Acoustic Emissions to Measure Drought-Induced Cavitation in Plants
Abstract
:1. Introduction
2. The Importance of Water
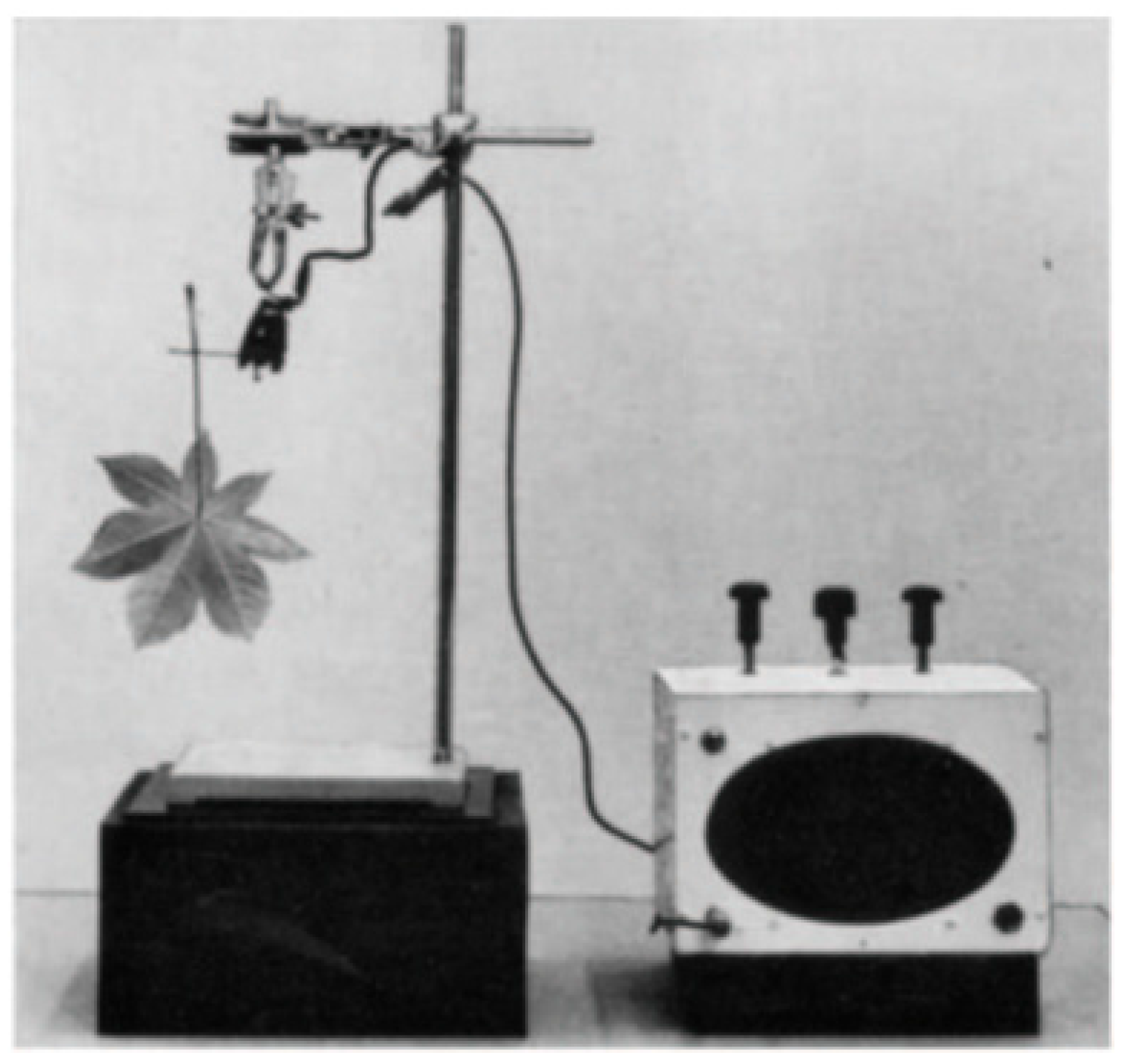
3. Acoustic Emission (AE) Application to Measure Drought-Induced Cavitation: From Past to Present
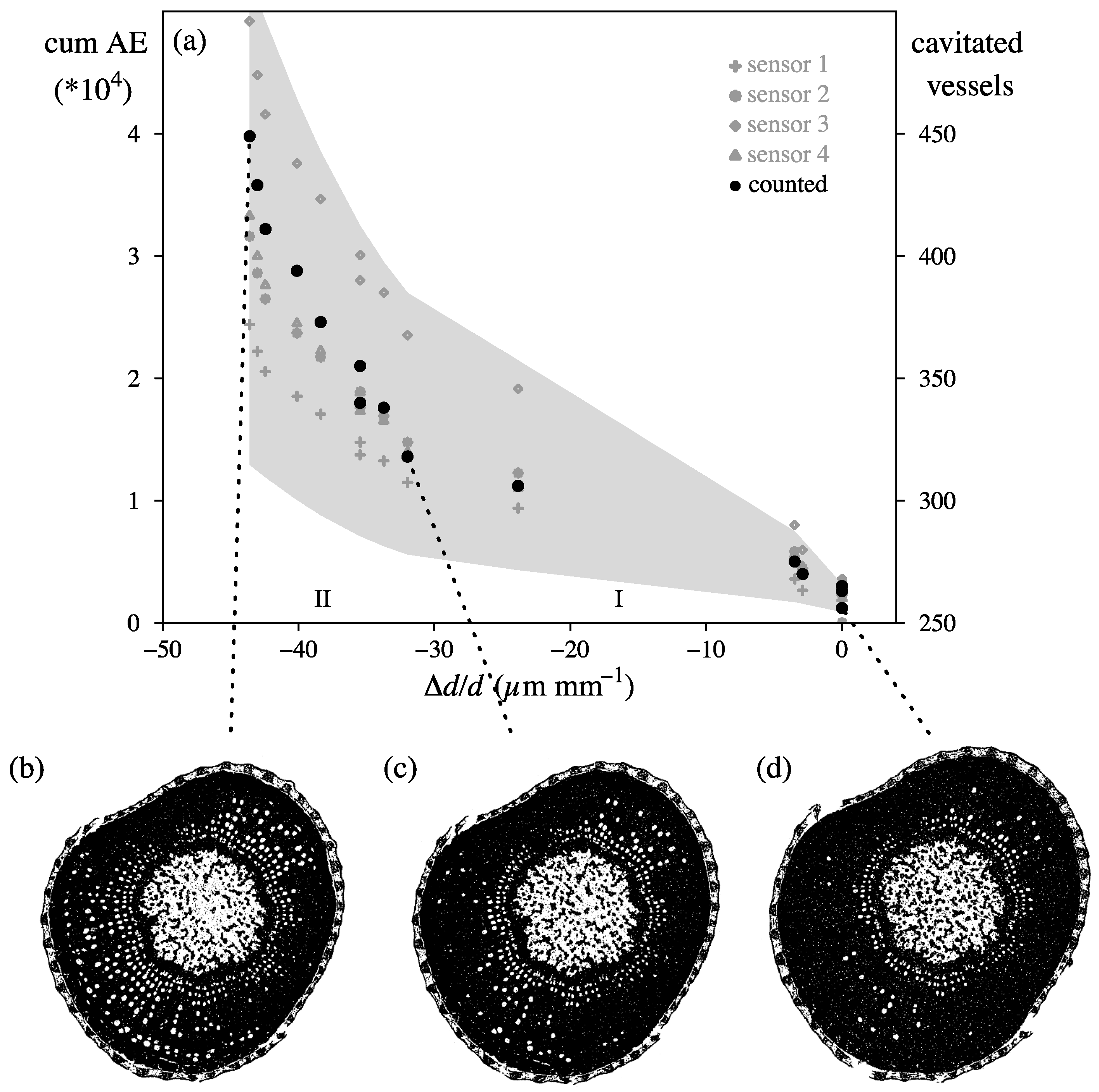
4. Current State-of-the-Art and Application of AE in Plant Sciences
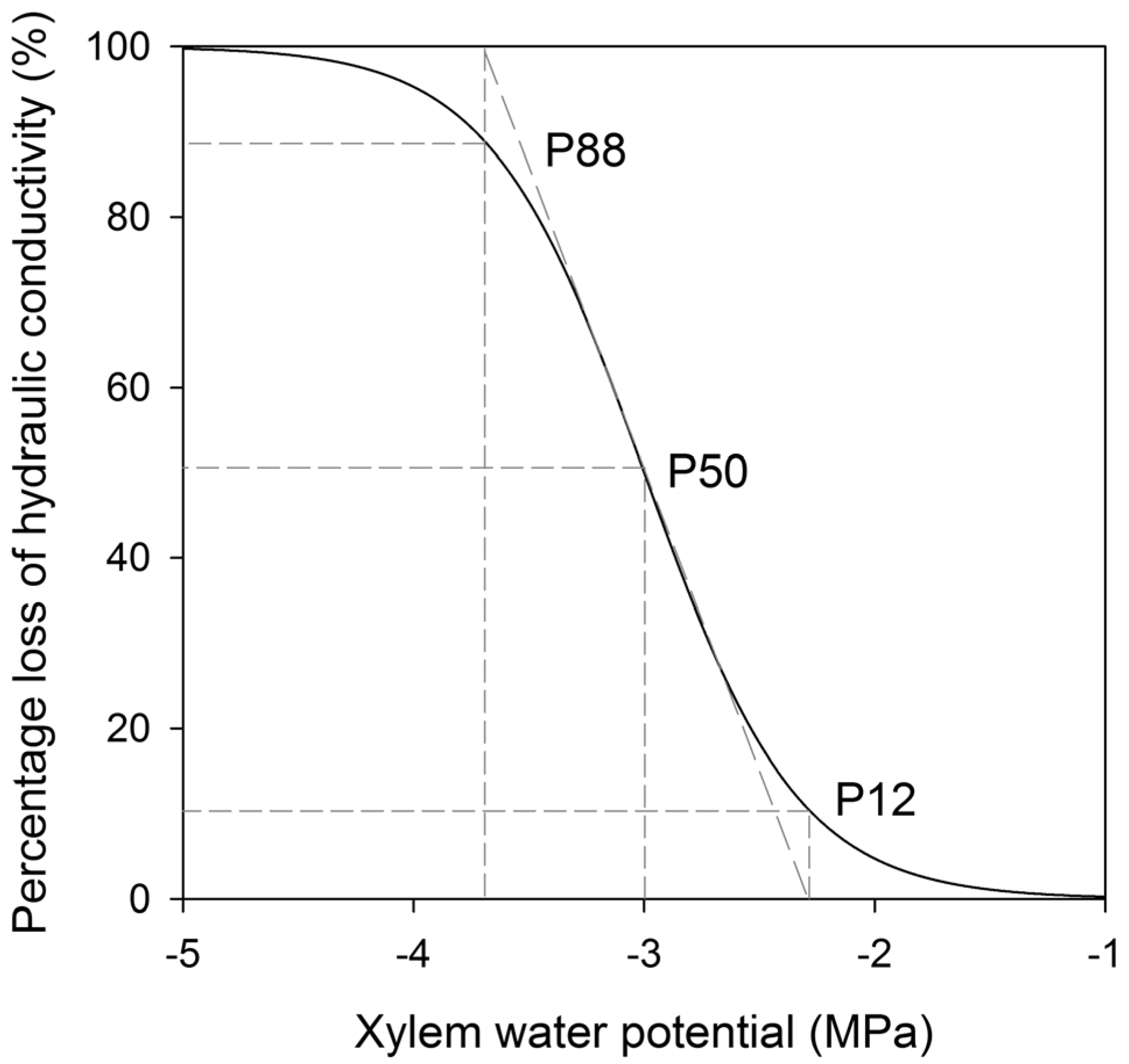
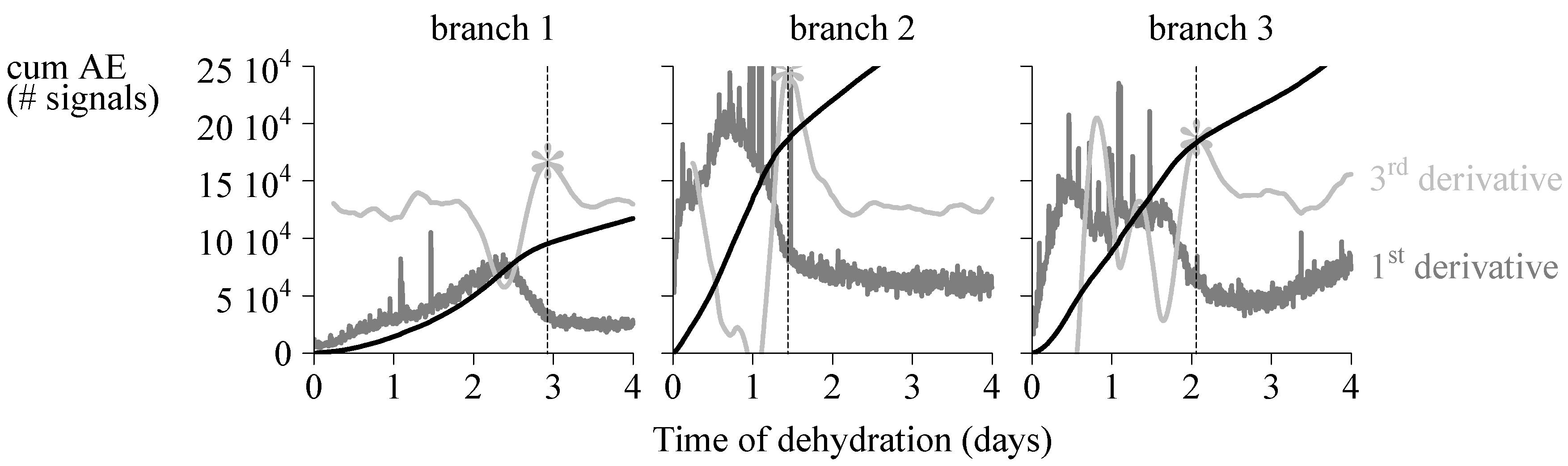
4.1. Endpoint Selection
4.2. AE Feature Extraction
4.3. In Vivo Measurements
5. Conclusions and Future Perspectives
- A combination of cavitation measurements with AE broadband sensors and µCT in a broad range of plant species: interpretation of the acoustic signals in combination with visuals will govern the largest insights in the mechanisms underlying cavitation.
- Feature extraction from the AE signals: this will allow a comprehensive analysis of the AE sources, and will deliver valuable information on cavitation and other AE producing processes that occur in drought-stressed plants.
- Detailed study of wave propagation and attenuation in plants, both in dehydrating and in frozen samples.
- Investigation of the effects of debarking prior to acoustic measurements: as the bark can be an additional AE source, this might influence the captured signals.
- Further validation of the use of broadband point-contact AE sensors in the field of plant hydraulics versus the 150 kHz resonance AE sensor.
