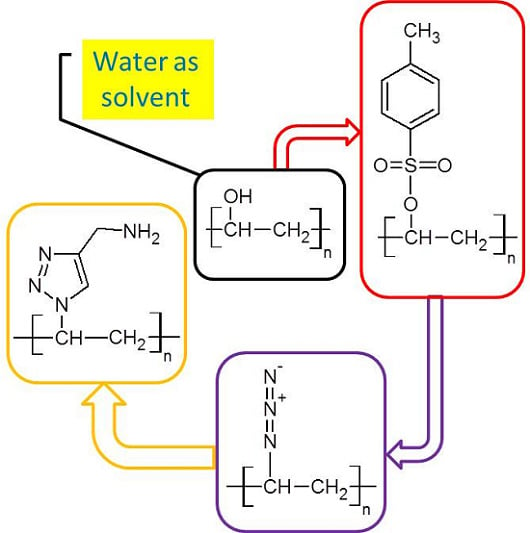
Chemical Modification of Poly(Vinyl Alcohol) in Water
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. Preparation of PVA Solution
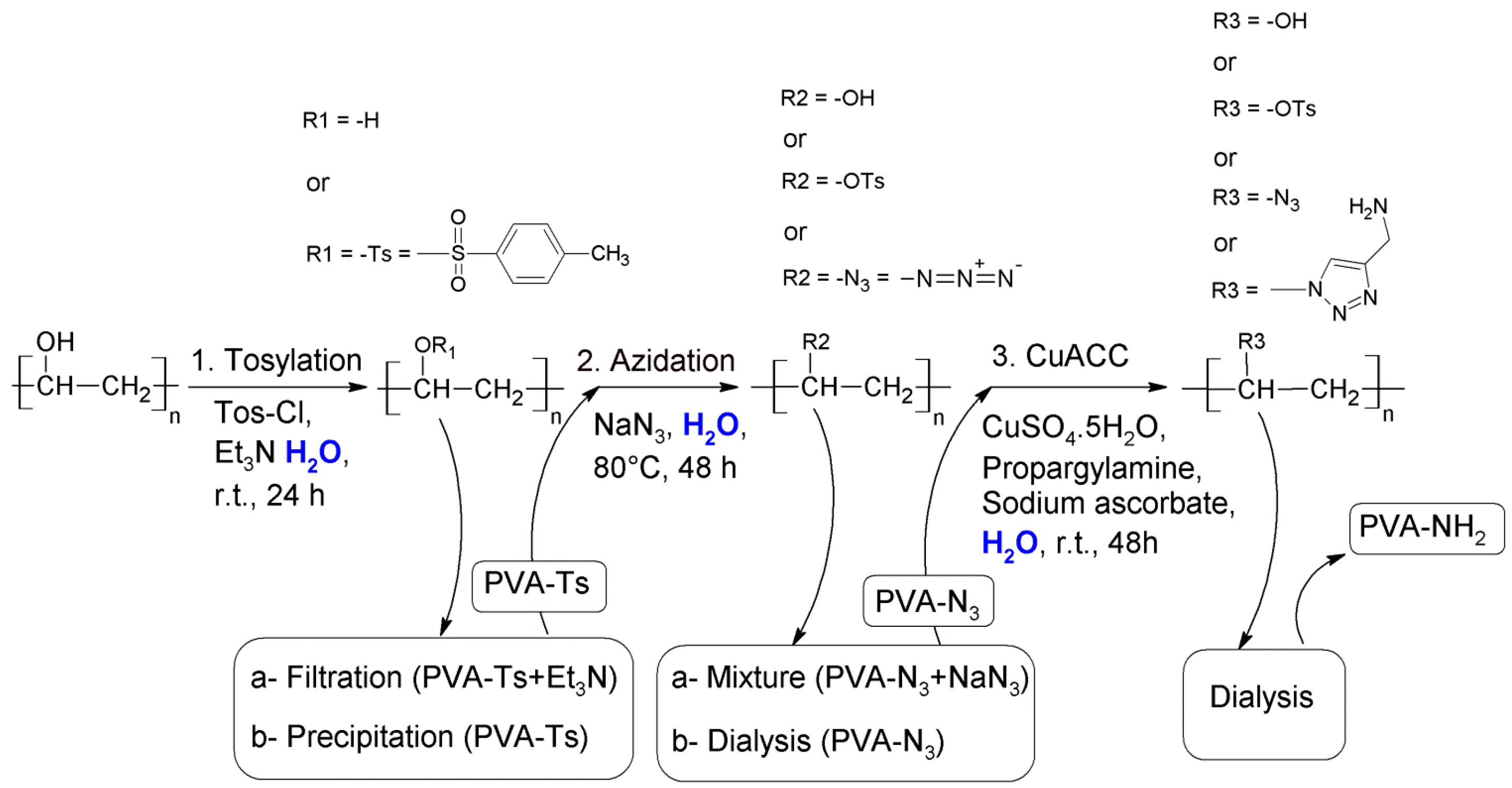
2.2.2. Synthesis of Tosyl Functionalized PVA (PVA-Ts)
- Filtration to remove excess of unreacted TsCl. The filtrated solution (PVA-Ts + Et3N) will be used for the azidation.
