1. Introduction
As the global economy and society continue to develop, energy consumption is steadily increasing. The Energy Institute (EI), in its
Statistical Review of World Energy [
1], reported that 2023 was marked by global energy shortages, with primary energy consumption rising by 2% compared to 2022. Renewable energy accounted for over 18% of total consumption, while the share of fossil fuels declined by 0.4%. Although the global energy transition remains gradual, different regions are adopting diverse approaches to energy transformation.
Hydrogen, recognized as a clean and efficient energy carrier, has garnered significant attention worldwide. In October 2024, the International Energy Agency (IEA) released the 2024 Global Hydrogen Review [
2], which indicated that global hydrogen demand reached 97 million tons in 2023, reflecting a 2.5% year-on-year increase. Despite planned projects aiming to produce up to 49 million tons of green hydrogen annually by 2030, supply is projected to fall short of demand. Consequently, the development of efficient, clean, and scaled-up hydrogen production technologies has become a research priority.
Among various hydrogen production methods, the thermochemical iodine–sulfur (I-S) cycle, initially proposed by General Atomics (GA) [
3,
4,
5], is regarded as one of the most promising technologies due to its industrial scalability and compatibility with diverse energy sources such as solar, nuclear, and industrial waste heat [
6,
7]. The I-S cycle comprises three key reactions:
In recent years, significant progress has been made in fundamental research on the three aforementioned reactions. However, challenges remain, including severe corrosion conditions, the high temperature required for sulfuric acid decomposition, and the continuous transport and circulation of gases and liquids. While researchers from various countries have made efforts to improve system construction and continuous operation, they continue to encounter difficulties in the practical application of the technology.
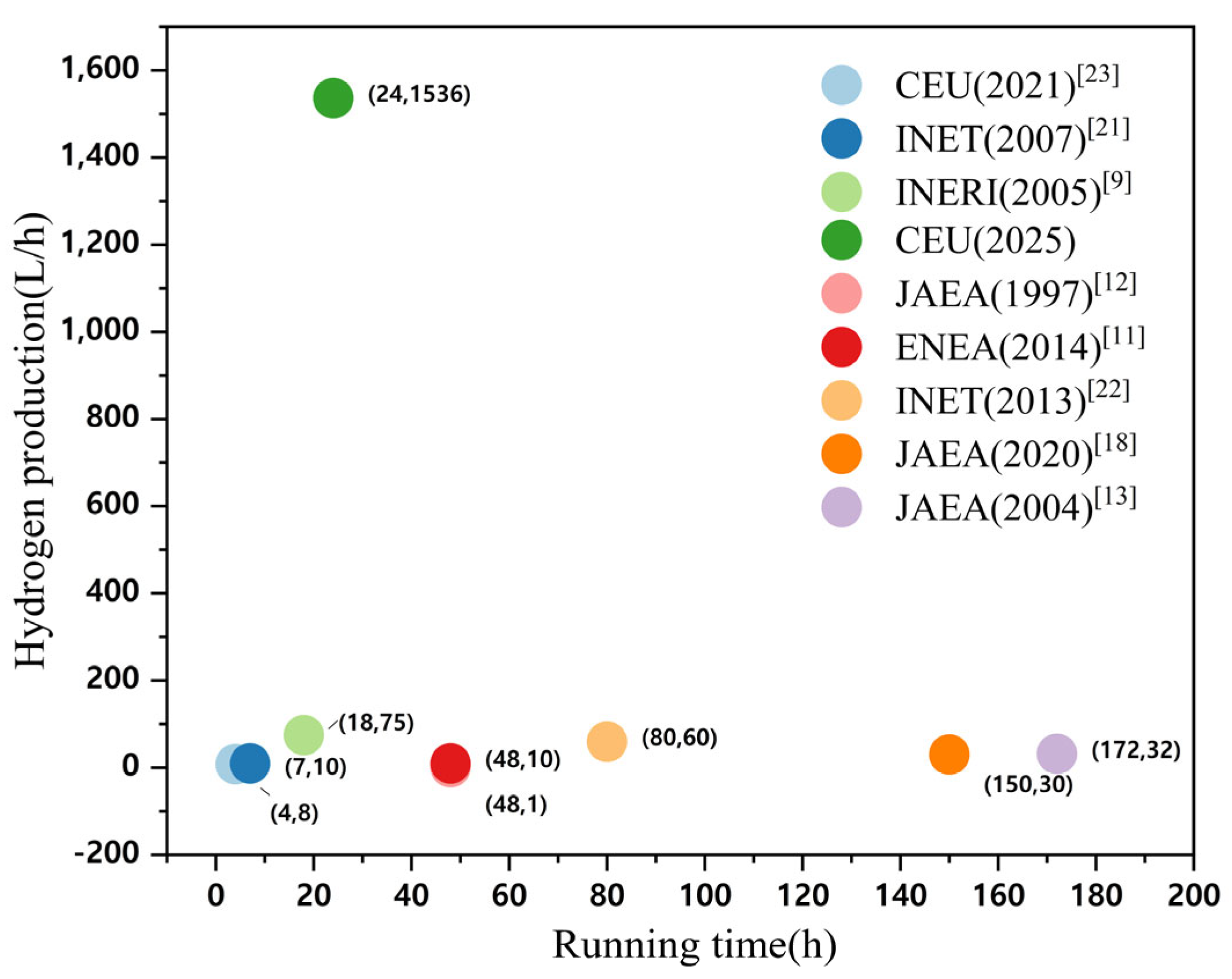
As the originator of the iodine–sulfur cycle process, General Atomics (GA) designed and built the world’s first thermochemical iodine–sulfur cycle hydrogen production system. The theoretical hydrogen production rate of the system was 4 L/min, with a thermal efficiency of 47% [
8]. However, due to various issues, the actual hydrogen production rate during intermittent operation was only 20 mL/min. Subsequently, with support from the U.S. Department of Energy’s Nuclear Energy Office and in collaboration with Sandia National Laboratories (SNL) and the French Commissariat à l’Énergie Atomique (CEA), GA undertook an International Nuclear Energy Research Initiative (INERI) [
9] to explore the feasibility of using the iodine–sulfur cycle for hydrogen production with nuclear energy. The system, constructed from glass-lined steel and tantalum–tungsten alloy, ultimately achieved continuous operation for 18 h, with hydrogen production rates ranging from 10 to 75 L/h.
The Italian National Agency for New Technologies, Energy, and Sustainable Economic Development (ENEA) designed and successfully operated a 10 L/h iodine–sulfur hydrogen production system constructed from glass and polytetrafluoroethylene (PTFE), achieving 48 h of operation [
10,
11].
The Japan Atomic Energy Agency (JAEA) successfully operated a 1 L/h hydrogen production system made of glass for 48 h in 1997, confirming the feasibility of the iodine–sulfur hydrogen production method [
12]. In 2004, JAEA implemented new measurement techniques and operational control technologies to develop and operate a 30 L/h laboratory-scale system [
13]. By 2014, JAEA had constructed a pilot-scale platform using industrial materials, achieving a hydrogen production rate of 100 L/h with continuous operation for 8 h [
14]. Later, JAEA addressed pump failure issues by developing an axial seal technology, which extended the pilot system’s operation time to 31 h and increased the hydrogen production rate to 20 L/h [
15]. Additionally, the agency introduced an improved glass-lined protective casing [
16] and proposed a method for estimating the construction strength of large-scale silicon carbide (SiC) based on effective volume theory [
17]. These enhancements, along with other improvements, enabled the pilot platform to achieve 150 h of continuous operation, with a hydrogen production rate ranging from 10 to 30 L/h [
18].
In 2009, the Institute of Nuclear Energy Technology (INET) at Tsinghua University constructed a glass-based hydrogen production system with a hydrogen production rate of 10 L/h [
19,
20]. This system was the first to employ electrodialysis (EED) technology, achieving continuous hydrogen production at 10 L/h for 7 h. Subsequently, through system optimization, the laboratory-scale system was scaled up, ultimately achieving continuous hydrogen production at 60 L/h for 60 h [
21,
22].