- Development of an in situ AE measurement protocol for living plants, and its translation to drought sensitivity.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ernst, R.; Zwimpfer, F.; Dual, J. One sensor acoustic emission localization in plates. Ultrasonics 2016, 64, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Robeyst, N.; Grosse, C.U.; de Belie, N. Measuring the change in ultrasonic p-wave energy transmitted in fresh mortar with additives to monitor the setting. Cem. Concrete Res. 2009, 39, 868–875. [Google Scholar] [CrossRef]
- Ohtsu, M. History and fundamentals. In Acoustic Emission Testing; Springer: Heidelberg, Germany, 2008; pp. 11–18. [Google Scholar]
- Ohtsu, M. Fundamentals and applications. In Acoustic Emission and Related Non-Destructive Evaluation Techniques in the Fracture Mechanics of Concrete; Woodhead Publishing: Cambridge, UK, 2015; Volume 1, pp. 11–18. [Google Scholar]
- Jayakumar, T.; Mukhopadhyay, C.K.; Venugopal, S.; Mannan, S.L.; Raj, B. A review of the application of acoustic emission techniques for monitoring forming and grinding processes. J. Mater. Process. Technol. 2005, 159, 48–61. [Google Scholar] [CrossRef]
- Hamstad, M.A. Thirty years of advances and some remaining challenges in the application of acoustic emission to composite materials. In Acoustic Emission beyond the Millennium; Kishi, T., Ohtsu, M., Yuyama, S., Eds.; Elsevier: Oxford, UK, 2000; pp. 77–91. [Google Scholar]
- Drouillard, T.F. Anecdotal history of acoustic emission from wood. J. Acoust. Emiss. 1990, 9, 155–176. [Google Scholar]
- Kishinouye, F. An experiment on the progression of fracture (a preliminary report). J. Acoust. Emiss. 1990, 9, 177–180. [Google Scholar]
- Anderegg, W.R.; Berry, J.A.; Smith, D.D.; Sperry, J.S.; Anderegg, L.D.; Field, C.B. The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proc. Natl. Acad. Sci. USA 2012, 109, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.T. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Flint, A.; Huang, C.-Y.; Flint, L.; Berry, J.A.; Davis, F.W.; Sperry, J.S.; Field, C.B. Tree mortality predicted from drought-induced vascular damage. Nat. Geosci. 2015, 8, 367–371. [Google Scholar] [CrossRef]
- McDowell, N.G.; Ryan, M.G.; Zeppel, M.J.; Tissue, D.T. Feature: Improving our knowledge of drought-induced forest mortality through experiments, observations, and modeling. New Phytol. 2013, 200, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vilalta, J.; Lloret, F.; Breshears, D.D. Drought-induced forest decline: Causes, scope and implications. Biol. Lett. 2012, 8, 689–691. [Google Scholar] [CrossRef] [PubMed]
- Zeppel, M.J.; Anderegg, W.R.; Adams, H.D. Forest mortality due to drought: Latest insights, evidence and unresolved questions on physiological pathways and consequences of tree death. New Phytol. 2013, 197, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Doughty, C.E.; Metcalfe, D.; Girardin, C.; Amézquita, F.F.; Cabrera, D.G.; Huasco, W.H.; Silva-Espejo, J.; Araujo-Murakami, A.; da Costa, M.; Rocha, W. Drought impact on forest carbon dynamics and fluxes in amazonia. Nature 2015, 519, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.; Adams, H.D.; Anderegg, W.R.; Jansen, S.; Zeppel, M.J. Research frontiers in drought-induced tree mortality: Crossing scales and disciplines. New Phytol. 2015, 205, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Vegetation stress: An introduction to the stress concept in plants. J. Plant Physiol. 1996, 148, 4–14. [Google Scholar] [CrossRef]
- Dixon, H.H. Transpiration and the Ascent of Sap in Plants; Macmillan and Co.: London, UK, 1914. [Google Scholar]
- Kozlowski, T.T.; Pallardy, S.G. Growth Control in Woody Plants; Elsevier: San Diego, CA, USA, 1997. [Google Scholar]
- Steppe, K.; Sterck, F.; Deslauriers, A. Diel growth dynamics in tree stems: Linking anatomy and ecophysiology. Trends Plant Sci. 2015, 20, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Sevanto, S.; McDowell, N.G.; Dickman, L.T.; Pangle, R.; Pockman, W.T. How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant Cell Environ. 2014, 37, 153–161. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, A.; Vincent, O.; Pietriga, A.; Cochard, H.; Badel, E.; Marmottant, P. Ultrasonic emissions reveal individual cavitation bubbles in water-stressed wood. J. R. Soc. Interface 2014, 11. [Google Scholar] [CrossRef] [PubMed]
- Nobel, P.S. Physicochemical and Environmental Plant Physiology; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- Jackson, G.; Grace, J. Cavitation and water transport in trees. Endeavour 1994, 18, 50–54. [Google Scholar] [CrossRef]
