Effects of Sterilization on Bioactives of Jatropha dioica and Opuntia oligacantha Extracts, and on Antimicrobial Capacity against Streptococcus mutans
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction Procedure
2.3. Non-Sterilized or Autoclave-Sterilized Extracts
2.4. Determination of Physicochemical Properties
2.5. Total Phenolic Analysis
2.6. Flavonoid Content
2.7. Quantification of Betalain
2.8. Identification of Extract Compounds by HPLC
2.9. Determination of Antioxidant Activity by the DPPH Method
2.10. Determination of Antioxidant Activity by the ABTS Method
2.11. Antimicrobial Activity
2.12. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Analyses
3.2. Total Phenolics and Flavonoids
3.3. Betalains in Xoconostle Extracts
3.4. Identification of Extracted Compounds by HPLC
3.5. Free Radical Scavenging Ability as Estimated by DPPH and ABTS
3.6. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ojeda-Garcés, J.C.; Oviedo-García, E.O.; Salas, L.A. Streptococcus mutans and dental caries. Revista CES Odontología 2013, 26, 44–52. [Google Scholar]
- Bascones-Martínez, A.; Mudarra-Morante, S. Antisépticos orales. Revisión de la literatura y prespectiva actual. Revista Clínica de Periodoncia Implantol 2006, 18, 11–39. [Google Scholar]
- Fernández-Riverón, F.; López-Hernández, J.; Ponce-Martínez, L.M.; Machado-Betarte, C. Resistencia bacteriana. Revista Cubana de Medicina Militar 2003, 32, 44–52. [Google Scholar]
- Benítez-Azaola, R.; García-Varela, R.; García-García, R.M.; Suárez-Jacobo, A.; Gardineau, G.A.; Altamirano, J.; Serna-Saldívar, S.O. Listeria monocytogenes growth inhibition in inoculated fresh panela cheese by the addition of Rhoeo discolor aqueous extracts combined with potassium sorbate. Revista Mexicana de Ingeniería Química 2017, 16, 425–434. [Google Scholar]
- Waizel-Bucay, J.; Martínez-Rico, I.M. Algunas plantas usadas en México en padecimientos periodontales. Revista Asociación Dental Mexicana 2011, 68, 73–88. [Google Scholar]
- Hayek, S.S.; Ibrahim, S.A. Antimicrobial activity of xoconostle pears (Opuntia matudae) against Escherichia coli O157:H7 in laboratory medium. Int. J. Microbiol. 2012, 2012, 368472. [Google Scholar] [CrossRef]
- Morales, P.; Ramírez-Moreno, E.; Sánchez-Mata, M.; Carvalho, A.M.; Ferreira, I. Nutricional and antioxidant properties of pulp and seeds of two xoconostles cultivars (Opuntiajoconostle F.A.C. Weber ex Diguet and Opuntia matudae Scheinvar) of hight consumption in Mexico. Food Res. Int. 2012, 46, 279–285. [Google Scholar] [CrossRef]
- Hernández-Fuentes, A.D.; Trapala-Islas, A.; Gallegos-Vázquez, C.; Campos-Montiel, R.G.; Pinedo-Espinoza, J.M.; Guzmán-Maldonado, S.H. Physicochemical variability and nutritional and functional characteristics of xoconostles (Opuntia spp.) accessions from Mexico. Fruits 2015, 70, 109–116. [Google Scholar] [CrossRef]
- Wong-Paz, J.E.; Castillo-Inungaray, M.L.; López-López, L.; Contreras-Esquivel, J.C.; Nevárez-Moorillon, G.V.; Aguilar, C.N. Jatropha dioica Fuente potencial de agentes antimicrobianos. Revista Científica de la Universidad Autónoma de Coahuila 2010, 2, 1–5. [Google Scholar]
- Mujumdar, A.M.; Misar, A.V. Anti-inflamatory activity of Jatropha curcas roots in mice and rats. J. Ethnopharmacol. 2004, 90, 11–16. [Google Scholar] [CrossRef]
- Silva-Belmares, Y.; Rivas-Morales, C.; Viveros-Valdez, E.; Cruz-Galicia, M.G.; Carranza-Rosales, P. Antimicrobial and cytotoxic activities from Jatropha dioica roots. Pak. J. Biol. Sci. 2014, 17, 748–750. [Google Scholar]
