Dynamic Heart Rate Variability Vector and Premature Ventricular Contractions Patterns in Adult Hemodialysis Patients: A 48 h Risk Exploration
Abstract
1. Introduction
2. Materials and Methods
2.1. Database and Population
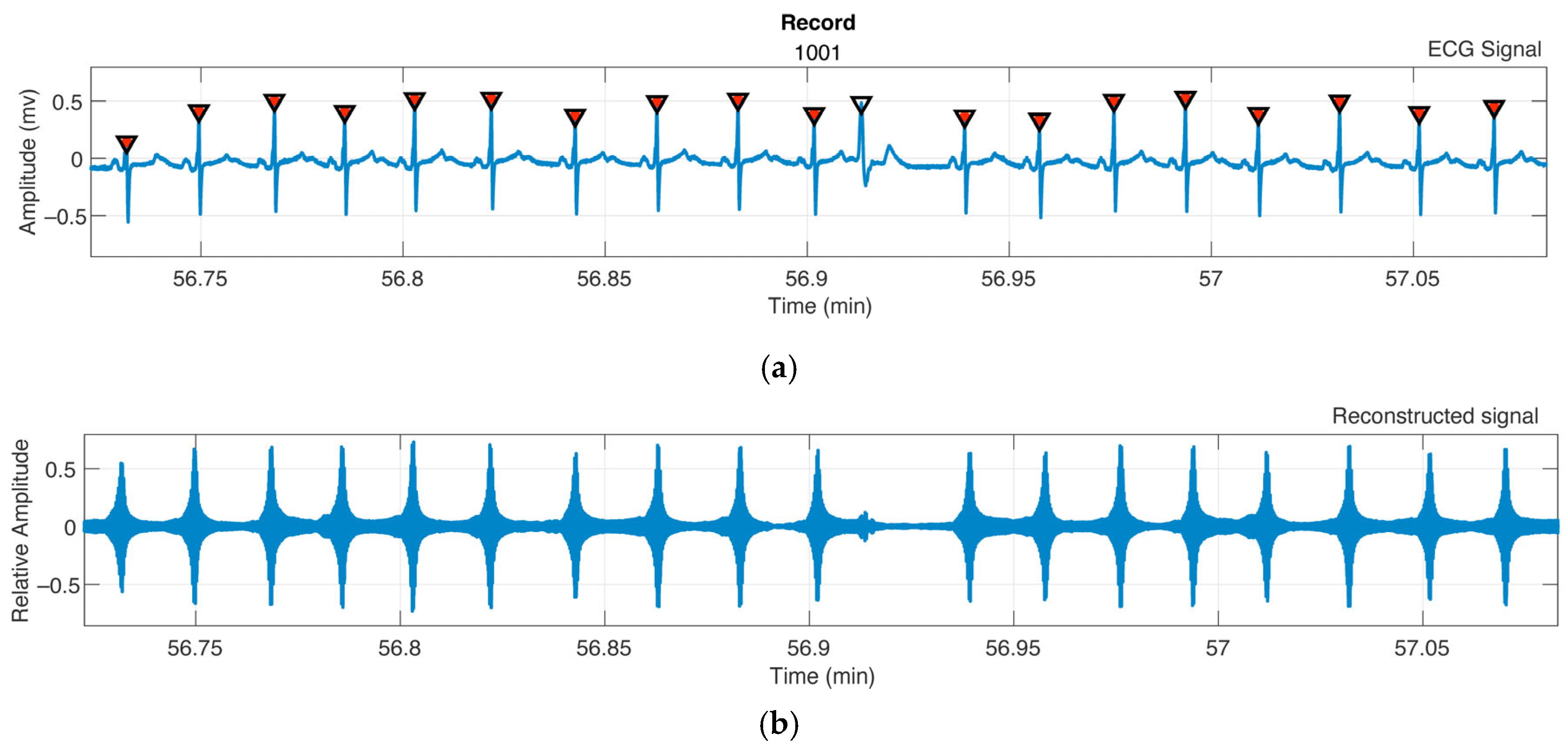
2.2. QRS and R-Wave Identification
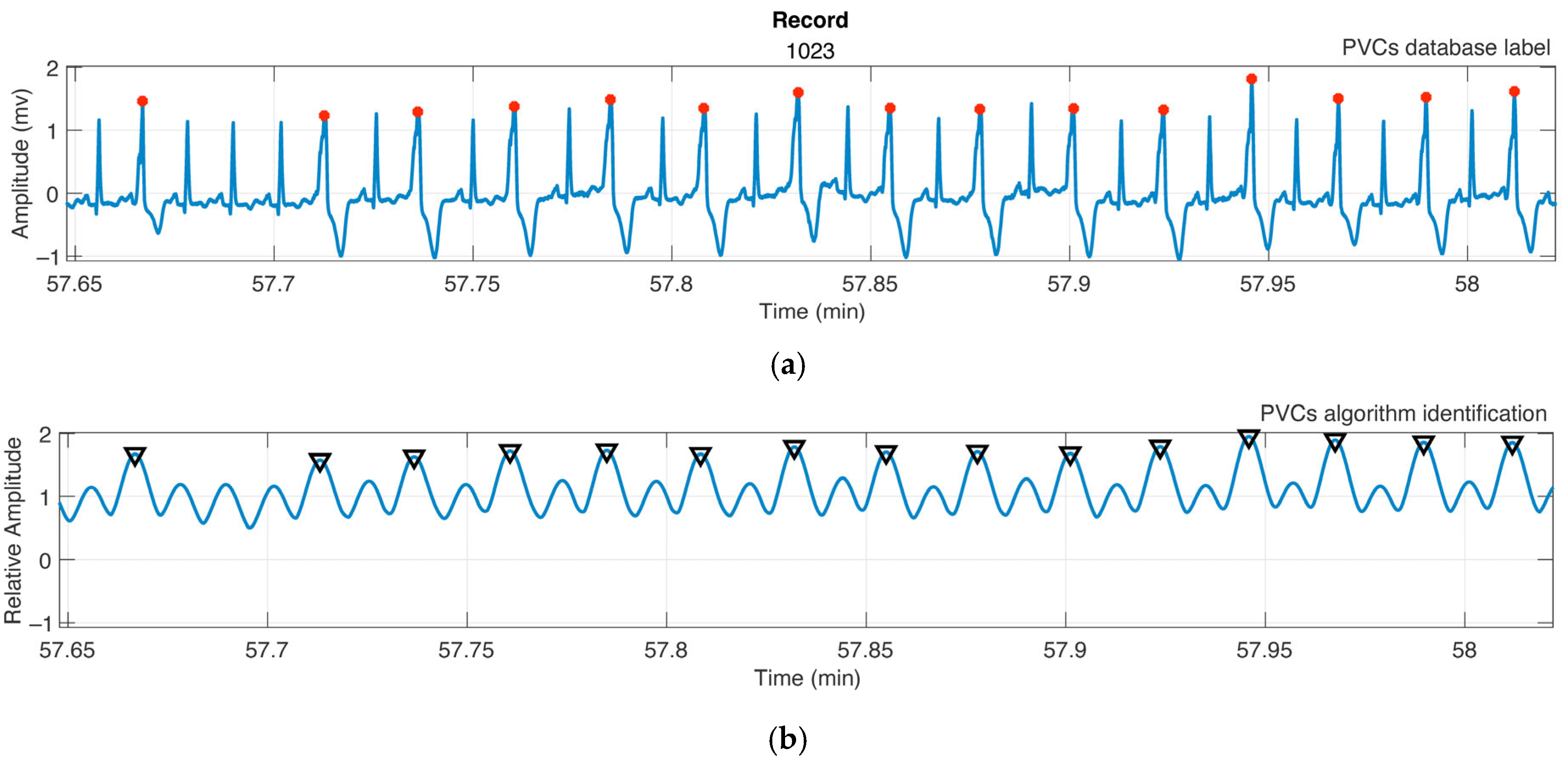
2.3. PVC Identification
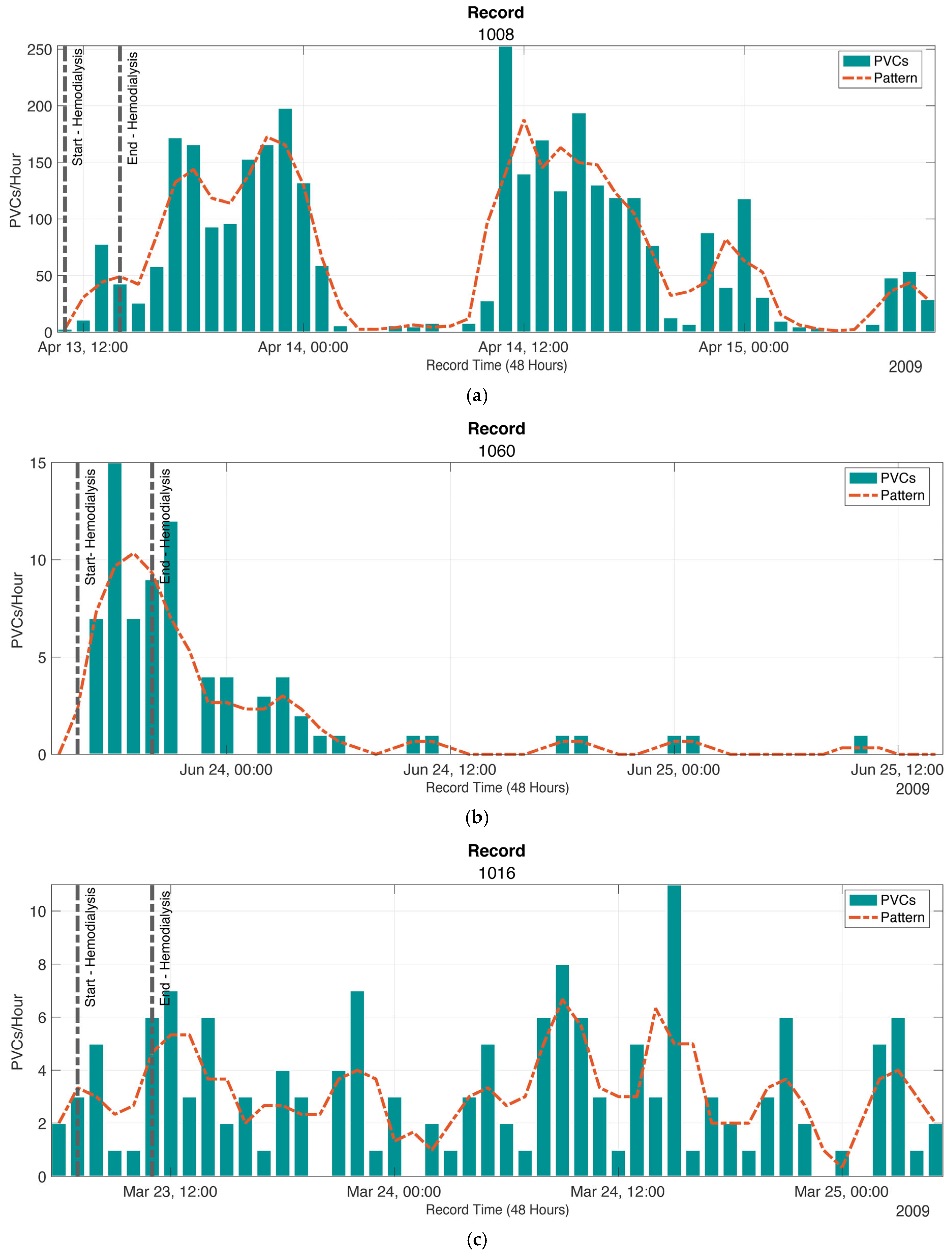
2.4. Patterns of the Presence of PVCs
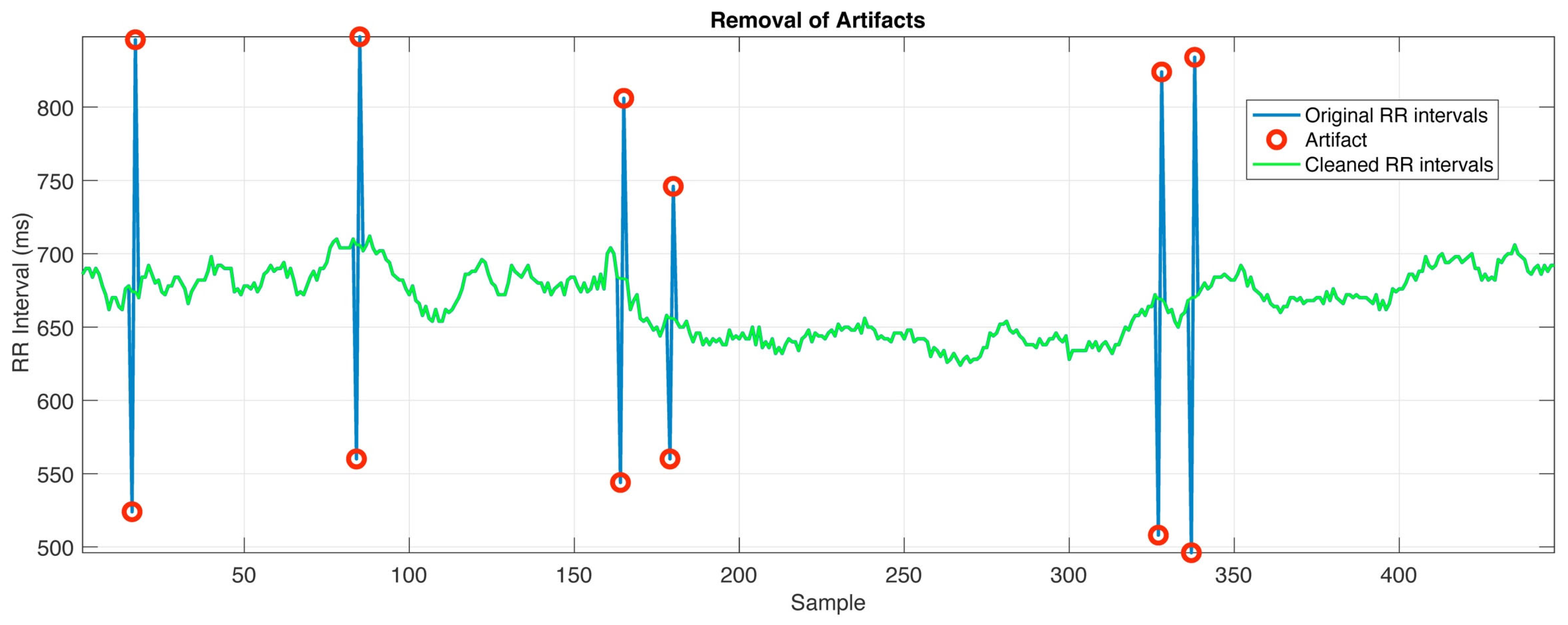
2.5. Tachogram
2.6. Heart Rate Variability Indices
2.7. Statistical Analysis
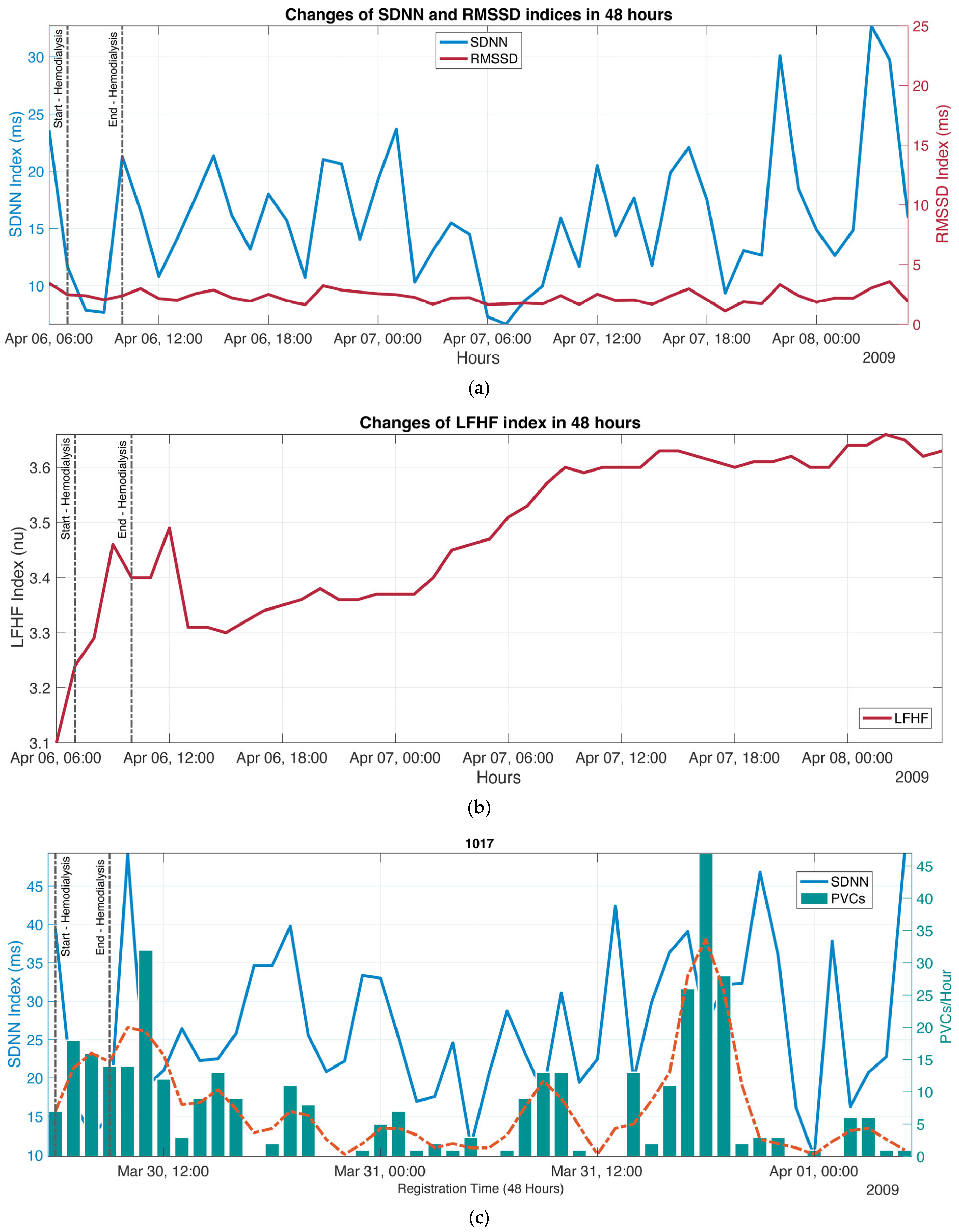
3. Results
3.1. QRS and R-Wave Identification
3.2. PVC Identification
3.3. Patterns of the Presence of PVCs
3.4. Tachogram
3.5. HRV Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECG | Electrocardiography |
| CKD | Chronic kidney disease |
| PVCs | Premature ventricular contractions |
| ANS | Autonomous nervous system |
| LF | Low frequency |
| HF | High frequency |
| LF/HF | Ratio between both bands |
| SDNN | Standard deviation of RR intervals |
| RMSSD | Square root of the mean square differences between consecutive RR intervals |
| SD1 | Poincaré SD1 Index |
| SD2 | Poincaré SD2 Index |
| THEW | Telemetric and Holter ECG Warehouse project |
| DWT | Discrete wavelet transform |
| CWT | Continuous wavelet transform |
| db4 | Wavelet order 4 Daubechies |
| PPV | Positive predictive value |
| F-Score | Precision measure for the test |
| ID Px | Patient identification number |
