Bacillus sp. GLN Laccase Characterization: Industry and Biotechnology Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Processing of Decaying Wood Samples
2.2. Bacterial Isolation and Purification
2.3. Initial Evaluation of the Bacterial Isolates for Laccase Activity
2.4. Quantitative Evaluation of the Bacterial Strains with Positive Laccase Activity
2.5. Determination of Laccase Activity and Protein Content
2.6. Identification of the Potent Laccase Producer
2.7. Construction of Optimal Physicochemical Variables
2.8. Characterization of the Produced Laccase
2.8.1. Determination of pH Optimal and Stability Profile of the Laccase
2.8.2. Determination of Temperature Optimal and Stability Profile of the Laccase
2.8.3. The Impact of Organic Solvents and Inhibitors on Laccase Stability
2.8.4. Determination of Metal Ions’ Influence on the Laccase Stability
2.9. Data Analysis
3. Results
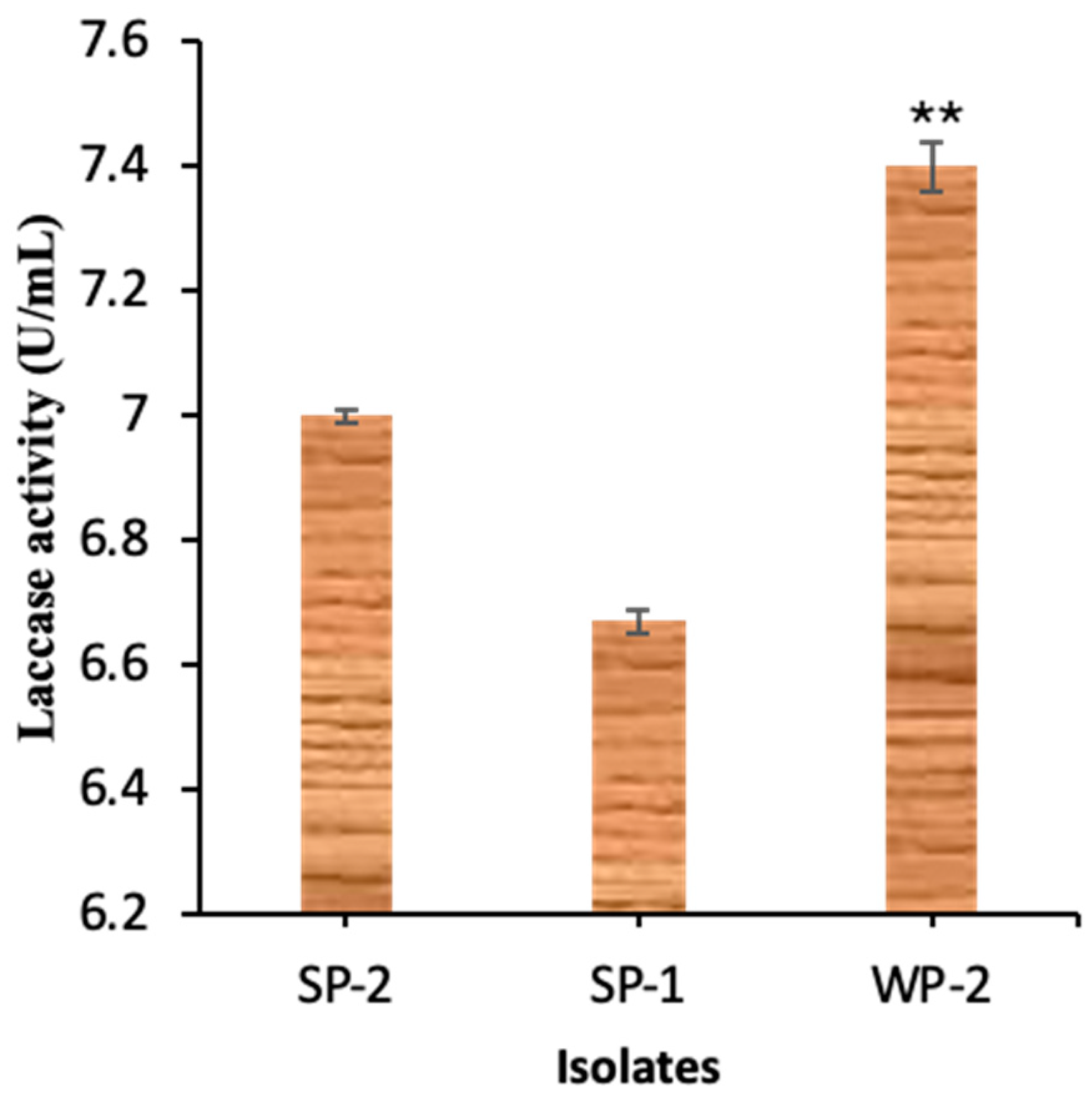
3.1. Bacteria Isolation, Laccase Production, and Identity Confirmation
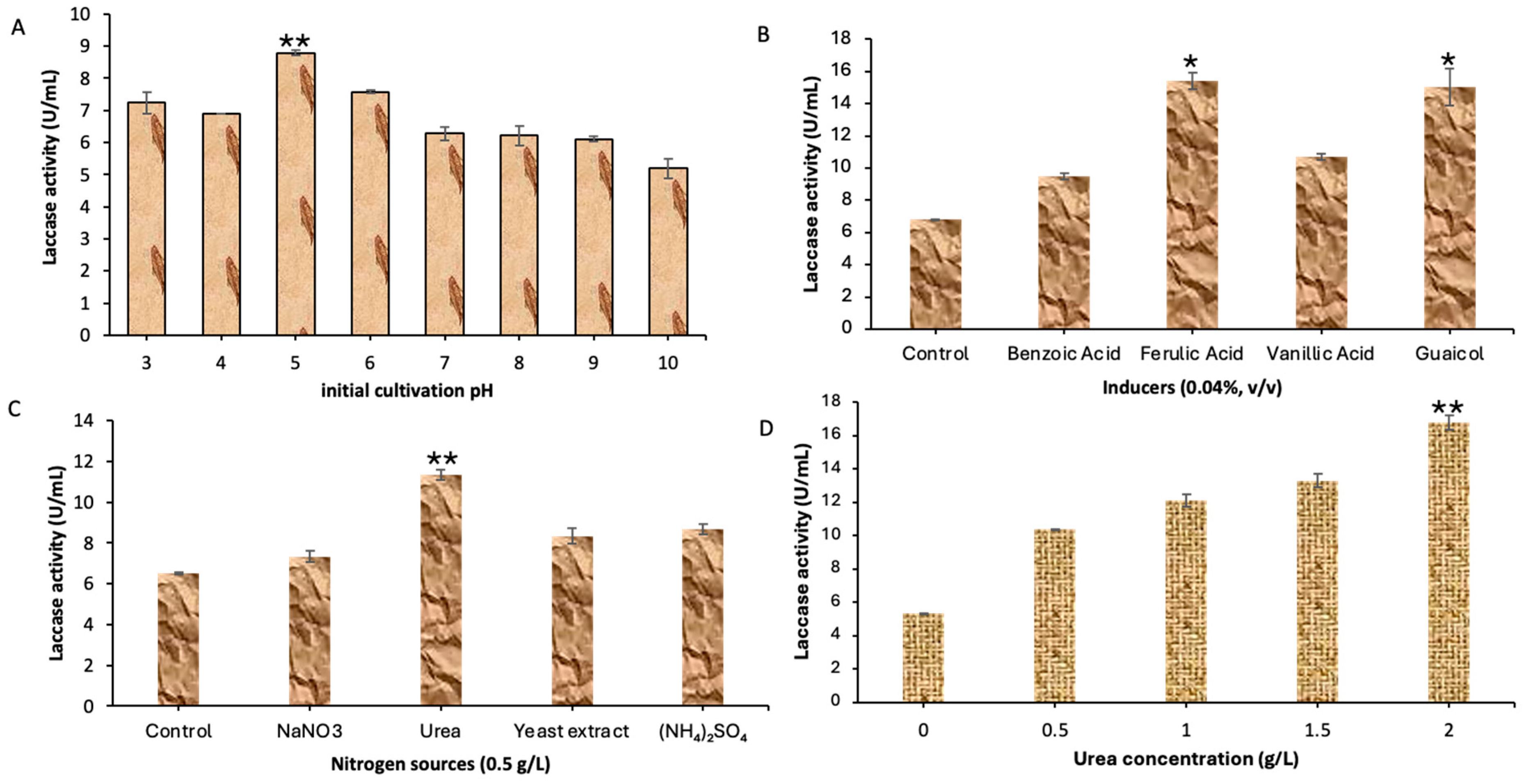
3.2. Process Variables Optimization for Enhanced Laccase Activity
3.3. Time-Dependent Profiling of Laccase Production by Bacillus sp. GLN
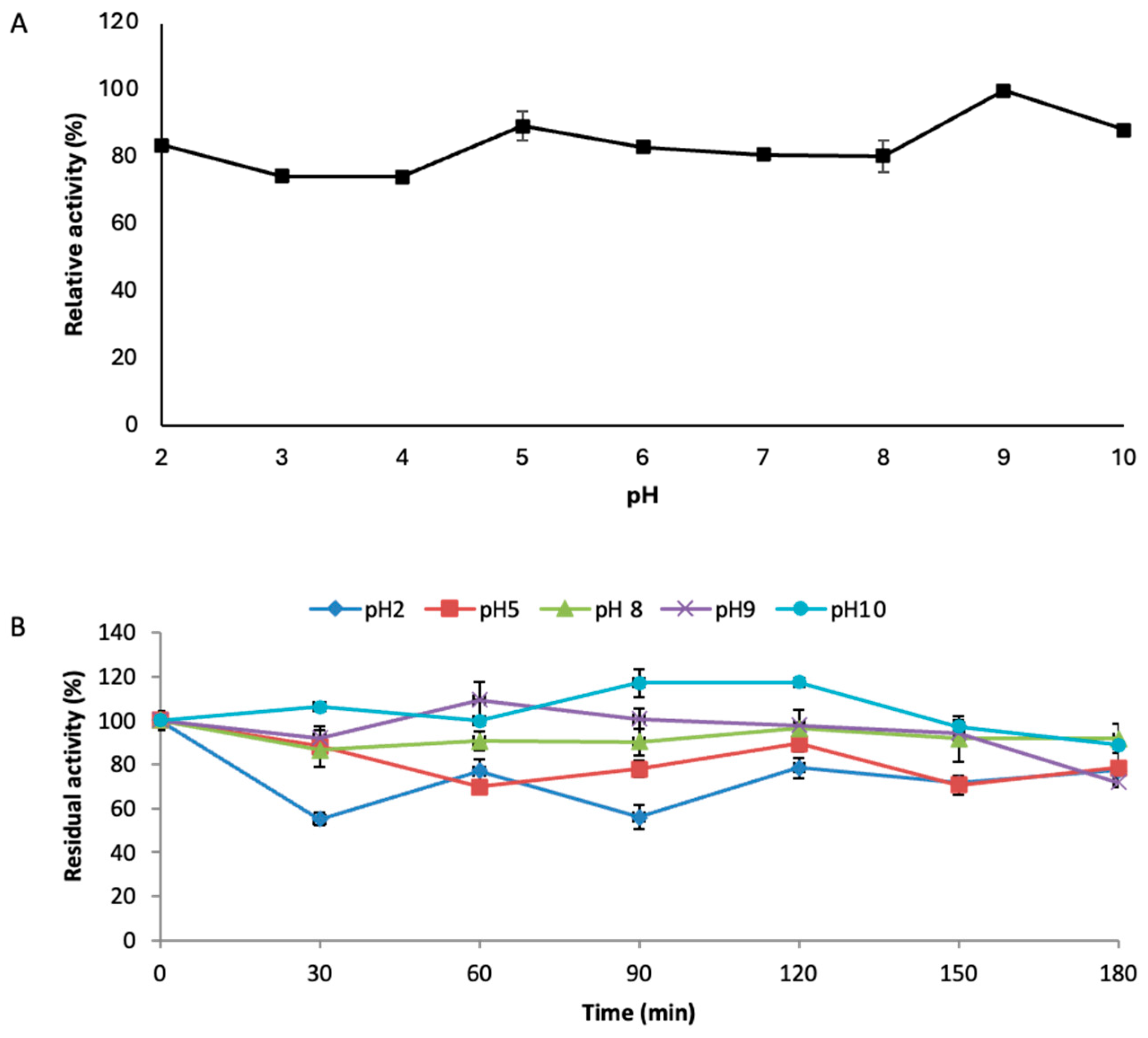
3.4. pH Optimal and Stability Profile of Strain GLN Laccase
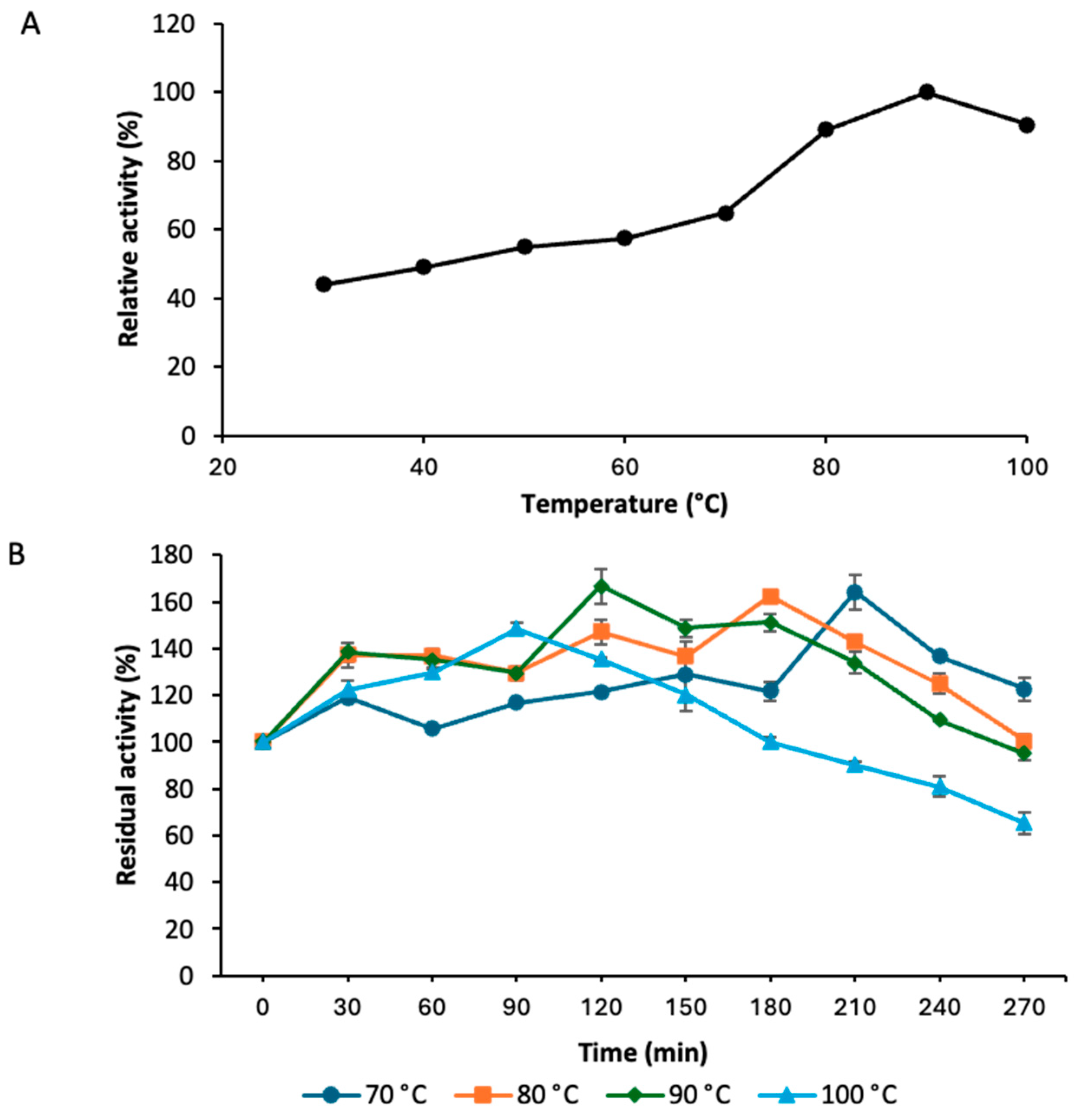
3.5. Temperature Optimal and Stability Profile of Strain GLN Laccase
3.6. Influence of Organic Solvents, Inhibitors, and Metal Ions on Laccase Stability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCR | Polymerase chain reaction |
| DNA | Deoxyribonucleic acid |
| SDS | Sodium dodecyl sulfate |
| EDTA | Ethylenediaminetetraacetic acid |
| SPSS | Statistical Package for the Social Sciences |
| DMP | 2,6-Dimethoxyphenol |
| BSM | Basal salt medium |
| ABTS | 2,2 amino-bis– (3-ethylbenzothiazoline 6 sulphonic acid) |
| U | Unit |
References
- Ahmad, M.; Pataczek, L.; Hilger, T.H.; Zahir, Z.A.; Hussain, A.; Rasche, F.; Schafleitner, R.; Solberg, S.Ø. Perspectives of microbial inoculation for sustainable development and environmental management. Front. Microbiol. 2018, 9, 2992. [Google Scholar] [CrossRef] [PubMed]
- Akinsemolu, A.A. Principles of green microbiology: The microbial blueprint for sustainable development. Environ. Adv. 2023, 14, 100440. [Google Scholar] [CrossRef]
- Timmis, K.; de Vos, W.M.; Ramos, J.L.; Vlaeminck, S.E.; Prieto, A.; Danchin, A.; Verstraete, W.; de Lorenzo, V.; Lee, S.Y.; Brüssow, H.; et al. The contribution of microbial biotechnology to sustainable development goals. Microb. Biotechnol. 2017, 10, 984–987. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, M.; Mittal, A.; Mehta, P.K. Microbial enzymes: Industrial progress in 21st century. 3 Biotech 2016, 6, 174. [Google Scholar] [CrossRef] [PubMed]
