Influence of Agronomic Practices on the Antioxidant Activity of Three Mediterranean Officinal Wild Plants: Silybum marianum, Achillea millefolium, and Trifolium pratense
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
Agronomical Factors
2.3. Evaluation of Growth Trend Using BBCH Scales
2.4. Plant Material
Reagents Employed for the Appraisal of the Total Antioxidant Capacity (TAC) with the Three Spectrophotometric Assays
2.5. Extraction of the Phenols Containing Phytocomplex
2.5.1. Evaluation of the TAC Using the FRAP Method
2.5.2. Evaluation of the Radical Scavenging Capacity by the DPPH Method
2.5.3. Evaluation of the Radical Scavenging Capacity Using the ABTS Method
2.6. Instrumentation
2.7. Statistics
3. Results and Discussion
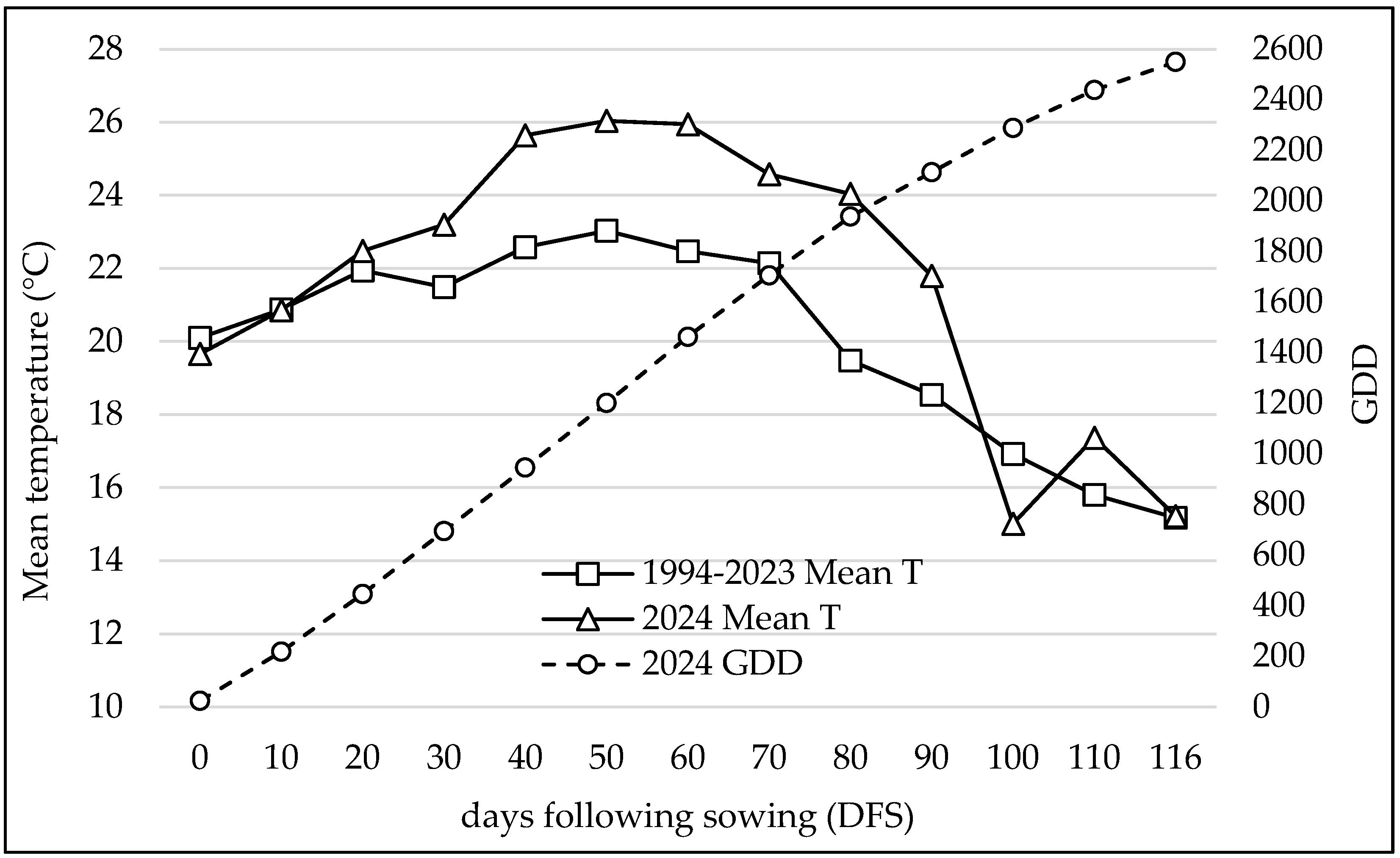
3.1. Study Area Climate Conditions
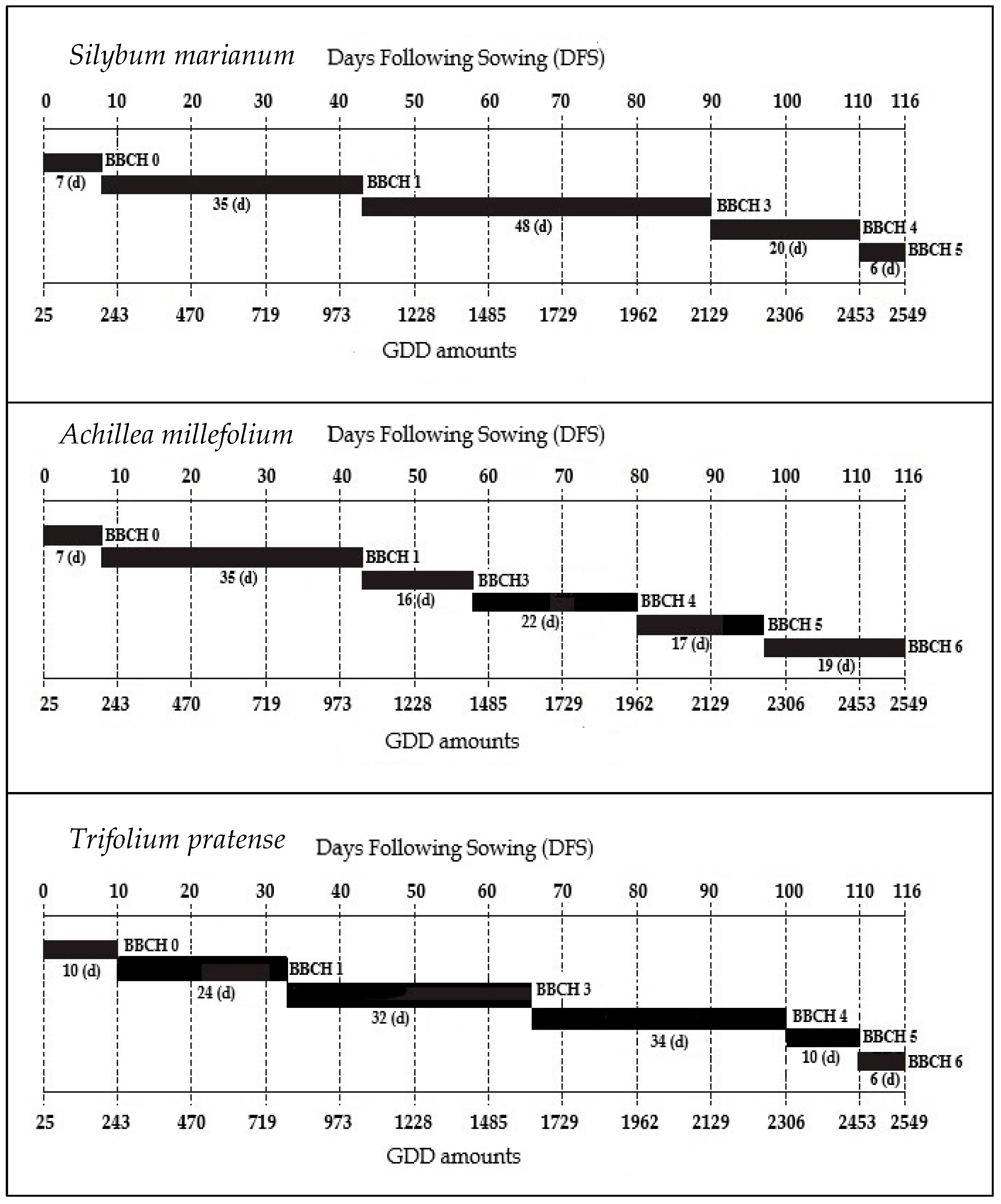
3.2. Phenological Observations
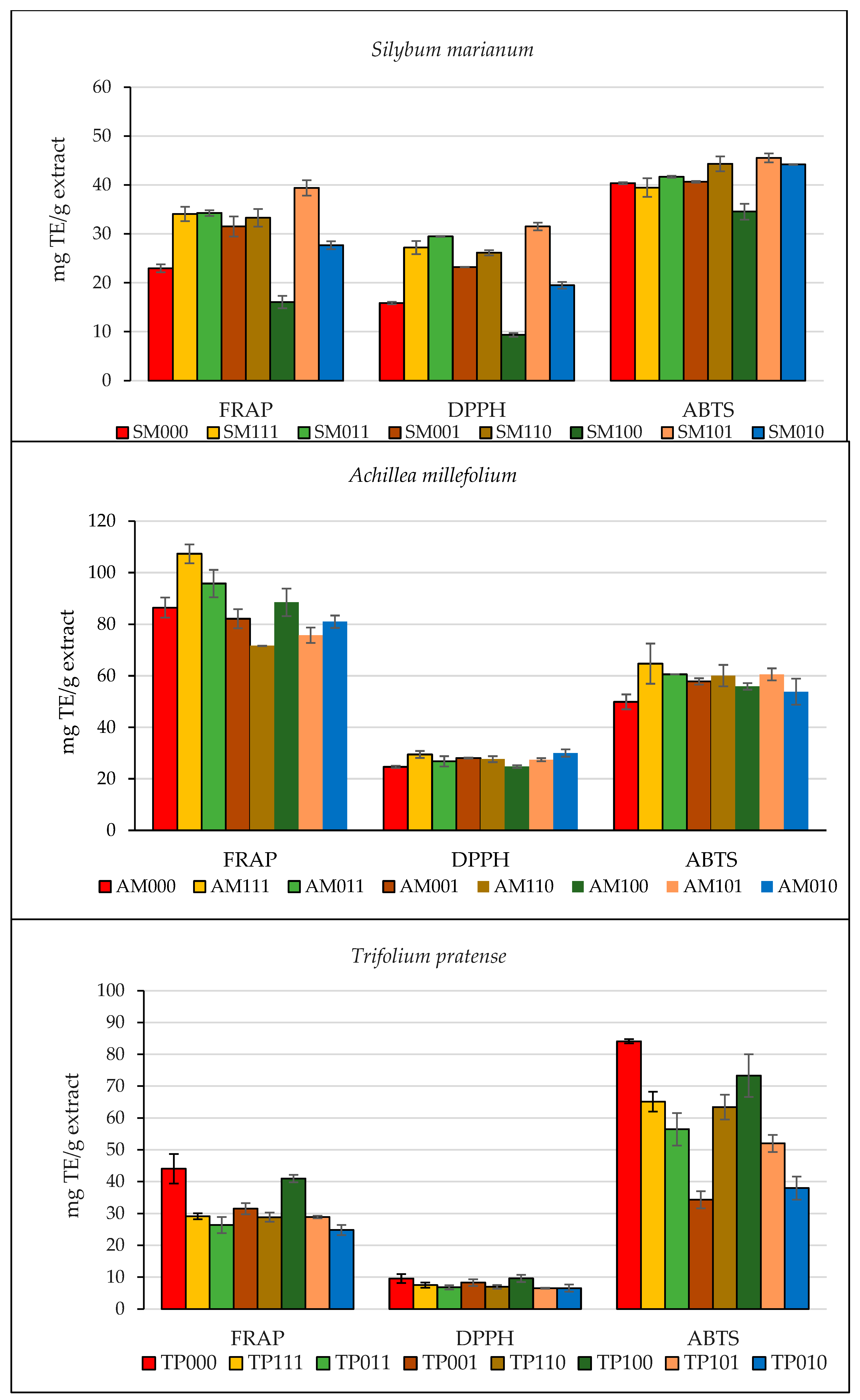
3.3. Evaluation of the Total Antioxidant Capacity (TAC) of Hydroalcoholic Extracts
3.4. Ultra High-Performance Liquid Chromatography Coupled to Mass Spectrometry (UHPLC-MS/MS) Analysis of the Three Extracts SM101, AM111, and TP000
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BBCH | Biologische Bundesanstalt, Bundessortenamt and CHemical industry |
| TAC | Total antioxidant capacity |
| TE | Trolox equivalent |
| PGPR | Plant growth-promoting rhizobacteria |
| K2O | Potassium oxide |
| P2O5 | Phosphorus pentoxide |
| MOWPs | Mediterranean Officinal Wild Plants |
| GDD | Growing degree day |
| DFS | Days following sowing |
| SM | Silybum marianum |
| AM | Achillea millefolium |
| TP | Trifolium pratense |
References
- Davis, C.C.; Choisy, P. Medicinal Plants Meet Modern Biodiversity Science. Curr. Biol. 2024, 34, R158–R173. [Google Scholar] [CrossRef] [PubMed]
- Orlandi Fabio, P.C. Nutraceutical Compounds of Edible Wild Plants Collected in Central Italy. 2024. Available online: https://zenodo.org/records/13820009 (accessed on 19 September 2024).
- Li, Z.; Li, W.; Zhang, Y.; Hu, Y.; Sheward, R.; Irwin, A.J.; Finkel, Z.V. Dynamic Photophysiological Stress Response of a Model Diatom to Ten Environmental Stresses. J. Phycol. 2021, 57, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Şeker, M.E.; Erdoğan, A. Phenolic and Carotenoid Composition of Rhododendron Luteum Sweet and Ferula communis L. Subsp. Communis Flowers. Front. Life Sci. Relat. Technol. 2023, 4, 37–42. [Google Scholar] [CrossRef]
- Rizvi, A.; Ahmed, B.; Khan, M.S.; El-Beltagi, H.S.; Umar, S.; Lee, J. Bioprospecting Plant Growth Promoting Rhizobacteria for Enhancing the Biological Properties and Phytochemical Composition of Medicinally Important Crops. Molecules 2022, 27, 1407. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Drouza, C.; Tzortzakis, N. Optimization of Potassium Fertilization/Nutrition for Growth, Physiological Development, Essential Oil Composition and Antioxidant Activity of Lavandula Angustifolia Mill. J. Soil Sci. Plant Nutr. 2017, 17, 291–306. [Google Scholar] [CrossRef]
