Gypsum-Dependent Effect of NaCl on Strength Enhancement of CaO-Activated Slag Binders
Abstract
1. Introduction
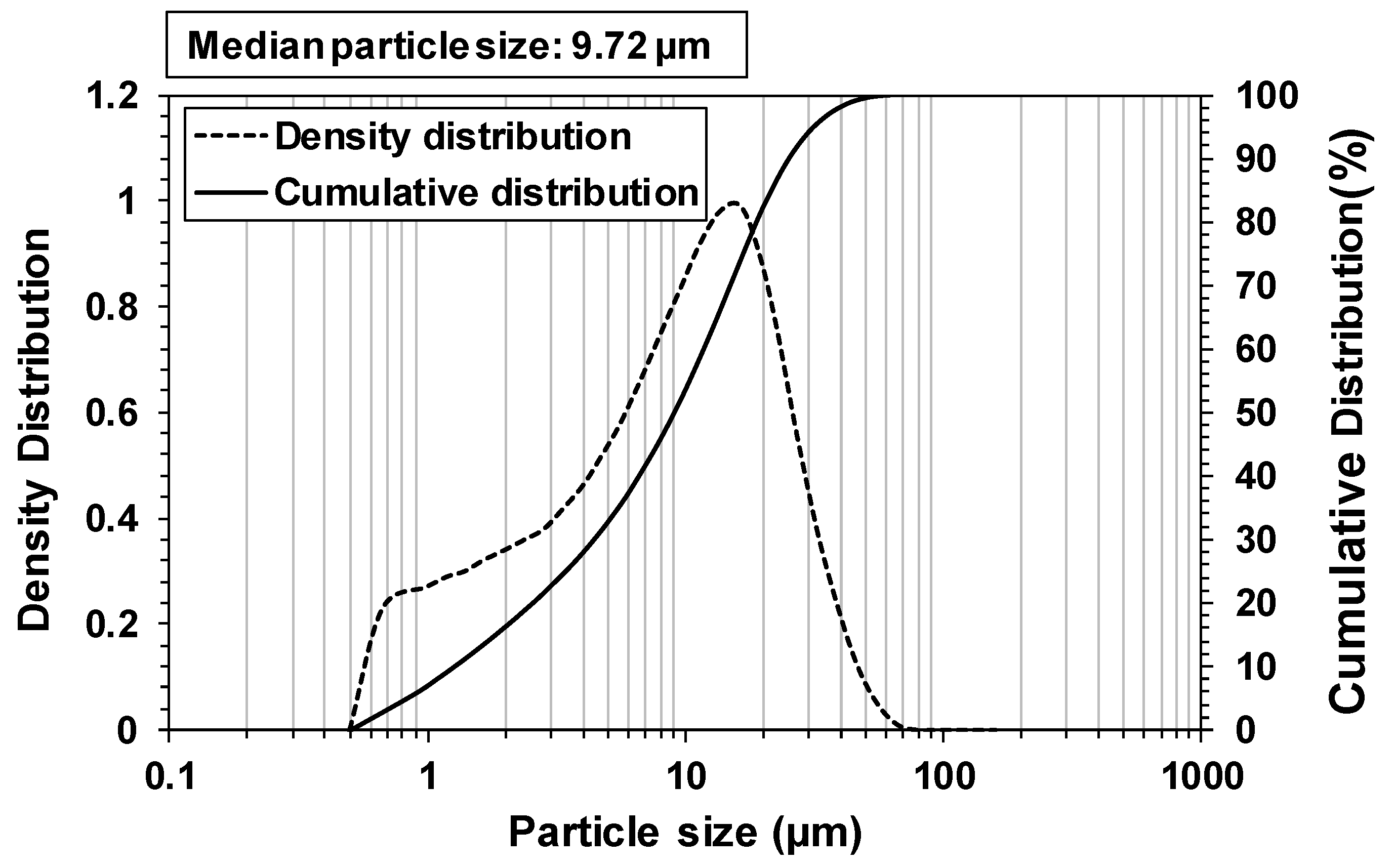
2. Materials and Methods
3. Results and Discussion
