L-DOPA Trends in Different Tissues at Early Stages of Vicia faba Growth: Effect of Tyrosine Treatment
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Plant Extracts
2.3. Enzyme Assays
2.3.1. TYROX
2.3.2. GPX
2.4. Growth Rate Record
2.5. L-DOPA Content in V. faba
2.6. Statistical Analysis
3. Results and Discussion
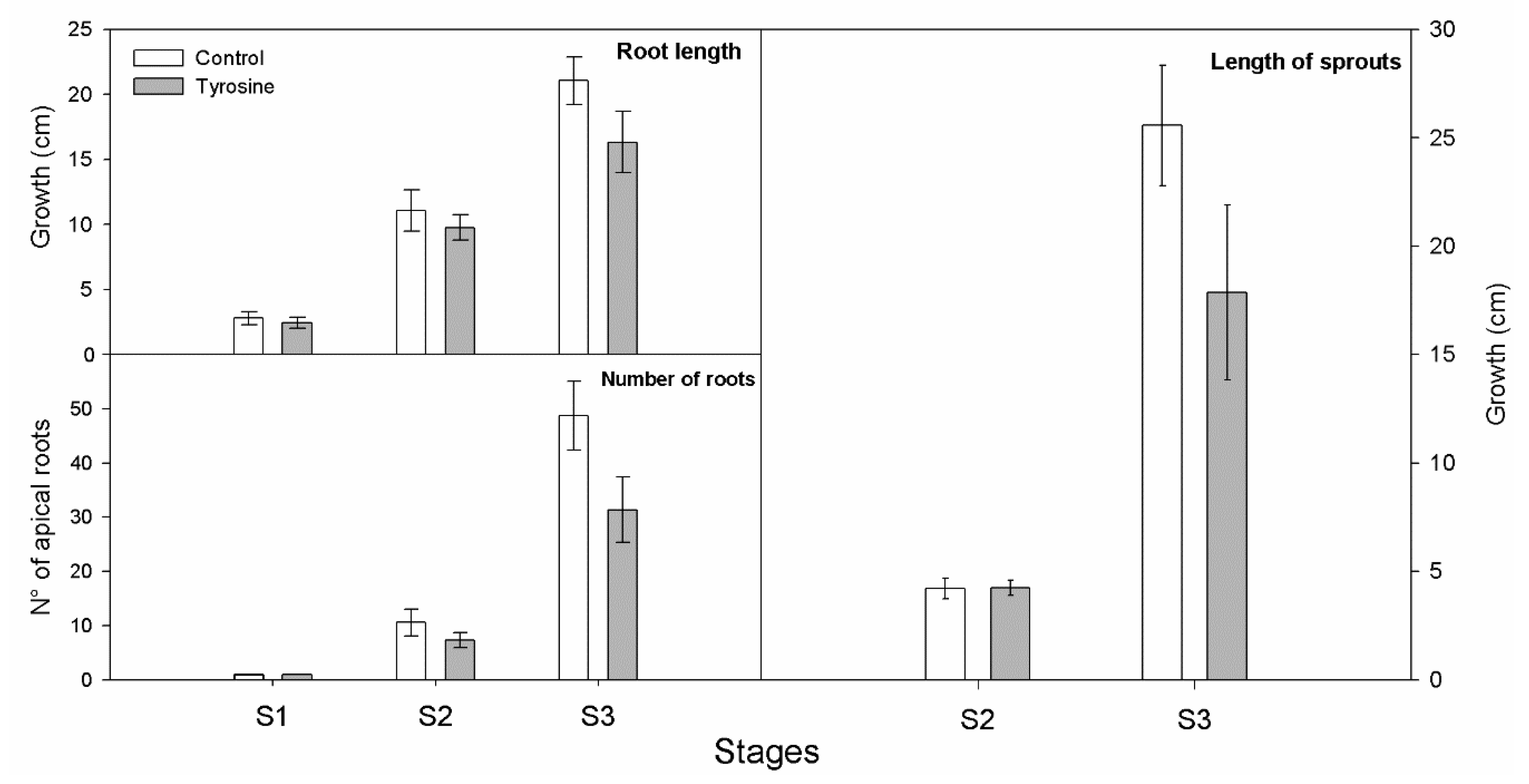
3.1. L-DOPA Content
3.2. Comparison of Methods for L-DOPA Determination
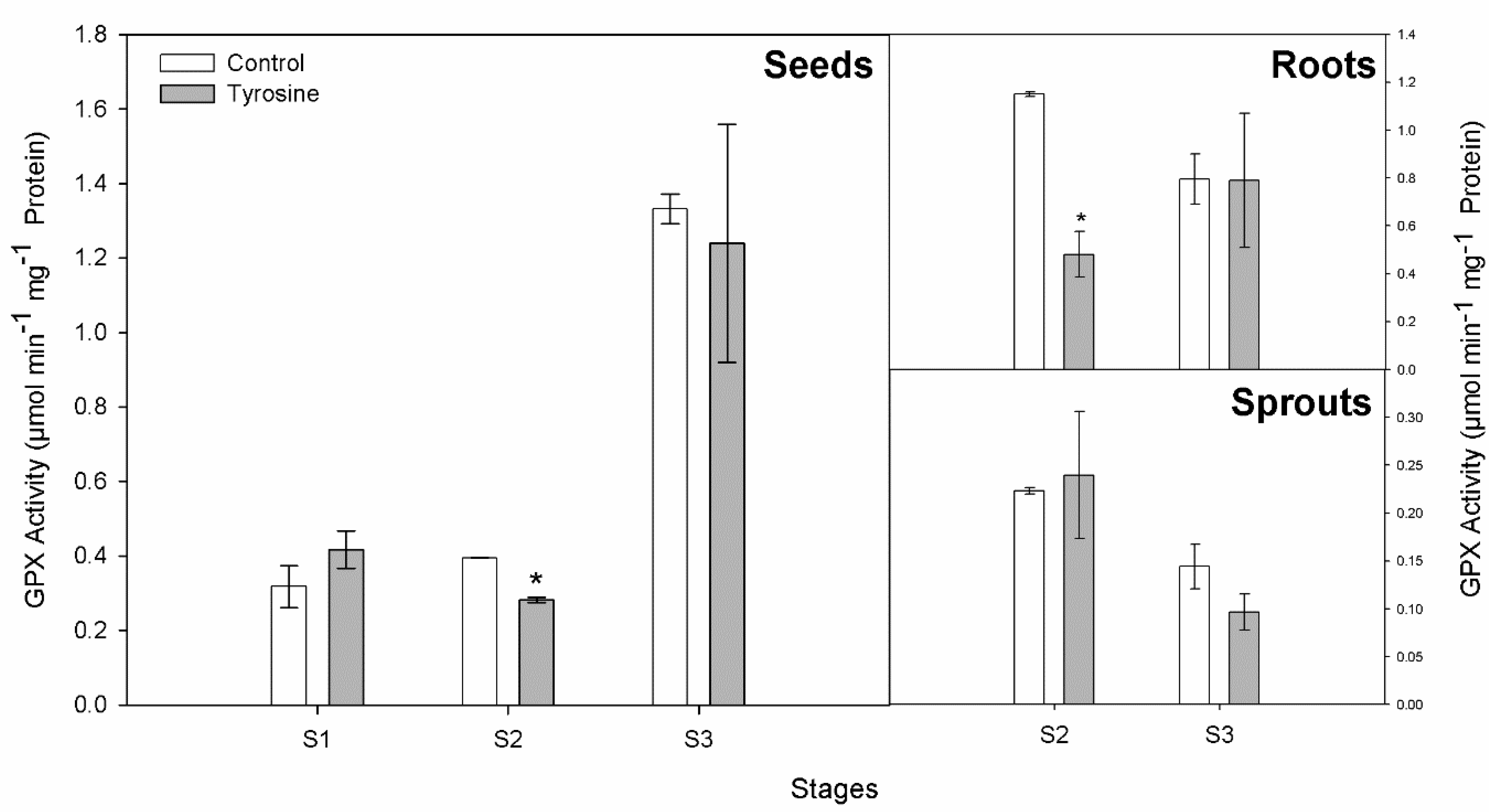
3.3. GPX and TYROX Activities
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Patil, S.A.; Apine, O.A.; Surwase, S.N.; Jadhav, J.P. Biological sources of L-DOPA: An alternative approach. Adv. Parkinson Dis. 2013, 2, 81–87. [Google Scholar] [CrossRef]
- Gautam, M.; Chandel, M.; Azmi, W. Therapeutic role of L-DOPA produced as a secondary metabolite from different legumes and plant sources. Ann. Phytomed. 2012, 1, 1–8. [Google Scholar]
- Rios, M.; Habecker, B.; Sasaoka, T.; Eisenhofer, G.; Tian, H.; Landis, S.; Roffler-Tarlov, S. Catecholamine synthesis is mediated by tyrosinase in the absence of tyrosine hydroxylase. J. Neurosci. 1999, 19, 3519–3526. [Google Scholar] [CrossRef] [PubMed]
- Gish, M.; Mescher, M.C.; De Moraes, C.M. Targeted predation of extrafloral nectaries by insects despite localized chemical defenses. Proc. R. Soc. B 2015, 282, 20151835. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N. L-DOPA Inhibited Early Root Growth in Rice Involves Biochemical Alterations in Macromolecules and Associated Hydrolytic Enzymes. Ann. Plant Sci. 2015, 4, 1109–1115. [Google Scholar]
- Soares, A.R.; Marchiosi, R.; Siqueira-Soares, R.D.; Barbosa de Lima, R.; Dantas dos Santos, W.; Ferrarese-Filho, O. The role of L-DOPA in plants. Plant Signal. Behav. 2014, 9, 28275. [Google Scholar] [CrossRef] [PubMed]