- Curran, L.M.L.K.; Sale, K.L.; Simmons, B.A. Review of advances in the development of laccases for the valorization of lignin to enable the production of lignocellulosic biofuels and bioproducts. Biotechnol. Adv. 2022, 54, 107809. [Google Scholar] [CrossRef]
- Tiwari, A.; Chen, C.W.; Haldar, D.; Patel, A.K.; Dong, C.D.; Singhania, R.R. Laccase in biorefinery of lignocellulosic biomass. Appl. Sci. 2023, 13, 4673. [Google Scholar] [CrossRef]
- Panwar, V.; Lzaod, S.; Dutta, T. Thermostable bacterial laccase: Catalytic properties and its application in biotransformation of emerging pollutants. ACS Omega 2023, 8, 34710–34719. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Goradia, B.; Saxena, A. Bacterial laccase: Recent update on production, properties and industrial applications. 3 Biotech 2017, 7, 323. [Google Scholar] [CrossRef]
- Lima, N.S.M.; Gomes-Pepe, E.S.; Campanharo, J.C.; de Macedo Lemos, E.G. Broad thermal spectrum metagenomic laccase with action for dye decolorization and fentin hydroxide treatment. AMB Express. 2022, 12, 38. [Google Scholar] [CrossRef]
- Sondhi, S.; Sharma, P.; Saini, S.; Puri, N.; Gupta, N. Purification and characterization of an extracellular, thermo-alkali-stable, metal tolerant laccase from Bacillus tequilensis SN4. PLoS ONE 2014, 9, e96951. [Google Scholar] [CrossRef]
- Sharma, V.; Ayothiraman, S.; Dhakshinamoorthy, V. Production of highly thermo-tolerant laccase from novel thermophilic bacterium Bacillus sp. PC-3 and its application in functionalization of chitosan film. J. Biosci. Bioeng. 2019, 127, 672–678. [Google Scholar] [CrossRef]
- Navas, L.E.; Martínez, F.D.; Taverna, M.E.; Fetherolf, M.M.; Eltis, L.D.; Nicolau, V.; Estenoz, D.; Campos, E.; Benintende, G.B.; Berretta, M.F. A thermostable laccase from Thermus sp. 2.9 and its potential for delignification of Eucalyptus biomass. AMB Expr. 2019, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.C.; Rodgers, D.W.; Dumon, C.; Shi, J. Characterization and enzyme engineering of a hyperthermophilic laccase toward improving its activity in ionic liquid. Front. Energy Res. 2020, 8, 158. [Google Scholar] [CrossRef]
- Miranda-Blancas, R.; Avelar, M.; Rodriguez-Arteaga, A.; Sinicropi, A.; Rudiño-Piñera, E. The β-hairpin from the Thermus thermophilus HB27 laccase works as a pH-dependent switch to regulate laccase activity. J. Struct. Biol. 2021, 213, 107740. [Google Scholar] [CrossRef]
- Gianolini, J.E.; Britos, C.N.; Mulreedy, C.B.; Trelles, J.A. Hyperstabilization of a thermophile bacterial laccase and its application for industrial dyes degradation. 3 Biotech 2020, 10, 288. [Google Scholar] [CrossRef]
- Sharma, N.; Leung, I.K.H. Novel thermophilic bacterial laccase for the degradation of aromatic organic pollutants. Front. Chem. 2021, 9, 711345. [Google Scholar] [CrossRef] [PubMed]
