Structural Pattern Analysis in Patella vulgata Shells Using Raman Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Variability and Environmental Influence on P. vulgata Shells
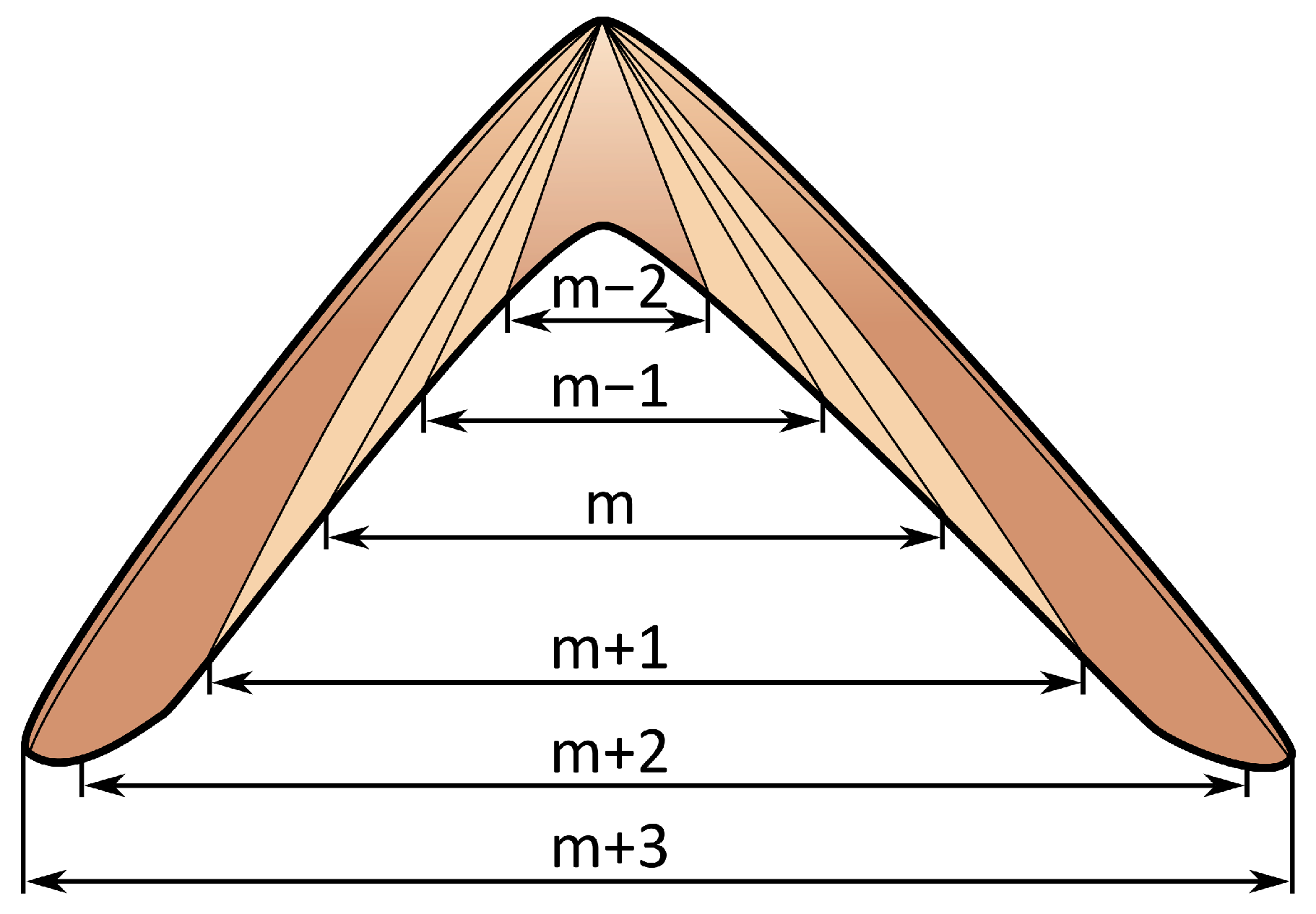
2.2. Shell Structure of P. vulgata
2.3. Sample Preparation
2.4. Raman Spectroscopy Measurements
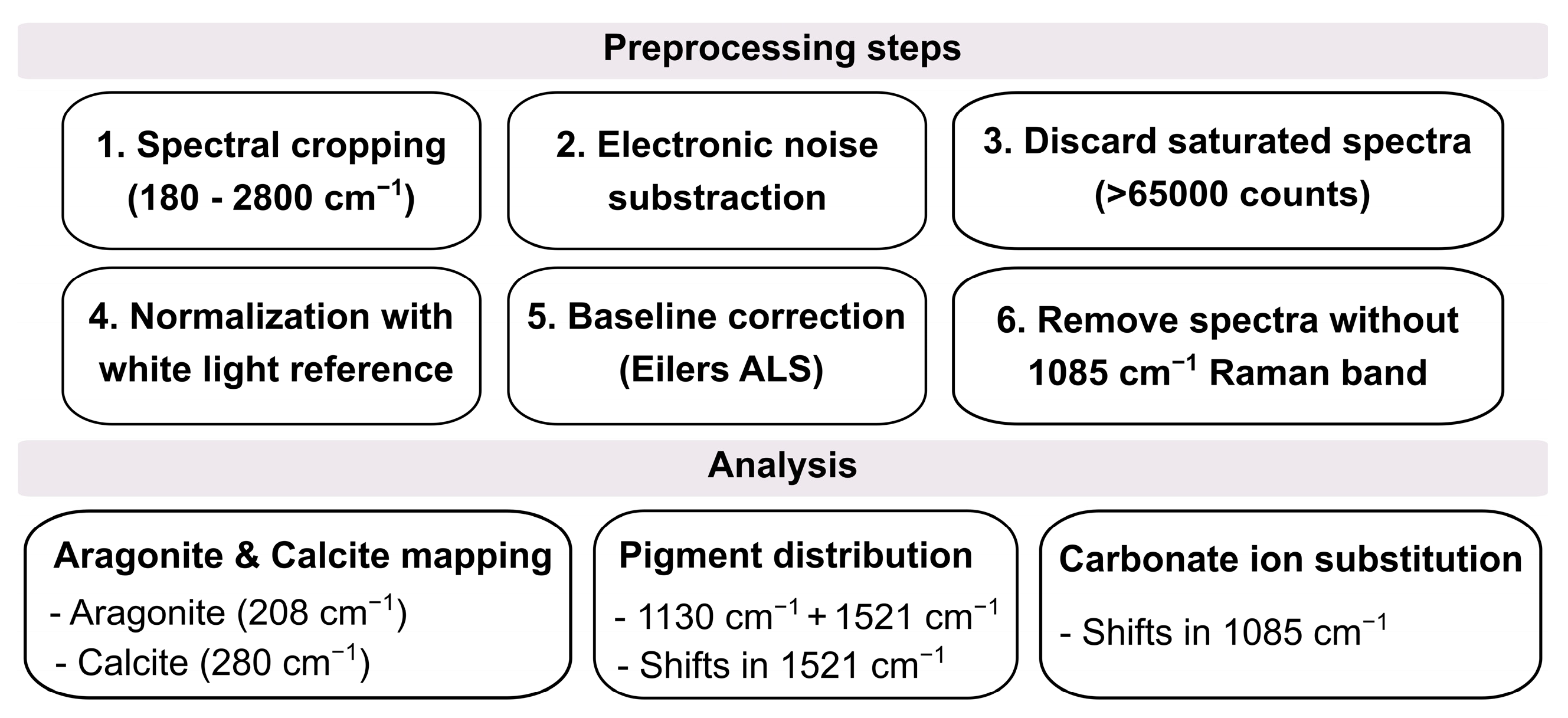
2.5. Data Analysis
3. Results and Discussion
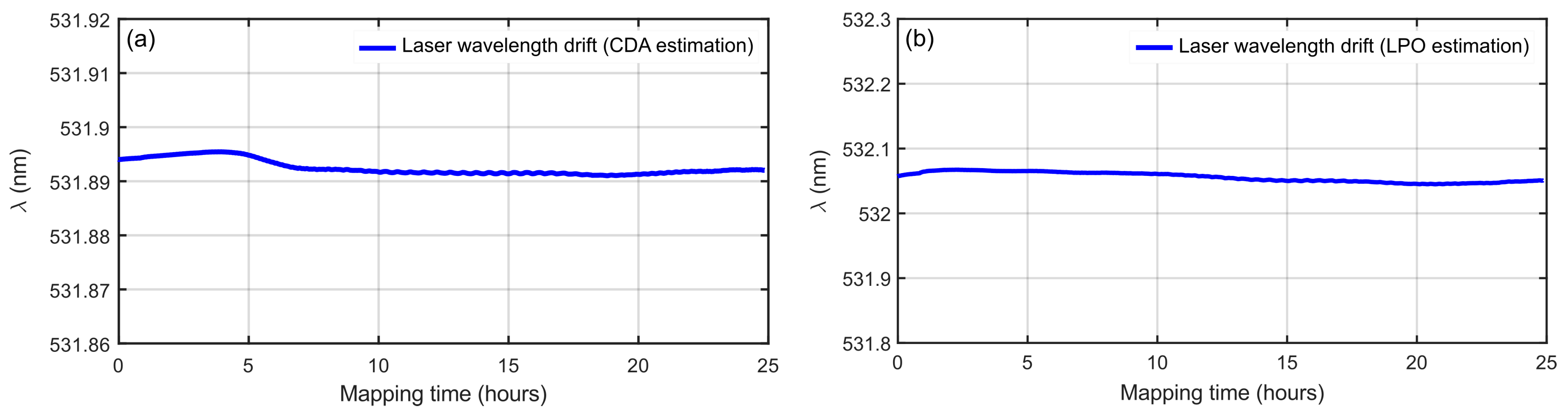
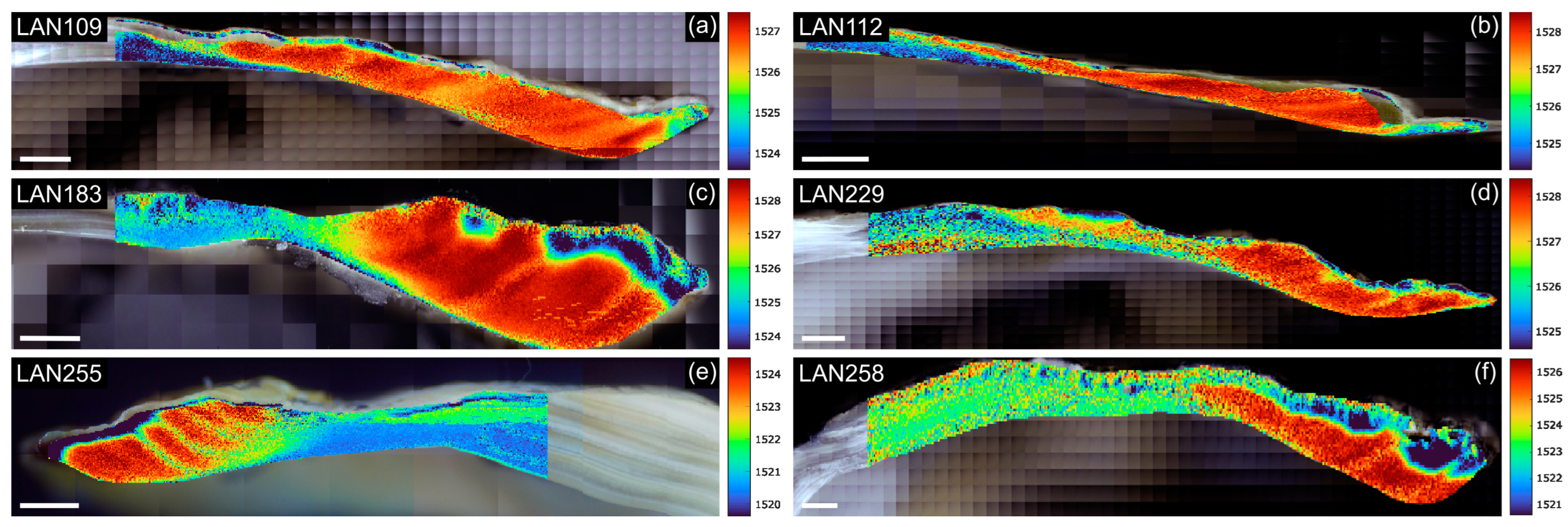
3.1. Laser Wavelength Stability
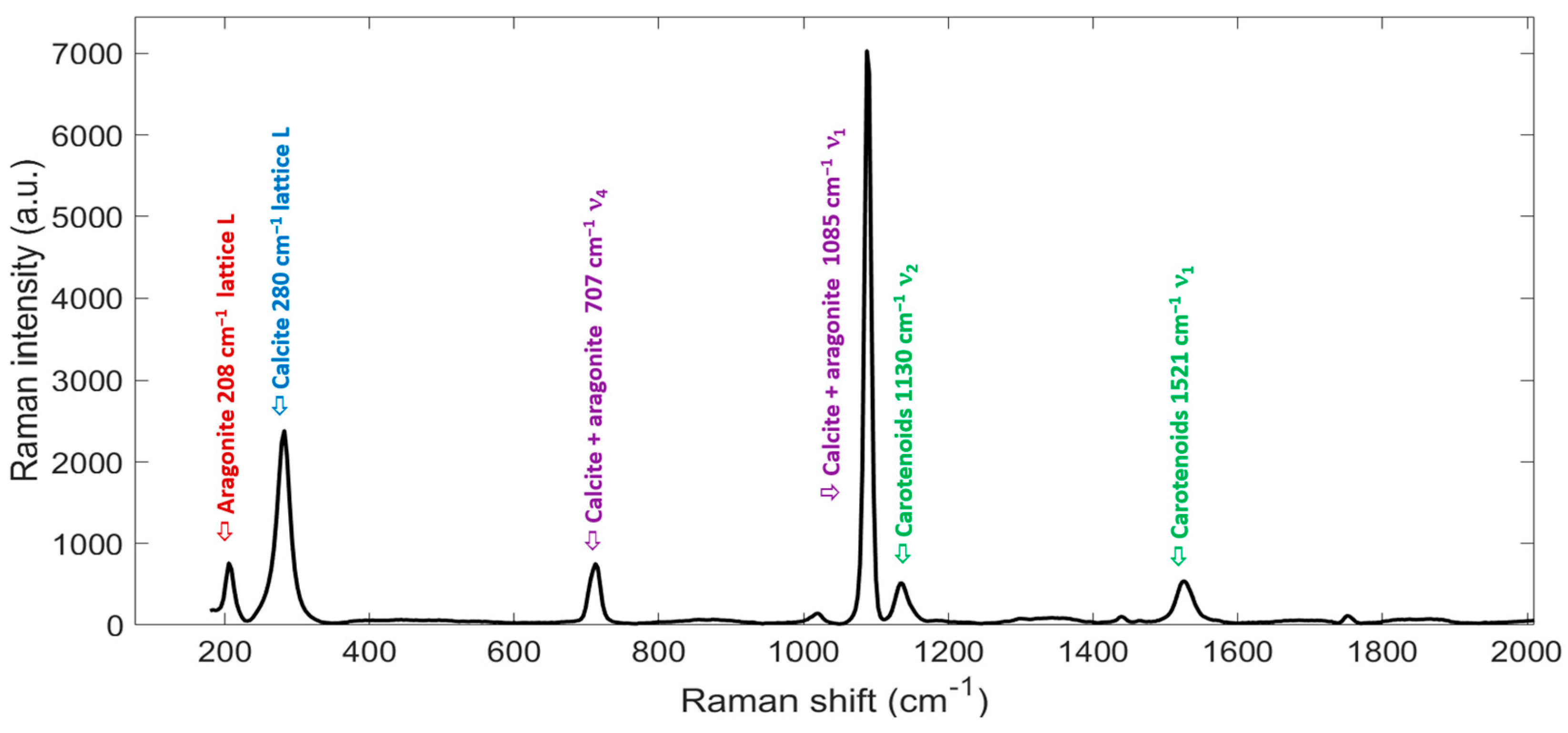
3.2. Raman Band Assignment in P. vulgata Shell
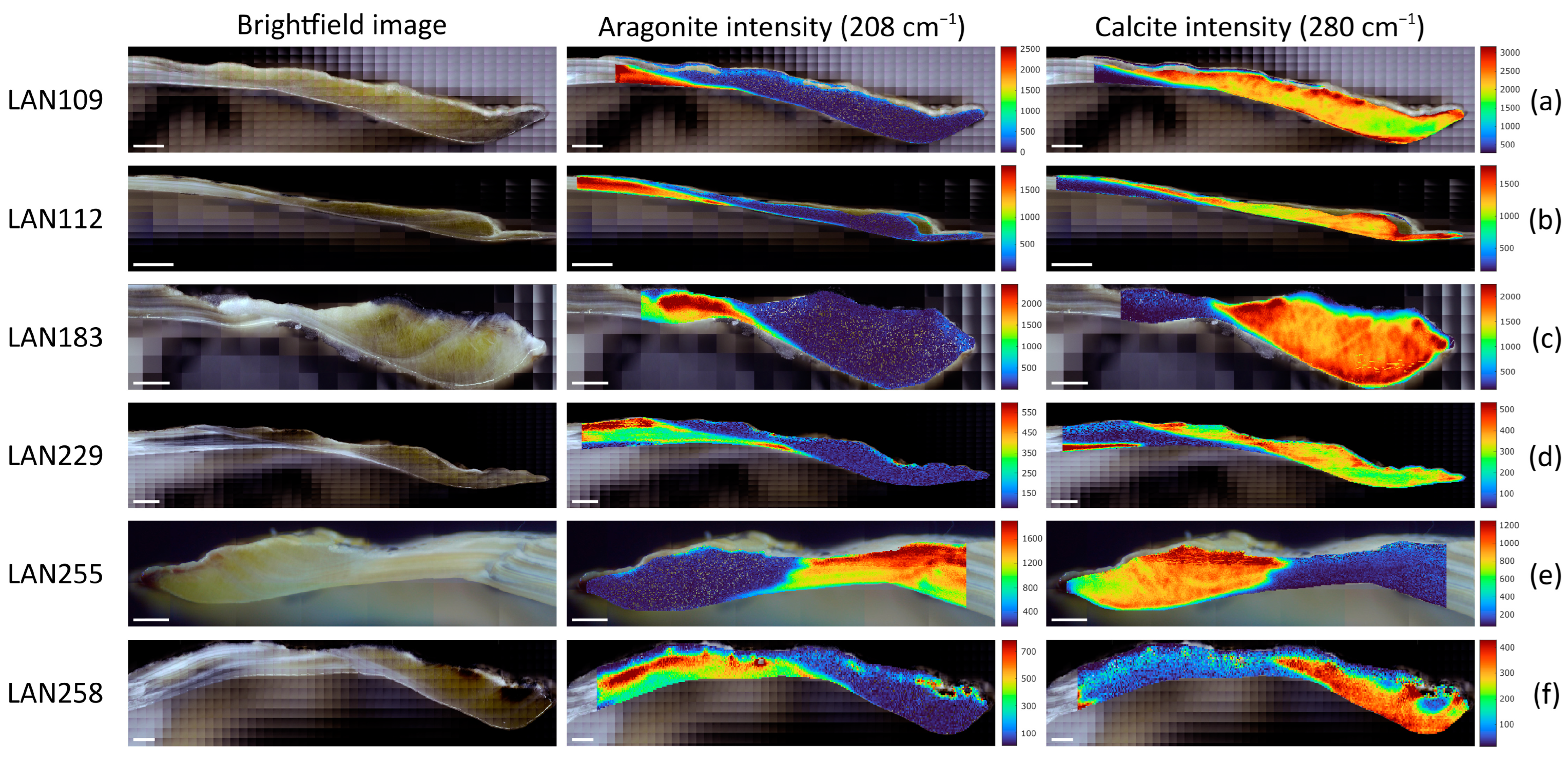
3.3. Spatial Distribution of Aragonite and Calcite
3.4. Pigment Distribution and Variations
3.5. Carbonate Ion Substitution in the CaCO3 Lattice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | Amorphous Calcium Carbonate |
| ALS | Asymmetric Least Squares |
| CCD | Charge-Coupled Device |
| CDA | Centroid Detection Algorithm |
| FTIR | Fourier Transform Infrared Spectroscopy |
| ICP-MS | Inductively Coupled Plasma Mass Spectrometry |
| LIBS | Laser-Induced Breakdown Spectroscopy |
| LPO | Linear Phase Operator |
| RGB | Red–Green–Blue |
| SEM | Scanning Electron Microscopy |
| XRD | X-Ray Diffraction |
| STEM | Scanning Transmission Electron Microscopy |
| APT | Atom Probe Tomography |
References
- Endo, K.; Kogure, T.; Nagasawa, H. Biomineralization: From Molecular and Nano-Structural Analyses to Environmental Science; Springer: Singapore, 2018; ISBN 978-981-13-1001-0. [Google Scholar]
- Marin, F.; Luquet, G. Molluscan Shell Proteins. Comptes Rendus Palevol 2004, 3, 469–492. [Google Scholar] [CrossRef]
- Song, X.; Liu, Z.; Wang, L.; Song, L. Recent Advances of Shell Matrix Proteins and Cellular Orchestration in Marine Molluscan Shell Biomineralization. Front. Mar. Sci. 2019, 6, 41. [Google Scholar] [CrossRef]
- Milano, S.; Schöne, B.R.; Witbaard, R. Changes of Shell Microstructural Characteristics of Cerastoderma Edule (Bivalvia)—A Novel Proxy for Water Temperature. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 465, 395–406. [Google Scholar] [CrossRef]
- Thomas, K.D. Molluscs Emergent, Part I: Themes and Trends in the Scientific Investigation of Mollusc Shells as Resources for Archaeological Research. J. Archaeol. Sci. 2015, 56, 133–140. [Google Scholar] [CrossRef]