- Wong-Paz, J.E.; Contreras-Esquivel, J.C.; Rodríguez-Herrera, R.; Carrillo-Inungaray, M.L.; López, L.; Nevárez-Moorillón, V.; Aguilar, C.N. Total phenolic content, in vitro antioxidant activity and chemical composition of plant extracts from semiarid Mexican region. Asian Pac. J. Trop. Dis. 2015, 8, 104–111. [Google Scholar] [CrossRef]
- Gutiérrez-Tlahque, J.; Aguirre-Mancilla, C.L.; Raya-Pérez, J.C.; Ramírez-Pimentel, J.G.; Jiménez-Alvarado, R.; Hernández-Fuentes, A.D. Effect of climate conditions on total phenolic content and antioxidant activity of Jatropha dioica Cerv. var. dioica. Ciencia e Investigación Agraria 2018, 45, 70–81. [Google Scholar] [CrossRef]
- Hashemi, S.R.; Zulkifli, I.; Zunita, Z.; Somchit, M.N. The effect of selected sterilization method on antibacterial activity of aqueous extract of herbal plants. Biol. Sci. 2008, 8, 1072–1076. [Google Scholar] [CrossRef]
- Yin, Q.; Mu, H.; Zeng, M.; Gao, D.; Qin, F.; Chen, J.; He, Z. Effects of heating of the total phenolic content, antioxidant activities and main functional components of simulated Chinese herb candy during boiling process. J. Food Meas. Charact. 2018, 1–11. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Dimler, R.J.; Schaefer, W.C.; Wise, C.S.; Rist, C.E. Quantitative paper chromatography of d-glucose and its oligosaccharides. Anal. Chem. 1952, 24, 1411–1414. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Jagota, S.K.; Dani, H.M. A new colorimetric technique for the estimation of vitamin C using folin phenol reagent. Anal. Biochem. 1982, 127, 178–182. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolic with phosphotungstic acid reagents. Am. J. Enol. Vitic. 1956, 16, 1444–1458. [Google Scholar]
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardization of propolis extract and identification of principal constituents. J. Pharmacie de Belgique 1994, 49, 462–470. [Google Scholar]
- Nilsson, T. Studies into the pigments in beetroot (Beta vulgaris L. ssp. vulgaris var. rubra L.). Langbrukshogskolans Ann. 1970, 36, 179–219. [Google Scholar]
- Aguiñiga-Sánchez, I.; Cadena-Iñiguez, J.; Santiago-Osorio, E.; Gómez-García, G.; Mendoza-Núñez, V.M.; Rosado-Pérez, J.; Ruíz-Ramos, M.; Cisneros-Solano, V.M.; Ledesma-Martínez, E.; Delgado-Bordanave, A.D.J.; et al. Chemical analyses and in vitro and in vivo toxicity of fruit methanol extract of Sechiumedule var. Pharm. Biol. 2017, 55, 1638–1645. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluated antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1238. [Google Scholar] [CrossRef]
- Domínguez, M.C.; de la Rosa, M.; Borobio, M.V. Application of a spectrophotometric method for the determination of post-antibiotic effect and comparison with viable counts in agar. J. Antimicrob. Chemother. 2011, 47, 391–400. [Google Scholar] [CrossRef]
- Aguilera, M.C.; Romano, E.; Ramos, N.; Rojas, L. Sensibilidad del Streptococcusmutans a tres enjuagues bucales comerciales (Estudio in vitro). ODOUS Cient. 2011, 12, 7–13. [Google Scholar]
- Salmon, S.A.; Watts, J.L.; Aarestrup, F.M.; Pankey, J.W.; Yancey, R.J. Minimum inhibitory concentrations for selected antimicrobial agents against organisms isolated from the mammary glands of dairy heifers in New Zealand and Denmark. J. Dairy Sci. 1998, 81, 570–578. [Google Scholar] [CrossRef]
- Cuastumal-Canacuan, H.G.; Valencia-Murillo, B.L.; Ordóñez-Santos, L.E. Efectos de los tratamientos térmicos en la concentración de vitamina C y color superficial en tres frutas tropicales. Revista Lasallista de Investigación 2016, 13, 85–93. [Google Scholar] [CrossRef]