- Atalah, J.; Blamey, J.M. Isolation and characterization of a novel laccase from an Antarctic thermophilic Geobacillus. Antarct. Sci. 2022, 34, 289–297. [Google Scholar] [CrossRef]
- Bibi, M.; Yasmin, A.; Blanford, C.; Safdar, N. Production and characterization of a highly stable laccase from extreme thermophile Geobacillus stearothermophilus MB600 isolated from hot spring of Gilgit Balitistan (Pakistan). J. Taibah Univ. Sci. 2023, 17, 2268903. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Okoh, A.I.; Nwodo, U.U. Recovery of laccase-producing gammaproteobacteria from wastewater. Biotechnol. Rep. 2019, 21, e00320. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Moubasher, H.A.; Okoh, A.I.; Nwodo, U.U. Production of polyextremotolerant laccase by Achromobacter xylosoxidans HWN16 and Citrobacter freundii LLJ16. Biotechnol. Rep. 2019, 22, e00337. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetic Analysis version 12 for adaptive and green computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef] [PubMed]
- Gogotya, A.; Nnolim, N.E.; Digban, T.O.; Okoh, A.I.; Nwodo, U.U. Characterization of a thermostable and solvent-tolerant laccase produced by Streptomyces sp. LAO. Biotechnol. Lett. 2021, 43, 1429–1442. [Google Scholar] [CrossRef] [PubMed]
- Tonin, F.; Melis, R.; Cordes, A.; Sanchez-Amat, A.; Pollegioni, L.; Rosini, E. Comparison of different microbial laccases as tools for industrial uses. New Biotechnol. 2016, 33, 387–398. [Google Scholar] [CrossRef]
- Martins, L.O.; Melo, E.P.; Sanchez-Amat, A.; Robalo, M.P. Bacterial laccases: Some recent advances and applications. In Laccases in Bioremediation and Waste Valorisation; Schlosser, D., Ed.; Microbiology Monographs; Springer: Cham, Switzerland, 2020; Volume 33, pp. 27–55. [Google Scholar] [CrossRef]
- Dhiman, K.; Shirkot, P. Bioprospecting and molecular characterization of laccase producing bacteria from paper mills of Himachal Pradesh. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 85, 1095–1103. [Google Scholar] [CrossRef]
- Ali, N.S.; Huang, F.; Qin, W.; Yang, T.C. Identification and characterization of a new Serratia proteamaculans strain that naturally produces significant amount of extracellular laccase. Front. Microbiol. 2022, 13, 878360. [Google Scholar] [CrossRef]
- Kumar, V.; Jamwal, A.; Kumar, V.; Singh, D. Green bioprocess for degradation of synthetic dyes mixture using consortium of laccase-producing bacteria from Himalayan niches. J. Environ. Manage. 2022, 310, 114764. [Google Scholar] [CrossRef]
- Kuddus, M.; Joseph, B.; Ramteke, P.W. Production of laccase from newly isolated Pseudomonas putida and its application in bioremediation of synthetic dyes and industrial effluents. Biocatal. Agric. Biotechnol. 2013, 2, 333–338. [Google Scholar] [CrossRef]