- Paleček, D.; Milano, S.; Gutiérrez-Zugasti, I.; Talamo, S. Stable Isotopes in the Shell Organic Matrix for (Paleo)Environmental Reconstructions. Commun. Chem. 2024, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Arniz-Mateos, R.; García-Escárzaga, A.; Fernandes, R.; González-Morales, M.R.; Gutiérrez-Zugasti, I. Living in the Shore: Changes in Coastal Resource Intensification during the Mesolithic in Northern Iberia. Archaeol. Anthropol. Sci. 2024, 16, 79. [Google Scholar] [CrossRef]
- Fenger, T.; Surge, D.; Schöne, B.; Milner, N. Sclerochronology and Geochemical Variation in Limpet Shells (Patella vulgata): A New Archive to Reconstruct Coastal Sea Surface Temperature. Geochem. Geophys. Geosyst. 2007, 8, 2006GC001488. [Google Scholar] [CrossRef]
- Gutiérrez-Zugasti, I.; Suárez-Revilla, R.; Clarke, L.J.; Schöne, B.R.; Bailey, G.N.; González-Morales, M.R. Shell Oxygen Isotope Values and Sclerochronology of the Limpet Patella vulgata Linnaeus 1758 from Northern Iberia: Implications for the Reconstruction of Past Seawater Temperatures. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 475, 162–175. [Google Scholar] [CrossRef]
- Orton, J.H. Observations on Patella uulgata. Part III. Habitat and Habits. J. Mar. Biol. Assoc. U. K. 1929, 16, 277–288. [Google Scholar] [CrossRef]
- García-Escárzaga, A.; Clarke, L.J.; Gutiérrez-Zugasti, I.; González-Morales, M.R.; Martinez, M.; López-Higuera, J.-M.; Cobo, A. Mg/Ca Profiles within Archaeological Mollusc (Patella vulgata) Shells: Laser-Induced Breakdown Spectroscopy Compared to Inductively Coupled Plasma-Optical Emission Spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2018, 148, 8–15. [Google Scholar] [CrossRef]
- Chateigner, D.; Hedegaard, C.; Wenk, H.-R. Mollusc Shell Microstructures and Crystallographic Textures. J. Struct. Geol. 2000, 22, 1723–1735. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Villar, S.E.J.; Jehlicka, J.; Munshi, T. FT–Raman Spectroscopic Study of Calcium-Rich and Magnesium-Rich Carbonate Minerals. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 61, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Peter, N.J.; Griesshaber, E.; Reisecker, C.; Hild, S.; Oliveira, M.V.G.; Schmahl, W.W.; Schneider, A.S. Biocrystal Assembly Patterns, Biopolymer Distribution and Material Property Relationships in Mytilus Galloprovincialis, Bivalvia, and Haliotis Glabra, Gastropoda, Shells. Materialia 2023, 28, 101749. [Google Scholar] [CrossRef]