- Shetty, P.; Atallah, M.T.; Shetty, K. Enhancement of total phenolic, L-DOPA and proline contents in germinating fava bean (Vicia faba) in response to bacterial elicitors. Food Biotechnol. 2001, 15, 47–67. [Google Scholar] [CrossRef]
- Inamdar, S.; Joshi, S.; Jadhav, J.; Bapat, V. Innovative use of intact seeds of Mucuna monosperma Wight for improved yield of L-DOPA. Nat. Prod. Bioprospecting 2012, 2, 16–20. [Google Scholar] [CrossRef]
- Mohseni Mehran, S.M.; Golshani, B. Simultaneous Determination of Levodopa and Carbidopa from Fava Bean, Green Peas and Green Beans by High Performance Liquid Gas Chromatography. J. Clin. Diagn. Res. 2013, 7, 1004–1007. [Google Scholar] [CrossRef]
- Burbano, C.; Cuadrado, C.; Muzquiz, M.; Cubero, J.I. Variation of favism-inducing factors (vicine, convicine and L-DOPA) during pod development in Vicia faba L. Plant Foods Hum. Nutr. 1995, 47, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Goyoaga, C.; Burbano, C.; Cuadrado, C.; Varela, A.; Guillamón, E.; Pedrosa, M.; Muzquiz, M. Content and distribution of vicine, convicine and L-DOPA during germination and seedling growth of two Vicia faba L. varieties. Eur. Food Res. Technol. 2008, 227, 1537–1542. [Google Scholar] [CrossRef]
- Raghavendra, S.; Ramesh, C.K.; Kumar, V.; Moinuddin Khan, M.H. Elicitors and precursor induced effect on L-DOPA production in suspension cultures of Mucuna pruriens L. Front. Life Sci. 2011, 5, 127–133. [Google Scholar] [CrossRef]
- Schenck, C.A.; Maeda, H.A. Tyrosine biosynthesis, metabolism, and catabolism in plants. Phytochemistry 2018, 149, 82–102. [Google Scholar] [CrossRef] [PubMed]