In 2015, the State Key Laboratory of Clean Energy Utilization (CEU) of Zhejiang University constructed a laboratory-scale hydrogen production system with a hydrogen production rate of 50 L/h. The system was validated through trial runs to ensure the normal operation of each module. In 2019, CEU developed a pilot-scale system capable of producing maximum hydrogen at 5 m
3/h using industrial materials. After a segmented operation to verify the normal functionality of each module, the system achieved continuous operation for 4 h in 2021, with a hydrogen production rate of 80 L/h [
23].
This article primarily focuses on the updating and optimization of the CEU’s system and reports the latest 24 h testing results. The redesign and manufacturing of several key reactors and components, as well as continuous operational performance, will be reported here. Compared to previous studies, this paper designs a three-stage sulfuric acid decomposition reaction module to address corrosion issues in high-temperature sulfuric acid decomposition, and develops a heat-recovery HI decomposition module to reduce system energy consumption, ultimately achieving 24 h continuous high-yield system operation. This demonstration trial achieves a 13% increase in thermal efficiency, extends hydrogen production stability from 4 h to 24 h, and improves hydrogen purity from 92% to 98%. It effectively resolves the corrosion issues and waste heat utilization problems existing in the prior system.
2. System Update and Optimization
In the previous system process, the undecomposed HI solution and H
2SO
4 solution from the decomposer outlet were recycled to the Bunsen tank to participate in the Bunsen reaction again. This significantly increased the system’s energy consumption and hindered the Bunsen reaction. Therefore, in the optimized system, the undecomposed HI and H
2SO
4 solutions are redirected to the purification tower, where they are mixed and purified with freshly separated liquid–liquid phases. As a result, a small cycle within the entire system was established to reduce the overall energy consumption. The undigested sulfuric acid and iodate solutions no longer return to the Bunsen reaction to participate in the overall system circulation, but undergo small cycles in the distillation and decomposition module circulation. Aspen Plus V12 software was utilized to design and optimize the process. For the thermochemical sulfur–iodine cycle, a typical polar non-ideal system, this study adopts a differentiated-property method selection strategy based on the material characteristics and processing conditions of different process modules. The research findings from [
24] demonstrate that the ELECNRTL electrolyte model exhibits significant advantages in simulating such systems. This model boasts broad applicability, as it can handle electrolyte solutions across various concentrations and is equally suitable for mixed-solution systems. Its theoretical foundation rests on two core assumptions: First, mutual repulsion exists between ions of the same charge, with ions of opposite charge distributed around any central ion. Second, local electroneutrality is maintained, meaning the total charge of surrounding ions balances that of the central ion within its immediate vicinity. In terms of specific model construction, long-range ion interactions are characterized using a modified Pitzer–Debye–Hückel asymmetric model combined with the Born correction equation [
25], while short-range molecular interactions are described by the Non-Random Two-Liquid (NRTL) model. This multi-scale modeling approach effectively integrates the influence mechanisms of long-range electrostatic interactions and short-range intermolecular forces. Consequently, the expression for calculating the excess Gibbs energy is formulated as follows:
The relationship between the corresponding activity coefficients can be obtained from this:
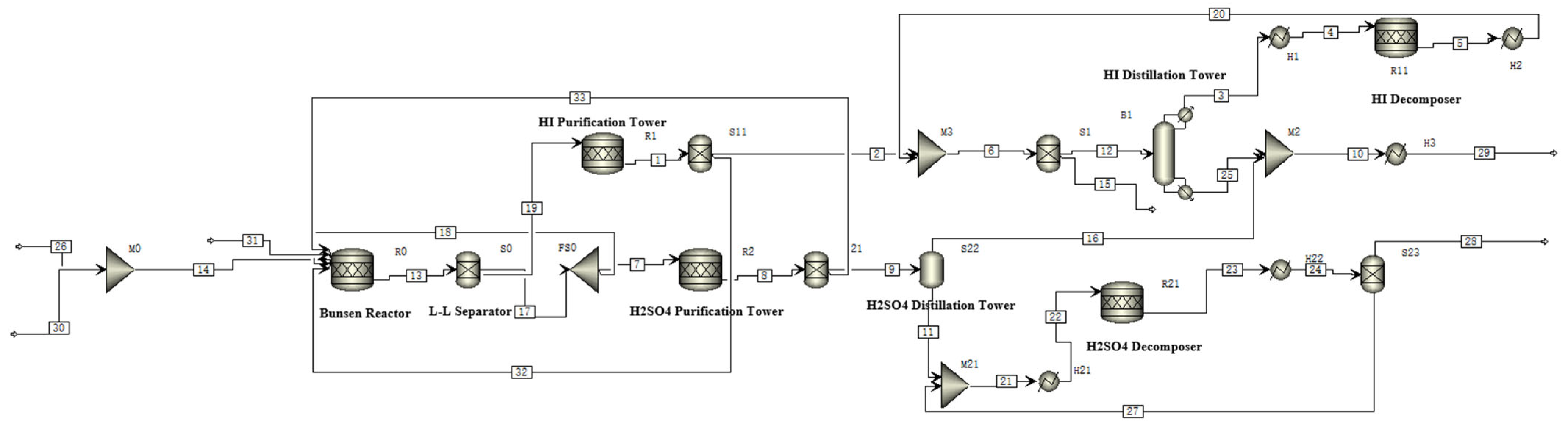
The system flow is illustrated in
Figure 1, while the corresponding stream components, temperature, and pressure are detailed in
Table 1.
For the entire system, a fully closed-loop operation is employed, requiring only 44.65 mol of water and an external energy input to produce 44.642 mol of hydrogen and 22.321 mol of oxygen. In the Bunsen reaction module, the reverse reaction products (streams 32 and 33) from the HI and H2SO4 purification towers, along with the circulating streams (28 and 29) from the H2SO4 and HI decomposition modules, are directed back to the Bunsen reaction. In the H2SO4 module, to match the hydrogen production target, a portion of H2SO4 stream 18 is recycled to the Bunsen reaction, while the remaining stream 7 is purified in the R2 purification tower at 418.15 K to remove HI and I2. The product is transferred to flash distillation tower S22 to obtain 87.75% H2SO4. It is then mixed with the remaining H2SO4 stream 27 and heated to 1123.15 K in H21. Subsequently, it enters reactor R21 for decomposition into SO2, O2, and H2O, achieving a conversion rate of 70%. The liquid-phase stream 27 is mixed in M21 with the H2SO4 from the flash distillation, while the gas-phase stream 28 is recycled to the Bunsen reaction. In the HI module, stream 19 is purified in R1 at 390.15 K to remove H2SO4. Afterward, the purified flow is transferred to B1 for distillation. At the top of the tower, HI vapor with a concentration of 91.44% is obtained. Stream 25 from the reboiler is condensed in condenser H3 before being transferred to the Bunsen reaction module. Stream 3 is heated to 773.15 K in H1 and undergoes decomposition in reactor R11, achieving a 15% conversion rate to produce H2 and I2. After condensing to 393.15 K in H2, it is mixed with stream 2 and transferred to S1 for gas–liquid separation. Liquid-phase stream 12 enters the distillation column for the small cycle, while gas-phase stream 15 is collected as the final product.
Table 2 shows the external thermal and electrical energy demands before and after the system optimization. In the absence of heat recovery utilization, the thermal efficiency is defined as
where
denotes the higher heating value of hydrogen, which is 286 kJ/mol-H
2;
represents the hydrogen production rate;
is the total external heat required;
is the electrical energy needed for the system; and
is the conversion efficiency of electrical energy, which is 40% here. The calculated thermal efficiency is 53%, representing a 12% increase compared to the efficiency before the update [
23].