- Pokorný, J. The impact of food processing in phytochemicals; the case of antioxidants. In Phytochemical Functional Foods, 1st ed.; Johnson, I., Williamson, G., Eds.; CRC Press: New York, NY, USA, 2003; Volume 1, pp. 298–312. [Google Scholar]
- López-Palestina, C.U.; Aguirre-Mancilla, C.L.; Raya-Pérez, J.C.; Ramírez-Pimentel, J.G.; Gutiérrez-Tlahque, J.; Hernández-Fuentes, A.D. The Effect of an Edible Coating with Tomato Oily Extract on the Physicochemical and Antioxidant Properties of Garambullo (Myrtillocactus geometrizans) Fruits. Agronomy 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Randhir, R.; Kwon, Y.I.; Shetty, K. Effect of thermal processing on phenolics, antioxidant activity and health-relevant functionality of select grain and sprouts and seedling. Innov. Food Sci. Emerg. Technol. 2008, 9, 355–364. [Google Scholar] [CrossRef]
- Beta, T.; Hwang, T. Influence of heat and moisture treatment on carotenoids, phenolic content, and antioxidant capacity of orange maize flour. Food Chem. 2018, 246, 58–64. [Google Scholar] [CrossRef]
- Randhir, R.; Shetty, K. Mung beans processed by solid-state bioconversion improves phenolic content and functionality relevant for diabetes and ulcer management. Innov. Sci. Emerg. Technol. 2007, 8, 197–204. [Google Scholar] [CrossRef]
- Felix, A.C.S.; Alvares, L.D.G.; Santana, R.A.; Valasques-Junior, G.L.; Bezerra, M.A.; Oliveira-Neto, N.M.; de Oliveira-Lima, E.; de Oliveira-Filho, A.A.; Franco, M.; do Nascimento-Junior, B.B. Application of experimental designs to evaluate the total phenolics content and antioxidant activity of cashew apple bagasse. Revista Mexicana de Ingeniería Química 2018, 17, 165–175. [Google Scholar]
- Altemimi, A.; Choudhary, R.; Watson, D.G.; Lighfoot, D.A. Effects of ultrasonic treatments on the polyphenol and antioxidant content of spinach extracts. Ultrason. Sonochem. 2015, 24, 247–272. [Google Scholar] [CrossRef]
- Elhamirad, H.A.; Zamanipoor, M.H. Thermal stability of some flavonoids and phenolic acids in sheep tallow olein. Eur. J. Lipid Sci. Technol. 2012, 114, 602–606. [Google Scholar] [CrossRef]
- Esquivel-Gonzales, B.E.; Ochoa-Martínez, L.A.; Rutiaga-Quiñones, O.M. Microencapsulación por secado por aspersión de compuestos bioactivos. Revista Latinoamericana Tecnología Postcosecha 2015, 16, 180–192. [Google Scholar]
- López-García, F.; Jiménez-Martínez, C.; Guzmán-Lucero, D.; Maciel-Cerda, A.; Delgado-Macuil, R.; Cabrero-Palomino, D.; Terrés-Rojas, E.; Arzate-Vázquez, I. Physical and chemical characterization of a biopolimer film made with corn starch and nopal xoconostle (Opuntia joconostle). Revista Mexicana de Ingeniería Química 2017, 16, 147–158. [Google Scholar]
- Shaimaa, G.A.; Mahmoud, M.S.; Mohamed, M.R.; Emam, A.A. Phytochemical screening, antioxidant activities and in vitro anticancer potential of Egyptian Capsicum spp. Biochem. Pharmacol. 2016, 5, 1–8. [Google Scholar]
- Eichner, H. Antioxidative effect of Maillard reaction intermediates. In Progress in Food Nutrition and Science; Ericksson, C., Ed.; Pergammon Press: Oxford, UK, 1981; pp. 441–451. [Google Scholar]
- Martínez, N.; Almaguer, G.; Vázquez-Alvarado, P.; Figueroa, A.; Zuñiga, C.; Hernández-Ceruelos, A. Análisis fitoquímico de Jatropha dioica y determinación de su efecto antioxidante y quimioprotector sobre el potencial genotóxico de ciclofosfamida, daunorrubicina y metilmetanosulfonato evaluado mediante el ensayo cometa. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas 2014, 13, 437–457. [Google Scholar]