- Bergamonti, L.; Bersani, D.; Mantovan, S.; Lottici, P.P. Micro-Raman Investigation of Pigments and Carbonate Phases in Corals and Molluscan Shells. Eur. J. Mineral. 2014, 25, 845–853. [Google Scholar] [CrossRef]
- Williams, S.T. Molluscan Shell Colour. Biol. Rev. 2017, 92, 1039–1058. [Google Scholar] [CrossRef]
- Williams, S.T.; Ito, S.; Wakamatsu, K.; Goral, T.; Edwards, N.P.; Wogelius, R.A.; Henkel, T.; De Oliveira, L.F.C.; Maia, L.F.; Strekopytov, S.; et al. Identification of Shell Colour Pigments in Marine Snails Clanculus Pharaonius and C. Margaritarius (Trochoidea; Gastropoda). PLoS ONE 2016, 11, e0156664. [Google Scholar] [CrossRef]
- Nance, J.R.; Armstrong, J.T.; Cody, G.D.; Fogel, M.L.; Hazen, R.M. Preserved Macroscopic Polymeric Sheets of Shell-Binding Protein in the Middle Miocene (8 to 18 Ma) Gastropod Ecphora. Geochem. Perspect. Lett. 2015, 1, 1–9. [Google Scholar] [CrossRef]
- Verdes, A.; Cho, W.; Hossain, M.; Brennan, P.L.R.; Hanley, D.; Grim, T.; Hauber, M.E.; Holford, M. Nature’s Palette: Characterization of Shared Pigments in Colorful Avian and Mollusk Shells. PLoS ONE 2015, 10, e0143545. [Google Scholar] [CrossRef]
- Hedegaard, C.; Bardeau, J.-F.; Chateigner, D. Molluscan shell pigments: An in situ resonance raman study. J. Molluscan Stud. 2006, 72, 157–162. [Google Scholar] [CrossRef]
- Segev, E.; Erez, J. Effect of Mg/Ca Ratio in Seawater on Shell Composition in Shallow Benthic Foraminifera. Geochem. Geophys. Geosyst. 2006, 7, 2005GC000969. [Google Scholar] [CrossRef]
- Tanaka, K.; Okaniwa, N.; Miyaji, T.; Murakami-Sugihara, N.; Zhao, L.; Tanabe, K.; Schöne, B.R.; Shirai, K. Microscale Magnesium Distribution in Shell of the Mediterranean Mussel Mytilus Galloprovincialis: An Example of Multiple Factors Controlling Mg/Ca in Biogenic Calcite. Chem. Geol. 2019, 511, 521–532. [Google Scholar] [CrossRef]