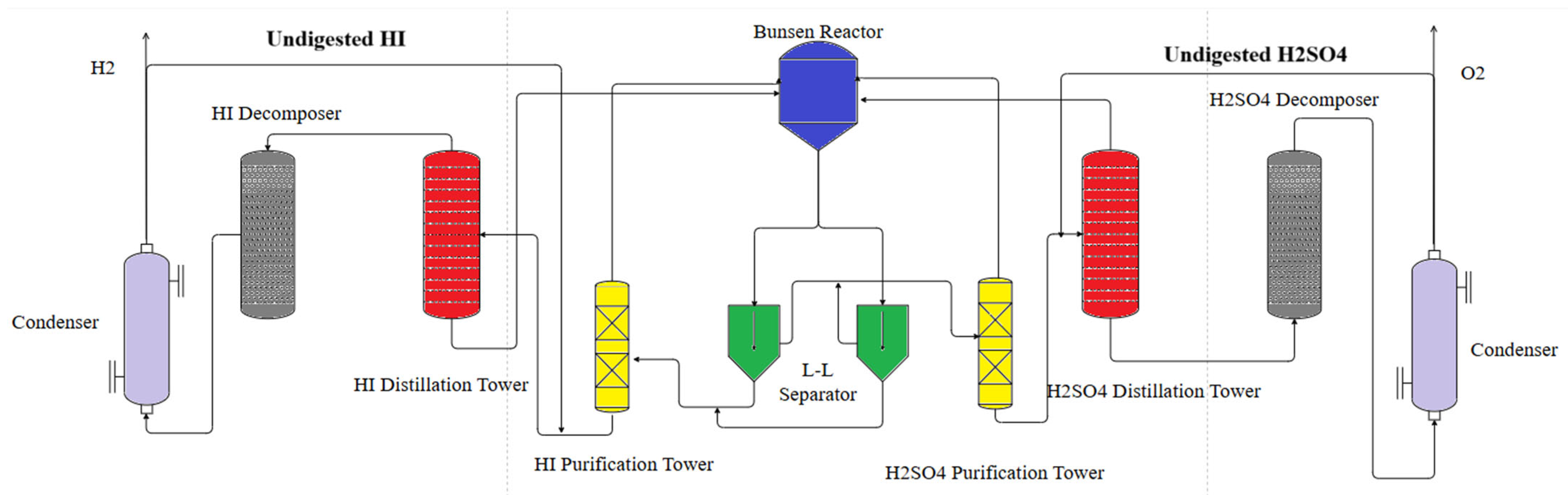
Based on the Aspen simulation results, the hydrogen production pilot platform was updated. The system utilizes industrial materials such as HC276, TC4, carbon steel lined with enamel, and SiC. All the thermal energy for the system is provided by electric heating.
Figure 2 is a schematic diagram of the modified hydrogen production pilot platform.
3. Key Component Update
3.1. Selection of Corrosion-Resistant Materials
The issue of material corrosion is particularly critical due to the involvement of media such as H2SO4 and HI. Earlier studies investigated the corrosion resistance of metallic materials, but these were limited to conventional single-metal materials, which exhibited severe corrosion in practical applications. In this study, we selected nine alloy materials (HC276, HC2000, HB-3, TA2, TC4, Monel400, Inconel617, Inconel601, Inconel690) from those that are commonly used and perform well on the market as research subjects for corrosion resistance testing. During previous pilot-scale tests, we observed significant metal corrosion during the phase transition of H2SO4. Therefore, in addition to traditional gaseous HI and H2SO4 corrosion tests, we specifically examined H2SO4 corrosion during phase transition. Furthermore, three ceramic materials (SiC, Al2O3, WC) were included in the corrosion experiments to provide guidance for subsequent reactor development.
In the corrosion testing, the samples were placed in quartz tubes and heated in a tubular furnace to specified temperatures: 500 °C for gaseous HI corrosion, 850 °C for gaseous H2SO4 corrosion, and 400 °C for H2SO4 phase transition corrosion. Upon reaching the target temperature, the corrosive solution was injected into the evaporator via a peristaltic pump at a constant rate (1 mL/min). The gasified materials were then tested under a stable nitrogen carrier gas flow of 200 mL/min.
The corrosion rates of different materials are shown in
Table 3. HC276, TC4, and TA2 exhibited negative corrosion rates under gaseous HI decomposition conditions, attributed to the formation of dense oxide protective films on their surfaces, which effectively resisted acidic gas corrosion. HC2000 and HB-3 demonstrated excellent corrosion resistance, with corrosion rates of 0.0171 mm/a and 0.0053 mm/a, respectively. Monel and Inconel alloys showed poor corrosion resistance in gaseous HI environments, leading to the selection of the TC4 alloy for the HI decomposer design.
In gaseous H2SO4 decomposition conditions, TA2 and Monel400 displayed negative corrosion rates. However, post-experiment analysis revealed spalling of brittle surface layers on TC4, TA2, and Monel400, with TC4 showing the most severe detachment. This indicates that although passivation films formed on these metals, the films were unstable and prone to mechanical failure. Hastelloy and Inconel alloys exhibited superior corrosion resistance in gaseous sulfuric acid, particularly Inconel690 with a corrosion rate of only 0.076 mm/a, demonstrating high stability. Considering material processability and cost, HC276 was selected for sulfuric acid module reactor development.
Hastelloy, titanium alloys, and Inconel alloys showed unsatisfactory corrosion resistance during H2SO4 phase transition, with titanium alloys being the most severely affected. In contrast, ceramic materials maintained excellent stability during sulfuric acid boiling and phase transition. SiC and WC performed exceptionally well, with WC exhibiting a corrosion rate of 0.0239 mm/a, making it suitable for long-term use in phase transition environments. However, Al2O3, being an amphoteric oxide, reacts with strong acids and bases, resulting in instability in industrial applications. Although WC is an ideal benchmark material, its scarcity, high cost, and processing challenges limit its current adoption. Therefore, SiC or ceramic-lined carbon steel heat exchange tubes were temporarily chosen as materials for sulfuric acid phase transition processes.
3.2. Three-Stage H2SO4 Decomposition Reactor
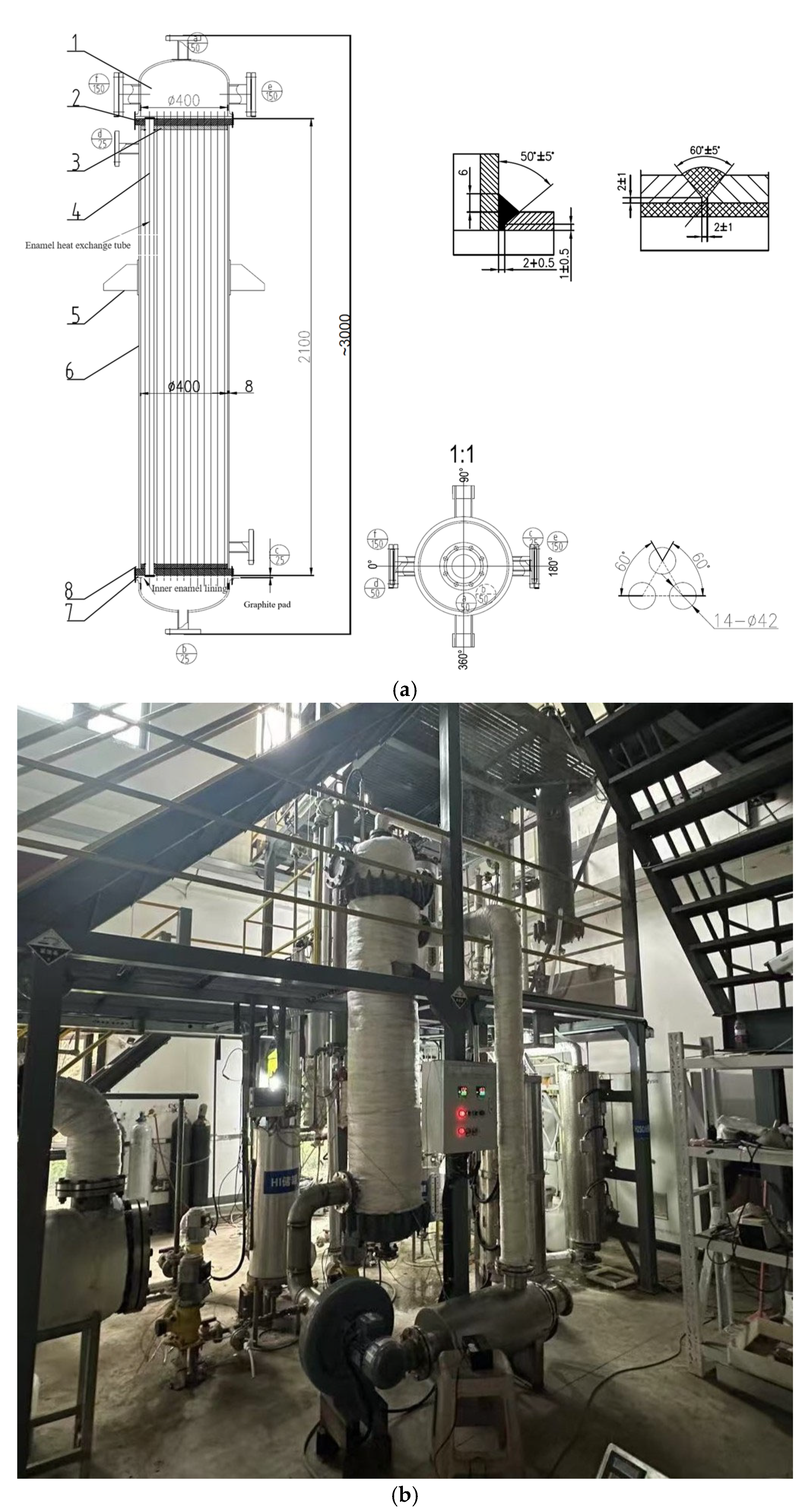
To ensure sufficient heat exchange, the H2SO4 catalytic decomposer is designed with dimensions of Φ108 × 1500 mm. Prior to using the H2SO4 decomposer, an HC276 alloy superheater is used as a preheating device to ensure that the preheating section outlet temperature reaches 850 °C, enabling efficient H2SO4 decomposition. The superheater dimensions are Φ98 × 1600 mm.