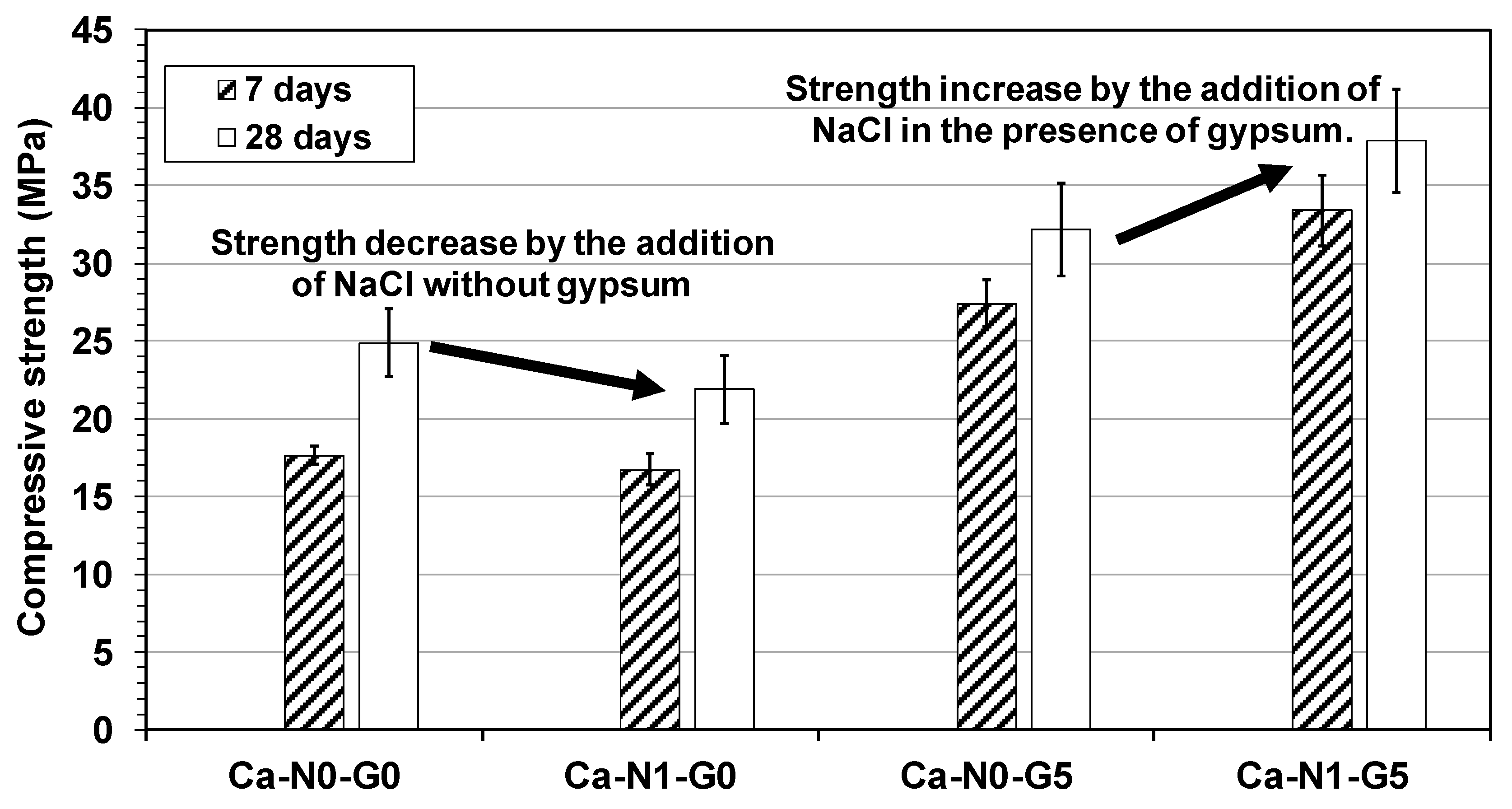
3.1. Compressive Strength
3.2. ANOVA Analysis
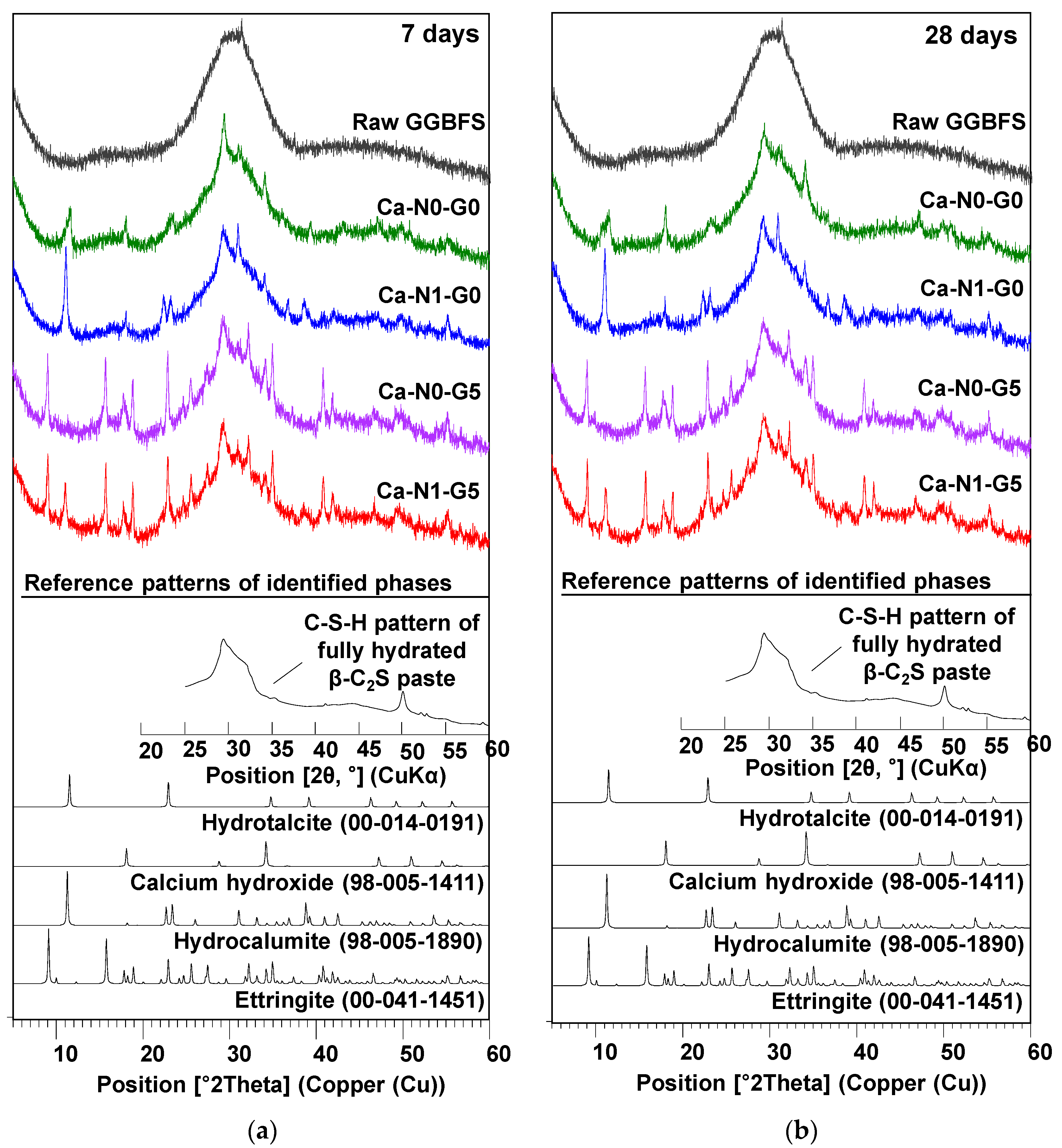
3.3. Powder X-Ray Diffraction (XRD)
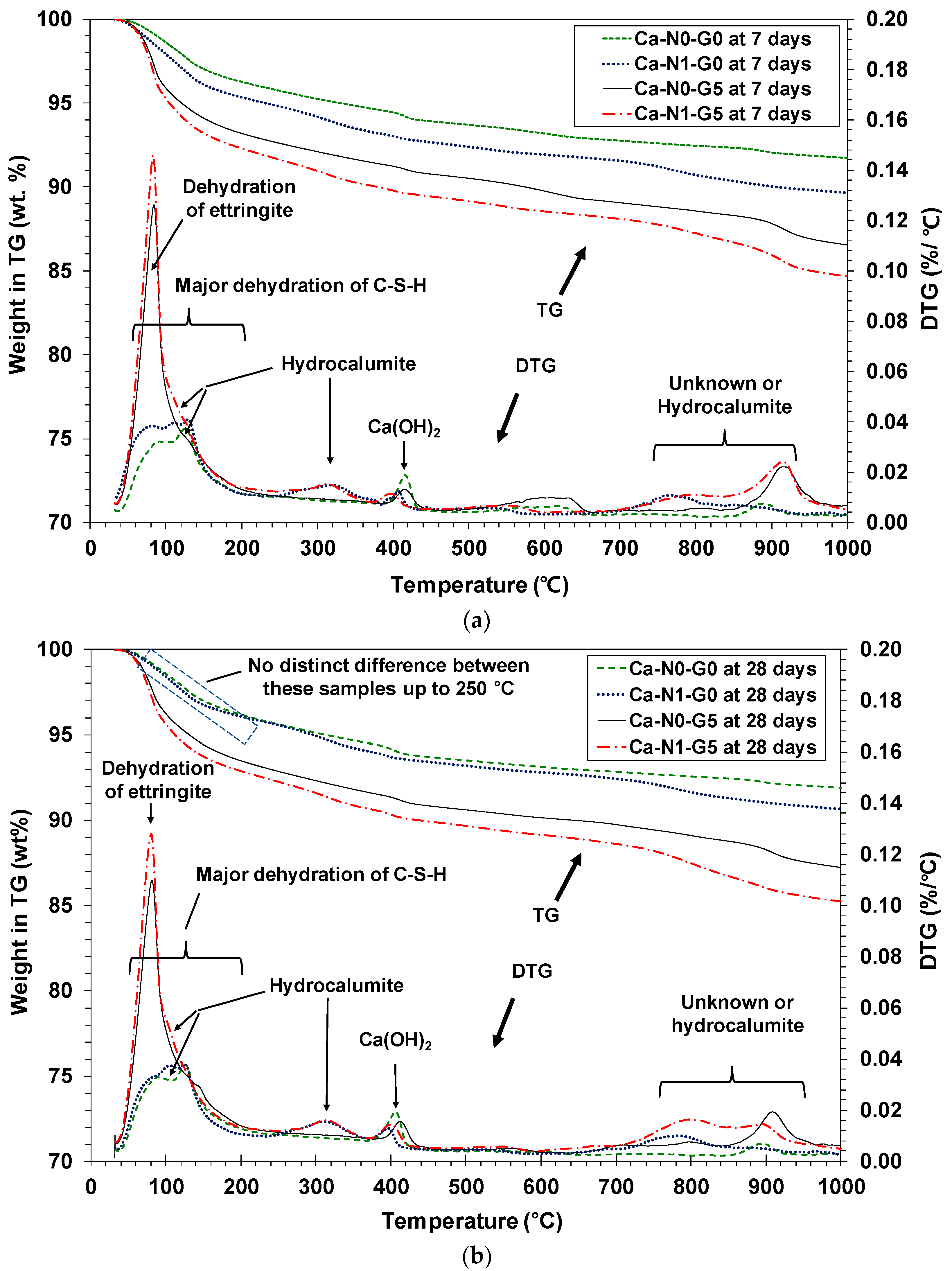