- Mouchi, V.; De Rafélis, M.; Lartaud, F.; Fialin, M.; Verrecchia, E. Chemical Labelling of Oyster Shells Used for Time-Calibrated High-Resolution Mg/Ca Ratios: A Tool for Estimation of Past Seasonal Temperature Variations. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 373, 66–74. [Google Scholar] [CrossRef]
- Mirapeix, J.; Arniz-Mateos, R.; García-Escárzaga, A.; Gutierrez-Zugasti, I.; López-Higuera, J.M.; Cobo, A. Virtual Sampling: Archaeological Implications of a New Technique for Elemental Mapping of Mg/Ca Ratios in Marine Mollusc Shells. J. Archaeol. Sci. 2025, 173, 106123. [Google Scholar] [CrossRef]
- Mills, J.V.; Barnhart, H.A.; DePaolo, D.J.; Lammers, L.N. New Insights into Mn2+ and Mg2+ Inhibition of Calcite Growth. Geochim. Cosmochim. Acta 2022, 334, 338–367. [Google Scholar] [CrossRef]
- Zhou, G.-T.; Yao, Q.-Z.; Ni, J.; Jin, G. Formation of Aragonite Mesocrystals and Implication for Biomineralization. Am. Mineral. 2009, 94, 293–302. [Google Scholar] [CrossRef]
- Seesanong, S.; Boonchom, B.; Chaiseeda, K.; Boonmee, W.; Laohavisuti, N. Conversion of Bivalve Shells to Monocalcium and Tricalcium Phosphates: An Approach to Recycle Seafood Wastes. Materials 2021, 14, 4395. [Google Scholar] [CrossRef]
- Zlotnikov, I.; Schoeppler, V. Thermodynamic Aspects of Molluscan Shell Ultrastructural Morphogenesis. Adv. Funct. Mater. 2017, 27, 1700506. [Google Scholar] [CrossRef]
- Durham, S.R.; Gillikin, D.P.; Goodwin, D.H.; Dietl, G.P. Rapid Determination of Oyster Lifespans and Growth Rates Using LA-ICP-MS Line Scans of Shell Mg/Ca Ratios. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 485, 201–209. [Google Scholar] [CrossRef]
- McMillan, N.J.; Harmon, R.S.; De Lucia, F.C.; Miziolek, A.M. Laser-Induced Breakdown Spectroscopy Analysis of Minerals: Carbonates and Silicates. Spectrochim. Acta Part B At. Spectrosc. 2007, 62, 1528–1536. [Google Scholar] [CrossRef]
- Ren, L.; Li, S.; Ye, W.; Lv, Q.; Sun, Y.; Zhou, X.; Lian, S.; Lv, J.; Wang, S.; Guo, J.; et al. Tracking Organic Matrix in the Seashell by Elemental Mapping under Laser-Induced Breakdown Spectroscopy. Talanta 2024, 271, 125658. [Google Scholar] [CrossRef]
- Peharda, M.; Schöne, B.R.; Markulin, K.; Uvanović, H.; Tanaka, K.; Shirai, K.; Goodwin, D.; Mihanović, H. Mytilus Galloprovincialis Shell Growth—Insights from Shell Geochemistry. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2024, 650, 112367. [Google Scholar] [CrossRef]
