; Lin, C.P.; Guo, Y.Z.; Liu, B.L. Radioiodinated benzyloxybenzene derivatives: A class of flexible ligands target to β-amyloid plaques in Alzheimer’s brains. J. Med. Chem. 2014, 57, 6030–6042. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhang, S.; Liu, S.; Jiang, L. Synthesis of novel 3,3-dimethyl-1-(pyridin-3-yl) butan-2-one oxime esters and evaluation of their antifungal activity. Chin. J. Org. Chem. 2017, 37, 2767–2771. [Google Scholar] [CrossRef]
- Song, X.J.; Tan, X.H. Synthesis, structure and biological activity of some new aroylurea derivatives containing 1,3,4-thiadiazole. Phosphorus Sulfur Silicon Relat. Elem. 2008, 183, 1755–1765. [Google Scholar] [CrossRef]
- Li, C.K.; Jiang, L.; Wang, Y.; Wan, F.X.; Zhang, P.Z.; Li, Y.; Cui, Z.N. Design, synthesis and biological activities of N-(substitutedbenzoyl)-N′-(5-methoxyl-2-methylsulfanylpyrimidin-4-amino) (thio) ureas. Chin. J. Org. Chem. 2014, 34, 2296–2303. [Google Scholar] [CrossRef]
- Sun, J.L.; Mu, W. Pesticide Science Experimental Techniques and Guidance; Chemical Industry Press: Beijing, China, 2009; pp. 228–229. ISBN 978-7-122-05509-5. [Google Scholar]
- Zhang, Z.H. Organic Spectroscopic Analysis; People’s Health Press: Beijing, China, 2009; p. 81. ISBN 978-7-117-11741-8. [Google Scholar]
- Zhang, Y.B.; Zhang, Y.; Wu, X.Y. Recent Progress in World Pesticides; Chemical Industry Press: Beijing, China, 2014; pp. 324–326. ISBN 978-7-122-18588-4. [Google Scholar]
| Compd. | R | EC50 (μg/mL), 95% CL | |
|---|---|---|---|
| S. sclerotiorum | B. cinerea | ||
| 4a | H | 12.67 (8.98–17.89) | 13.03 (12.02–14.11) |
| 4b | 2-CH3 | 14.96 (10.42–19.48) | 10.50 (8.49–14.03) |
| 4c | 3-CH3 | 15.89 (13.19–19.40) | 8.04 (6.54–11.68) |
| 4d | 4-CH3 | 13.18 (9.06–18.21) | 6.70 (4.03–11.02) |
| 4e | 2-OCH3 | 14.06 (10.82–19.74) | 7.84 (5.50–10.90) |
| 4f | 2-Cl | 11.84 (8.04–17.90) | 6.98 (6.24–7.79) |
| 4g | 3-Cl | 12.41 (8.19–18.80) | 6.81 (5.39–9.60) |
| 4h | 4-Cl | 10.85 (6.57–17.92) | 6.45 (5.44–7.65) |
| 4i | 2-Br | 12.98 (9.26–16.64) | 9.80 (6.48–13.16) |
| 4j | 4-Br | 11.25 (7.40–17.78) | 7.95 (5.21–12.15) |
| Diethofencarb | 2.95 (1.24–5.08) | 4.72 (2.49–7.15) | |
| Chlorothalonil | 9.97 (7.51–13.22) | 6.56 (4.95–9.70) | |
| Carbendazim | 0.24 (0.20–0.28) | 19.20 (13.27–27.89) | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, F.-X.; Wang, J.-H.; Shi, Y.-H.; Niu, L.-Z.; Jiang, L. Design, Synthesis and Antifungal Activity of Novel Benzoylcarbamates Bearing a Pyridine Moiety. Appl. Sci. 2018, 8, 2577. https://doi.org/10.3390/app8122577
Wan F-X, Wang J-H, Shi Y-H, Niu L-Z, Jiang L. Design, Synthesis and Antifungal Activity of Novel Benzoylcarbamates Bearing a Pyridine Moiety. Applied Sciences. 2018; 8(12):2577. https://doi.org/10.3390/app8122577
Chicago/Turabian StyleWan, Fu-Xian, Jian-Hua Wang, Yan-Hua Shi, Li-Zhi Niu, and Lin Jiang. 2018. "Design, Synthesis and Antifungal Activity of Novel Benzoylcarbamates Bearing a Pyridine Moiety" Applied Sciences 8, no. 12: 2577. https://doi.org/10.3390/app8122577
APA StyleWan, F.-X., Wang, J.-H., Shi, Y.-H., Niu, L.-Z., & Jiang, L. (2018). Design, Synthesis and Antifungal Activity of Novel Benzoylcarbamates Bearing a Pyridine Moiety. Applied Sciences, 8(12), 2577. https://doi.org/10.3390/app8122577