- El-Bendary, M.A.; Ezzat, S.M.; Ewais, E.A.; Al-Zalama, M.A. Optimization of spore laccase production by Bacillus amyloliquefaciens isolated from wastewater and its potential in green biodecolorization of synthetic textile dyes. Prep. Biochem. Biotechnol. 2020, 51, 16–27. [Google Scholar] [CrossRef]
- Sheikhi, F.; Roayaei Ardakani, M.; Enayatizamir, N.; Rodriguez-Couto, S. The determination of assay for laccase of Bacillus subtilis WPI with two classes of chemical compounds as substrates. Indian J. Microbiol. 2012, 52, 701–707. [Google Scholar] [CrossRef]
- Babinskas, J.; Sabotič, J.; Matijošytė, I. Synthesis and application of a phenazine class substrate for high-throughput screening of laccase activity. Appl. Microbiol. Biotechnol. 2024, 108, 66. [Google Scholar] [CrossRef] [PubMed]
- Abioye, O.P.; Umaru, S.; Aransiola, S.A.; Oyewole, O.A.; Maddela, N.R.; Prasad, R. Production of laccase by Bacillus subtilis and Aspergillus niger for treatment of textile effluent. Sustain. Chem. Environ. 2025, 9, 100222. [Google Scholar] [CrossRef]
- Fayad, N.; Kallassy Awad, M.; Mahillon, J. Diversity of Bacillus cereus sensu lato mobilome. BMC Genom. 2019, 20, 436. [Google Scholar] [CrossRef]
- Azizoglu, U.; Argentel-Martínez, L.; Peñuelas-Rubio, O.; Herrera-Sepúlveda, A.; Ibal, J.C.; Sharafi, R.; Salehi Jouzani, G.; Ortiz, A.; Vaca, J.; Sansinenea, E. Natural products produced by the species of Bacillus cereus group: Recent updates. J. Basic Microbiol. 2024, 65, e2400666. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.A.M.; Aguilar, C.E.G.; Silva, H.O.; Vidal, A.M.C. Bacillus cereus group: Genetic aspects related to food safety and dairy processing. Arq. Inst. Biol. 2018, 85, e0232017. [Google Scholar] [CrossRef]
- Edoamodu, C.E.; Nwodo, U.U. Optimization and physicochemical characterization of a thermo-alkali stable laccase produced by wastewater associated Bacillus sp. NU2. Environ. Technol. 2023, 45, 4441–4456. [Google Scholar] [CrossRef]
- Deepa, T.; Gangwane, A.; Sayyed, R.Z.; Jadhav, H.P. Optimization and scale-up of laccase production by Bacillus sp. BAB-4151 isolated from the waste of the soap industry. Environ. Sustain. 2020, 3, 471–479. [Google Scholar] [CrossRef]
- Kaushik, G.; Thakur, I.S. Production of laccase and optimization of its production by Bacillus sp. using distillery spent wash as inducer. Bioremediat. J. 2014, 18, 28–37. [Google Scholar] [CrossRef]
- Srinivasan, P.; Selvankumar, T.; Kamala-Kannan, S.; Mythili, R.; Sengottaiyan, A.; Govarthanan, M.; Selvam, K. Production and purification of laccase by Bacillus sp. using millet husks and its pesticide degradation application. 3 Biotech 2019, 9, 396. [Google Scholar] [CrossRef]