- Concentration of the mixture in a rotary evaporator and precipitation of PVA-Ts with ethanol followed by filtration and washing with hot ethanol. A part of the product was dried at room temperature for further analysis. The modified PVA was dissolved in hot water directly after precipitation.
2.2.3. Synthesis of Azide Functionalized PVA (PVA-N3)
2.2.4. Synthesis of Amine Functionalized PVA (PVA-NH2)
2.3. Characterization
3. Results and Discussion
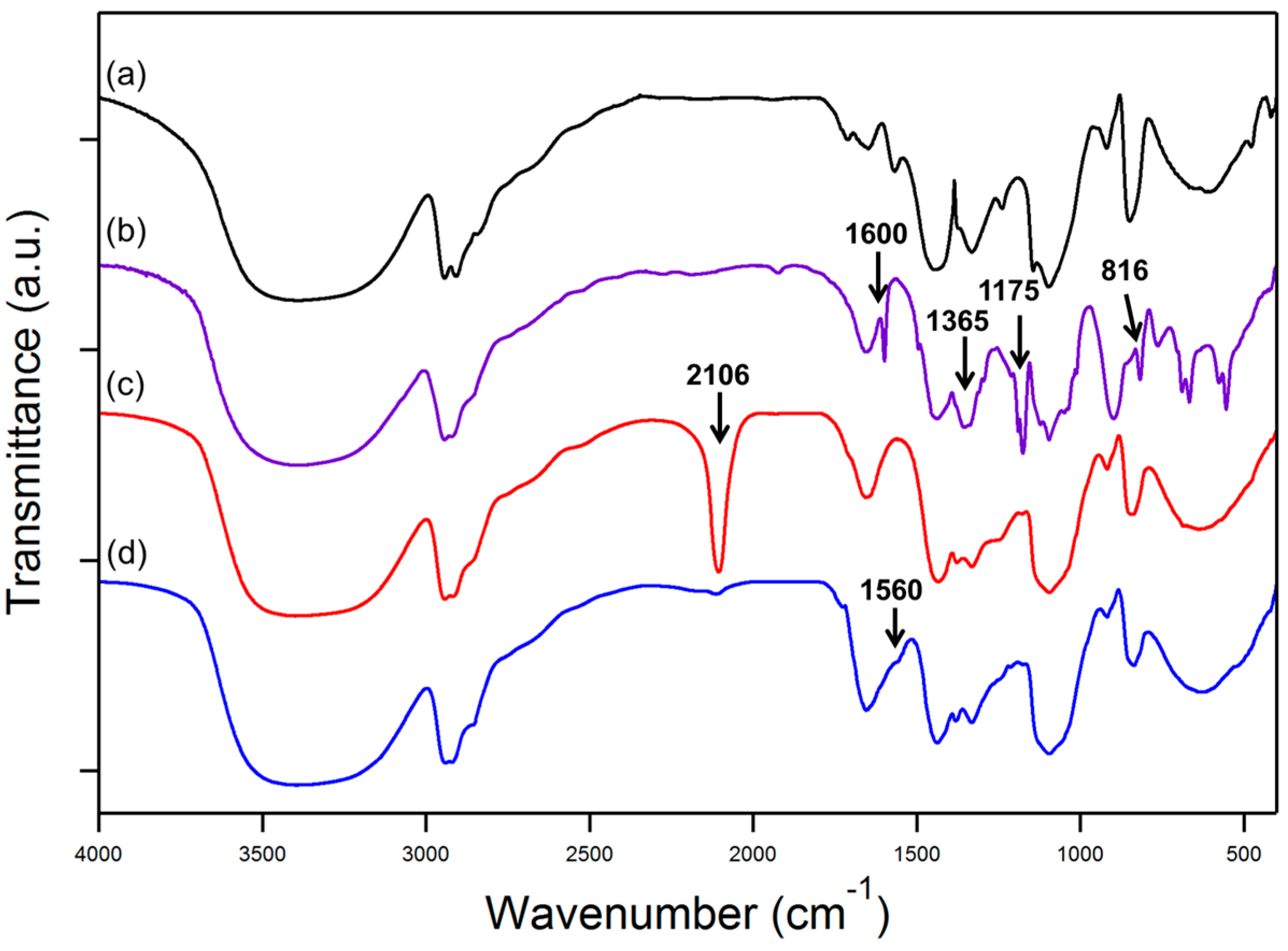
3.1. FTIR Analysis
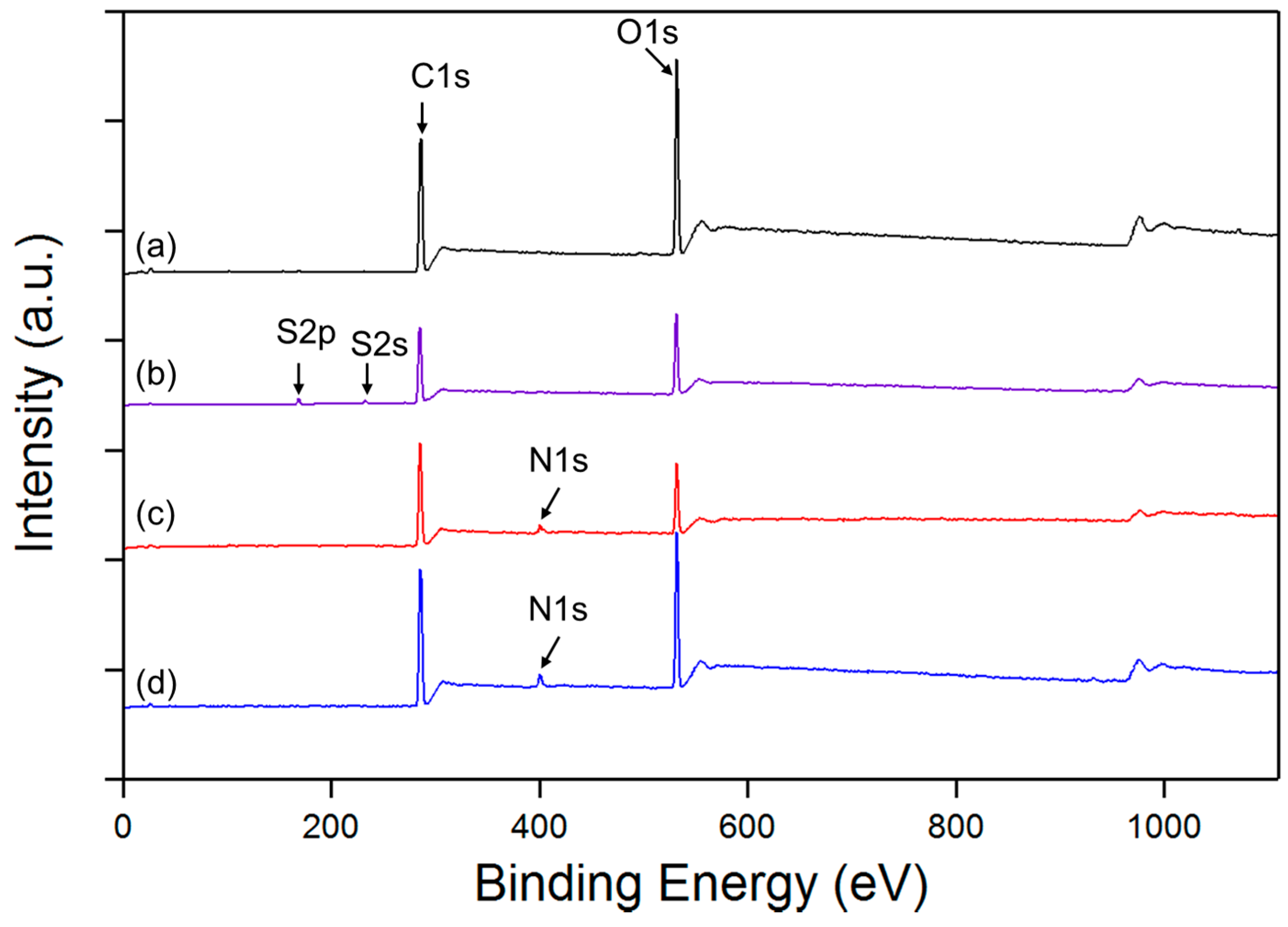
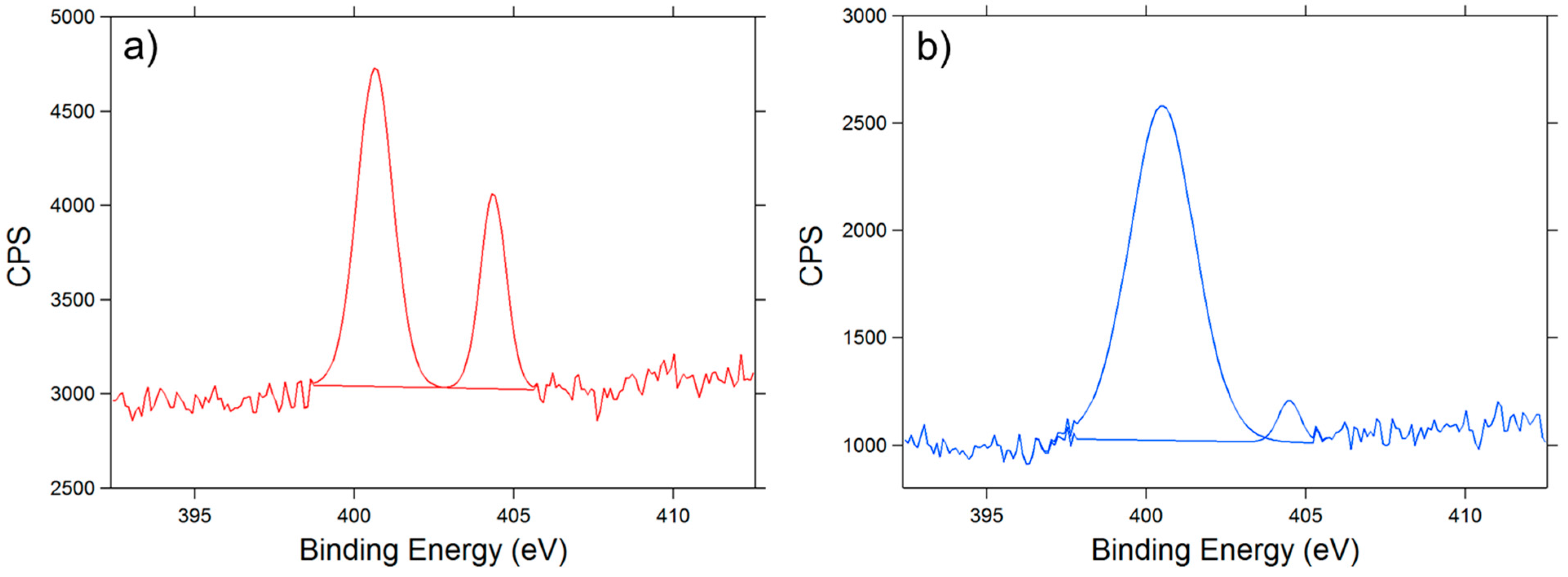
3.2. XPS Analysis
- % [Ts] = % [S] = 3.27
- % [OTs] = 2 × 3.27 = 6.54 (each molecule of Ts contains 2 Oxygen atoms)
- % [OVA] = % [1 VA] = 24.81 − 6.54 = 18.27 (VA = unit of polyvinyl alcohol; each molecule of VA contains 1 Oxygen atom)
- Finally the DS of tosylation is obtained by dividing % [tos] per % [One VA]
- DS = [Ts]/[1 VA] = 0.1789
| Sample | C% | O% | S% | N% |
|---|---|---|---|---|
| PVA | 69.95 | 30.05 | - | - |
| PVA-Ts | 71.92 | 24.81 | 3.27 | - |
| PVA-N3 | 77.42 | 17.74 | - | 4.84 |
| PVA-NH2 | 72.15 | 23.82 | - | 4.03 |
3.3. TGA Analysis
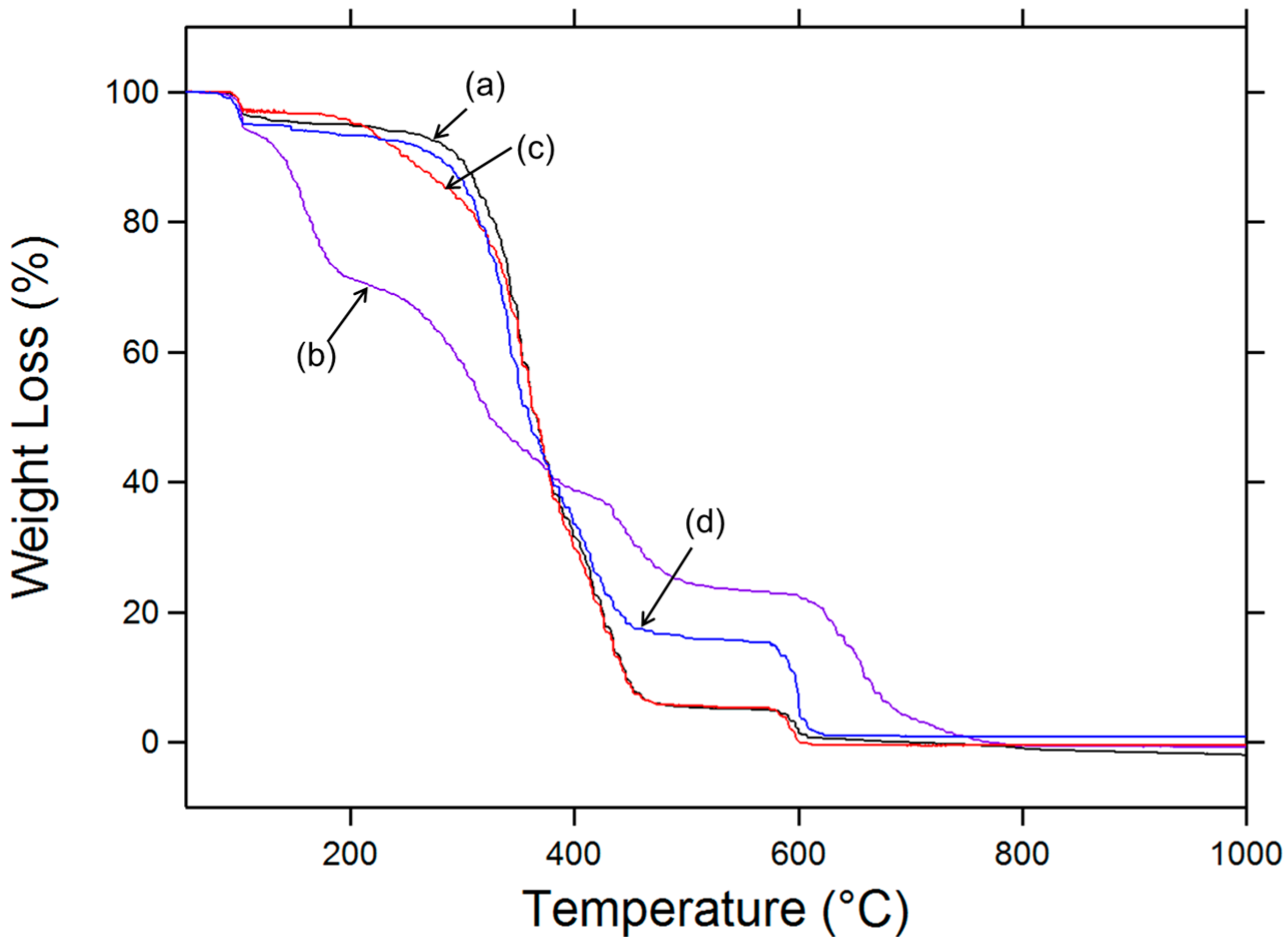
| Sample | TWeight loss (10%) (°C) * | Tpeak I (°C) | Tpeak II (°C) | Tpeak III (°C) |