- Yoruk, R.; Marshall, M.R. A survey on the potential mode of inhibition for oxalic acid on polyphenol oxidase. J. Food Sci. 2003, 68, 2479–2485. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Siqueira-Soares, R.D.; Soares, A.R.; Parizotto, A.V.; Ferrarese, M.D.; Ferrarese-Filho, O. Root growth and enzymes related to the lignification of maize seedlings exposed to the allelochemical L-DOPA. Sci. World J. 2013, 2013, 134237. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Sulaiman, M.; Beckett, R.P.; Minibayeva, F.V. Cell wall peroxidases in the liverwort Dumortiera hirsuta are responsible for extracellular superoxide production, and can display tyrosinase activity. Physiol. Plant. 2010, 138, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef]
- Saeidian, S. Kinetics properties of polyphenol oxidase in hawthorn (Crataegus spp.). Int. Lett. Nat. Sci. 2014, 25, 29–38. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Daudi, A.; Butt, V.S.; Bolwell, G.P. Reactive oxygen species and their role inplant defense and cell wall metabolism. Planta 2012, 236, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Randhir, R.; Shetty, P.; Shetty, K. L-DOPA and total phenolic stimulation in dark germinated fava bean in response to peptide and phytochemical elicitors. Process Biochem. 2002, 37, 1247–1256. [Google Scholar] [CrossRef]
- Kampmann, M.; Boll, S.; Kossuch, J.; Bielecki, J.; Uhl, S.; Kleiner, B.; Wichmann, R. Efficient immobilization of mushroom tyrosinase utilizing whole cells from Agaricus bisporus and its application for degradation of bisphenol A. Water Res. 2014, 57, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Kao, C.H. NaCl induced changes in ionically bound peroxidase activity in roots of rice seedlings. Plant Soil 1999, 216, 147. [Google Scholar] [CrossRef]