Previous experiments revealed severe corrosion in the HC276 alloy during phase changing of the H
2SO
4 stream. To address this problem, a two-step strategy was utilized here. Firstly, an enamel-lined air heat exchanger was used to evaporate liquid-phase H
2SO
4 into a gas phase, followed by HC276 superheating. The enamel-lined air heat exchanger is shown in
Figure 3. The evaporator has a shell-and-tube heat exchanger structure with dimensions of Φ400 × 2100 mm and a heat exchange tube diameter of 42 mm. Electrically heated air was used as a heat source for evaporation.
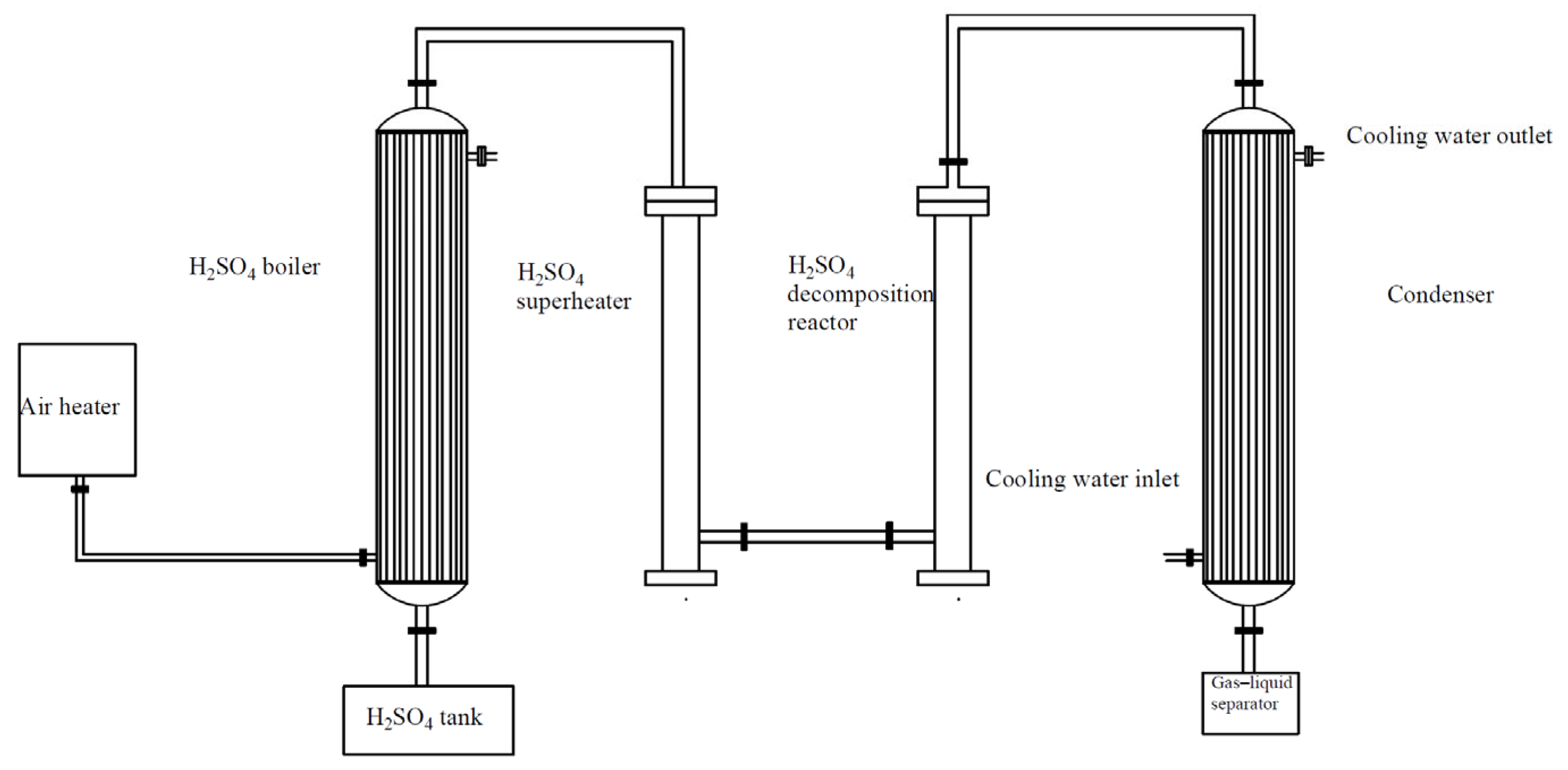
As shown in
Figure 4, H
2SO
4 is pumped from the bottom of the air heat exchanger into the H
2SO
4 evaporator for evaporation. The gaseous H
2SO
4 then enters the top inlet of the H
2SO
4 superheater from the top. Heat exchange is enhanced using small silicon carbide balls in a cylindrical reactor chamber, where the first step of decomposition occurs, decomposing H
2SO
4 into SO₃ and H
2O, with heat supplied by external electric heating.
Finally, gaseous SO₃ enters the H2SO4 catalytic decomposition reactor from the superheater’s base. The reactor cavity is filled with iron oxide-based catalysts, where catalytic decomposition occurs, producing SO2 and O2.