- Manzocco, L.; Calligaris, S.; Mastrocola, D.; Nicoli, M.; Lerici, C. Review of nonenzymatic browning and antioxidant capacity in processed food. Trends Food Sci. Technol. 2011, 11, 340–346. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Betalain stability and degradation products from purified betanin, phyllocactin and hylocerenin by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 19, 2603–2616. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Herbach, K.M.; Mosshammer, M.R.; Carle, R.; Yi, W.; Sellapan, S.; Akoh, C.C.; Bunch, R.; Felker, P. Color, betalain pattern, and antioxidant properties of cactus pear (Opuntia spp.) clones. J. Agric. Food Chem. 2005, 53, 442–451. [Google Scholar] [CrossRef]
- Rodríguez-Sauceda, E.N.; Ximhai, R. Natural antimicrobial agent use in the preservation of fruits and vegetables. Res. Gate 2011, 7, 153–170. [Google Scholar]
- González-Cruz, L.; Hernández-Castillo, J.B.E.; Juárez-Gpiz, J.M.S.; Flores-Martínez, N.L.; Bernardino-Nicanor, A. Effect of traditional thermal treatment on the antioxidant activity and carotenoids content of nopalitos. Revista Mexicana de Ingeniería Química 2018, 17, 823–833. [Google Scholar] [CrossRef]
- Pastene, E.R. Estado actual de la búsqueda de plantas con actividad antioxidante. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas 2009, 8, 449–455. [Google Scholar]
- Bondet, W.; Williams, B.; Berset, C. Kinetics and mechanism of antioxidant activity using the DPPH free radical method. Lebensmittel Wissenschaft und Technologie 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Pérez-Grijalva, B.; García-Zabadúa, J.C.; Ruíz-Pérez, V.M.; Téllez-Medina, D.I.; García-Pinilla, S.; Guzmán-Gerónimo, R.I.; Mora-Escobedo, R. Biofunctionality, colorimetric coefficients and microbiological stability of blackberry (Rubusfructicosusvar. Himalaya) juice under microwave/ultrasound processing. Revista Mexicana de Ingeniería Química 2018, 8, 13–28. [Google Scholar]
- Yu, X.; Zhao, M.; Hu, J.; Zeng, S.; Bai, X. Correspondence analysis of antioxidant activity and UV-Vis absorbance of Maillard reaction products as related to reactants. Food Sci. Technol. 2012, 46, 1–9. [Google Scholar] [CrossRef]
- Kuskoski, M.; Asuero, A.G.; Troncoso, A.M.; Mancini-Filho, J.; Fett, R. Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos. Ciência e Tecnologia de Alimentos 2005, 25, 726–732. [Google Scholar] [CrossRef]
- Hu, J.; Wang, J.; Li, S.; Yang, B.; Gong, M.; Li, X.; Zhang, L.; Tian, J. Phytochemical compositions, antioxidant and antimicrobial activities analysis of extracts from Vaccinium bracteatum Thunb. Leaves. J. Appl. Bot. Food Qual. 2016, 89, 150–155. [Google Scholar]
- Poveda-Romero, M.; Sánchez-García, S.; Medina-García, E.; Espinel-Bermúdez, M.C.; Ríos-Szalay, E.; Fernández-Pedrero, J.A. Gluconato de clorhexidina al 0.12% en la inhibición de la adherencia del Streptococcus mutans en restauraciones provisionales de polimetil metacrilato in vitro. Revista Odontológica Mexicana 2006, 10, 24–29. [Google Scholar]
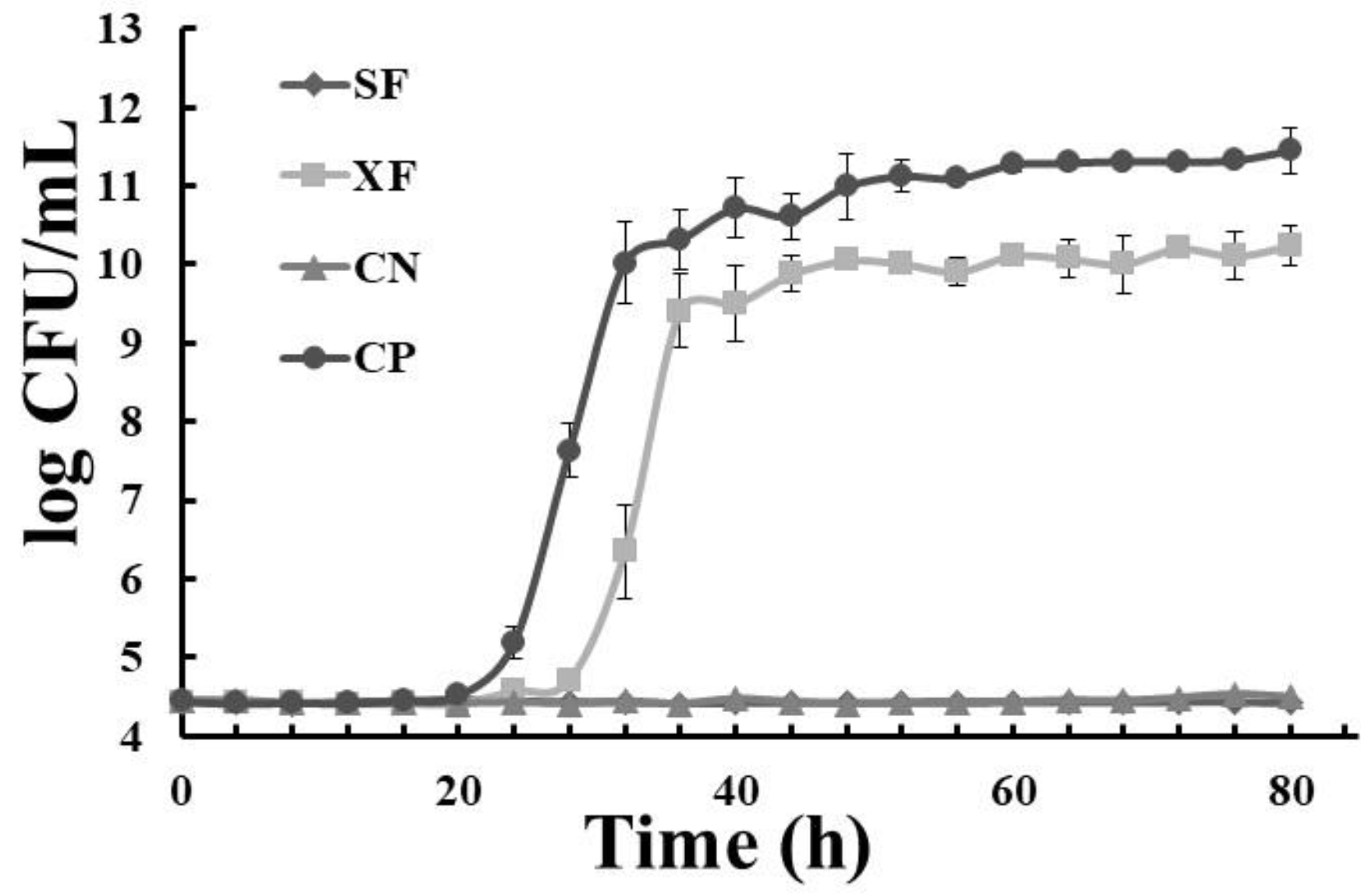
 ) dragon’s blood rhizomes (5 mg/mL); (
) dragon’s blood rhizomes (5 mg/mL); ( ) xoconostle (5 mg/mL); (
) xoconostle (5 mg/mL); ( ) negative control (chlorhexidine); (●) positive control, SB: autoclave-sterilized dragon’s blood rhizome extract, XA: autoclave-sterilized xoconoxtle fruit extract; CN: control negative; CP: control positive.