| SD | Standard deviation |
References
- Ronco, C.; McCullough, P.; Anker, S.D.; Anand, I.; Aspromonte, N.; Bagshaw, S.M.; Bellomo, R.; Berl, T.; Bobek, I.; Cruz, D.N.; et al. Cardio-renal syndromes: Report from the consensus conference of the acute dialysis quality initiative. Eur. Heart J. 2010, 31, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Quarti-Trevano, F.; Seravalle, G.; Dell’Oro, R.; Mancia, G.; Grassi, G. Autonomic Cardiovascular Alterations in Chronic Kidney Disease: Effects of Dialysis, Kidney Transplantation, and Renal Denervation. Curr. Hypertens. Rep. 2021, 23, 10. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Iñiguez, J.S.; Sánchez-Villaseca, S.J.; García-Macías, L.A. Síndrome cardiorrenal: Clasificación, fisiopatología, diagnóstico y tratamiento. Una revisión de las publicaciones médicas. Arch. Cardiol. Mex. 2021, 92, 253–263. [Google Scholar] [CrossRef]
- Roig Minguell, E. Utilidad clínica de los marcadores neurohormonales en la insuficiencia cardíaca. Rev. Esp. Cardiol. 2004, 57, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Seibert, E.; Zohles, K.; Ulrich, C.; Kluttig, A.; Nuding, S.; Kors, J.A.; Swenne, C.A.; Werdan, K.; Fiedler, R.; Girndt, M. Association between autonomic nervous dysfunction and cellular inflammation in end-stage renal disease. BMC Cardiovasc. Disord. 2016, 16, 210. [Google Scholar] [CrossRef]