- Kuhar, F.; Papinutti, L. Optimization of laccase production by two strains of Ganoderma lucidum using phenolic and metallic inducers. Rev. Argent. Microbiol. 2014, 46, 144–149. [Google Scholar] [CrossRef]
- Zhuo, R.; Yuan, P.; Yang, Y.; Zhang, S.; Ma, F.; Zhang, X. Induction of laccase by metal ions and aromatic compounds in Pleurotus ostreatus HAUCC 162 and decolorization of different synthetic dyes by the extracellular laccase. Biochem. Eng. J. 2017, 117, 62–72. [Google Scholar] [CrossRef]
- Afreen, S.; Anwer, R.; Singh, R.K.; Fatma, T. Extracellular laccase production and its optimization from Arthrospira maxima catalyzed decolorization of synthetic dyes. Saudi J. Biol. Sci. 2018, 25, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Chen, Y.; Sun, S.; Hu, Y. Interaction among multiple microorganisms and effects of nitrogen and carbon supplementations on lignin degradation. Bioresour. Technol. 2014, 155, 144–151. [Google Scholar] [CrossRef]
- Muthukumarasamy, N.P.; Jackson, B.; Joseph Raj, A.; Sevanan, M. Production of extracellular laccase from Bacillus subtilis MTCC 2414 using agroresidues as a potential substrate. Biochem. Res. Int. 2015, 2015, 765190. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, A.K.; Ahmad, S.; Chandra, R. Optimization of laccase production by Bacillus sp. strain AKRC01 in presence of agro-waste as effective substrate using response surface methodology. J. Pure Appl. Microbiol. 2020, 14, 351–362. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.K.; Bilal, M.; Chandra, R. Sustainable production of thermostable laccase from agro-residues waste by Bacillus aquimaris AKRC02. Catal. Lett. 2022, 152, 1784–1800. [Google Scholar] [CrossRef]
- Tamilvanan, N. Optimization, production, purification of laccase enzyme from Bacillus sp. J. New Dev. Chem. 2020, 2, 35–43. [Google Scholar] [CrossRef]
- Sharma, A.; Muthupriya, M.; Raj, R.; Shameen, Z.; SM, V.; Niyonzima, F.N.; More, S.S. Properties of laccase of Bacillus marisflavi strain BB4 and its synthetic dyes decolorization analysis. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2021, 91, 477–485. [Google Scholar] [CrossRef]
- Rajeswari, M.; Bhuvaneswari, V. Optimization of laccase production from Bacillus sp. PK4 through statistical design of experiments. Microbiol. Biotechnol. Lett. 2017, 45, 330–342. [Google Scholar] [CrossRef]
- Edoamodu, C.E.; Nwodo, U.U. Decolourization of synthetic dyes by laccase produced from Bacillus sp. NU2. Biotechnol. Biotechnol. Equip. 2022, 36, 95–106. [Google Scholar] [CrossRef]
- Mohammed, M.K.; Khudhair, S.H.; Jabber, A.D. Production, purification, and characterization of laccase enzyme from local isolate Bacillus cereus TY10 isolated from soil samples. J. Nat. Sci. Biol. Med. 2024, 15, 90–96. [Google Scholar]