) negative control (chlorhexidine); (●) positive control, SB: autoclave-sterilized dragon’s blood rhizome extract, XA: autoclave-sterilized xoconoxtle fruit extract; CN: control negative; CP: control positive.
 ) dragon’s blood rhizomes (5 mg/mL); (
) dragon’s blood rhizomes (5 mg/mL); ( ) xoconostle (5 mg/mL); (
) xoconostle (5 mg/mL); ( ) negative control (chlorhexidine); (●) positive control, SB: autoclave-sterilized dragon’s blood rhizome extract, XA: autoclave-sterilized xoconoxtle fruit extract; CN: control negative; CP: control positive.
) negative control (chlorhexidine); (●) positive control, SB: autoclave-sterilized dragon’s blood rhizome extract, XA: autoclave-sterilized xoconoxtle fruit extract; CN: control negative; CP: control positive.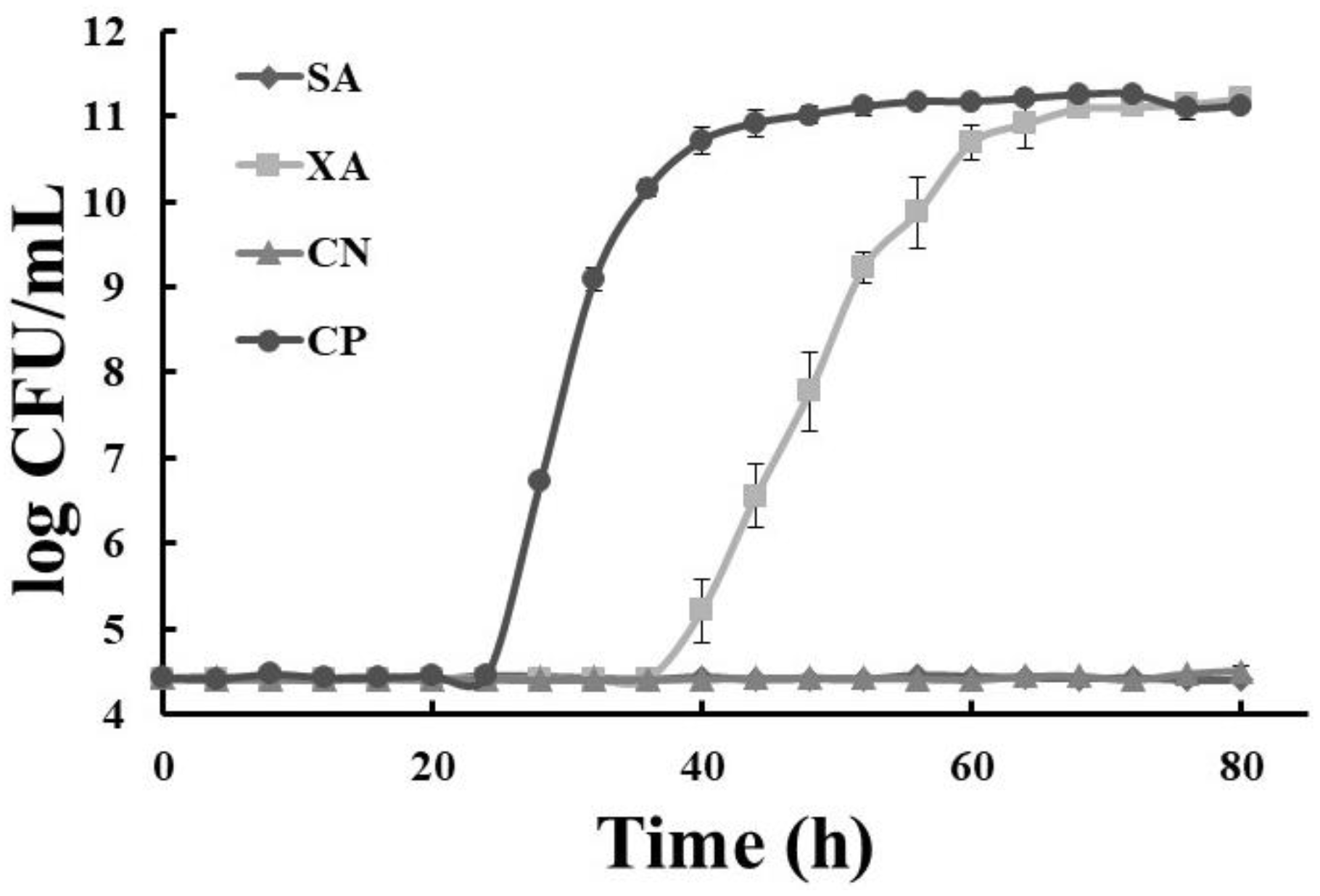
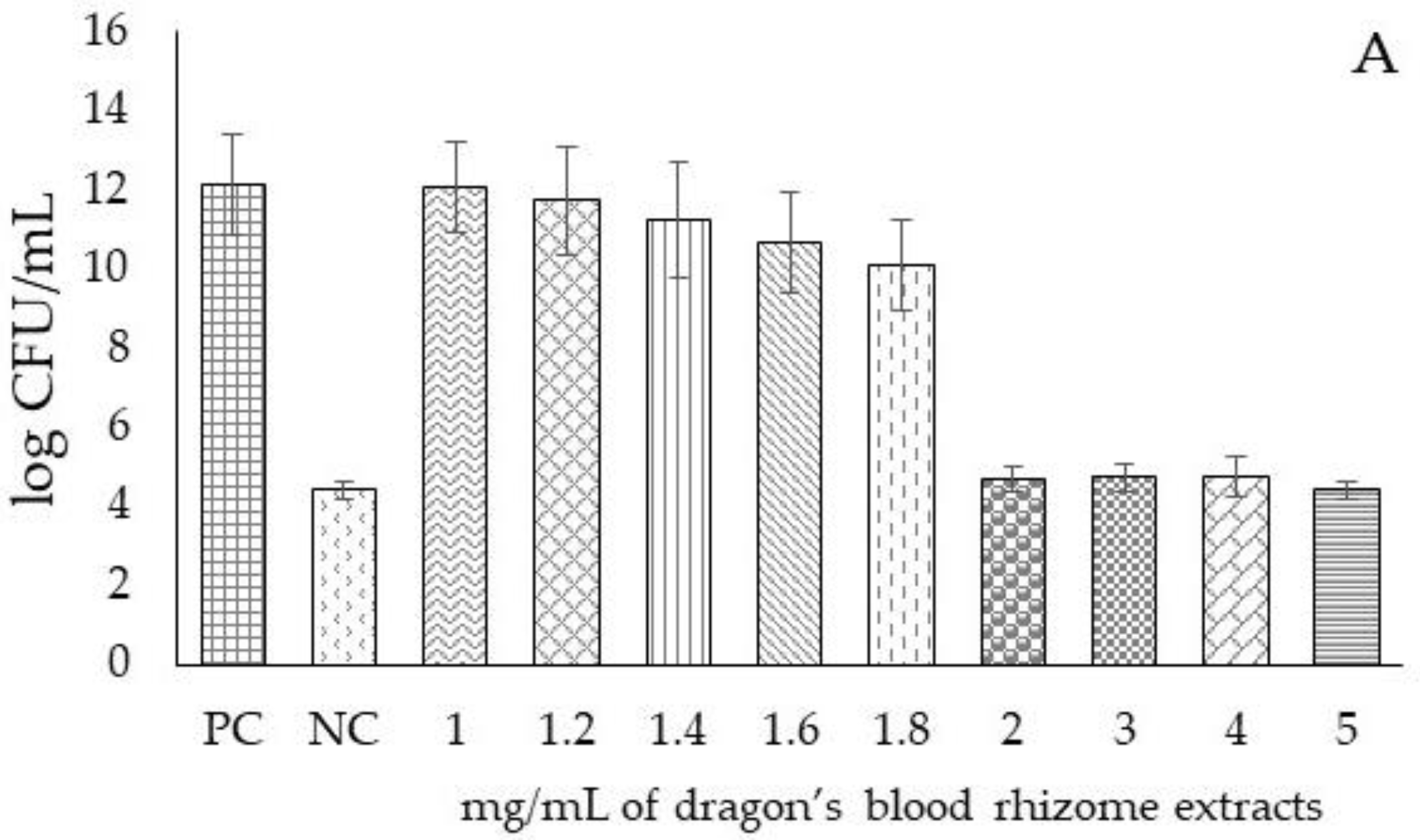
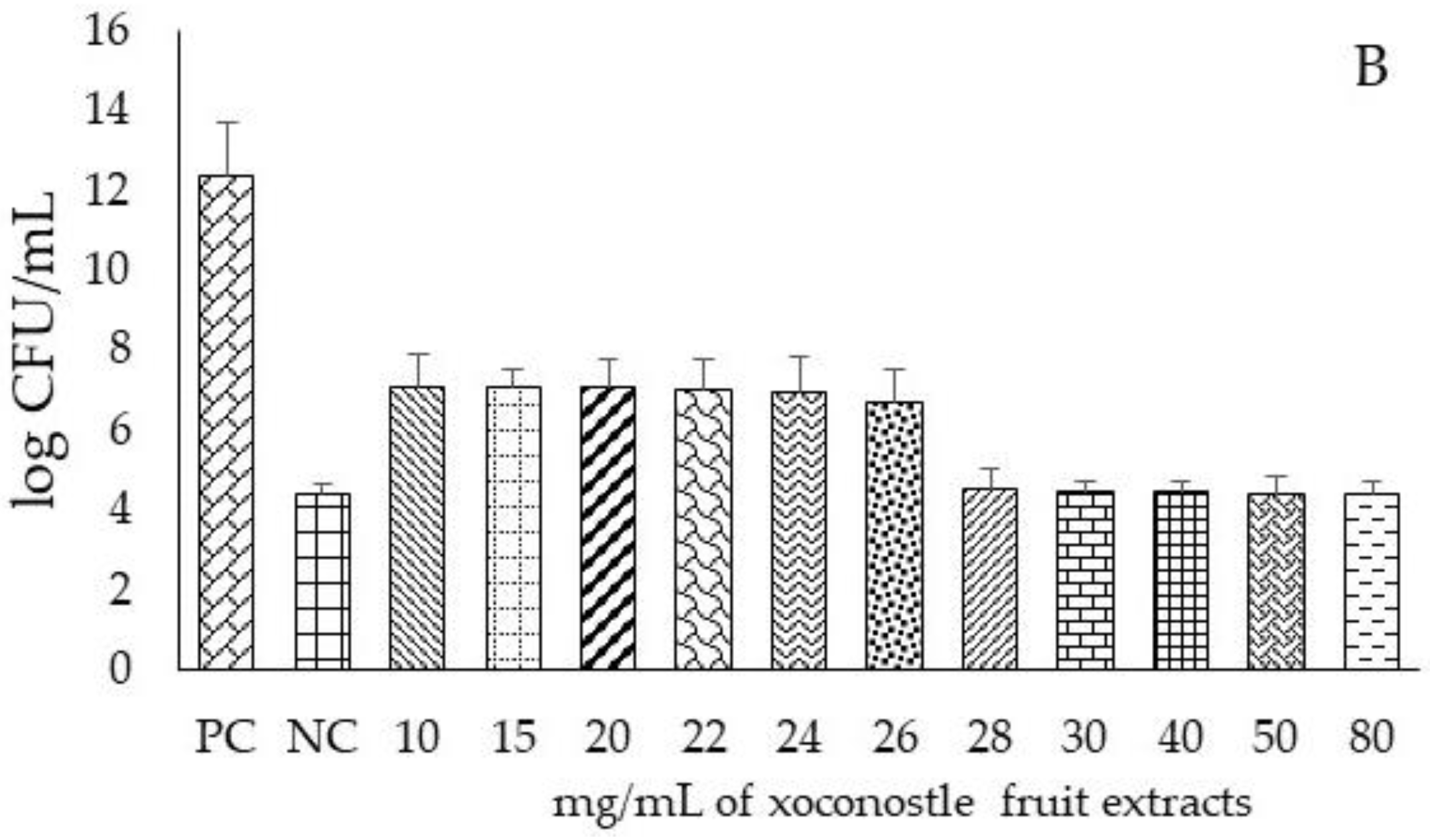
| Samples | pH | TSC (mgGluE/g DW) | RS (mgGluE/g DW) | ASC (mgASCE/100 g DW) |
|---|---|---|---|---|
| SA | 5.60 ± 0.17b | 158.20 ± 6.40c | 87.10 ± 1.28c | 267.30 ± 30.07b |
| SB | 6.55 ± 0.50a | 179.44 ± 7.02c | 116.01 ± 6.40c | 99.53 ± 24.73d |