- Biesiada, A. The Effect of Nitrogen Fertilization on Yielding and Antioxidant Activity of Lavender (Lavandula Angustifolia Mill. Acta Sci. Pol. Hortorum Cultus 2008, 7, 33–40. [Google Scholar]
- Nguyen, P.M.; Niemeyer, E.D. Effects of Nitrogen Fertilization on the Phenolic Composition and Antioxidant Properties of Basil (Ocimum basilicum L.). J. Agric. Food Chem. 2008, 56, 8685–8691. [Google Scholar] [CrossRef]
- Peng, L.-C.; Ng, L.-T. Impacts of Nitrogen and Phosphorus Fertilization on Biomass, Polyphenol Contents, and Essential Oil Yield and Composition of Vitex Negundo Linn. Agriculture 2022, 12, 859. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In Vitro Bio-Accessibility and Antioxidant Activity of Grape Polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Christodoulou, M.C.; Orellana Palacios, J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric Methods for Measurement of Antioxidant Activity in Food and Pharmaceuticals. Antioxidants 2022, 11, 2213. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical Evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu Assays to Assess the Antioxidant Capacity of Lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef] [PubMed]
- Puri, C.; Pucciarini, L.; Tiecco, M.; Brighenti, V.; Volpi, C.; Gargaro, M.; Germani, R.; Pellati, F.; Sardella, R.; Clementi, C. Use of a Zwitterionic Surfactant to Improve the Biofunctional Properties of Wool Dyed with an Onion (Allium cepa L.) Skin Extract. Antioxidants 2020, 9, 1055. [Google Scholar] [CrossRef] [PubMed]
- Volpi, C.; Bartolini, D.; Brighenti, V.; Galli, F.; Tiecco, M.; Pellati, F.; Clementi, C.; Sardella, R. Antioxidant Power on Dermal Cells by Textiles Dyed with an Onion (Allium cepa L.) Skin Extract. Antioxidants 2021, 10, 1655. [Google Scholar] [CrossRef] [PubMed]
- Fanoudi, S.; Alavi, M.S.; Karimi, G.; Hosseinzadeh, H. Milk Thistle (Silybum Marianum) as an Antidote or a Protective Agent against Natural or Chemical Toxicities: A Review. Drug Chem. Toxicol. 2020, 43, 240–254. [Google Scholar] [CrossRef]
- Ayhan, N.K.; Tunc, M.G.K.; Noma, S.A.A.; Kurucay, A.; Ates, B. Characterization of the Antioxidant Activity, Total Phenolic Content, Enzyme Inhibition, and Anticancer Properties of Achillea millefolium L. (Yarrow). Instrum. Sci. Technol. 2022, 50, 654–667. [Google Scholar] [CrossRef]
- Khorasani Esmaeili, A.; Mat Taha, R.; Mohajer, S.; Banisalam, B. Antioxidant Activity and Total Phenolic and Flavonoid Content of Various Solvent Extracts from In Vivo and In Vitro Grown Trifolium pratense L. (Red Clover). BioMed Res. Int. 2015, 2015, 643285. [Google Scholar] [CrossRef]
- Marmouzi, I.; Bouyahya, A.; Ezzat, S.M.; El Jemli, M.; Kharbach, M. The Food Plant Silybum marianum (L.) Gaertn.: Phytochemistry, Ethnopharmacology and Clinical Evidence. J. Ethnopharmacol. 2021, 265, 113303. [Google Scholar] [CrossRef]