- Jebara, S.; Jebara, M.; Limam, F.; Aouani, M.E. Changes in ascorbate peroxidase, catalase, guaiacol peroxidase and superoxide dismutase activities in common bean (Phaseolus vulgaris) nodules under salt stress. J. Plant Physiol. 2005, 162, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Arnow, L.E. Colorimetric determination of the components of 3,4-dihydroxyphenylalanine-tyrosine mixtures. J. Biol. Chem. 1937, 118, 531–537. [Google Scholar]
- Shetty, P.; Atallah, M.T.; Shetty, K. Effects of UV treatment on the proline-linked pentose phosphate pathway for phenolics and L-DOPA synthesis in dark germinated Vicia faba. Process Biochem. 2002, 37, 1285–1295. [Google Scholar] [CrossRef]
- Vattem, D.; Randhir, R.; Shetty, K. Cranberry phenolics-mediated elicitation of antioxidant enzyme response in fava bean (Vicia faba) sprouts. J. Food Biochem. 2005, 29, 41–70. [Google Scholar] [CrossRef]
- Ray, H.; Georges, F. A genomic approach to nutritional, pharmacological and genetic issues of faba bean (Vicia faba): Prospects for genetic modifications. GM Crops 2010, 1, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Randhir, R.; Shetty, K. Light-mediated fava bean (Vicia faba) response to phytochemical and protein elicitors and consequences on nutraceutical enhancement and seed vigour. Process Biochem. 2003, 38, 945–952. [Google Scholar] [CrossRef]
- Wichers, H.J.; Wijnsma, R.; Visser, J.F.; Malingré, T.M.; Huizing, H.J. Production of L-DOPA by cell suspension cultures of Mucuna pruriens. Plant Cell Tissue Organ Cult. 1985, 4, 75–82. [Google Scholar] [CrossRef]