| XA | 3.20 ± 0.35d | 503.00 ± 26.19b | 466.60 ± 16.81b | 443.70 ± 20.84a |
| XB | 3.90 ± 0.25c | 568.46 ± 19.20a | 517.84 ± 9.30a | 280.02 ± 16.18b |
| Samples | CIELAB Color Space | |||
|---|---|---|---|---|
| L* | a* | b* | ΔE | |
| SA | 35.39 ± 0.48a | 11.12 ± 0.12c | 43.23 ± 0.51b | 10.24 ± 0.43b |
| SB | 29.88 ± 0.25b | 14.88 ± 0.72b | 47.49 ± 0.71a | |
| XA | 24.38 ± 0.52c | 32.91 ± 0.25a | 25.32 ± 0.48c | 33.06 ± 1.76a |
| XB | 34.83 ± 0.38a | 11.33 ± 0.4c | 49.78 ± 0.99a | |
| Samples | TPC (mgGAE/g) | Flavonoids (mgQE/g) | Total Betalains | Antioxidant Activity | ||
|---|---|---|---|---|---|---|
| BC (mg/100 g) | VXC (mg/100 g) | DPPH (mgTE/g) | ABTS (mgTE/g) | |||
| SA | 99.25 ± 2.50b | 23.59 ± 0.5 b | ND | ND | 273.69 ± 2.93a | 310.68 ± 6.81b |
| SB | 194.31 ± 7.04a | 45.30 ± 0.24a | ND | ND | 269.38 ± 2.71b | 345.02 ± 10.57a |
| XA | 24.11 ± 1.21c | 13.62 ± 0.66d | 28.64 ± 0.96a | 11.92 ± 0.13b | 42.87 ± 0.96b | 26.72 ± 1.21c |
| XB | 23.53 ± 0.23c | 15.92 ± 0.24c | 4.24 ± 0.09b | 14.67 ± 0.40a | 31.81 ± 0.96c | 21.83 ± 1.49c |
| Samples | Phenolic Acid Standards | |||||||
|---|---|---|---|---|---|---|---|---|
| GA | CGA | SA | VA | PHA | CA | FA | PCA | |