- Borromeo, L.; Zimmermann, U.; Andò, S.; Coletti, G.; Bersani, D.; Basso, D.; Gentile, P.; Schulz, B.; Garzanti, E. Raman Spectroscopy as a Tool for Magnesium Estimation in Mg-calcite. J. Raman Spectrosc. 2017, 48, 983–992. [Google Scholar] [CrossRef]
- Barnard, W.; De Waal, D. Raman Investigation of Pigmentary Molecules in the Molluscan Biogenic Matrix. J. Raman Spectrosc. 2006, 37, 342–352. [Google Scholar] [CrossRef]
- Nehrke, G.; Nouet, J. Confocal Raman Microscope Mapping as a Tool to Describe Different Mineral and Organic Phases at High Spatial Resolution Within Marine Biogenic Carbonates: Case Study on Nerita undata (Gastropoda, Neritopsina). Biogeosciences 2011, 8, 3761–3769. [Google Scholar] [CrossRef]
- Stemmer, K.; Nehrke, G. The Distribution of Polyenes in the Shell of Arctica Islandica from North Atlantic Localities: A Confocal Raman Microscopy Study. J. Molluscan Stud. 2014, 80, 365–370. [Google Scholar] [CrossRef]
- DeCarlo, T.M.; Comeau, S.; Cornwall, C.E.; Gajdzik, L.; Guagliardo, P.; Sadekov, A.; Thillainath, E.C.; Trotter, J.; McCulloch, M.T. Investigating Marine Bio-calcification Mechanisms in a Changing Ocean with in Vivo and High-resolution Ex Vivo Raman Spectroscopy. Glob. Change Biol. 2019, 25, 1877–1888. [Google Scholar] [CrossRef]
- Jehlička, J.; Culka, A. Critical Evaluation of Portable Raman Spectrometers: From Rock Outcrops and Planetary Analogs to Cultural Heritage—A Review. Anal. Chim. Acta 2022, 1209, 339027. [Google Scholar] [CrossRef]
- Nehrke, G.; Poigner, H.; Wilhelms-Dick, D.; Brey, T.; Abele, D. Coexistence of Three Calcium Carbonate Polymorphs in the Shell of the Antarctic Clam Laternula elliptica. Geochem. Geophys. Geosyst. 2012, 13. [Google Scholar] [CrossRef]
- Badou, A.; Pont, S.; Auzoux-Bordenave, S.; Lebreton, M.; Bardeau, J.-F. New Insight on Spatial Localization and Microstructures of Calcite-Aragonite Interfaces in Adult Shell of Haliotis Tuberculata: Investigations of Wild and Farmed Abalones by FTIR and Raman Mapping. J. Struct. Biol. 2022, 214, 107854. [Google Scholar] [CrossRef]
- Langer, G.; Nehrke, G.; Baggini, C.; Rodolfo-Metalpa, R.; Hall-Spencer, J.M.; Bijma, J. Limpets Counteract Ocean Acidification Induced Shell Corrosion by Thickening of Aragonitic Shell Layers. Biogeosciences 2014, 11, 7363–7368. [Google Scholar] [CrossRef]