- Selvam, K.; Ameen, F.; Amirul Islam, M.; Sudhakar, C.; Selvankumar, T. Laccase production from Bacillus aestuarii KSK using Borassus flabellifer empty fruit bunch waste as a substrate and assessing their malachite green dye degradation. J. Appl. Microbiol. 2022, 133, 3288–3295. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chandra, R. Biodegradation and toxicity reduction of pulp paper mill wastewater by isolated laccase producing Bacillus cereus AKRC03. Clean. Eng. Technol. 2021, 4, 100193. [Google Scholar] [CrossRef]
- Yang, J.; Xu, H.; Zhao, J.; Zhang, N.; Xie, J.; Jiang, J. Isolation of Bacillus sp. with high laccase activity for green biodecolorization of synthetic textile dyes. Water Sci. Technol. 2022, 86, 777–786. [Google Scholar] [CrossRef]
- Liu, J.; Li, B.; Li, Z.; Yang, F.; Chen, B.; Chen, J.; Li, H.; Jiang, Z. Deciphering the alkaline stable mechanism of bacterial laccase from Bacillus pumilus by molecular dynamics simulation can improve the decolorization of textile dyes. J. Hazard. Mater. 2023, 443, 130370. [Google Scholar] [CrossRef]
- Omeje, K.O.; Nnolim, N.E.; Ezema, B.O.; Ozioko, J.N.; Eze, S.O. Synthetic dyes decolorization potential of agroindustrial waste-derived thermo-active laccase from Aspergillus species. Biocatal. Agric. Biotechnol. 2020, 29, 101800. [Google Scholar] [CrossRef]
- Aza, P.; Camarero, S. Fungal laccases: Fundamentals, engineering and classification update. Biomolecules 2023, 13, 1716. [Google Scholar] [CrossRef]
- Zhang, Y.; Plesner, T.J.; Ouyang, Y.; Zheng, Y.C.; Bouhier, E.; Berentzen, E.I.; Zhang, M.; Zhou, P.; Zimmermann, W.; Andersen, G.R.; et al. Computer-aided discovery of a novel thermophilic laccase for low-density polyethylene degradation. J. Hazard. Mater. 2023, 458, 131986. [Google Scholar] [CrossRef]
- Martins, L.O.; Soares, C.M.; Pereira, M.M.; Teixeira, M.; Costa, T.; Jones, G.H.; Henriques, A.O. Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J. Biol. Chem. 2002, 277, 18849–18859. [Google Scholar] [CrossRef]
- Verma, A.; Shirkot, P. Purification and characterization of thermostable laccase from thermophilic Geobacillus thermocatenulatus MS5 and its applications in removal of textile dyes. Sch. Acad. J. Biosci. 2014, 2, 479–485. [Google Scholar]
- Espina, G.; Cáceres-Moreno, P.; Mejías-Navarrete, G.; Ji, M.; Sun, J.; Blamey, J.M. A novel and highly active recombinant spore-coat bacterial laccase, able to rapidly biodecolorize azo, triarylmethane and anthraquinonic dyestuffs. Int. J. Biol. Macromol. 2021, 170, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Trubitsina, L.I.; Abdullatypov, A.V.; Larionova, A.P.; Trubitsin, I.V.; Alferov, S.V.; Ponamoreva, O.N.; Leontievsky, A.A. Expression of thermophilic two-domain laccase from Catenuloplanes japonicus in Escherichia coli and its activity against triarylmethane and azo dyes. PeerJ 2021, 9, e11646. [Google Scholar] [CrossRef]