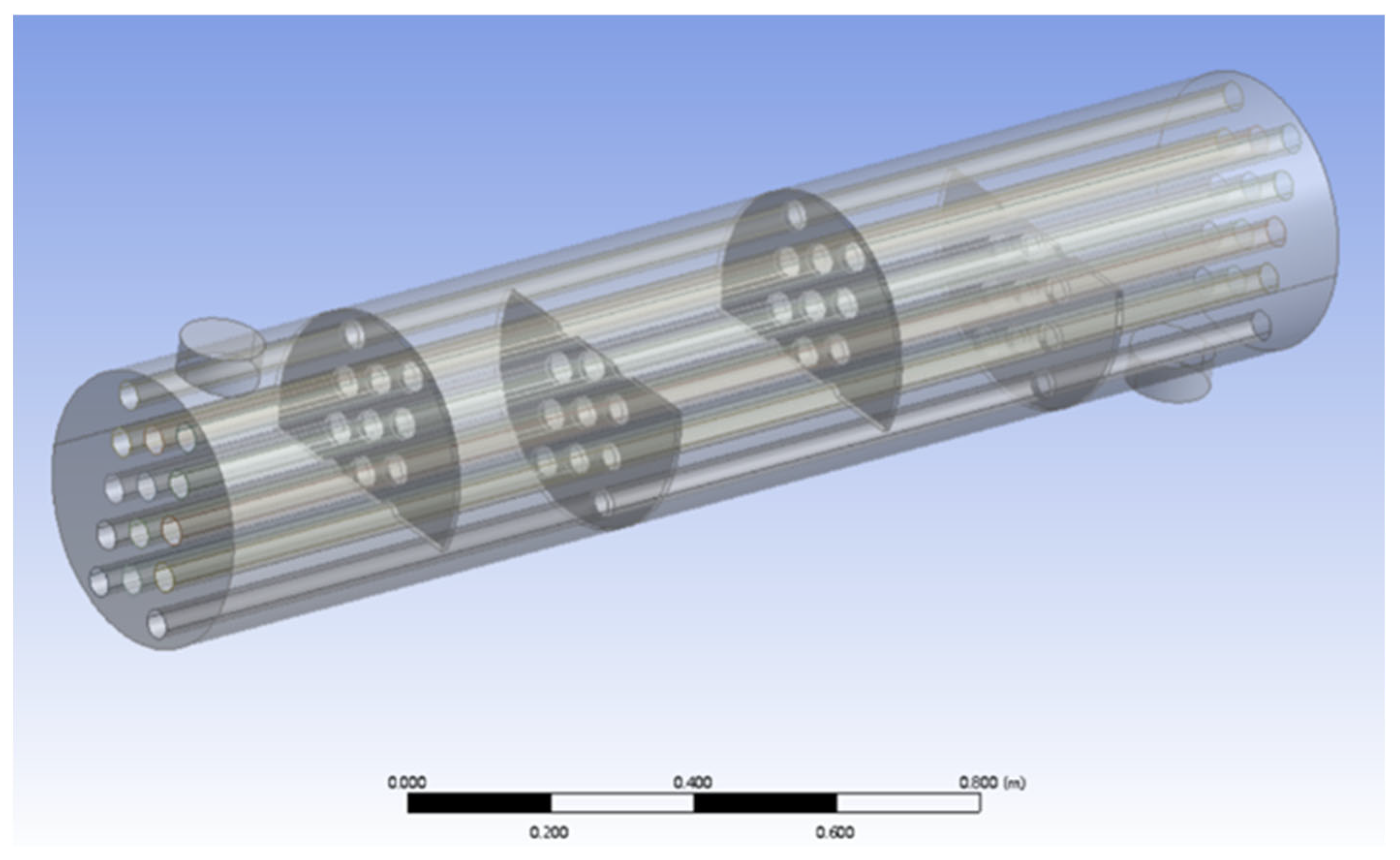
Before conducting the experiments, a Computational Fluid Dynamics (CFD) simulation was performed for the H
2SO
4 evaporator to ensure that H
2SO
4 would be evaporated properly and prevent corrosion in the subsequent HC276 reactor. Due to the complexity of the H
2SO
4 evaporator structure, this simulation mainly focused on the primary heat exchange region, ignoring the upper and lower heads of the evaporator. It was assumed that the mass flow rate of H
2SO
4 was uniform across all 14 heat exchange tubes, the external surface of the evaporator had good insulation with no heat loss, and the heat transfer through the baffles and shell was neglected. A geometric model of the H
2SO
4 evaporator is shown in
Figure 5.
The heat exchange tubes of the H
2SO
4 evaporator are made of enamel-lined carbon steel. Using parameters from the network database, the thermal resistance of the heat exchange tube walls was calculated. The specific parameter settings are shown in
Table 4.
Due to the complexity of simulating H
2SO
4 boiling and the primary goal of evaluating heat exchanger performance, an equivalent heat capacity method was employed. The latent heat of H
2SO
4 vaporization was converted into the heat capacity of sulfuric acid. This simplification avoids the complex calculations of the boiling process. Additionally, since the heat transfer coefficient during boiling is much higher than that of normal convective heat conduction, using the equivalent heat capacity method to calculate the heat exchange power will yield better results in practice than using the calculated values. The relevant boundary conditions are shown in
Table 5.
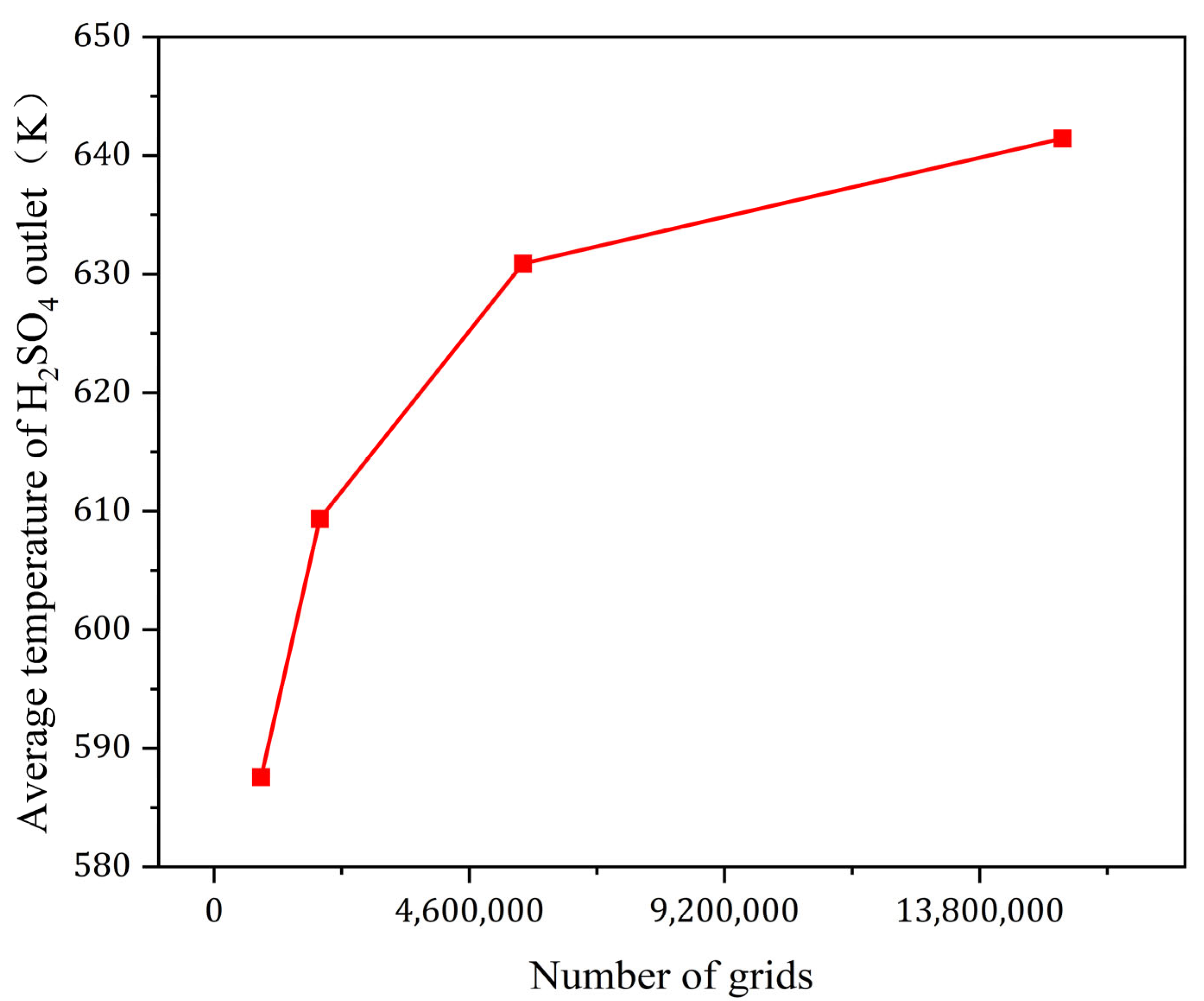
This model primarily uses quadrilateral meshing for grid division. To validate this approach, four mesh densities were selected: fine (15,294,678 cells), normal (5,565,668 cells), medium (1,906,694 cells), and coarse (846,135 cells). The verification was conducted by comparing the average H
2SO
4 outlet temperature, which is a critical parameter for the final results. As shown in
Figure 6, which illustrates the mesh independence verification, the fine mesh model was ultimately chosen for the calculations.