3.4. Thermogravimetric (TG) Analysis
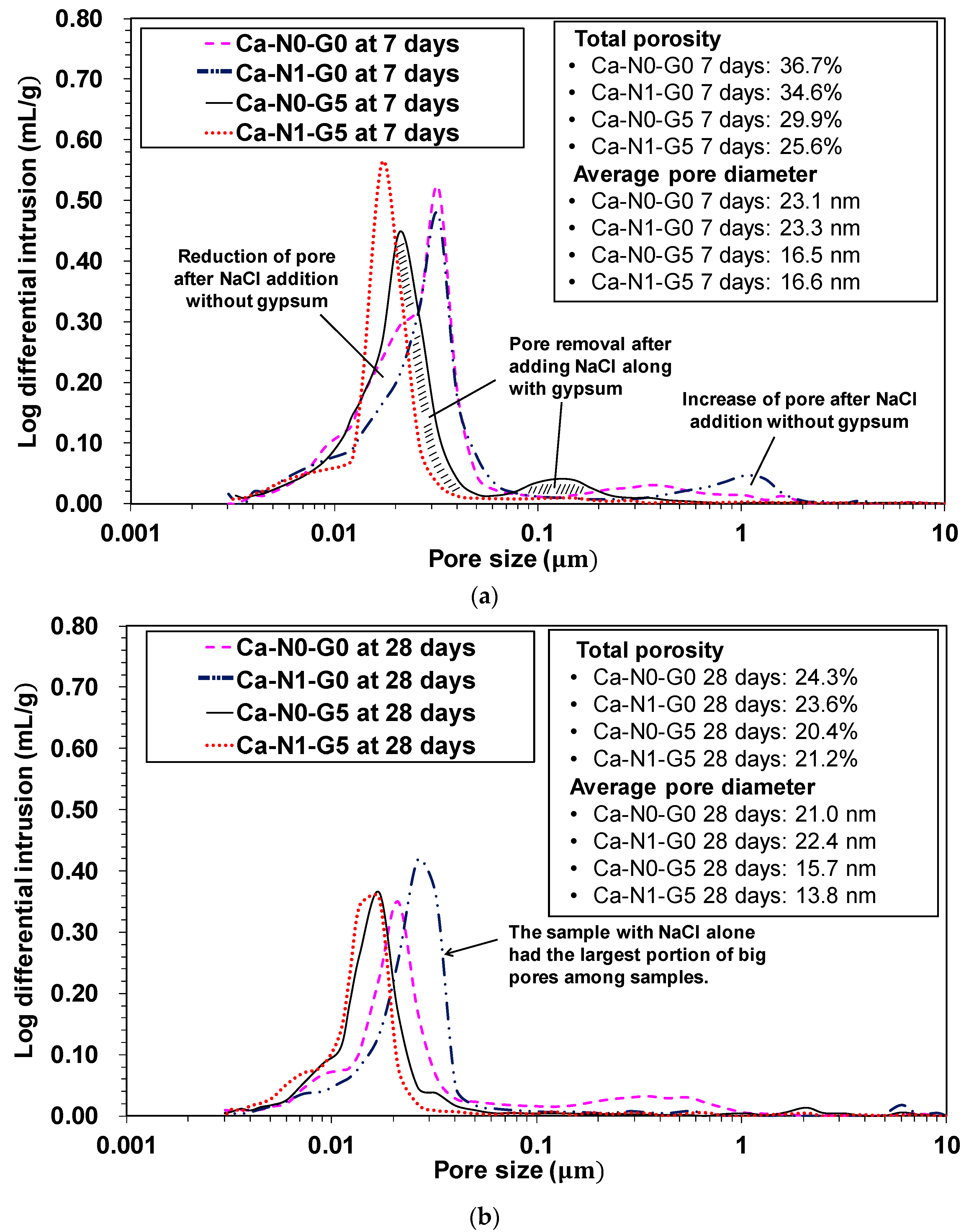
3.5. Mercury Intrusion Porosimetry (MIP)
4. Conclusions
- Considering that most strength gains of samples with gypsum were obtained by 7 days and the 7-day strength of Ca-N1-G5 was approximately 22% higher than that of Ca-N0-G5, the combined effect of NaCl with gypsum on strength seems to have mostly occurred before three days.