- Pignatti, S. Flora d’Italia, 3rd ed.; Edagricole: Bologna, Italy, 1982; pp. 154–159. [Google Scholar]
- Vargas-Mendoza, N. Hepatoprotective Effect of Silymarin. World J. Hepatol. 2014, 6, 144. [Google Scholar] [CrossRef]
- Freedman, N.D.; Curto, T.M.; Morishima, C.; Seeff, L.B.; Goodman, Z.D.; Wright, E.C.; Sinha, R.; Everhart, J.E.; the HALT-C Trial Group. Silymarin Use and Liver Disease Progression in the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis Trial. Aliment. Pharmacol. Ther. 2011, 33, 127–137. [Google Scholar] [CrossRef]
- Székely-Szentmiklósi, I.; Rédai, E.M.; Kovács, B.; Gergely, A.-L.; Albert, C.; Szabó, Z.-I.; Székely-Szentmiklósi, B.; Sipos, E. Investigation of Yarrow Essential Oil Composition and Microencapsulation by Complex Coacervation Technology. Appl. Sci. 2024, 14, 7867. [Google Scholar] [CrossRef]
- Saviranta, N.M.; Anttonen, M.J.; Von Wright, A.; Karjalainen, R.O. Red Clover (Trifolium pratense L.) Isoflavones: Determination of Concentrations by Plant Stage, Flower Colour, Plant Part and Cultivar. J. Sci. Food Agric. 2008, 88, 125–132. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger Climate Classification Updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Meier, U.; Bleiholder, H.; Buhr, L.; Feller, C.; Hack, H.; Heß, M.; Lancashire, P.D.; Schnock, U.; Stauß, R.; Van Den Boom, T.; et al. The BBCH System to Coding the Phenological Growth Stages of Plants—History and Publications. J. Cultiv. Plants 2009, 61, 41–52. [Google Scholar] [CrossRef]
- Pucciarini, L.; Ianni, F.; Petesse, V.; Pellati, F.; Brighenti, V.; Volpi, C.; Gargaro, M.; Natalini, B.; Clementi, C.; Sardella, R. Onion (Allium cepa L.) Skin: A Rich Resource of Biomolecules for the Sustainable Production of Colored Biofunctional Textiles. Molecules 2019, 24, 634. [Google Scholar] [CrossRef]
- Abualzulof, G.W.A.; Scandar, S.; Varfaj, I.; Dalla Costa, V.; Sardella, R.; Filippini, R.; Piovan, A.; Marcotullio, M.C. The Effect of Maturity Stage on Polyphenolic Composition, Antioxidant and Anti-Tyrosinase Activities of Ficus Rubiginosa Desf. Ex Vent. Extracts. Antioxidants 2024, 13, 1129. [Google Scholar] [CrossRef]
- Acito, M.; Varfaj, I.; Brighenti, V.; Cengiz, E.C.; Rondini, T.; Fatigoni, C.; Russo, C.; Pietrella, D.; Pellati, F.; Bartolini, D.; et al. A Novel Black Poplar Propolis Extract with Promising Health-Promoting Properties: Focus on Its Chemical Composition, Antioxidant, Anti-Inflammatory, and Anti-Genotoxic Activities. Food Funct. 2024, 15, 4983–4999. [Google Scholar] [CrossRef]
- Marceddu, R.; Dinolfo, L.; Carrubba, A.; Sarno, M.; Di Miceli, G. Milk Thistle (Silybum marianum L.) as a Novel Multipurpose Crop for Agriculture in Marginal Environments: A Review. Agronomy 2022, 12, 729. [Google Scholar] [CrossRef]
- Porwal, O.; Mohammed Ameen, M.S.; Anwer, E.T.; Uthirapathy, S.; Ahamad, J.; Tahsin, A. Silybum Marianum (Milk Thistle): Review on Its Chemistry, Morphology, Ethno Medical Uses, Phytochemistry and Pharmacological Activities. J. Drug Delivery Ther. 2019, 9, 199–206. [Google Scholar] [CrossRef]