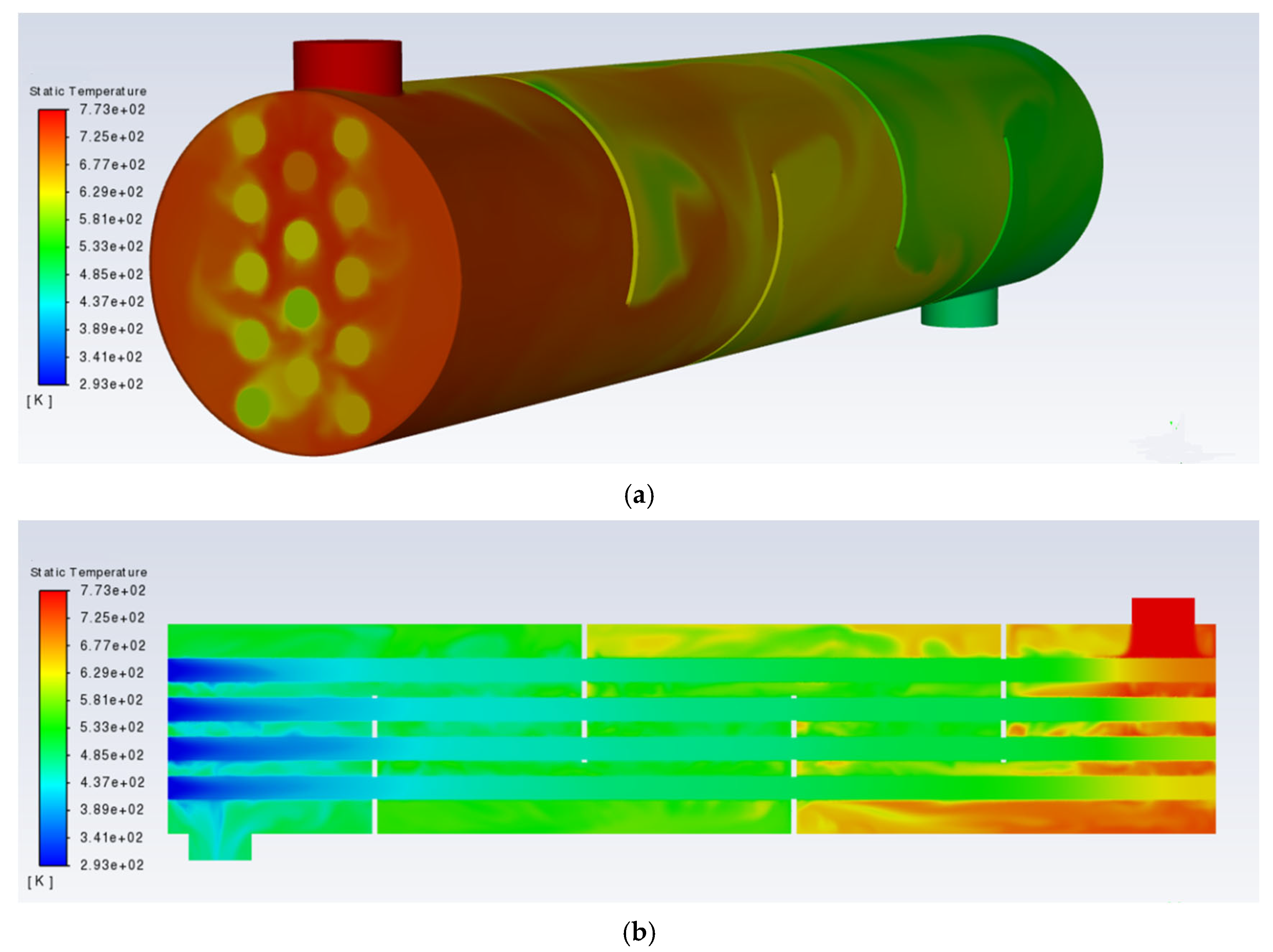
The overall temperature distribution of the evaporator after calculation is shown in
Figure 7a. To more clearly present the overall temperature distribution inside the evaporator, the central plane of the evaporator was selected, and its temperature distribution is shown in
Figure 7b. It can be seen that the H
2SO
4 evaporator performs well in heat exchange, with the temperature of the hot air dropping from 773.15 K to 461.59 K, and the temperature of the H
2SO
4 rising from 293.15 K to an average of 641.44 K. The boiling point of sulfuric acid is a maximum of 603.15 K, so it can be concluded that the sulfuric acid in the evaporator fully boiled under the designed operating conditions. Using Fluent’s built-in surface integration function, the heat exchange power of the 14 heat exchange tubes was calculated, with a total heat exchange power of 5041.01 W.
The simulation’s accuracy was validated through energy conservation analysis, comparing heat released by cooling hot air, heat absorbed by H
2SO
4 during heating/vaporization, and the heat exchange tubes’ power. Using Aspen Plus, the thermophysical properties of hot air and H
2SO
4 from the parameter library were used for energy conservation calculations. The calculation results are shown in
Table 6. It can be seen that the error calculated using the energy conservation method is within a reasonable range, indicating that the calculation results are reliable and the designed evaporator meets the sulfuric acid gasification requirements.
3.3. HI Decomposer with Heat Recovery Utilization
During early experiments, it was found that activated carbon had poor thermal conductivity, making it difficult for a large flow of HI to achieve both superheating and decomposition within the reactor. Therefore, an evaporator was added before the HI catalytic decomposer to gasify HI, and a superheater was installed to heat HI to the reaction temperature (773.15 K). In previous experiments, the decomposed HI was directly cooled by a cooler, resulting in significant heat loss. In this modification, to verify the feasibility of internal heat recovery, the high-temperature gas from decomposition is used to preheat the HI vapor before entering the superheater. This optimization aims for a hydrogen production rate of 1000 L/h, based on relevant data obtained from prior experiments. The relevant parameters for the HI decomposer are shown in
Table 7.
Through thermodynamic calculations, the heat absorption and release for heating the distilled HI solution to 773.15 K and cooling the high-temperature gas from decomposition to 473.15 K were determined, as shown in
Table 8.