|---|---|---|---|---|
| PVA | 295 | - | 351 | 423 |
| PVA-Ts | 139 | 161 | 320 | 434 |
| PVA-N3 | 244 | - | 352 | 422 |
| PVA-NH2 | 275 | - | 337 | 405 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ding, B.; Kim, H.Y.; Lee, S.C.; Shao, C.L.; Lee, D.R.; Park, S.J.; Kwag, G.B.; Choi, K.J. Preparation and characterization of a nanoscale poly(vinyl alcohol) fiber aggregate produced by an electrospinning method. J. Polym. Sci. Part B 2002, 40, 1261–1268. [Google Scholar] [CrossRef]
- Shi, R.; Bi, J.; Zhang, Z.; Zhu, A.; Chen, D.; Zhou, X.; Zhang, L.; Tian, W. The effect of citric acid on the structural properties and cytotoxicity of the polyvinyl alcohol/starch films when molding at high temperature. Carbohydr. Polym. 2008, 74, 763–770. [Google Scholar] [CrossRef]
- Chiellini, E.; Corti, A.; D’Antone, S.; Solaro, R. Biodegradation of poly(vinyl alcohol) based materials. Prog. Polym. Sci. 2008, 28, 963–1014. [Google Scholar] [CrossRef]
- Thomas, L.V.; Arun, U.; Remya, S.; Nair, P.D. A biodegradable and biocompatible PVA—Citric acid polyester with potential applications as matrix for vascular tissue engineering. J. Mater. Sci. Mater. Med. 2009, 20, S259–S269. [Google Scholar] [CrossRef] [PubMed]