- Rockwell, F.E.; Wheeler, J.K.; Holbrook, N.M. Cavitation and its discontents: Opportunities for resolving current controversies. Plant Physiol. 2014, 164, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M. Xylem structure and the ascent of sap. Xylem Structure and the Ascent of Sap; Springer-Verlag: Berlin, Germany, 1983. [Google Scholar]
- Schenk, H.J.; Steppe, K.; Jansen, S. Nanobubbles: A new paradigm for air-seeding in xylem. Trends Plant Sci. 2015, 20, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Domec, J.-C.; Gartner, B.L. Cavitation and water storage capacity in bole xylem segments of mature and young douglas-fir trees. Trees 2001, 15, 204–214. [Google Scholar] [CrossRef]
- Rosner, S. A new type of vulnerability curve: Is there truth in vine? Tree Physiol. 2015, 35, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Cochard, H.; Badel, E.; Herbette, S.; Delzon, S.; Choat, B.; Jansen, S. Methods for measuring plant vulnerability to cavitation: A critical review. J. Exp. Bot. 2013, 64, 4779–4791. [Google Scholar] [CrossRef] [PubMed]
- Vergeynst, L.L.; Dierick, M.; Bogaerts, J.A.; Cnudde, V.; Steppe, K. Cavitation: A blessing in disguise? New method to establish vulnerability curves and assess hydraulic capacitance of woody tissues. Tree Physiol. 2015, 35, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Mayr, S.; Rosner, S. Cavitation in dehydrating xylem of picea abies: Energy properties of ultrasonic emissions reflect tracheid dimensions. Tree Physiol. 2011, 31, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Gullo, M.L.; Salleo, S. Different vulnerabilities of Quercus ilex L. To freeze-and summer drought-induced xylem embolism: An ecological interpretation. Plant Cell Environ. 1993, 16, 511–519. [Google Scholar] [CrossRef]
- Fichot, R.; Brignolas, F.; Cochard, H.; Ceulemans, R. Vulnerability to drought-induced cavitation in poplars: Synthesis and future opportunities. Plant Cell Environ. 2015, 38, 1233–1251. [Google Scholar] [CrossRef] [PubMed]
- Dixon, H.H.; Joly, J. On the ascent of sap. Philos. Trans. R. Soc. Lond. B 1895. [Google Scholar] [CrossRef]
- Steudle, E. The cohesion-tension mechanism and the acquisition of water by plant roots. Annu. Rev. Plant Biol. 2001, 52, 847–875. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.; Schuldt, B.; Choat, B. Current controversies and challenges in applying plant hydraulic techniques. New Phytol. 2015, 205, 961–964. [Google Scholar] [CrossRef]
- Wheeler, J.K.; Huggett, B.A.; Tofte, A.N.; Rockwell, F.E.; Holbrook, N.M. Cutting xylem under tension or supersaturated with gas can generate plc and the appearance of rapid recovery from embolism. Plant Cell Environ. 2013, 36, 1938–1949. [Google Scholar] [CrossRef] [PubMed]
- Cochard, H.; Delzon, S.; Badel, E. X-ray microtomography (micro-CT): A reference technology for high-resolution quantification of xylem embolism in trees. Plant Cell Environ. 2015, 38, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Milburn, J.; Johnson, R. The conduction of sap: II. Detection of vibrations produced by sap cavitation in ricinus xylem. Planta 1966, 69, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Tyree, M.T.; Dixon, M.A. Cavitation events in Thuja occidentalis L.? Utrasonic acoustic emissions from the sapwood can be measured. Plant Physiol. 1983, 72, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Temperley, H.; Chambers, L.G. The behaviour of water under hydrostatic tension: I. Proc. Phys. Soc. 1946, 58, 420. [Google Scholar] [CrossRef]
- Milburn, J.A. Cavitation in Ricinus by acoustic detection: Induction in excised leaves by various factors. Planta 1973, 110, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Tyree, M.T.; Dixon, M.A.; Thompson, R.G. Ultrasonic acoustic emissions from the sapwood of Thuja occidentalis measured inside a pressure bomb. Plant Physiol. 1984, 74, 1046–1049. [Google Scholar] [CrossRef] [PubMed]
- Tyree, M.T.; Dixon, M.A.; Tyree, E.L.; Johnson, R. Ultrasonic acoustic emissions from the sapwood of cedar and hemlock an examination of three hypotheses regarding cavitations. Plant Physiol. 1984, 75, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Milburn, J.A. Cavitation studies on whole ricinus plants by acoustic detection. Planta 1973, 112, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Kikuta, S.B. Ultrasound acoustic emissions from bark samples differing in anatomical characteristics. Phyton 2003, 43, 161–178. [Google Scholar]
- Raschi, A.; Mugnozza, G.S.; Surace, R.; Valentini, R.; Vazzana, C. The use of ultrasound technique to monitor freezing and thawing of water in plants. Agric. Ecosyst. Environ. 1989, 27, 411–418. [Google Scholar] [CrossRef]