| SA | ND | ND | ND | 1.98 ± 0.02b | ND | 0.08 ± 0.01b | ND | ND |
| SB | ND | ND | ND | 3.42 ± 0.13a | ND | 0.22 ± 0.04a | ND | ND |
| XA | 1.53 ± 0.06a | ND | ND | 1.63 ± 0.02c | ND | ND | ND | 15.52 ± 0.32a |
| XB | 1.42 ± 0.04b | ND | ND | 1.24 ± 0.08d | ND | ND | ND | 14.41 ± 0.21b |
| RP | 2.5 | 4.29 | 5.10 | 5.5 | 5.71 | 6.63 | 9.36 | 9.74 |
| Samples | Flavonoid Standards | |||||||
|---|---|---|---|---|---|---|---|---|
| RT | PHR | MC | QE | NG | PHT | AP | GLG | |
| SA | ND | ND | ND | ND | 25.45 ± 1.05b | ND | 0.96 ± 0.03b | 5.83 ± 1.09b |
| SB | ND | ND | ND | ND | 30.66 ± 2.96a | ND | 1.31 ± 0.08a | 8.69 ± 1.72a |
| XA | ND | ND | ND | ND | ND | ND | ND | 2.91 ± 0.05d |
| XB | ND | ND | ND | ND | ND | ND | ND | 3.58 ± 0.25c |
| RP | 4.4 | 6.77 | 7.57 | 10.5 | 12.25 | 13.18 | 14.63 | 22.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terrazas-Hernández, J.; Santos-López, E.M.; Cariño-Cortés, R.; Jiménez-Alvarado, R.; López-Palestina, C.U.; Hernández-Fuentes, A.D. Effects of Sterilization on Bioactives of Jatropha dioica and Opuntia oligacantha Extracts, and on Antimicrobial Capacity against Streptococcus mutans. Appl. Sci. 2018, 8, 2516. https://doi.org/10.3390/app8122516