- Rescignanoa, N.; Fortunati, E.; Montesano, S.; Emiliani, C.; Kenny, J.M.; Martino, S.; Armentano, I. PVA bio-nanocomposites: A new take-off using cellulose nanocrystals and PLGA nanoparticles. Carbohydr. Polym. 2014, 99, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Gaina, C.; Ursache, O.; Gaina, V.; Ionita, D. Study on the Chemical Modification of Poly(Vinyl Alcohol) with 4-Maleimidophenyl Isocyanate. Polym. Plast. Technol. Eng. 2012, 51, 65–70. [Google Scholar] [CrossRef]
- Erdmenger, T.; Sanchez, C.G.; Vitz, J.; Hoogenboom, R.; Schubert, U.S. Recent developments in the utilization of green solvents in polymer chemistry. Chem. Soc. Rev. 2010, 39, 3317–3333. [Google Scholar] [CrossRef] [PubMed]
- Gacal, B.N.; Koz, B.; Gacal, B.; Kiskan, B.; Erdogan, M.; Yaggi, Y. Pyrene Functional Poly(vinyl alcohol) by “Click” Chemistry. J. Polym. Sci. Part A 2009, 47, 1317–1326. [Google Scholar] [CrossRef]
- Kara, S.; Gacal, B.; Tunc, D.; Yagci, Y.; Pekcan, O. Sorption and desorption of PVA-pyrene chains in and out of agarose Gel. J. Fluoresc. 2012, 22, 1073–1080. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elchinger, P.-H.; Faugeras, P.-A.; Zerrouki, C.; Montplaisir, D.; Brouillette, F.; Zerrouki, R. Tosylcellulose synthesis in aqueous medium. Green Chem. 2012, 14, 3126–3131. [Google Scholar] [CrossRef]