- Mayr, S.; Sperry, J.S. Freeze-thaw-induced embolism in pinus contorta: Centrifuge experiments validate the “thaw-expansion hypothesis” but conflict with ultrasonic emission data. New Phytol. 2010, 185, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Charrier, G.; Charra-Vaskou, K.; Kasuga, J.; Cochard, H.; Mayr, S.; Ameglio, T. Freeze-thaw stress: Effects of temperature on hydraulic conductivity and ultrasonic activity in ten woody angiosperms. Plant Physiol. 2014, 164, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Moliński, W.; Raczkowski, J.; Poliszko, S.; Ranachowski, Z. Mechanism of acoustic emission in wood soaked in water. Holzforschung 1991, 45, 13–17. [Google Scholar] [CrossRef]
- Rosner, S. Characteristics of acoustic emissions from dehydrat-ing wood related to shrinkage processes. J. Acoustic Emission 2007, 25, 149–157. [Google Scholar]
- Gagliano, M.; Mancuso, S.; Robert, D. Towards understanding plant bioacoustics. Trends Plant Sci. 2012, 17, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Kikuta, S.; Gullo, M.; Nardini, A.; Richter, H.; Salleo, S. Ultrasound acoustic emissions from dehydrating leaves of deciduous and evergreen trees. Plant Cell Environ. 1997, 20, 1381–1390. [Google Scholar] [CrossRef]
- Hacke, U.G.; Sperry, J.S.; Pittermann, J. Drought experience and cavitation resistance in six shrubs from the great basin, utah. Basic Appl. Ecol. 2000, 1, 31–41. [Google Scholar] [CrossRef]
- Sperry, J.S.; Tyree, M.T.; Donnelly, J.R. Vulnerability of xylem to embolism in a mangrove vs. an inland species of rhizophoraceae. Physiol. Plant. 1988, 74, 276–283. [Google Scholar] [CrossRef]
- Cochard, H.; Tyree, M.T. Xylem dysfunction in quercus: Vessel sizes, tyloses, cavitation and seasonal changes in embolism. Tree Physiol. 1990, 6, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Tyree, M.; Sperry, J. Characterization and propagation of acoustic emission signals in woody plants: Towards an improved acoustic emission counter. Plant Cell Environ. 1989, 12, 371–382. [Google Scholar] [CrossRef]
- Ritman, K.; Milburn, J. Acoustic emissions from plants: Ultrasonic and audible compared. J. Exp. Bot. 1988, 39, 1237–1248. [Google Scholar] [CrossRef]
- Kasuga, J.; Charrier, G.; Uemura, M.; Améglio, T. Characteristics of ultrasonic acoustic emissions from walnut branches during freeze-thaw-induced embolism formation. J. Exp. Bot. 2015, 66, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Rosner, S.; Konnerth, J.; Plank, B.; Salaberger, D.; Hansmann, C. Radial shrinkage and ultrasound acoustic emissions of fresh versus pre-dried norway spruce sapwood. Trees 2010, 24, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Wolkerstorfer, S.V.; Rosner, S.; Hietz, P. An improved method and data analysis for ultrasound acoustic emissions and xylem vulnerability in conifer wood. Physiol. Plant 2012, 146, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Rosner, S. Waveform features of acoustic emission provide information about reversible and irreversible processes during spruce sapwood drying. BioResources 2012, 7, 1253–1263. [Google Scholar]
- Rosner, S.; Klein, A.; Wimmer, R.; Karlsson, B. Extraction of features from ultrasound acoustic emissions: A tool to assess the hydraulic vulnerability of norway spruce trunkwood? New Phytol. 2006, 171, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Mayr, S.; Schmid, P.; Laur, J.; Rosner, S.; Charra-Vaskou, K.; Dämon, B.; Hacke, U.G. Uptake of water via branches helps timberline conifers refill embolized xylem in late winter. Plant Physiol. 2014, 164, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Laschimke, R.; Burger, M.; Vallen, H. Acoustic emission analysis and experiments with physical model systems reveal a peculiar nature of the xylem tension. J. Plant Physiol. 2006, 163, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Tyree, M.T.; Fiscus, E.L.; Wullschleger, S.; Dixon, M. Detection of xylem cavitation in corn under field conditions. Plant Physiol. 1986, 82, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Ohtsu, M. Detection of xylem cavitation in field-grown pine trees using the acoustic emission technique. Ecol. Res. 1992, 7, 391–395. [Google Scholar] [CrossRef]
- Jackson, G.; Grace, J. Field measurements of xylem cavitation: Are acoustic emissions useful? J. Exp. Bot. 1996, 47, 1643–1650. [Google Scholar] [CrossRef]
- Perks, M.P.; Irvine, J.; Grace, J. Xylem acoustic signals from mature pinus sylvestris during an extended drought. Ann. For. Sci. 2004, 61, 1–8. [Google Scholar] [CrossRef]
- Hölttä, T.; Vesala, T.; Nikinmaa, E.; Perämäki, M.; Siivola, E.; Mencuccini, M. Field measurements of ultrasonic acoustic emissions and stem diameter variations. New insight into the relationship between xylem tensions and embolism. Tree Physiol. 2005, 25, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Quarles, S.L.; Association, W.D.K.; Association, W.D.K. Acoustic Emission Generated during Drying. In Proceedings of the 41st Western Dry Kiln Association Meeting, Corvallis, OR, USA, 1990; p. 53.