Terrazas-Hernández J, Santos-López EM, Cariño-Cortés R, Jiménez-Alvarado R, López-Palestina CU, Hernández-Fuentes AD. Effects of Sterilization on Bioactives of Jatropha dioica and Opuntia oligacantha Extracts, and on Antimicrobial Capacity against Streptococcus mutans. Applied Sciences. 2018; 8(12):2516. https://doi.org/10.3390/app8122516
Chicago/Turabian StyleTerrazas-Hernández, Jorge, Eva M. Santos-López, Raquel Cariño-Cortés, Rubén Jiménez-Alvarado, César Uriel López-Palestina, and Alma Delia Hernández-Fuentes. 2018. "Effects of Sterilization on Bioactives of Jatropha dioica and Opuntia oligacantha Extracts, and on Antimicrobial Capacity against Streptococcus mutans" Applied Sciences 8, no. 12: 2516. https://doi.org/10.3390/app8122516
APA StyleTerrazas-Hernández, J., Santos-López, E. M., Cariño-Cortés, R., Jiménez-Alvarado, R., López-Palestina, C. U., & Hernández-Fuentes, A. D. (2018). Effects of Sterilization on Bioactives of Jatropha dioica and Opuntia oligacantha Extracts, and on Antimicrobial Capacity against Streptococcus mutans. Applied Sciences, 8(12), 2516. https://doi.org/10.3390/app8122516