- Diop, A.; Awada, H.; Zerrouki, R.; Daneault, C.; Montplaisir, D. Tosylation and Characterization of Lignin in Water. Ind. Eng. Chem. Res. 2014, 53, 16771–16776. [Google Scholar] [CrossRef]
- Schmidt, S.; Liebert, T.; Heinze, T. Synthesis of soluble cellulose tosylates in an eco-friendly medium. Green Chem. 2014, 16, 1941–1946. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Kaneko, Y.; Matsuda, S.; Kadokawa, J. Chemoenzymatic synthesis of amylose-grafted poly(vinyl alcohol). Polym. Chem. 2010, 1, 193–197. [Google Scholar] [CrossRef]
- Pramanik, S.; Reddy, R.R.; Ghorai, P. Transition Metal-Free Generation of N-Unsubstituted Imines from Benzyl Azides: Synthesis of N-Unsubstituted Homoallylic Amines. J. Org. Chem. 2014, 80, 3656–3663. [Google Scholar] [CrossRef] [PubMed]
- Elchinger, P.-H.; Awada, H.; Zerrouki, C.; Montplaisir, D.; Zerrouki, R. Kraft Pulp-Starch Covalent Linking: A Promising Route to a New Material. Ind. Eng. Chem. Res. 2014, 53, 7604–7610. [Google Scholar] [CrossRef]