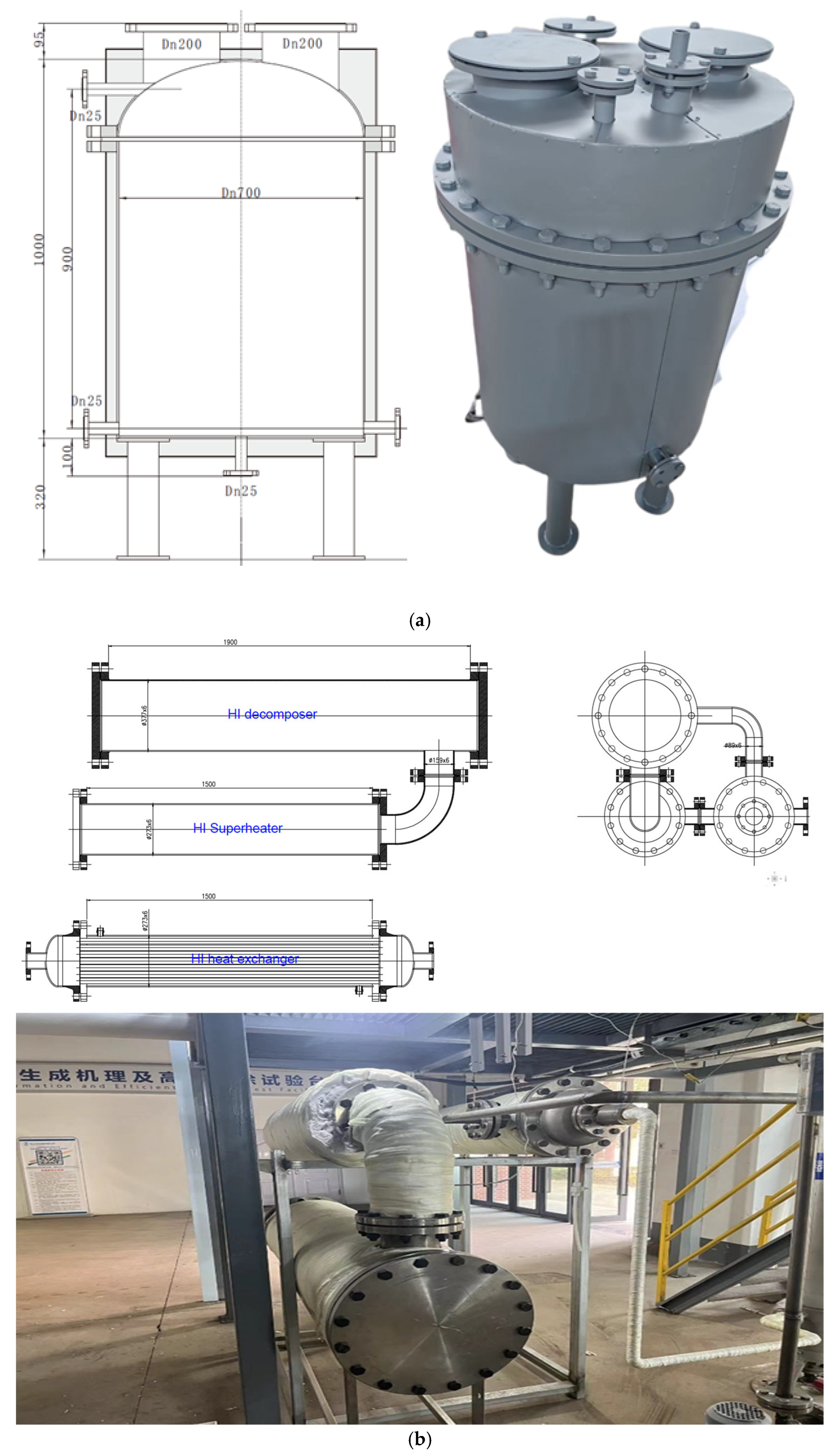
Based on the thermodynamic calculation results, the HI decomposition reaction module was redesigned and manufactured as shown in
Figure 8. All equipment is made of TC4 alloy. The distilled HI solution first enters the evaporator from the top. The evaporator has dimensions of Φ700 × 900 mm and is equipped with 54 kW adjustable-power titanium heating rods (108 kW at full load). In the evaporator, the H
2 solution undergoes heating and vaporization. The resulting gas then enters the HI shell-and-tube heat exchanger (Φ273 × 1500 mm), where it exchanges heat with the high-temperature gas flow from decomposition, heating the HI to about 653.15 K. The fluid then enters a Φ273 × 1500 mm superheater to heat to the required decomposition temperature of 773.15 K before entering the catalytic decomposition reactor filled with an activated carbon-based catalyst, where it decomposes to produce H
2 and I
2. The dimensions of the catalytic decomposer are Φ377 × 1900 mm. The generated products and undecomposed HI solution enter the heat exchanger tube side, exchanging heat with the gaseous HI solution in the shell side, cooling to about 473.15 K, and then entering the condenser and gas–liquid separator to produce hydrogen as the product.
5. Conclusions and Outlook
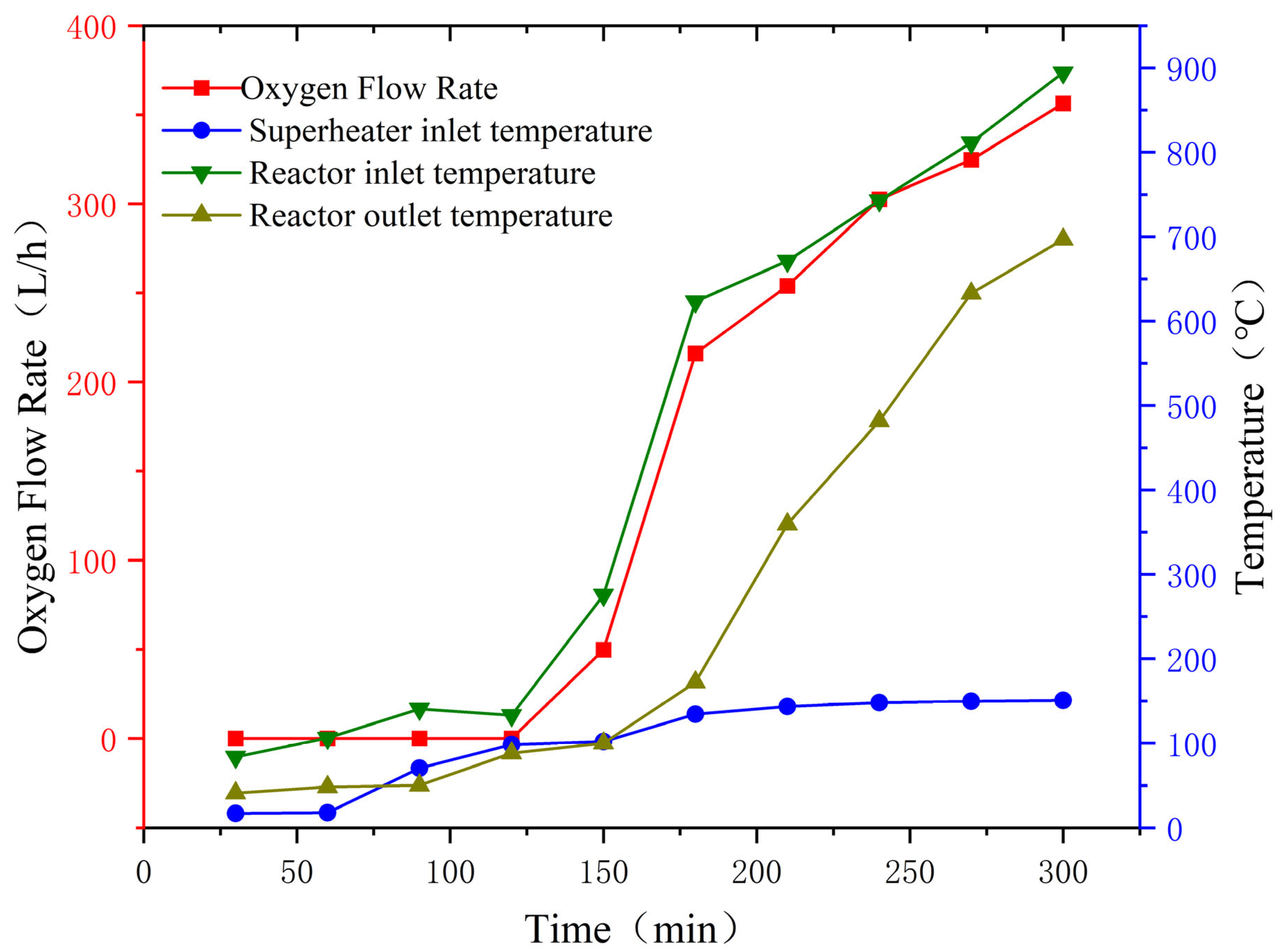
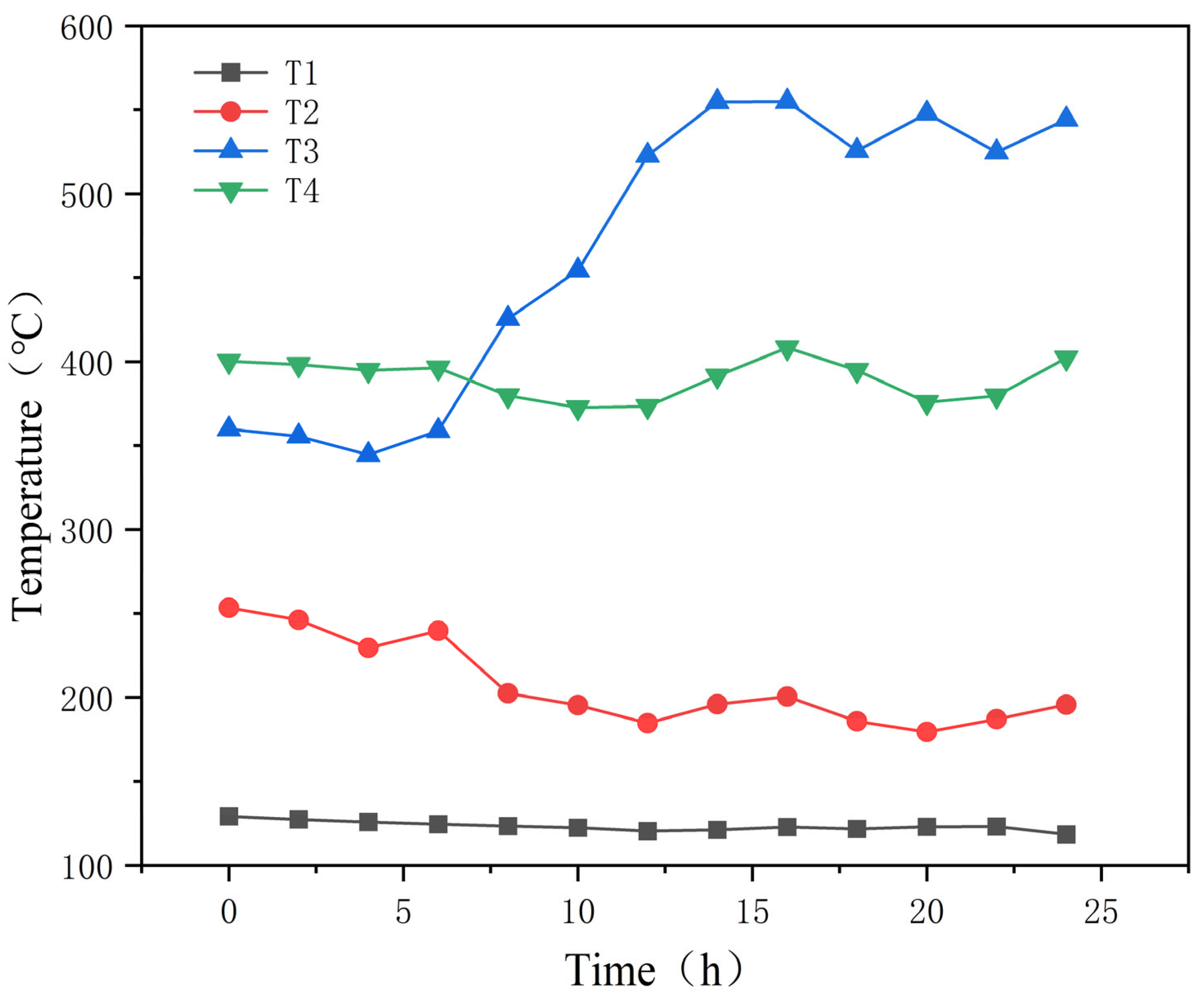
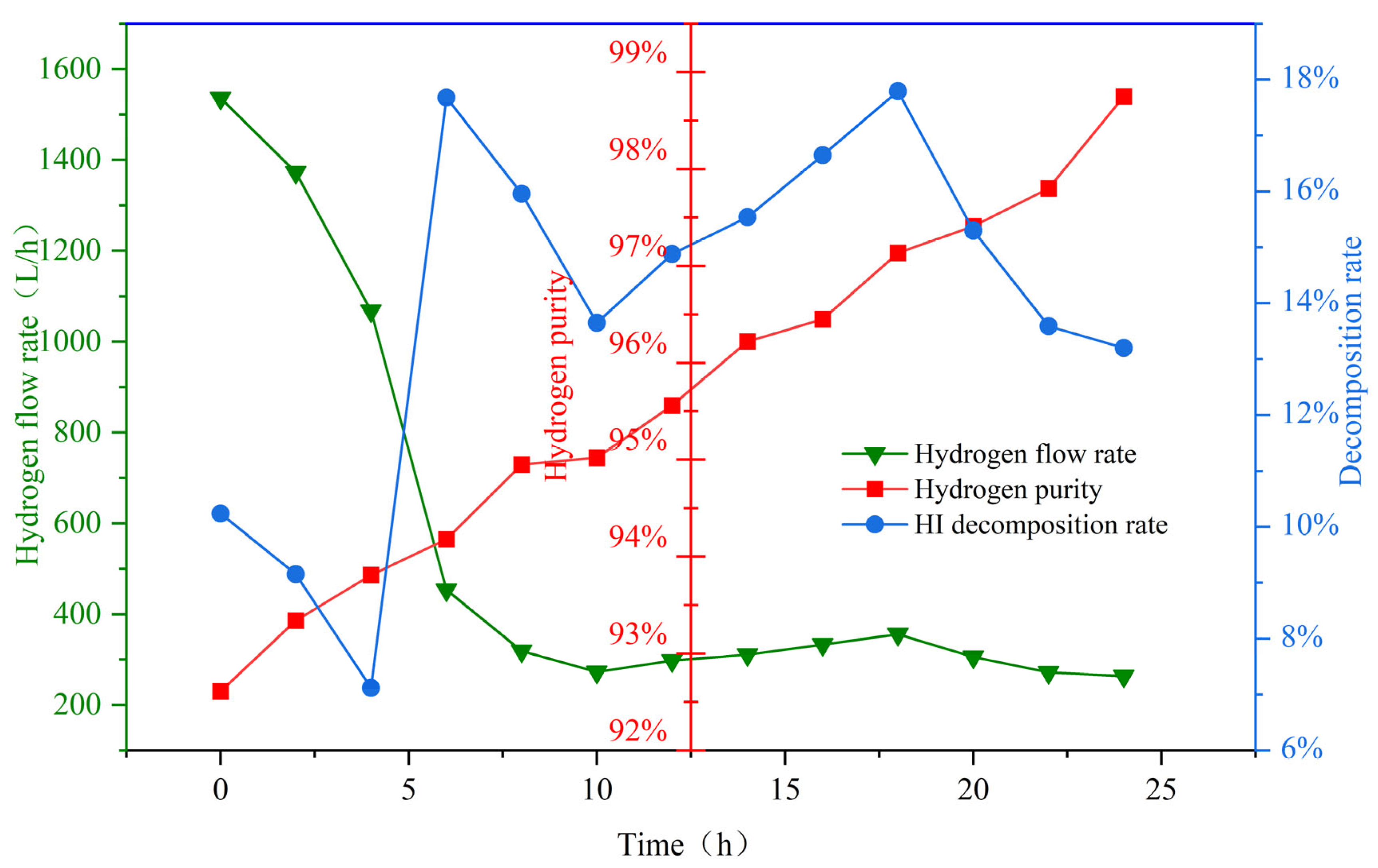
This paper details the optimization of traditional thermochemical iodine–sulfur cycle water-splitting hydrogen production technology. It introduces the concept of a small cycle, which enhances the thermal efficiency of hydrogen production to 53% under conditions where the electrical energy conversion efficiency is 40%. Based on this optimization, the hydrogen production pilot platform at Qingshan Lake Energy Research Base of Zhejiang university was modified. To address the corrosion problem during sulfuric acid evaporation, an innovative three-stage H2SO4 reactor was developed and validated through CFD simulations, preventing the corrosive issues that occur during high-temperature H2SO4 decomposition. A heat recovery-utilizing HI decomposition reactor was adopted to improve heat recovery efficiency and increase hydrogen production efficiency. Subsequently, in January 2025, a successful 24 h thermochemical iodine–sulfur cycle hydrogen production test was conducted, with a peak hydrogen production rate of 1536 L/h, stabilizing at approximately 300 L/h in the long term.
In future industrialization processes, the use of electric heating elements in small-scale and pilot tests is not advisable. The standard for industrialization is to use actual high-temperature heat sources for heat exchange. Therefore, the development of reactors for high-temperature actual heat source heat exchange and the design of fully industrial automation control are future research directions.