- Sahin, M.; Kayatas, M.; Urun, Y.; Sennaroglu, E.; Akdur, S. Performing only one cardiovascular reflex test has a high positive predictive value for diagnosing autonomic neuropathy in patients with chronic renal failure on hemodialysis. Ren. Fail. 2006, 28, 383–387. [Google Scholar] [CrossRef]
- Masuo, K.; Lambert, G.W.; Esler, M.D.; Rakugi, H.; Ogihara, T.; Schlaich, M.P. The role of sympathetic nervous activity in renal injury and end-stage renal disease. Hypertens. Res. 2010, 33, 521–528. [Google Scholar] [CrossRef]
- Calvo, C.; Maule, S.; Mecca, F.; Quadri, R.; Martina, G.; Perin, P.C. The influence of autonomic neuropathy on hypotension during hemodialysis. Clin. Auton. Res. 2002, 12, 84–87. [Google Scholar] [CrossRef]
- Rubinger, D.; Backenroth, R.; Sapoznikov, D. Sympathetic nervous system function and dysfunction in chronic hemodialysis patients. Semin. Dial. 2013, 26, 333–343. [Google Scholar] [CrossRef]
- Sztajzel, J. Heart rate variability: A noninvasive electrocardiographic method to measure the autonomic nervous system. Swiss Med. Wkly. 2004, 134, 514–522. [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Hoshi, R.A.; Pastre, C.M.; Vanderlei, L.C.M.; Godoy, M.F. Poincaré plot indexes of heart rate variability: Relationships with other nonlinear variables. Auton. Neurosci. 2013, 177, 271–274. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public. Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, M.; Zheng, Y.; Li, G. Toward Capturing Momentary Changes of Heart Rate Variability by a Dynamic Analysis Method. PLoS ONE 2015, 10, e0133148. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dantas, E.M.; Kemp, A.H.; Andreão, R.V.; da Silva, V.J.D.; Brunoni, A.R.; Hoshi, R.A.; Bensenor, I.M.; Lotufo, P.A.; Ribeiro, A.L.P.; Mill, J.G. Reference values for short-term resting-state heart rate variability in healthy adults: Results from the Brazilian Longitudinal Study of Adult Health-ELSA-Brasil study. Psychophysiology 2018, 55, e13052. [Google Scholar] [CrossRef]
- Zeid, S.; Buch, G.; Velmeden, D.; Söhne, J.; Schulz, A.; Schuch, A.; Tröbs, S.-O.; Heidorn, M.W.; Müller, F.; Strauch, K.; et al. Heart rate variability: Reference values and role for clinical profile and mortality in individuals with heart failure. Clin. Res. Cardiol. 2024, 113, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, Y.; Qiao, B.; Wang, Y.; Zhang, L.; Cui, T.; Fu, P. Heart Rate Variability and Prognosis in Hemodialysis Patients: A Meta-Analysis. Blood Purif. 2021, 50, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Jhen, R.-N.; Wang, P.-C.; Chang, Y.-M.; Kao, J.-L.; Wu, E.C.-H.; Shiao, C.-C. The Clinical Significance and Application of Heart Rate Variability in Dialysis Patients: A Narrative Review. Biomedicines 2024, 12, 1547. [Google Scholar] [CrossRef]
- Chen, S.-C.; Huang, J.-C.; Tsai, Y.-C.; Mai, R.N.H.-C.; Chen, R.N.J.-H.; Kuo, P.-L.; Chang, J.-M.; Hwang, S.-J.; Chen, H.-C. Heart Rate Variability Change Before and After Hemodialysis is Associated with Overall and Cardiovascular Mortality in Hemodialysis. Sci. Rep. 2016, 6, 20597. [Google Scholar] [CrossRef][Green Version]