- Michlmayr, G.; Cohen, D.; Or, D. Sources and characteristics of acoustic emissions from mechanically stressed geologic granular media—A review. Earth Sci. Rev. 2012, 112, 97–114. [Google Scholar] [CrossRef]
- Tyree, M.T.; Sperry, J.S. Vulnerability of xylem to cavitation and embolism. Annu. Rev. Plant Biol. 1989, 40, 19–36. [Google Scholar] [CrossRef]
- Hacke, U.; Sauter, J. Xylem dysfunction during winter and recovery of hydraulic conductivity in diffuse-porous and ring-porous trees. Oecologia 1996, 105, 435–439. [Google Scholar] [CrossRef]
- Nardini, A.; Tyree, M.T.; Salleo, S. Xylem cavitation in the leaf of prunus laurocerasusand its impact on leaf hydraulics. Plant Physiol. 2001, 125, 1700–1709. [Google Scholar] [CrossRef] [PubMed]
- Salleo, S.; Gullo, L.; Raimondo, F.; Nardini, A. Vulnerability to cavitation of leaf minor veins: Any impact on leaf gas exchange? Plant Cell Environ. 2001, 24, 851–859. [Google Scholar] [CrossRef]
- Vergeynst, L.L.; Sause, M.G.R.; Steppe, K. Clustering reveals cavitation-related acoustic emission signals from dehydrating branches. Tree Physiol. 2016, in press. [Google Scholar]
- Aggelis, D.; Matikas, T.; Shiotani, T. Advanced acoustic techniques for health monitoring of concrete structures. In The Song’s Handbook of Concrete Durability; Kim, S.H., Ann, K.Y., Eds.; Middleton Publishing Inc: Middleton, WI, USA, 2010; pp. 331–378. [Google Scholar]
- Choat, B.; Drayton, W.M.; Brodersen, C.; Matthews, M.A.; Shackel, K.A.; Wada, H.; Mcelrone, A.J. Measurement of vulnerability to water stress-induced cavitation in grapevine: A comparison of four techniques applied to a long-vesseled species. Plant Cell Environ. 2010, 33, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Brodersen, C.R.; McElrone, A.J.; Choat, B.; Lee, E.F.; Shackel, K.A.; Matthews, M.A. In vivo visualizations of drought-induced embolism spread in vitis vinifera. Plant Physiol. 2013, 161, 1820–1829. [Google Scholar] [CrossRef] [PubMed]
- Nolf, M.; Beikircher, B.; Rosner, S.; Nolf, A.; Mayr, S. Xylem cavitation resistance can be estimated based on time-dependent rate of acoustic emissions. New Phytol. 2015, 208, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Vergeynst, L.L.; Sause, M.G.; Hamstad, M.A.; Steppe, K. Deciphering acoustic emission signals in drought stressed branches: The missing link between source and sensor. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Lo, G.M.; Salleo, S. Three different methods for measuring xylem cavitation and embolism: A comparison. Ann. Bot. 1991, 67, 417–424. [Google Scholar]
- Cochard, H. Vulnerability of several conifers to air embolism. Tree Physiol. 1992, 11, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Niemz, P.; Emmler, R.; Pridöhl, E.; Fröhlich, J.; Lühmann, A. Vergleichende untersuchungen zur Anwendung von piezoelektrischen und Schallemissionssignalen bei der trocknung von Holz. Holz als Roh und Werkstoff 1994, 52, 162–168. [Google Scholar] [CrossRef]
- Lee, S.-H.; Quarles, S.; Schniewind, A. Wood fracture, acoustic emission, and the drying process part 2. Acoustic emission pattern recognition analysis. Wood Sci. Technol. 1996, 30, 283–292. [Google Scholar] [CrossRef]
- Beall, F. Overview of the use of ultrasonic technologies in research on wood properties. Wood Sci. Technol. 2002, 36, 197–212. [Google Scholar] [CrossRef]
- Kawamoto, S.; Williams, R.S. Acoustic Emission and Acousto-Ultrasonic Techniques for Wood and Wood-Based Composites: A Review; US Department of Agriculture: Madison, WI, USA, 2002.