- In XRD and TG testing, the use of NaCl alone notably formed hydrocalumite, while the incorporation of only gypsum clearly produced ettringite. When NaCl and gypsum were used together, hydrocalumite and ettringite were simultaneously formed; however, no other type of new reaction product was identified.
- In TG/DTG testing, the use of NaCl likely boosted the formation of ettringite by notably increasing the concentration of sulfate and calcium ions in early days (i.e., before 7 days), resulting in the improvement of strength early in the process.
- In MIP testing, the use of NaCl did not yield a beneficial influence on pore size distribution for strength improvement at either 7 or 28 days when no gypsum was present. Rather, the use of NaCl hindered pore size refinement from 7 to 28 days. These observations are fairly consistent with their corresponding strength results. However, when gypsum was present, the use of NaCl yielded a significantly advantageous influence on pore size distribution; in particular, at 7 days, the addition of NaCl had removed relatively large pores, possibly due to the increased formation of ettringite.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bellmann, F.; Stark, J. Activation of blast furnace slag by a new method. Cem. Concr. Res. 2009, 39, 644–650. [Google Scholar] [CrossRef]
- Gu, K.; Jin, F.; Al-Tabbaa, A.; Shi, B. Activation of ground granulated blast furnace slag by using calcined dolomite. Constr. Build. Mater. 2014, 68, 252–258. [Google Scholar] [CrossRef]
- Shi, C.; Day, R.L. Chemical Activation of Lime-Slag Blends. Spec. Publ. 1995, 153, 1165–1178. [Google Scholar]
- Qing, Y.; Huxing, C.; Yuqing, W.; Shangxian, W.; Zonghan, L. Effect of MgO and gypsum content on long-term expansion of low heat Portland slag cement with slight expansion. Cem. Concr. Compos. 2004, 26, 331–337. [Google Scholar] [CrossRef]
- Wang, S.-D.; Scrivener, K.L. Hydration products of alkali activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Kim, M.S.; Jun, Y.; Lee, C.; Oh, J.E. Use of CaO as an activator for producing a price-competitive non-cement structural binder using ground granulated blast furnace slag. Cem. Concr. Res. 2013, 54, 208–214. [Google Scholar] [CrossRef]
- Taylor, H.F. Cement Chemistry; Thomas Telford: London, UK, 1997. [Google Scholar]
- Metha, K.; Monteiro, P. Concrete: Microstructure, Properties, and Materials, 3rd ed.; McGraw-Hill Publishing: New York, NY, USA, 2006. [Google Scholar]
- Wang, S.-D.; Scrivener, K.L.; Pratt, P. Factors affecting the strength of alkali-activated slag. Cem. Concr. Res. 1994, 24, 1033–1043. [Google Scholar] [CrossRef]
- Brough, A.; Holloway, M.; Sykes, J.; Atkinson, A. Sodium silicate-based alkali-activated slag mortars: Part II. The retarding effect of additions of sodium chloride or malic acid. Cem. Concr. Res. 2000, 30, 1375–1379. [Google Scholar] [CrossRef]
- Yang, K.-H.; Cho, A.-R.; Song, J.-K.; Nam, S.-H. Hydration products and strength development of calcium hydroxide-based alkali-activated slag mortars. Constr. Build. Mater. 2012, 29, 410–419. [Google Scholar] [CrossRef]
- Park, H.; Jeong, Y.; Jun, Y.; Jeong, J.-H.; Oh, J.E. Strength enhancement and pore-size refinement in clinker-free CaO-activated GGBFS systems through substitution with gypsum. Cem. Concr. Compos. 2016, 68, 57–65. [Google Scholar] [CrossRef]
- Yum, W.S.; Jeong, Y.; Yoon, S.; Jeon, D.; Jun, Y.; Oh, J.E. Effects of CaCl2 on hydration and properties of lime (CaO)-activated slag/fly ash binder. Cem. Concr. Compos. 2017, 84, 111–123. [Google Scholar] [CrossRef]
- Jeong, Y.; Yum, W.S.; Jeon, D.; Oh, J.E. Strength development and microstructural characteristics of barium hydroxide-activated ground granulated blast furnace slag. Cem. Concr. Compos. 2017, 79, 34–44. [Google Scholar] [CrossRef]