- Santoro, A.; Mancini, E.; London, G.; Mercadal, L.; Fessy, H.; Perrone, B.; Cagnoli, L.; Grandi, E.; Severi, S.; Cavalcanti, S. Patients with complex arrhythmias during and after haemodialysis suffer from different regimens of potassium removal. Nephrol. Dial. Transplant. 2008, 23, 1415–1421. [Google Scholar] [CrossRef]
- Thio, C.H.M.; van Roon, A.M.; Lefrandt, J.D.; Gansevoort, R.T.; Snieder, H. Heart Rate Variability and Its Relation to Chronic Kidney Disease: Results from the PREVEND Study. Psychosom. Med. 2018, 80, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Rantanen, J.M.; Riahi, S.; Schmidt, E.B.; Johansen, M.B.; Søgaard, P.; Christensen, J.H. Arrhythmias in Patients on Maintenance Dialysis: A Cross-sectional Study. Am. J. Kidney Dis. 2020, 75, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Bozbas, H.; Atar, I.; Yildirir, A.; Ozgul, A.; Uyar, M.; Ozdemir, N.; Muderrisoglu, H.; Ozin, B. Prevalence and predictors of arrhythmia in end stage renal disease patients on hemodialysis. Ren. Fail. 2007, 29, 331–339. [Google Scholar] [CrossRef]
- Dong, Y.; Li, X.; Zheng, W.; Man, Y.; Liu, J.; Yu, P.; Zhang, F.; Yang, B.; Cao, K. Prevalence and heart rate variability characteristics of premature ventricular contractions detected by 24-hour Holter among outpatients with palpitations in China: A cross-sectional study. BMJ Open 2022, 12, e059337. [Google Scholar] [CrossRef]
- Telemetric and ECG Holter Warehouse Project. Available online: https://thew-project.org/index.htm (accessed on 7 December 2024).