- Andrzejewska, J.; Martinelli, T.; Sadowska, K. Silybum Marianum: Non-Medical Exploitation of the Species: Silybum Marianum: Non-Medical Exploitation of the Species. Ann. Appl. Biol. 2015, 167, 285–297. [Google Scholar] [CrossRef]
- BourdôT, G.W.; Field, R.J. Review on Ecology and Control of Achillea millefolium L. (Yarrow) on Arable Land in New Zealand. New Zealand J. Exp. Agric. 1988, 16, 99–108. [Google Scholar] [CrossRef]
- Booth, N.L.; Overk, C.R.; Yao, P.; Totura, S.; Deng, Y.; Hedayat, A.S.; Bolton, J.L.; Pauli, G.F.; Farnsworth, N.R. Seasonal Variation of Red Clover (Trifolium pratense L., Fabaceae) Isoflavones and Estrogenic Activity. J. Agric. Food Chem. 2006, 54, 1277–1282. [Google Scholar] [CrossRef] [PubMed]
- Amtmann, A.; Troufflard, S.; Armengaud, P. The Effect of Potassium Nutrition on Pest and Disease Resistance in Plants. Physiol. Plant. 2008, 133, 682–691. [Google Scholar] [CrossRef]
- Talbi, S.; Rojas, J.A.; Sahrawy, M.; Rodríguez-Serrano, M.; Cárdenas, K.E.; Debouba, M.; Sandalio, L.M. Effect of Drought on Growth, Photosynthesis and Total Antioxidant Capacity of the Saharan Plant Oudeneya Africana. Environ. Exp. Bot. 2020, 176, 104099. [Google Scholar] [CrossRef]
- Francis, B.; Aravindakumar, C.T.; Brewer, P.B.; Simon, S. Plant Nutrient Stress Adaptation: A Prospect for Fertilizer Limited Agriculture. Environ. Exp. Bot. 2023, 213, 105431. [Google Scholar] [CrossRef]
- Vardharajula, S.; Zulfikar Ali, S.; Grover, M.; Reddy, G.; Bandi, V. Drought-Tolerant Plant Growth Promoting Bacillus spp.: Effect on Growth, Osmolytes, and Antioxidant Status of Maize under Drought Stress. J. Plant Interact. 2011, 6, 1–14. [Google Scholar] [CrossRef]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A Review on Drought Stress in Plants: Implications, Mitigation and the Role of Plant Growth Promoting Rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Simova-Stoilova, L.; Demirevska, K.; Petrova, T.; Tsenov, N.; Feller, U. Antioxidative Protection in Wheat Varieties under Severe Recoverable Drought at Seedling Stag. Plant Soil Environ. 2008, 54, 529–536. [Google Scholar] [CrossRef]
- Kohler, J.; Hernández, J.A.; Caravaca, F.; Roldán, A. Plant-Growth-Promoting Rhizobacteria and Arbuscular Mycorrhizal Fungi Modify Alleviation Biochemical Mechanisms in Water-Stressed Plants. Funct. Plant Biol. 2008, 35, 141–151. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. S. Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, Y.; Yan, F.; Dong, M.; Ren, Y. Research progress of quercetin in cardiovascular disease. Front. Cardiovasc. Med. 2023, 10, 1203713. [Google Scholar] [CrossRef]