- Delagrave, A.; Marchand, J.; Ollivier, J.-P.; Julien, S.; Hazrati, K. Chloride binding capacity of various hydrated cement paste systems. Adv. Cem. Based Mater. 1997, 6, 28–35. [Google Scholar] [CrossRef]
- Yuan, Q.; Shi, C.; De Schutter, G.; Audenaert, K.; Deng, D. Chloride binding of cement-based materials subjected to external chloride environment–a review. Constr. Build. Mater. 2009, 23, 1–13. [Google Scholar] [CrossRef]
- Al-Akhras, N.M. Durability of metakaolin concrete to sulfate attack. Cem. Concr. Res. 2006, 36, 1727–1734. [Google Scholar] [CrossRef]
- Al-Amoudi, O.S.B.; Maslehuddin, M.; Saadi, M.M. Effect of magnesium sulfate and sodium sulfate on the durability performance of plain and blended cements. ACI Mater. J. 1995, 92, 15–24. [Google Scholar]
- Papadakis, V.G. Effect of supplementary cementing materials on concrete resistance against carbonation and chloride ingress. Cem. Concr. Res. 2000, 30, 291–299. [Google Scholar] [CrossRef]
- Al-Dulaijan, S.U.; Maslehuddin, M.; Al-Zahrani, M.; Sharif, A.; Shameem, M.; Ibrahim, M. Sulfate resistance of plain and blended cements exposed to varying concentrations of sodium sulfate. Cem. Concr. Compos. 2003, 25, 429–437. [Google Scholar] [CrossRef]
- Al-Amoudi, O.S.B.; Maslehuddin, M.; Abdul-Al, Y.A. Role of chloride ions on expansion and strength reduction in plain and blended cements in sulfate environments. Constr. Build. Mater. 1995, 9, 25–33. [Google Scholar] [CrossRef]
- Geng, J.; Easterbrook, D.; Li, L.-Y.; Mo, L.-W. The stability of bound chlorides in cement paste with sulfate attack. Cem. Concr. Res. 2015, 68, 211–222. [Google Scholar] [CrossRef]
- Sotiriadis, K.; Nikolopoulou, E.; Tsivilis, S. Sulfate resistance of limestone cement concrete exposed to combined chloride and sulfate environment at low temperature. Cem. Concr. Compos. 2012, 34, 903–910. [Google Scholar] [CrossRef]
- Madgin, W.; Swales, D. Solubilities in the system CaSO4-NaCl-H2O at 25° and 35°. J. Chem. Technol. Biotechnol. 1956, 6, 482–487. [Google Scholar] [CrossRef]
- Johnston, J.; Grove, C. The solubility of calcium hydroxide in aqueous salt solutions. J. Am. Chem. Soc. 1931, 53, 3976–3991. [Google Scholar] [CrossRef]
- American Society for Testing Materials. Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens); ASTM C109/C109M-13; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Olson, R.A.; Neubauer, C.M.; Jennings, H.M. Damage to the pore structure of hardened Portland cement paste by mercury intrusion. J. Am. Ceram. Soc. 1997, 80, 2454–2458. [Google Scholar] [CrossRef]
- Diamond, S. Mercury porosimetry: An inappropriate method for the measurement of pore size distributions in cement-based materials. Cem. Concr. Res. 2000, 30, 1517–1525. [Google Scholar] [CrossRef]
- Gallé, C. Effect of drying on cement-based materials pore structure as identified by mercury intrusion porosimetry: A comparative study between oven-, vacuum-, and freeze-drying. Cem. Concr. Res. 2001, 31, 1467–1477. [Google Scholar] [CrossRef]
- Morandeau, A.; Thiéry, M.; Dangla, P. Impact of accelerated carbonation on OPC cement paste blended with fly ash. Cem. Concr. Res. 2015, 67, 226–236. [Google Scholar] [CrossRef]
- Gallé, C. Reply to the discussion by S. Diamond of the paper “Effect of drying on cement-based materials pore structure as identified by mercury intrusion porosimetry: A comparative study between oven-, vacuum- and freeze-drying”. Cem. Concr. Res. 2003, 33, 171–172. [Google Scholar] [CrossRef]
- Neter, J.; Kutner, M.H.; Nachtsheim, C.J.; Wasserman, W. Applied Linear Statistical Models; Irwin: Chicago, IL, USA, 1996; Volume 4. [Google Scholar]
- PANalytical, B. X’Pert HighScore Plus Software; Version 3.0 e; PANanalytical: Almelo, The Netherlands, 2012. [Google Scholar]