- Martinek, R.; Ladrova, M.; Sidikova, M.; Jaros, R.; Behbehani, K.; Kahankova, R.; Kawala-Sterniuk, A. Advanced Bioelectrical Signal Processing Methods: Past, Present and Future Approach—Part I: Cardiac Signals. Sensors 2021, 21, 5186. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.; Soliman, E.Z.; Wu, H.-T. An adaptive QRS detection algorithm for ultra-long-term ECG recordings. J. Electrocardiol. 2020, 60, 165–171. [Google Scholar] [CrossRef]
- Sharmila, V.; Reddy, K.A. Identification of Premature Ventricular Cycles of Electrocardiogram Using Discrete Cosine Transform-Teager Energy Operator Model. J. Med. Eng. 2015, 2015. [Google Scholar] [CrossRef][Green Version]
- Kaya, Y. Classification of PVC Beat in ECG Using Basic Temporal Features. Balk. J. Electr. Comput. Eng. 2018, 6, 78–82. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, T.; Shen, Y.; Xing, Y.; Yan, R.; Li, J.; Liu, C. Robust PVC Identification by Fusing Expert System and Deep Learning. Biosensors 2022, 12, 185. [Google Scholar] [CrossRef]
- Fang, S.-C.; Wu, Y.-L.; Tsai, P.-S. Heart Rate Variability and Risk of All-Cause Death and Cardiovascular Events in Patients With Cardiovascular Disease: A Meta-Analysis of Cohort Studies. Biol. Res. Nurs. 2020, 22, 45–56. [Google Scholar] [CrossRef]
- Mccraty, R.; Shaffer, F. Heart Rate Variability: New Perspectives on Physiological Mechanisms, Assessment of Self-regulatory Capacity, and Health Risk. Glob. Adv. Health Med. 2015, 4, 46–61. [Google Scholar] [CrossRef] [PubMed]
| Index (Unit) | Equation | Clinical Significance | |
|---|---|---|---|
| 1. SDNN (ms) | (1) | Reflects the total variability over the recording period, capturing both sympathetic and parasympathetic modulations. | |
| 2. RMSSD (ms) | (2) | Measures short-term variability associated primarily with parasympathetic activity. Indicates vagal tone in the autonomic nervous system. | |
| 3. LF (ms2) | Power in the [0.04, 0.15] Hz band calculated by spectral analysis (Welch) | Represents sympathetic and parasympathetic modulations, with sympathetic predominance. | |
| 4. HF (ms2) | Power in the [0.15, 0.4] Hz band calculated by spectral analysis (Welch) | Reflects parasympathetic activity and vagal modulation of heart rate. Indicator of vagal tone in the autonomic system. | |
| 5. LF/HF Ratio | (3) | Indicates autonomic balance, where a high value suggests sympathetic predominance and a low value, parasympathetic predominance. | |
| 6. SD1–Poincaré (ms) | (4) | Measures the dispersion perpendicular to the identity line on the Poincaré plot. It represents the short-term variability associated with parasympathetic activity. | |
| 7. SD2–Poincaré (ms) | (5) | Measures the dispersion along the identity line on the Poincaré plot. It reflects both sympathetic and parasympathetic activity, indicating short- and long-term variability. | |
| Algorithm | Description | How | |
|---|---|---|---|
| 1. QRS and R-wave identification | ECG bandpass filtering | (6) | |
| Discrete wavelet decomposition (DWT) using Daubechies 4 | (7) | ||
| QRS signal reconstruction from level 2 | (8) | ||
| Definition of the adaptive threshold | (9) | ||
| Detection of time-restricted QRS spikes | (10) | ||
| 2. PVCs identification | Apply CWT to obtain a matrix of W coefficients with associated f frequencies | (11) | |
| where is the ECG sampling rate. | |||
| Extracting energy in the frequency band of PVCs | (12) | ||
| (13) | |||
| Define adaptive threshold for PVCs identification | (14) | ||
| Detect significant spikes in the PVCs energy series using the criterion of prominence | (t)) | (15) | |
| 3. Tachogram | Calculating RR Intervals | (16) | |
| (17) | |||
| Moving average calculation | (18) | ||
| Calculating the deviation from the moving average | (19) | ||