- Nakajima, N.; Ikada, Y. Mechanism of Amide Formation by Carbodiimide for Bioconjugation in Aqueous Media. Bioconjugate Chem. 1995, 6, 123–130. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Zhang, Y.; Zhu, Y. Preparation and characterization of graphene oxide reinforced PVA film with boric acid as crosslinker. J. Appl. Polym. Sci. 2015, 132, 42000–42008. [Google Scholar] [CrossRef]
- Kavitha, A.S.; Kiruthika, R.; Kalaivani, A.; Synthesis, S. Characterization and Antimicrobial Studies of Tosyl Esters of Carboxylic Acid. Int. J. Sci. Res. Publ. 2014, 4, 1–4. [Google Scholar]
- Gallardo, I.F.; Webb, L.J. Tethering Hydrophobic Peptides to Functionalized Self-Assembled Monolayers on Gold through Two Chemical Linkers Using the Huisgen Cycloaddition. Langmuir 2010, 26, 18959–18966. [Google Scholar] [CrossRef] [PubMed]
- Daugaard, A.E.; Hvilsted, S.; Hansen, T.S.; Larsen, N.B. Conductive Polymer Functionalization by Click Chemistry. Macromolecules 2008, 41, 4321–4327. [Google Scholar] [CrossRef]
- Darlatta, E.; Traulsen, C.H.-H.; Poppenberg, J.; Richter, S.; Kühna, J.; Schalley, C.A.; Unger, W.E.S. Evidence of click and coordination reactions on a self-assembled monolayer by synchrotron radiation based XPS and NEXAFS. J. Electron Spectrosc. Relat. Phenom. 2012, 185, 85–89. [Google Scholar] [CrossRef]