- Allmann, R.; Hinek, R. The introduction of structure types into the Inorganic Crystal Structure Database ICSD. Acta Crystallogr. Sect. A Found. Crystallogr. 2007, 63, 412–417. [Google Scholar] [CrossRef]
- Mohan, K.; Taylor, H. Analytical Electron Microscopy of Cement Pastes: IV, β-Dicalcium Silicate Pastes. J. Am. Ceram. Soc. 1981, 64, 717–719. [Google Scholar] [CrossRef]
- Ke, X.; Bernal, S.A.; Provis, J.L. Uptake of chloride and carbonate by Mg-Al and Ca-Al layered double hydroxides in simulated pore solutions of alkali-activated slag cement. Cem. Concr. Res. 2017, 100, 1–13. [Google Scholar] [CrossRef]
- Gruskovnjak, A.; Lothenbach, B.; Winnefeld, F.; Figi, R.; Ko, S.-C.; Adler, M.; Mäder, U. Hydration mechanisms of super sulphated slag cement. Cem. Concr. Res. 2008, 38, 983–992. [Google Scholar] [CrossRef]
- Andrejkovičová, S.; Alves, C.; Velosa, A.; Rocha, F. Bentonite as a natural additive for lime and lime–metakaolin mortars used for restoration of adobe buildings. Cem. Concr. Compos. 2015, 60, 99–110. [Google Scholar] [CrossRef]
- Jun, Y.; Yoon, S.; Oh, J.E. A Comparison Study for Chloride-Binding Capacity between Alkali-Activated Fly Ash and Slag in the Use of Seawater. Appl. Sci. 2017, 7, 971. [Google Scholar] [CrossRef]
- Jeon, D.; Yum, W.S.; Jeong, Y.; Oh, J.E. Properties of quicklime (CaO)-activated Class F fly ash with the use of CaCl2. Cem. Concr. Res. 2018, 11, 147–156. [Google Scholar] [CrossRef]
- Michel, M.; Georgin, J.-F.; Ambroise, J.; Péra, J. The influence of gypsum ratio on the mechanical performance of slag cement accelerated by calcium sulfoaluminate cement. Constr. Build. Mater. 2011, 25, 1298–1304. [Google Scholar] [CrossRef]
- Neto, A.A.M.; Cincotto, M.A.; Repette, W. Mechanical properties, drying and autogenous shrinkage of blast furnace slag activated with hydrated lime and gypsum. Cem. Concr. Compos. 2010, 32, 312–318. [Google Scholar] [CrossRef]
- Shi, C.; Day, R.L. Chemical activation of blended cements made with lime and natural pozzolans. Cem. Concr. Res. 1993, 23, 1389–1396. [Google Scholar] [CrossRef]
- Khan, M.; Kayali, O.; Troitzsch, U. Chloride binding capacity of hydrotalcite and the competition with carbonates in ground granulated blast furnace slag concrete. Mater. Struct. 2016, 49, 4609–4619. [Google Scholar] [CrossRef]
- Bejoy, N. Hydrotalcite. Resonance 2001, 6, 57–61. [Google Scholar] [CrossRef]
- Kayali, O.; Khan, M.; Ahmed, M.S. The role of hydrotalcite in chloride binding and corrosion protection in concretes with ground granulated blast furnace slag. Cem. Concr. Compos. 2012, 34, 936–945. [Google Scholar] [CrossRef]
- Glasser, F.; Kindness, A.; Stronach, S. Stability and solubility relationships in AFm phases: Part I. Chloride, sulfate and hydroxide. Cem. Concr. Res. 1999, 29, 861–866. [Google Scholar] [CrossRef]
- Zhang, L.; Glasser, F. Critical examination of drying damage to cement pastes. Adv. Cem. Res. 2000, 12, 79–88. [Google Scholar] [CrossRef]
- Galan, I.; Beltagui, H.; García-Maté, M.; Glasser, F.P.; Imbabi, M.S. Impact of drying on pore structures in ettringite-rich cements. Cem. Concr. Res. 2016, 84, 85–94. [Google Scholar] [CrossRef]
- Zhou, Q.; Glasser, F.P. Thermal stability and decomposition mechanisms of ettringite at <120 °C. Cem. Concr. Res. 2001, 31, 1333–1339. [Google Scholar]
- Bakolas, A.; Aggelakopoulou, E.; Moropoulou, A.; Anagnostopoulou, S. Evaluation of pozzolanic activity and physicomechanical characteristics in metakaolin-lime pastes. J. Therm. Anal. Calorim. 2006, 84, 157–163. [Google Scholar] [CrossRef]
- Sha, W.; Pereira, G. Differential scanning calorimetry study of hydrated ground granulated blast-furnace slag. Cem. Concr. Res. 2001, 31, 327–329. [Google Scholar] [CrossRef]