- Ambrose, W.G.; Locke, V.W.L.; Bigelow, G.F.; Renaud, P.E. Deposition of Annual Growth Lines in the Apex of the Common Limpet (Patella vulgata) from Shetland Islands, UK and Norway: Evidence from Field Marking and Shell Mineral Content of Annual Line Deposition. Environ. Archaeol. 2016, 21, 79–87. [Google Scholar] [CrossRef]
- Lewis, J.R.; Bowman, R.S. Local Habitat-Induced Variations in the Population Dynamics of Patella vulgata L. J. Exp. Mar. Biol. Ecol. 1975, 17, 165–203. [Google Scholar] [CrossRef]
- Surge, D.; Wang, T.; Gutierrez-Zugasti, I.; Kelley, P.H. Isotope Sclerochronolog and Season of Annual Growth Line Formation in Limpet Shells (Patella vulgata) from Warm- and Cold-Temperature Zones in the Easten North Atlantic. Palaios 2013, 28, 386–393. [Google Scholar] [CrossRef]
- Moisez, E.; Spilmont, N.; Seuront, L. Microhabitats Choice in Intertidal Gastropods Is Species-, Temperature- and Habitat-Specific. J. Therm. Biol. 2020, 94, 102785. [Google Scholar] [CrossRef] [PubMed]
- Boaventura, D.; Cancela Da Fonseca, L.; Hawkins, S.J. Analysis of Competitive Interactions between the Limpets Patella Depressa Pennant and Patella vulgata L. on the Northern Coast of Portugal. J. Exp. Mar. Biol. Ecol. 2002, 271, 171–188. [Google Scholar] [CrossRef]
- MacClintock, C. Shell Structure of Patelloid and Bellerophontoid Gastropods (Mollusca). Peabody Mus. Nat. Hist. Yale Univ. Bull. 1967, 22, 1–140. [Google Scholar]
- Demarchi, B.; Rogers, K.; Fa, D.A.; Finlayson, C.J.; Milner, N.; Penkman, K.E.H. Intra-Crystalline Protein Diagenesis (IcPD) in Patella vulgata. Part I: Isolation and Testing of the Closed System. Quat. Geochronol. 2013, 16, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.L.; Branch, G.M. Environmentally Controlled Variation in the Structure and Mineralogy of Patella Granularis Shells from the Coast of Southern Africa: Implications for Palaeotemperature Assessments. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1992, 91, 49–57. [Google Scholar] [CrossRef]
- Best, R.J.; Stier, D.; Kuhrts, L.; Zlotnikov, I. Classical View on Nonclassical Crystal Growth in a Biological Setting. J. Am. Chem. Soc. 2025, 147, 1–9. [Google Scholar] [CrossRef]