- Wang, J.; Chang, F.; Tang, X.; Li, W.; Yin, Q.; Yang, Y.; Hu, Y. Bacterial laccase of Anoxybacillus ayderensis SK3-4 from hot springs showing potential for industrial dye decolorization. Ann. Microbiol. 2020, 70, 51. [Google Scholar] [CrossRef]
- Chen, C.Y.; Huang, Y.C.; Wei, C.M.; Meng, M.; Liu, W.H.; Yang, C.H. Properties of the newly isolated extracellular thermo-alkali-stable laccase from thermophilic actinomycetes, Thermobifida fusca and its application in dye intermediates oxidation. AMB Express 2013, 3, 49. [Google Scholar] [CrossRef]
- Das, R.; Li, G.; Mai, B.; An, T. Spore cells from BPA degrading bacteria Bacillus sp. GZB displaying high laccase activity and stability for BPA degradation. Sci. Total Environ. 2018, 640, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Britos, C.N.; Gianolini, J.E.; Portillo, H.; Trelles, J.A. Biodegradation of industrial dyes by a solvent, metal and surfactant-stable extracellular bacterial laccase. Biocatal. Agric. Biotechnol. 2018, 14, 221–227. [Google Scholar] [CrossRef]
- Bhankar, U.; Gupta, V.K. Purification and characterization of an extracellular thermostable laccase from Bacillus cereus UV25 and its potential in enrichment of fruit juices. Lett. Appl. NanoBioSci. 2023, 12, 144. [Google Scholar]
- Kaushik, G.; Thakur, I.S. Purification, characterization and usage of thermotolerant laccase from Bacillus sp. for biodegradation of synthetic dyes. Appl. Biochem. Microbiol. 2013, 49, 352–359. [Google Scholar] [CrossRef]
- Suarez, F.J.; Santillán, S.O.; Vazquez-Duhalt, R.; Graeve, O.A. Enhanced catalytic stability of laccase immobilized on copper oxide nanoparticles. ChemCatChem 2025, 17, e202401232. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, R.; Zhu, G.; Wang, L.; Bai, H.; Qian, Y.; Zhou, X.; Yin, Q.; Zhang, Y. Expression of a deep-sea bacterial laccase from Halomonas alkaliantartica and its application in dyes decolorization. Ann. Microbiol. 2023, 73, 19. [Google Scholar] [CrossRef]



| S/N | Isolate | Guaiacol | α-Naphthol |
|---|---|---|---|
| 1. | SP-2 | + | + |
| 2. | WP-3 | + | − |
| 3. | WP-1 | + | − |
| 4. | WP-4 | + | − |
| 5. | SP-1 | + | + |
| 6. | WP-2 | + | + |
| 7. | WP-5 | − | − |
| 8. | SP-3 | − | − |
| Organic Solvent | Concentration (%) | Residual Activity (%) a | ||
|---|---|---|---|---|
| 1 h | 8 h | 16 h | ||
| Control | - | 100 ± 1.01 | 100 ± 1.32 | 100 ± 1.08 |
| Acetone | 10 | 80.18 ± 0.73 | 84.41 ± 0.09 | 92.53 ± 6.06 |
| 20 | 90.77 ± 4.14 | 81.42 ± 1.01 | 86.61 ± 0.83 | |
| Ethanol | 10 | 84.99 ± 1.1 | 78.95 ± 1.19 | 82.0 ± 1.29 |
| 20 | 91.94 ± 0.46 | 88.56 ± 2.11 | 82.26 ± 1.10 | |
| Methanol | 10 | 86.35 ± 1.01 | 89.67 ± 4.59 | 84.47 ± 0 |
| 20 | 81.81 ± 1.56 | 87.52 ± 3.77 | 93.11 ± 2.66 | |
| Propanol | 10 | 89.21 ± 3.58 | 84.47 ± 0 | 82.33 ± 2.30 |