- Etemadi, F.; Hashemi, M.; Randhir, R.; ZandVakili, O.; Ebadi, A. Accumulation of L-DOPA in various organs of faba bean and influence of drought, nitrogen stress, and processing methods on L-DOPA yield. Crop J. 2018. [CrossRef]
- Fujii, Y.; Shibuya, T.; Yasuda, T. L-3, 4-dihydroxyphenylalanine as an allelochemical candidate from Mucuna pruriens (L.) DC. var. utilis. Agric. Boil. Chem. 1991, 55, 617–618. [Google Scholar] [CrossRef]
- Prakash, D.; Abhishek Niranjan, S.K.; Tewari, D. Some nutritional properties of the seeds of three Mucuna species. Int. J. Food Sci. Nutr. 2001, 52, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Siddhuraju, P.; Becker, K. Rapid reversed-phase high performance liquid chromatographic method for the quantification of L-DOPA (L-3,4-dihydroxyphenylalanine), non-methylated and methylated tetrahydroisoquinoline compounds from Mucuna beans. Food Chem. 2001, 72, 389–394. [Google Scholar] [CrossRef]
- Ramya, K.B.; Thaakur, S. Herbs containing L-DOPA: An update. Anc. Sci. Life 2007, 27, 50. [Google Scholar] [PubMed]
- Hildebrandt, T.M.; Nesi, A.N.; Araújo, W.L.; Braun, H.P. Amino acid catabolism in plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Ellis, B.E. Catabolic ring-cleavage of tyrosine in plant cell cultures. Planta 1973, 111, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Rani, N.; Joy, B.; Abraham, T.E. Cell suspension cultures of Portulaca grandiflora. as potent catalysts for biotransformation of L-tyrosine into L-DOPA, an anti-Parkinson’s drug. Pharm. Boil. 2007, 45, 48–53. [Google Scholar] [CrossRef]