- Hausmann, N.; Surge, D.; Briz I Godino, I. Confirmation of Mg/Ca Ratios as Palaeothermometers in Atlantic Limpet Shells. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2024, 655, 112538. [Google Scholar] [CrossRef]
- Mirapeix, J.; Cobo, A.; Jaúregui, C.; López-Higuera, J.M. Fast Algorithm for Spectral Processing with Application to On-Line Welding Quality Assurance. Meas. Sci. Technol. 2006, 17, 2623–2629. [Google Scholar] [CrossRef]
- Long, D.A. The Raman Effect: A Unified Treatment of the Theory of Raman Scattering by Molecules; Wiley: Chichester, UK; New York, NY, USA, 2002; ISBN 978-0-471-49028-9. [Google Scholar]
- Urmos, J.; Snnnrvr, S.K.; Mackenzie, F.T. Characterization of Some Biogenic Carbonates with Raman Spectroscopy. Am. Mineral. 1991, 76, 641–646. [Google Scholar]
- Nouet, J.; Chevallard, C.; Farre, B.; Nehrke, G.; Campmas, E.; Stoetzel, E.; El Hajraoui, M.A.; Nespoulet, R. Limpet Shells from the Aterian Level 8 of El Harhoura 2 Cave (Témara, Morocco): Preservation State of Crossed-Foliated Layers. PLoS ONE 2015, 10, e0137162. [Google Scholar] [CrossRef] [PubMed]
- Urashima, S.; Nishioka, T.; Yui, H. Micro-Raman Spectroscopic Analysis on Natural Carbonates: Linear Relations Found via Biaxial Plotting of Peak Frequencies for Cation Substituted Species. Anal. Sci. 2022, 38, 921–929. [Google Scholar] [CrossRef]
- Alves, J.F.; Edwards, H.G.M.; Korsakov, A.; De Oliveira, L.F.C. Revisiting the Raman Spectra of Carbonate Minerals. Minerals 2023, 13, 1358. [Google Scholar] [CrossRef]
- Foster, P.; Chacko, J. Minor and Trace Elements in the Shell of Patella vulgata (L.). Mar. Environ. Res. 1995, 40, 55–76. [Google Scholar] [CrossRef]
- Schleinkofer, N.; Raddatz, J.; Evans, D.; Gerdes, A.; Flögel, S.; Voigt, S.; Büscher, J.V.; Wisshak, M. Compositional Variability of Mg/Ca, Sr/Ca, and Na/Ca in the Deep-Sea Bivalve Acesta Excavata (Fabricius, 1779). PLoS ONE 2021, 16, e0245605. [Google Scholar] [CrossRef]
- Yarra, T.; Ramesh, K.; Blaxter, M.; Hüning, A.; Melzner, F.; Clark, M.S. Transcriptomic Analysis of Shell Repair and Biomineralization in the Blue Mussel, Mytilus Edulis. BMC Genom. 2021, 22, 437. [Google Scholar] [CrossRef]