- Bispo da Silva, A.; Cerqueira Coelho, P.L.; Neves Oliveira, M.; Luz Oliveira, J.; Oliveira Amparo, J.A.; Costa da Silva, K.; Pereira Soares, J.R.; Seara Pitanga, B.P.; Santos Souza, C.; Faria Lopes, G.P.; et al. The flavonoid rutin and its aglycone quercetin modulate the microglia inflammatory profile improving antiglioma activity. Brain Behav. Immun. 2020, 85, 170–185. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, M.I.; Pasupuleti, V.R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front. Oncol. 2022, 12, 860508. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Sampaio, C.I.; Ševčovičová, A.; Dias, A.M.; Oliveira, R. Cynaropicrin- and chlorogenic acid-rich extracts easily prepared from Cynara cardunculus var. scolymus: Antioxidant and antigenotoxic properties. Biocatal. Agric. Biotechnol. 2023, 53, 102808. [Google Scholar] [CrossRef]
- Gebhardt, R.; Fausel, M. Antioxidant and hepatoprotective effects of artichoke extracts and constituents in cultured rat hepatocytes. Toxicol. Vitr. 1997, 11, 669–672. [Google Scholar] [CrossRef]


| Combination | PGPR | Fertilization | Water Supply |
|---|---|---|---|
| Inoculation | (K2O-P2O5) | (Field Capacity) | |
| 0,0,0 | 0 | 0 | 0 |
| 0,1,0 | 0 | 1 | 0 |
| 0,1,1 | 0 | 1 | 1 |
| 0,0,1 | 0 | 0 | 1 |
| 1,1,0 | 1 | 1 | 0 |
| 1,0,0 | 1 | 0 | 0 |
| 1,0,1 | 1 | 0 | 1 |
| 1,1,1 | 1 | 1 | 1 |
| DFS | BBCH Code | Description |
|---|---|---|
| 0 | 0 | Germination—Dry seeds |
| 7 | 12 | Leaf development—First pair of elliptic true leaves visible |
| 21 | 15 | Leaf development—Five leaves visible |
| 34 | 18 | Leaf development—Eight leaves visible |
| 42 | 31 | Rosette growth—10% of ground covered by leaves |
| 67 | 37 | Rosette growth—70% of ground covered by leaves |
| 76 | 39 | Rosette growth—90% of ground covered by leaves |
| 90 | 42 | 20% of the maximum biomass reached |
| 97 | 47 | 70% of the maximum biomass reached |
| 105 | 49 | 90% of the maximum biomass reached |
| 110 | 50 | Flower central head present (3–5 mm ∅) but completely enclosed between developing leaves |
| 116 | 51 | Flower central head clearly visible between leaves, vertical bract tips, and internodes of main stem clearly elongating |
| DFS | BBCH Code | Description |
|---|---|---|
| 0 | 0 | Germination—Dry seeds |
| 7 | 10 | Leaf development—Cotyledons completely unfolded |
| 21 | 12 | Leaf development—2 true leaves |
| 34 | 18 | Leaf development—8 or more true leaves |
| 42 | 32 | Steam elongation—Stem 20% of final length |
| 67 | 47 | Harvestable vegetative plant parts have reached 70% of final size |
| 76 | 49 | Harvestable vegetative plant parts have reached final size |
| 90 | 59 | Inflorescence emergence—First flower petals visible |
| 97 | 60 | Flowering—First flowers open |
| 105 | 61 | Flowering—10% of flowers open |
| 110 | 62 | Flowering—20% of flowers open |
| 116 | 64 | Flowering—40% of flowers open |
| DFS | BBCH Code | Description |
|---|---|---|
| 0 | 0 | Germination—Dry seeds |
| 7 | 9 | Germination—Emergence of cotyledons break through soil surface |
| 21 | 16 | Leaf development—Six true leaf |
| 34 | 30 | Steam elongation- Start of steam elongation |
| 42 | 33 | Steam elongation—Three visible nodes |
| 67 | 39 | Steam elongation—nine or more visible nodes |