| 20 | 86.55 ± 1.84 | 88.17 ± 3.03 | 86.29 ± 5.70 | |
| Chemical Agents | Concentration (mM) | Residual Activity (%) a |
|---|---|---|
| Control | - | 100 ± 0.76 |
| SDS | 100 | 53.67 ± 0.13 |
| EDTA | 100 | 58.33 ± 0.51 |
| NaN3 | 100 | 65.14 ± 1.26 |
| Urea | 100 | 63.08 ± 3.17 |
| NaCl | 100 | 63.98 ± 0 |
| Metal Ions | Concentration (mM) | Residual Activity (%) a |
|---|---|---|
| Control | - | 100 ± 2.65 |
| CaCl2 | 1 | 86.37 ± 3.337 |
| MgCl2 | 1 | 96.42 ± 6.34 |
| FeCl2 | 1 | 104.32 ± 1.53 |
| MnSO4 | 1 | 96.76 ± 0.32 |
| ZnSO4 | 1 | 92.67 ± 0.56 |
| CuSO4 | 1 | 110.68 ± 2.17 |
| NiCl | 1 | 93.87 ± 0.64 |
| KCl | 1 | 93.70 ± 6.02 |
| Bacterial Source | Optimal Prod. Time (h) | Assay Substrate | Optimal pH | Optimal Temp. (°C) | Half-Life of GLN Laccase | Activity Enhancing Metal | References |
|---|---|---|---|---|---|---|---|
| Bacillus sp. GLN | 48 | ABTS | 9 | 90 | >270 min at 100 °C | Cu2+ | This study |
| Geobacillus stearothermophilus MB600 | 72 | Guaiacol | 5 | 90 | - | Cu2+, Ca2+, Cd2+, Li+ | [18] |
| Bacillus tequilensis SN4 | 96 | ABTS | 5.5 | 85 | 1 h at 55 °C | Cu2+, Co2+ | [10] |
| Lysinibacillus fusiformis | - | DMP | 10.4 | 70 | 15.9 h at 60 °C | Cu2+ | [59] |
| Bacillus sp. PC-3 | 36 | ABTS | 7 | 60 | 3.75 h at 60 °C | - | [11] |
| Bacillus subtilis | - | ABTS | 3 | 75 | 2 h at 80 °C | - | [60] |
| Geobacillus thermocatenulatus MS5 | 96 | ABTS | 4–5 | 55–60 | - | - | [61] |
| Geobacillus yumthangensis | - | ABTS | 5 | 60 | - | Cu2+ | [16] |
| Geobacillus sp. ID17 | Syringaldazine | 7 | 55 | 30 min at 60 °C | Cu2+ | [17] | |
| Bacillus sp. FNT | - | Syringaldazine | 6 | 70 | >2 h at 60 °C | - | [62] |
| Catenuloplanes japonicus | - | DMP | 9.2 | 70 | 60 min at 90 °C | - | [63] |
| Anoxybacillus ayderensis SK3-4 | - | Syringaldazine | 7 | 75 | 155 min at 65 °C | Cu2+, Mg2+ | [64] |
| Thermobifida fusca | 36 | DMP | 8 | 60 | >3 h at 50 °C | - | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gogotya, A.; Nnolim, N.E.; Nwodo, U.U. Bacillus sp. GLN Laccase Characterization: Industry and Biotechnology Implications. Appl. Sci. 2025, 15, 5144. https://doi.org/10.3390/app15095144
Gogotya A, Nnolim NE, Nwodo UU. Bacillus sp. GLN Laccase Characterization: Industry and Biotechnology Implications. Applied Sciences. 2025; 15(9):5144. https://doi.org/10.3390/app15095144
Chicago/Turabian StyleGogotya, Asemahle, Nonso E. Nnolim, and Uchechukwu U. Nwodo. 2025. "Bacillus sp. GLN Laccase Characterization: Industry and Biotechnology Implications" Applied Sciences 15, no. 9: 5144. https://doi.org/10.3390/app15095144
APA StyleGogotya, A., Nnolim, N. E., & Nwodo, U. U. (2025). Bacillus sp. GLN Laccase Characterization: Industry and Biotechnology Implications. Applied Sciences, 15(9), 5144. https://doi.org/10.3390/app15095144