- Beall, F.C.; Breiner, T.A.; Wang, J. Closed-loop control of lumber drying based on acoustic emission peak amplitude. For. Prod. J. 2005, 55, 167–174. [Google Scholar]
- Johnson, D.M.; Meinzer, F.C.; Woodruff, D.R.; McCulloh, K.A. Leaf xylem embolism, detected acoustically and by cryo-sem, corresponds to decreases in leaf hydraulic conductance in four evergreen species. Plant Cell Environ. 2009, 32, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Rosner, S.; Karlsson, B.; Konnerth, J.; Hansmann, C. Shrinkage processes in standard-size norway spruce wood specimens with different vulnerability to cavitation. Tree Physiol. 2009, 29, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Sause, M.G.R.; Gribov, A.; Unwin, A.R.; Horn, S. Pattern recognition approach to identify natural clusters of acoustic emission signals. Pattern Recognit. Lett. 2012, 33, 17–23. [Google Scholar] [CrossRef]
- Zelenyak, A.M.; Hamstad, M.A.; Sause, M.G. Modeling of acoustic emission signal propagation in waveguides. Sensors 2015, 15, 11805–11822. [Google Scholar] [CrossRef] [PubMed]
- Sause, M.; Horn, S. Simulation of acoustic emission in planar carbon fiber reinforced plastic specimens. J. Nondestruct. Eval. 2010, 29, 123–142. [Google Scholar] [CrossRef]
- Haacs, R.; Blank, R.W. Acoustic emission from drought-stressed red pine (Pinus resinosa). J. Acoust. Emiss. 1990, 9, 181–187. [Google Scholar]
- Lewis, A.M. Two Mechanisms for the Initiation of Embolism in Tracheary Elements and Other Dead Plant Cells under Water Stress; Harvard University: Cambridge, MA, USA, 1987. [Google Scholar]
- Sandford, A.; Grace, J. The measurement and interpretation of ultrasound from woody stems. J. Exp. Bot. 1985, 36, 298–311. [Google Scholar] [CrossRef]
- Ritman, K.; Milburn, J. Monitoring of ultrasonic and audible emissions from plants with or without vessels. J. Exp. Bot. 1991, 42, 123–130. [Google Scholar] [CrossRef]
- Kikuta, S.; Lo Gullo, M.; Kartusch, B.; Rosner, S.; Richter, H. Ultrasound Acoustic Emissions from Conifer Sapwood Sections: Relationship between Number of Events Detected and Number of Tracheids. In Proceedings of the IAWA International Symposium on Wood Sciences, Montpellier, France, 24–29 October 2004; p. 29.
- Vergeynst, L.L. Investigation and Application of the Acoustic Emission Technique to Measure Drought-Induced Cavitation in Woody Plants; Ghent University: Ghent, Belgium, 2015. [Google Scholar]
- Charrier, G.; Pramsohler, M.; Charra-Vaskou, K.; Saudreau, M.; Ameglio, T.; Neuner, G.; Mayr, S. Ultrasonic emissions during ice nucleation and propagation in plant xylem. New Phytol. 2015, 207, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Torres-Ruiz, J.M.; Jansen, S.; Choat, B.; McElrone, A.J.; Cochard, H.; Brodribb, T.J.; Badel, E.; Burlett, R.; Bouche, P.S.; Brodersen, C.R.; et al. Direct X-ray microtomography observation confirms the induction of embolism upon xylem cutting under tension. Plant Physiol. 2015, 167, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, R.; Zeugin, F. Ultrasonic acoustic emissions in drought-stressed trees—More than signals from cavitation? New Phytol. 2008, 179, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Steppe, K.; Zeugin, F.; Zweifel, R. Low-decibel ultrasonic acoustic emissions are temperature-induced and probably have no biotic origin. New Phytol. 2009, 183, 928–931. [Google Scholar] [CrossRef] [PubMed]
- Brodersen, C.R.; McElrone, A.J. Maintenance of xylem network transport capacity: A review of embolism repair in vascular plants. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Zwieniecki, M.A.; Holbrook, N.M. Confronting maxwell′s demon: Biophysics of xylem embolism repair. Trends Plant Sci. 2009, 14, 530–534. [Google Scholar] [CrossRef] [PubMed]
- McCulloh, K.A.; Meinzer, F.C. Further evidence that some plants can lose and regain hydraulic function daily. Tree Physiol. 2015, 35, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Meinzer, F.C.; McCulloh, K.A. Xylem recovery from drought-induced embolism: Where is the hydraulic point of no return? Tree Physiol. 2013, 33, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Zwieniecki, M.A.; Secchi, F. Threats to xylem hydraulic function of trees under “new climate normal” conditions. Plant Cell Environ. 2015, 38, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- McElrone, A.; Brodersen, C.; Alsina, M.; Drayton, W.; Matthews, M.; Shackel, K.; Wada, H.; Zufferey, V.; Choat, B. Centrifuge technique consistently overestimates vulnerability to water stress-induced cavitation in grapevines as confirmed with high-resolution computed tomography. New Phytol. 2012, 196, 661–665. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Roo, L.; Vergeynst, L.L.; De Baerdemaeker, N.J.F.; Steppe, K. Acoustic Emissions to Measure Drought-Induced Cavitation in Plants. Appl. Sci. 2016, 6, 71. https://doi.org/10.3390/app6030071
De Roo L, Vergeynst LL, De Baerdemaeker NJF, Steppe K. Acoustic Emissions to Measure Drought-Induced Cavitation in Plants. Applied Sciences. 2016; 6(3):71. https://doi.org/10.3390/app6030071
Chicago/Turabian StyleDe Roo, Linus, Lidewei L. Vergeynst, Niels J.F. De Baerdemaeker, and Kathy Steppe. 2016. "Acoustic Emissions to Measure Drought-Induced Cavitation in Plants" Applied Sciences 6, no. 3: 71. https://doi.org/10.3390/app6030071
APA StyleDe Roo, L., Vergeynst, L. L., De Baerdemaeker, N. J. F., & Steppe, K. (2016). Acoustic Emissions to Measure Drought-Induced Cavitation in Plants. Applied Sciences, 6(3), 71. https://doi.org/10.3390/app6030071