- Zorn, G.; Liu, L.H.; Árnadóttir, L.; Wang, H.; Gamble, L.J.; Castner, D.G.; Yan, M. X-ray Photoelectron Spectroscopy Investigation of the Nitrogen Species in Photoactive Perfluorophenylazide-Modified Surfaces. J. Phys. Chem. C 2014, 118, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, S.M.; Brown, A.H.; Ashby, V.S. It Is the Outside That Counts: Chemical and Physical Control of Dynamic Surfaces. J. Am. Chem. Soc. 2013, 135, 3067–3072. [Google Scholar] [CrossRef] [PubMed]
- Cimenez, V.; Mantecon, A.; Cadiz, V. Modification of Poly(vinyl alcohol) with Acid Chlorides and Crosslinking with Difunctional Hardeners. J. Polym. Sci. Part A 1996, 34, 925–934. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awada, H.; Daneault, C. Chemical Modification of Poly(Vinyl Alcohol) in Water. Appl. Sci. 2015, 5, 840-850. https://doi.org/10.3390/app5040840
Awada H, Daneault C. Chemical Modification of Poly(Vinyl Alcohol) in Water. Applied Sciences. 2015; 5(4):840-850. https://doi.org/10.3390/app5040840
Chicago/Turabian StyleAwada, Houssein, and Claude Daneault. 2015. "Chemical Modification of Poly(Vinyl Alcohol) in Water" Applied Sciences 5, no. 4: 840-850. https://doi.org/10.3390/app5040840
APA StyleAwada, H., & Daneault, C. (2015). Chemical Modification of Poly(Vinyl Alcohol) in Water. Applied Sciences, 5(4), 840-850. https://doi.org/10.3390/app5040840