| Definition of the variability threshold | (20) | ||
| Ectopic beat or artifact detection | (21) | ||
| Ectopic beat or artifact correction | (22) | ||
| ID Px | Premature Ventricular Contractions (PVCs) | Metrics | ||
|---|---|---|---|---|
| PPV (%) | Sensitivity (%) | F-Score | ||
| F1001 | 147 | 90.35 | 94.77 | 0.9151 |
| F1005 | 75 | 90.47 | 70.25 | 0.8869 |
| F1008 | 253 | 93.29 | 72.69 | 0.7936 |
| F1013 | 35 | 84.26 | 87.5 | 0.8350 |
| F1015 | 80 | 67.25 | 76.45 | 0.8540 |
| F1017 | 47 | 96.42 | 84.26 | 0.9184 |
| F1020 | 33 | 96.18 | 76.6 | 0.8869 |
| F1028 | 6 | 92.85 | 69.04 | 0.8781 |
| F1029 | 37 | 72.16 | 44.28 | 0.5573 |
| F1035 | 20 | 100.00 | 92.26 | 0.9687 |
| F1044 | 93 | 69.72 | 100.00 | 0.7819 |
| F1060 | 15 | 94.64 | 57.59 | 0.7692 |
| M1002 | 31 | 85.11 | 62.24 | 0.6371 |
| M1007 | 488 | 52.11 | 89.34 | 0.6378 |
| M1014 | 48 | 98.97 | 92.14 | 0.9580 |
| M1016 | 11 | 75.00 | 40.61 | 0.7484 |
| M1018 | 145 | 65.78 | 92.47 | 0.7412 |
| M1022 | 62 | 96.42 | 93.56 | 0.9758 |
| M1023 | 1970 | 96.17 | 99.89 | 0.9850 |
| M1030 | 63 | 89.28 | 96.42 | 0.9596 |
| M1041 | 37 | 97.14 | 82.47 | 0.9221 |
| M1046 | 4 | 77.77 | 100.00 | 0.9333 |
| M1049 | 56 | 96.42 | 100.00 | 0.9862 |
| M1051 | 23 | 82.14 | 67.97 | 0.8572 |
| Mean ± SD | 85.830 ± 12.88 | 80.95 ± 17.38 | 0.8495 ± 0.1193 | |
| Women Population | ||||||||
|---|---|---|---|---|---|---|---|---|
| HRV Indices | ||||||||
| Block No. | SDNN [ms] | RMSSD [ms] | LF [ms2] | HF [ms2] | LF/HF | SD1 [ms] | SD2 [ms] | |
| Class 1 Dynamic HRV Vector | B1 | 29.45 | 23.04 | 627.58 | 343.39 | 2.35 | 16.32 | 38.20 |
| B2 | 27.60 | 21.22 | 698.32 | 311.87 | 2.42 | 15.03 | 35.92 | |
| B3 | 42.97 | 24.31 | 1603.61 | 292.66 | 2.81 | 17.22 | 57.93 | |
| B4 | 39.90 | 21.16 | 604.80 | 239.53 | 2.69 | 14.98 | 54.01 | |
| B5 | 30.43 | 20.90 | 393.15 | 226.61 | 2.54 | 14.80 | 40.20 | |
| B6 | 23.19 | 23.04 | 600.36 | 271.94 | 2.48 | 16.32 | 28.12 | |
| B7 | 30.13 | 19.64 | 706.79 | 268.65 | 2.47 | 13.91 | 40.21 | |
| B8 | 29.81 | 21.02 | 798.40 | 282.22 | 2.49 | 14.89 | 39.04 | |
| B9 | 52.01 | 27.87 | 2580.33 | 468.62 | 2.78 | 19.74 | 70.28 | |
| B10 | 25.96 | 24.20 | 1124.93 | 364.36 | 2.76 | 17.14 | 32.28 | |
| B11 | 38.10 | 25.61 | 1490.94 | 336.91 | 2.81 | 18.14 | 50.09 | |
| B12 | 32.13 | 24.24 | 1310.01 | 292.10 | 2.83 | 17.17 | 41.65 | |
| B13 | 25.07 | 24.84 | 1092.00 | 454.44 | 2.67 | 17.59 | 30.46 | |
| B14 | 28.42 | 26.63 | 729.23 | 454.59 | 2.57 | 18.86 | 34.85 | |
| Mean ± SD | 32.51 ± 7.98 | 23.41 ± 2.41 | 1025.75 ± 576.96 | 329.13 ± 79.94 | 2.62 ± 0.16 | 16.58 ± 1.71 | 42.37 ± 11.77 | |
| Class 2 Dynamic HRV Vector | B1 | 30.40 | 16.92 | 313.88 | 162.56 | 2.11 | 11.98 | 41.28 |
| B2 | 28.71 | 17.01 | 281.50 | 173.61 | 2.00 | 12.05 | 38.58 | |
| B3 | 23.68 | 15.12 | 242.87 | 144.94 | 1.96 | 10.70 | 31.67 | |
| B4 | 25.31 | 14.92 | 240.96 | 152.87 | 1.93 | 10.56 | 34.06 | |
| B5 | 28.21 | 14.74 | 261.92 | 151.40 | 1.96 | 10.44 | 38.44 | |
| B6 | 31.32 | 15.82 | 275.41 | 150.09 | 1.98 | 11.20 | 42.78 | |
| B7 | 28.75 | 16.37 | 274.45 | 181.13 | 1.98 | 11.59 | 38.91 | |
| B8 | 29.69 | 15.53 | 251.05 | 166.26 | 1.98 | 10.99 | 40.21 | |
| B9 | 32.92 | 18.39 | 296.61 | 184.88 | 1.99 | 13.02 | 44.40 | |
| B10 | 31.23 | 16.07 | 246.67 | 151.71 | 1.99 | 11.38 | 42.58 | |
| B11 | 36.10 | 23.43 | 555.10 | 269.60 | 1.89 | 16.59 | 48.25 | |
| B12 | 34.79 | 17.50 | 307.41 | 174.26 | 1.91 | 12.39 | 47.18 | |
| B13 | 37.50 | 12.99 | 238.72 | 95.22 | 1.92 | 9.19 | 52.11 | |
| B14 | 19.97 | 10.28 | 210.19 | 77.07 | 1.92 | 7.28 | 27.24 | |
| Mean ± SD | 29.90 ± 4.78 | 16.08 ± 2.92 | 285.48 ± 82.85 | 159.68 ± 43.94 | 1.97 ± 0.05 | 11.38 ± 2.07 | 40.55 ± 6.63 | |
| Class 3 Dynamic HRV Vector | B1 | 26.73 | 19.44 | 425.43 | 228.74 | 2.07 | 13.77 | 34.85 |