- Nadgórska-Socha, A.; Kafel, A.; Kandziora-Ciupa, M.; Gospodarek, J.; Zawisza-Raszka, A. Accumulation of heavy metals and antioxidant responses in Vicia faba plants grown on monometallic contaminated soil. Environ. Sci. Pollut. Res. 2013, 20, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Kabbadj, A.; Makoudi, B.; Mouradi, M.; Pauly, N.; Frendo, P.; Ghoulam, C. Physiological and biochemical responses involved in water deficit tolerance of nitrogen-fixing Vicia faba. PLoS ONE 2017, 12, e0190284. [Google Scholar] [CrossRef] [PubMed]
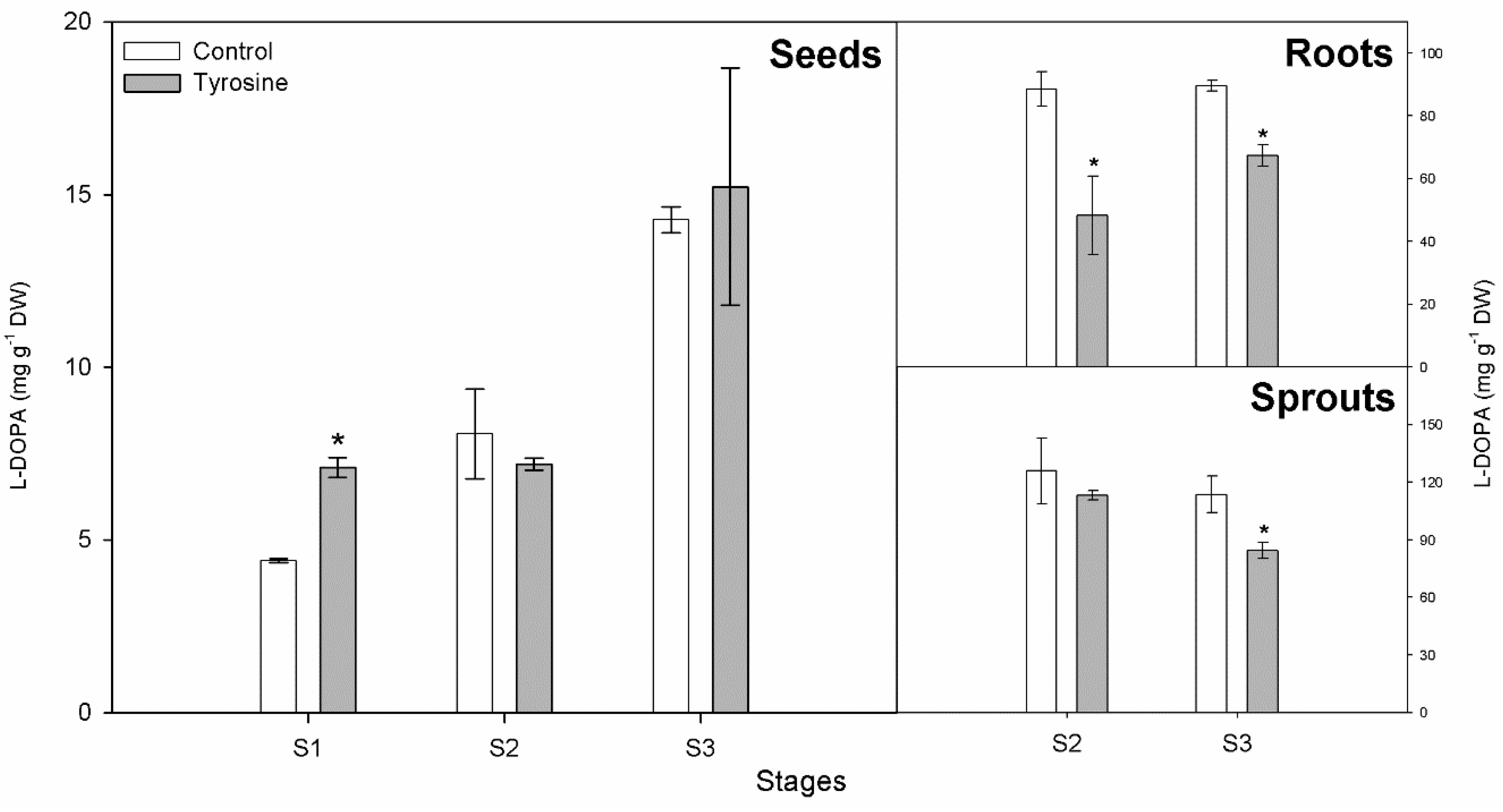
| Stages | Arnow | HPTLC | ||||
|---|---|---|---|---|---|---|
| Seed | Root | Sprout | Seed | Root | Sprout | |
| S1 | 0.90 ± 0.11 | * | * | 4.40 ± 0.06 | * | * |
| S2 | 1.90 ± 0.16 | 69.43 ± 3.05 | 81.25 ± 2.91 | 8.07 ± 1.30 | 88.50 ± 5.54 | 125.87 ± 17.18 |
| S3 | 5.69 ± 0.26 | 69.45 ± 5.33 | 89.28 ± 2.06 | 14.27 ± 0.38 | 89.67 ± 1.76 | 113.60 ± 9.53 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oviedo-Silva, C.A.; Elso-Freudenberg, M.; Aranda-Bustos, M. L-DOPA Trends in Different Tissues at Early Stages of Vicia faba Growth: Effect of Tyrosine Treatment. Appl. Sci. 2018, 8, 2431. https://doi.org/10.3390/app8122431
Oviedo-Silva CA, Elso-Freudenberg M, Aranda-Bustos M. L-DOPA Trends in Different Tissues at Early Stages of Vicia faba Growth: Effect of Tyrosine Treatment. Applied Sciences. 2018; 8(12):2431. https://doi.org/10.3390/app8122431
Chicago/Turabian StyleOviedo-Silva, Claudia A., Mhartyn Elso-Freudenberg, and Mario Aranda-Bustos. 2018. "L-DOPA Trends in Different Tissues at Early Stages of Vicia faba Growth: Effect of Tyrosine Treatment" Applied Sciences 8, no. 12: 2431. https://doi.org/10.3390/app8122431
APA StyleOviedo-Silva, C. A., Elso-Freudenberg, M., & Aranda-Bustos, M. (2018). L-DOPA Trends in Different Tissues at Early Stages of Vicia faba Growth: Effect of Tyrosine Treatment. Applied Sciences, 8(12), 2431. https://doi.org/10.3390/app8122431