| 76 | 43 | 30% of maximum biomass reached |
| 90 | 47 | 70% of maximum biomass reached |
| 97 | 49 | 90% of maximum biomass reached |
| 105 | 53 | Inflorescence emergence—Flower buds enlarged; petals visible |
| 110 | 60 | Flowering—Beginning of anthesis: First open flower |
| 116 | 62 | Flowering—20% of flowers open |
| Sample | Type of Assay | ||
|---|---|---|---|
| FRAP (mg TE/g Extract) | DPPH (mg TE/g Extract) | ABTS (mg TE/g Extract) | |
| SM000 | 22.94 ± 0.82 d | 15.87 ± 0.27 f | 40.37 ± 0.21 c |
| SM111 | 34.08 ± 1.46 b | 27.20 ± 1.33 c | 39.46 ± 1.91 c |
| SM011 | 34.27 ± 0.58 b | 29.48 ± 0.08 b | 41.67 ± 0.21 cb |
| SM001 | 31.50 ± 2.04 b | 23.23 ± 0.04 d | 40.64 ± 0.17 c |
| SM110 | 33.28 ± 1.80 b | 26.15 ± 0.53 b | 44.32 ± 1.54 ab |
| SM100 | 16.07 ± 1.28 e | 9.34 ± 0.38 g | 34.55 ± 1.62 d |
| SM101 | 39.39 ± 1.59 a | 31.50 ± 0.80 a | 45.55 ± 0.91 a |
| SM010 | 27.67 ± 0.83 c | 19.49 ± 0.68 e | 44.20 ± 0.08 bc |
| AM000 | 86.47 ± 3.85 bc | 24.64 ± 0.43 d | 49.90 ± 2.89 c |
| AM111 | 107.31 ± 3.66 a | 29.46 ± 1.34 ba | 64.74 ± 7.80 a |
| AM011 | 95.76 ± 5.31 b | 26.79 ± 2.00 dcb | 60.60 ± 0.04 ba |
| AM001 | 82.19 ± 3.72 cd | 28.06 ± 0.18 ba | 57.85 ± 1.23 ba |
| AM110 | 71.67 ± 0.06 e | 27.65 ± 1.15 ba | 60.09 ± 4.16 ba |
| AM100 | 88.52 ± 5.31 bc | 24.77 ± 0.54 dc | 55.92 ± 1.27 cb |
| AM101 | 75.79 ± 3.00 ed | 27.44 ± 0.59 cba | 60.60 ± 2.34 ba |
| AM010 | 81.04 ± 2.43 cd | 30.02 ± 1.41 a | 53.85 ± 5.07 cb |
| TP000 | 44.02 ± 4.67 a | 9.57 ± 1.37 a | 84.11 ± 0.67 a |
| TP111 | 29.14 ± 0.91 cb | 7.51 ± 0.79 ba | 65.13 ± 3.13 cb |
| TP011 | 26.39 ± 2.51 cb | 6.81 ± 0.68 b | 56.45 ± 5.11 dc |
| TP001 | 31.54 ± 1.73 b | 8.30 ± 1.05 ba | 34.28 ± 2.65 e |
| TP110 | 28.86 ± 1.44 cb | 6.95 ± 0.61 b | 63.40 ± 3.92 c |
| TP100 | 40.93 ± 1.15 a | 9.60 ± 1.15 a | 73.34 ± 6.73 b |
| TP101 | 28.93 ± 0.38 cb | 6.51 ± 0.16 b | 52.00 ± 2.69 d |
| TP010 | 24.83 ± 1.59 c | 6.55 ± 1.14 b | 37.95 ± 3.64 e |
| Possible Candidate | Formula | Rt (min) | m/z | MS/MS Fragments (m/z) | Exp Mass | Detected Mass | Score | Error (in ppm) |
|---|---|---|---|---|---|---|---|---|
| Apigenin | C15H10O5 | 12.07 | 269.03 | 117.03 | 270.0528 | 270.0528 | 89.56 | 1.88 |
| Chlorogenic Acid | C16H18O9 | 10.58 | 353.0883 | 191.0548, 137.02 | 354.3100 | 354.0939 | 98.81 | 2.19 |
| Chrysin | C15H10O4 | 11.29, 12.20 | 253.059 | 254.2400 | 254.0583 | 88.52 | −3.10 | |
| Cinnamic Acid | C9H8O2 | 8.28 | 147.04 | 148.1586 | 148.0524 | 99.77 | 0.90 | |
| Coumaric Acid | C9H8O3 | 5.44, 6.53 | 163.0405 | 119.05 | 164.1600 | 164.0473 | 99.84 | 0.41 |
| Cynarin | C25H24O12 | 10.58 | 515.27 | 516.4500 | 516.1280 | 96.53 | 2.43 | |
| Ferulic Acid | C10H10O4 | 7.15 | 193.01 | 117.03, 134.03, 175.78 | 194.1800 | 194.0579 | 86.00 | 2.48 |
| Fumaric Acid | C7H6O3 | 2.75 | 137.02 | 93.03 | 138.1200 | 138.0318 | 85.96 | 0.45 |
| Hydroxybenzoic Acid | C7H6O3 | 3.19 | 137.02 | 93.0329 | 138.1200 | 138.0317 | 99.34 | −0.79 |