- Song, H.; Jeong, Y.; Bae, S.; Jun, Y.; Yoon, S.; Eun Oh, J. A study of thermal decomposition of phases in cementitious systems using HT-XRD and TG. Constr. Build. Mater. 2018, 169, 648–661. [Google Scholar] [CrossRef]
- Vieille, L.; Rousselot, I.; Leroux, F.; Besse, J.-P.; Taviot-Guého, C. Hydrocalumite and its polymer derivatives. 1. Reversible thermal behavior of Friedel’s salt: A direct observation by means of high-temperature in situ powder X-ray diffraction. Chem. Mater. 2003, 15, 4361–4368. [Google Scholar] [CrossRef]
- Wei, Y.; Yao, W.; Xing, X.; Wu, M. Quantitative evaluation of hydrated cement modified by silica fume using QXRD, 27 Al MAS NMR, TG–DSC and selective dissolution techniques. Constr. Build. Mater. 2012, 36, 925–932. [Google Scholar] [CrossRef]
- Singh, M.; Garg, M. Calcium sulfate hemihydrate activated low heat sulfate resistant cement. Constr. Build. Mater. 2002, 16, 181–186. [Google Scholar] [CrossRef]
- Glasser, F.; Pedersen, J.; Goldthorpe, K.; Atkins, M. Solubility reactions of cement components with NaCl solutions: I. Ca(OH)2 and CSH. Adv. Cem. Res. 2005, 17, 57–64. [Google Scholar] [CrossRef]
- Nyame, B.; Illston, J. Capillary pore structure and permeability of hardened cement paste. In Proceedings of the 7th International Congress on the Chemistry of Cement, Paris, France, 30 June–4 July 1980. [Google Scholar]
- Kendall, K.; Howard, A.; Birchall, J.D. The relation between porosity, microstructure and strength, and the approach to advanced cement-based materials. Philos. Trans. R. Soc. Lond. A 1983, 310, 139–153. [Google Scholar] [CrossRef]
- Luping, T. A study of the quantitative relationship between strength and pore-size distribution of porous materials. Cem. Concr. Res. 1986, 16, 87–96. [Google Scholar] [CrossRef]
| Formula | CaO | SiO2 | Al2O3 | MgO | SO3 | TiO2 | K2O | Fe2O3 | Na2O | MnO |
|---|---|---|---|---|---|---|---|---|---|---|
| Oxide content | 44.3 | 34.3 | 14.3 | 3.3 | 1.4 | 0.7 | 0.6 | 0.5 | 0.3 | 0.3 |
| Sample Label | Binder | Water | |||
|---|---|---|---|---|---|
| GGBFS | CaO | NaCl | Gypsum | ||
| Ca-N0-G0 (Control) | 95 | 5 | 0 | 0 | 35 |
| Ca-N0-G5 | 90 | 5 | 0 | 5 | 35 |
| Ca-N1-G0 | 94 | 5 | 1 | 0 | 35 |
| Ca-N1-G5 | 89 | 5 | 1 | 5 | 35 |
| Factor | p-Value | |
|---|---|---|
| 7 Days | 28 Days | |
| NaCl content | 0.000461 | 0.2349 |
| Gypsum content | <0.00001 | <0.00001 |
| Gypsum–NaCl interaction | 0.00014 | 0.00082 |
| Sample Label | Reaction Products | ||||
|---|---|---|---|---|---|
| C-S-H | Hydrotalcite | Ca(OH)2 | Ettringite | Hydrocalumite | |
| Ca-N0-G0 (Control) | O | O | O | X | X |
| Ca-N1-G0 | O | X | O | X | O |
| Ca-N0-G5 | O | X | O | O | X |
| Ca-N1-G5 | O | X | O | O | O |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, S.; Lee, H.; Jeon, D.; Song, H.; Yum, W.S.; Kim, D.; Suh, J.-I.; Oh, J.E. Gypsum-Dependent Effect of NaCl on Strength Enhancement of CaO-Activated Slag Binders. Appl. Sci. 2018, 8, 2515. https://doi.org/10.3390/app8122515
Sim S, Lee H, Jeon D, Song H, Yum WS, Kim D, Suh J-I, Oh JE. Gypsum-Dependent Effect of NaCl on Strength Enhancement of CaO-Activated Slag Binders. Applied Sciences. 2018; 8(12):2515. https://doi.org/10.3390/app8122515
Chicago/Turabian StyleSim, Sungwon, Hwan Lee, Dongho Jeon, Haemin Song, Woo Sung Yum, Dohoon Kim, Jung-Il Suh, and Jae Eun Oh. 2018. "Gypsum-Dependent Effect of NaCl on Strength Enhancement of CaO-Activated Slag Binders" Applied Sciences 8, no. 12: 2515. https://doi.org/10.3390/app8122515
APA StyleSim, S., Lee, H., Jeon, D., Song, H., Yum, W. S., Kim, D., Suh, J.-I., & Oh, J. E. (2018). Gypsum-Dependent Effect of NaCl on Strength Enhancement of CaO-Activated Slag Binders. Applied Sciences, 8(12), 2515. https://doi.org/10.3390/app8122515