- Graniero, L.E.; Surge, D.; Gillikin, D.P.; Briz I Godino, I.; Álvarez, M. Assessing Elemental Ratios as a Paleotemperature Proxy in the Calcite Shells of Patelloid Limpets. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 465, 376–385. [Google Scholar] [CrossRef]
- Hausmann, N.; Prendergast, A.L.; Lemonis, A.; Zech, J.; Roberts, P.; Siozos, P.; Anglos, D. Extensive Elemental Mapping Unlocks Mg/Ca Ratios as Climate Proxy in Seasonal Records of Mediterranean Limpets. Sci. Rep. 2019, 9, 3698. [Google Scholar] [CrossRef]
- García-Escárzaga, A.; Martínez-Minchero, M.; Cobo, A.; Gutiérrez-Zugasti, I.; Arrizabalaga, A.; Roberts, P. Using Mg/Ca Ratios from the Limpet Patella Depressa Pennant, 1777 Measured by Laser-Induced Breakdown Spectroscopy (LIBS) to Reconstruct Paleoclimate. Appl. Sci. 2021, 11, 2959. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Manteca, M.G.; García García, B.; Gómez-Galdós, C.; Mirapeix, J.; Arniz-Mateos, R.; García-Escárzaga, A.; Gutiérrez-Zugasti, I.; Algorri, J.F.; López-Higuera, J.M.; Ocampo-Sosa, A.A.; et al. Structural Pattern Analysis in Patella vulgata Shells Using Raman Imaging. Appl. Sci. 2025, 15, 5180. https://doi.org/10.3390/app15095180
Fernández-Manteca MG, García García B, Gómez-Galdós C, Mirapeix J, Arniz-Mateos R, García-Escárzaga A, Gutiérrez-Zugasti I, Algorri JF, López-Higuera JM, Ocampo-Sosa AA, et al. Structural Pattern Analysis in Patella vulgata Shells Using Raman Imaging. Applied Sciences. 2025; 15(9):5180. https://doi.org/10.3390/app15095180
Chicago/Turabian StyleFernández-Manteca, María Gabriela, Borja García García, Celia Gómez-Galdós, Jesús Mirapeix, Rosa Arniz-Mateos, Asier García-Escárzaga, Igor Gutiérrez-Zugasti, José Francisco Algorri, José Miguel López-Higuera, Alain A. Ocampo-Sosa, and et al. 2025. "Structural Pattern Analysis in Patella vulgata Shells Using Raman Imaging" Applied Sciences 15, no. 9: 5180. https://doi.org/10.3390/app15095180
APA StyleFernández-Manteca, M. G., García García, B., Gómez-Galdós, C., Mirapeix, J., Arniz-Mateos, R., García-Escárzaga, A., Gutiérrez-Zugasti, I., Algorri, J. F., López-Higuera, J. M., Ocampo-Sosa, A. A., Rodríguez-Cobo, L., & Cobo, A. (2025). Structural Pattern Analysis in Patella vulgata Shells Using Raman Imaging. Applied Sciences, 15(9), 5180. https://doi.org/10.3390/app15095180