| Kaempferol (or luteolin) | C15H10O6 | 11.38 | 285.0411 | 184.23 | 286.2300 | 286.0477 | 98.44 | 1.01 |
| Malic Acid | C4H6O5 | 5.56 | 133.1442 | 115.0013 | 184.8400 | 134.0215 | 96.53 | 5.07 |
| Naringenin | C15H12O5 | 12.03 | 271.062 | 151.41 | 272.2500 | 272.0685 | 99.57 | 0.22 |
| Protechatecuic Acid | C7H6O4 | 5.01 | 153.0181 | 109.02 | 154.1200 | 154.0266 | 86.06 | −2.14 |
| Quercetin | C15H10O7 | 10.18 | 301.0362 | 151.0037 | 302.2360 | 302.0427 | 82.88 | 3.31 |
| Quercitrin | C21H20O11 | 10.68 | 447.0946 | 284.0326, 255.02 | 448.3800 | 448.0070 | 98.85 | 1.07 |
| Quinic Acid | C7H12O6 | 5.48, 7.53 | 191.0563 | 192.1700 | 192.0635 | 99.46 | 1.38 | |
| Rutin | C27H30O16 | 10.21 | 609.14 | 300.1 | 610.5200 | 610.1543 | 89.52 | −3.46 |
| Possible Candidate | Formula | Rt (min) | m/z | MS/MS Fragments (m/z) | Exp Mass | Detected Mass | Score | Error (in ppm) |
|---|---|---|---|---|---|---|---|---|
| Apigenin | C15H10O5 | 12.27 | 269.03 | 225 | 270.0528 | 270.0528 | 96.79 | 1.27 |
| Caffeic Acid | C9H8O4 | 6.34 | 179.034 | 135.179 | 180.1600 | 180.0423 | 99.48 | 1.44 |
| Chlorogenic Acid | C16H18O9 | 7.74, 9.74, 10.63 | 353.0883 | 191.0548 | 354.3100 | 354.0939 | 98.55 | 1.66 |
| Chrysin | C15H10O4 | 11.29, 12.19 | 253.059 | 254.2400 | 254.0583 | 99.53 | 0.53 | |
| Cinnamic Acid | C9H8O2 | 10.73, 11.65 | 147.04 | 148.1586 | 148.0524 | 97.93 | 3.54 | |
| Coumaric Acid | C9H8O3 | 5.44, 6.53 | 163.0405 | 119.05 | 164.1600 | 164.0473 | 98.54 | 0.07 |
| Fumaric Acid | C7H6O3 | 3.26 | 137.02 | 93.03 | 138.1200 | 138.0318 | 99.83 | 0.30 |
| Gallic Acid | C7H6O5 | 1.18 | 169.0138 | 170.1200 | 170.0215 | 95.83 | −0.91 | |
| Hydroxybenzoic Acid | C7H6O3 | 3.19 | 137.02 | 93.0329 | 138.1200 | 138.0317 | 98.85 | 0.59 |
| Kaempferol | C15H10O6 | 11.53 | 285.0411 | 184.23 | 286.2300 | 286.0477 | 85.64 | 0.30 |
| Malic Acid | C4H6O5 | 5.92 | 133.1442 | 115.87 | 184.8400 | 134.0215 | 97.50 | 3.07 |
| Naringenin | C15H12O5 | 10.67 | 271.062 | 317.0667, 331.0823 | 272.2500 | 272.0685 | 96.99 | 2.53 |
| Protechatecuic Acid | C7H6O4 | 4.97 | 153.0181 | 109.02 | 154.1200 | 154.0266 | 86.64 | −0.44 |
| Quercitrin | C21H20O11 | 10.30 | 447.0946 | 284.0326, 255.02 | 448.3800 | 448.0070 | 97.02 | 2.93 |
| Quinic Acid | C7H12O6 | 5.56 | 191.0563 | 85.03 | 192.1700 | 192.0635 | 99.81 | 0.78 |
| Rutin | C27H30O16 | 10.16 | 609.14 | 300.1 | 610.5200 | 610.1543 | 97.07 | 2.11 |
| Possible Candidate | Formula | Rt (min) | m/z | MS/MS Fragments (m/z) | Exp Mass | Detected Mass | Score | Error (in ppm) |
|---|---|---|---|---|---|---|---|---|
| Apigenin | C15H10O5 | 12.06 | 269.15 | 117.03 | 270.2300 | 270.0528 | 98.52 | 2.13 |
| Apigetrin | C21H20O10 | 12.10 | 431.0995 | 432.3810 | 432.1267 | 97.02 | 2.41 | |
| Caffeic Acid | C9H8O4 | 8.58 | 179.034 | 135, 179 | 180.1600 | 180.0423 | 99.84 | 0.56 |
| Chrlorogenic Acid | C16H18O9 | 9.74 | 353.0883 | 191.05, 137.02 | 354.3100 | 354.0939 | 98.81 | −2.78 |
| Chrysin | C15H10O4 | 11.28 | 235.059 | 254.2400 | 254.0583 | 98.24 | 1.69 | |
| Cinnamic Acid | C9H8O2 | 10.73, 11.65 | 147.04 | 148.1586 | 148.0524 | 99.04 | 0.52 | |
| Coumaric Acid | C9H8O3 | 5.44, 10.13 | 163.0405 | 119.05 | 164.1600 | 164.0473 | 99.37 | 0.41 |
| Ferulic Acid | C10H10O4 | 10.24 | 193.01 | 105.46, 106.04, 117.03, 134.03, 175.78 | 194.1800 | 194.0579 | 99.30 | 1.70 |
| Fumaric Acid | C7H6O3 | 2.75 | 137.02 | 93.03 | 138.1200 | 138.0318 | 99.91 | 0.43 |
| Gallic Acid | C7H6O5 | 1.63 | 169.0138 | 127.002 | 170.1200 | 170.0215 | 95.83 | −0.91 |
| Hydroxybenzoic Acid | C7H6O3 | 3.18 | 137.02 | 93.0329 | 138.1200 | 138.0317 | 99.91 | 0.43 |
| Kaempferol (or lueolin) | C15H10O6 | 11.38 | 285.0411 | 184.23 | 286.2300 | 286.0477 | 85.64 | 0.30 |
| Malic Acid | C4H6O5 | 8.78 | 133.1442 | 115.0013 | 184.8400 | 134.0215 | 99.86 | 1.10 |
| Naringenin | C15H12O5 | 12.63 | 271.062 | 151.41 | 272.2500 | 272.0685 | 97.61 | 2.39 |
| Protechatecuic Acid | C7H6O4 | 4.92 | 153.0181 | 109.02 | 154.1200 | 154.0266 | 99.13 | −1.00 |
| Quercetin | C15H10O7 | 11.60 | 301.0362 | 151.0037, 135.65 | 302.2360 | 302.0427 | 93.68 | 2.14 |
| Quercitrin | C21H20O11 | 10.61 | 447.0946 | 284.0326, 255.02 | 448.3800 | 448.0070 | 96.46 | 0.28 |
| Quinic Acid | C7H12O6 | 6.18 | 191.0563 | 192.1700 | 192.0635 | 81.90 | −6.78 | |
| Rutin | C27H30O16 | 10.14 | 609.14 | 300.1 | 610.5200 | 610.1543 | 97.91 | 1.50 |
| Vanillic Acid | C8H8O4 | 6.87 | 167.0348 | 168.1400 | 168.0420 | 86.42 | −0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trabalzini, A.; Fornaciari, M.; Bartolini, D.; Varfaj, I.; Sardella, R.; Paiella, M.; Sorci, G.; Orlandi, F. Influence of Agronomic Practices on the Antioxidant Activity of Three Mediterranean Officinal Wild Plants: Silybum marianum, Achillea millefolium, and Trifolium pratense. Appl. Sci. 2025, 15, 5153. https://doi.org/10.3390/app15095153
Trabalzini A, Fornaciari M, Bartolini D, Varfaj I, Sardella R, Paiella M, Sorci G, Orlandi F. Influence of Agronomic Practices on the Antioxidant Activity of Three Mediterranean Officinal Wild Plants: Silybum marianum, Achillea millefolium, and Trifolium pratense. Applied Sciences. 2025; 15(9):5153. https://doi.org/10.3390/app15095153
Chicago/Turabian StyleTrabalzini, Andrea, Marco Fornaciari, Desirée Bartolini, Ina Varfaj, Roccaldo Sardella, Martina Paiella, Guglielmo Sorci, and Fabio Orlandi. 2025. "Influence of Agronomic Practices on the Antioxidant Activity of Three Mediterranean Officinal Wild Plants: Silybum marianum, Achillea millefolium, and Trifolium pratense" Applied Sciences 15, no. 9: 5153. https://doi.org/10.3390/app15095153
APA StyleTrabalzini, A., Fornaciari, M., Bartolini, D., Varfaj, I., Sardella, R., Paiella, M., Sorci, G., & Orlandi, F. (2025). Influence of Agronomic Practices on the Antioxidant Activity of Three Mediterranean Officinal Wild Plants: Silybum marianum, Achillea millefolium, and Trifolium pratense. Applied Sciences, 15(9), 5153. https://doi.org/10.3390/app15095153