| B2 | 17.76 | 15.27 | 296.36 | 170.87 | 2.06 | 10.81 | 22.54 | |
| B3 | 18.96 | 16.73 | 256.74 | 213.94 | 2.00 | 11.85 | 23.63 | |
| B4 | 19.85 | 17.49 | 275.65 | 237.45 | 1.97 | 12.39 | 24.60 | |
| B5 | 18.16 | 15.43 | 291.03 | 190.46 | 2.01 | 10.93 | 22.73 | |
| B6 | 22.46 | 16.92 | 305.79 | 202.87 | 1.99 | 11.98 | 28.68 | |
| B7 | 22.66 | 18.11 | 305.87 | 219.33 | 2.00 | 12.82 | 28.78 | |
| B8 | 25.80 | 16.92 | 345.37 | 155.74 | 2.04 | 11.98 | 34.05 | |
| B9 | 18.58 | 16.29 | 284.12 | 151.85 | 2.03 | 11.53 | 23.32 | |
| B10 | 23.23 | 17.98 | 329.73 | 187.78 | 2.06 | 12.73 | 29.86 | |
| B11 | 18.11 | 17.92 | 277.80 | 195.70 | 2.05 | 12.69 | 21.78 | |
| B12 | 20.50 | 17.73 | 270.71 | 193.49 | 2.04 | 12.56 | 25.47 | |
| B13 | 21.17 | 14.10 | 302.98 | 132.31 | 2.06 | 9.99 | 27.89 | |
| B14 | 23.05 | 15.90 | 285.38 | 144.06 | 2.06 | 11.26 | 30.48 | |
| Mean ± SD | 21.22 ± 2.88 | 16.87 ± 1.39 | 303.78 ± 41.99 | 187.47 ± 35.52 | 2.03 ± 0.03 | 11.95 ± 0.99 | 27.05 ± 4.27 | |
| Normality Test (Shapiro-Wilk) | p = 0.024 | p = 0.178 | p < 0.001 | p < 0.020 | p < 0.063 | p = 0.179 | p = 0.030 | |
| Significance | *1 p < 0.001 | *2 p < 0.001 | *1 p < 0.001 | *1 p < 0.001 | *1 p < 0.001 | *2 p < 0.001 | *1 p < 0.001 | |
| Men Population | ||||||||
|---|---|---|---|---|---|---|---|---|
| HRV Indices | ||||||||
| Block No. | SDNN [ms] | RMSSD [ms] | LF [ms2] | HF [ms2] | LF/HF | SD1 [ms] | SD2 [ms] | |
| Class 1 Dynamic HRV Vector | B1 | 50.32 | 17.08 | 566.79 | 138.56 | 4.66 | 12.09 | 69.95 |
| B2 | 68.07 | 21.77 | 741.29 | 196.78 | 4.81 | 15.41 | 94.72 | |
| B3 | 83.41 | 23.93 | 958.38 | 246.35 | 5.15 | 16.94 | 116.51 | |
| B4 | 40.06 | 23.45 | 355.70 | 181.69 | 4.45 | 16.60 | 53.76 | |
| B5 | 45.98 | 30.42 | 462.95 | 236.18 | 4.18 | 21.54 | 59.71 | |
| B6 | 51.76 | 31.00 | 607.10 | 266.20 | 4.05 | 21.94 | 67.98 | |
| B7 | 67.28 | 35.76 | 441.87 | 229.01 | 3.98 | 25.31 | 88.89 | |
| B8 | 111.09 | 44.95 | 948.73 | 433.45 | 3.30 | 31.82 | 151.31 | |
| B9 | 39.88 | 31.38 | 371.28 | 190.20 | 3.23 | 22.21 | 50.58 | |
| B10 | 70.66 | 38.68 | 517.53 | 283.20 | 3.14 | 27.38 | 93.01 | |
| B11 | 87.64 | 39.29 | 761.10 | 292.68 | 3.19 | 27.82 | 117.92 | |
| B12 | 40.10 | 27.66 | 314.23 | 155.67 | 3.16 | 19.59 | 52.32 | |
| B13 | 28.09 | 18.88 | 245.38 | 125.41 | 3.14 | 13.37 | 36.88 | |
| B14 | 27.45 | 20.26 | 290.06 | 115.85 | 3.13 | 14.34 | 35.08 | |
| Mean ± SD | 57.98 ± 24.39 | 28.89 ± 8.52 | 541.60 ± 234.59 | 220.80 ± 83.88 | 3.83 ± 0.73 | 20.45 ± 6.03 | 77.76 ± 34.09 | |
| Class 2 Dynamic HRV Vector | B1 | 25.17 | 14.22 | 188.71 | 144.91 | 1.82 | 10.07 | 33.49 |
| B2 | 24.28 | 15.95 | 203.44 | 166.59 | 1.78 | 11.29 | 31.75 | |
| B3 | 28.59 | 17.75 | 277.37 | 214.57 | 1.82 | 12.56 | 37.61 | |
| B4 | 32.75 | 18.07 | 284.24 | 197.87 | 1.90 | 12.80 | 43.90 | |
| B5 | 19.56 | 14.30 | 202.92 | 149.26 | 1.88 | 10.12 | 25.48 | |
| B6 | 16.48 | 13.17 | 186.48 | 138.32 | 1.89 | 9.32 | 21.03 | |
| B7 | 21.50 | 15.86 | 178.73 | 162.09 | 1.87 | 11.23 | 27.38 | |
| B8 | 26.74 | 16.16 | 265.28 | 171.43 | 1.88 | 11.44 | 35.42 | |
| B9 | 24.70 | 20.22 | 229.44 | 223.60 | 1.86 | 14.32 | 30.84 | |
| B10 | 29.65 | 25.40 | 522.71 | 289.68 | 1.94 | 17.98 | 37.07 | |
| B11 | 23.20 | 20.55 | 401.32 | 202.06 | 1.98 | 14.55 | 28.74 | |
| B12 | 21.22 | 17.93 | 244.24 | 190.13 | 1.97 | 12.70 | 26.52 | |
| B13 | 17.85 | 17.34 | 194.44 | 156.64 | 1.96 | 12.27 | 21.54 | |
| B14 | 19.23 | 15.03 | 191.63 | 140.20 | 1.96 | 10.64 | 24.08 | |
| Mean ± SD | 23.64 ± 4.71 | 17.28 ± 3.18 | 255.07 ± 97.38 | 181.95 ± 41.69 | 1.89 ± 0.06 | 12.24 ± 2.25 | 30.35 ± 6.64 | |
| Class 3 Dynamic HRV Vector | B1 | 23.79 | 9.50 | 211.03 | 106.75 | 2.51 | 6.73 | 32.86 |
| B2 | 29.73 | 11.86 | 275.04 | 92.48 | 2.68 | 8.40 | 40.54 | |
| B3 | 17.41 | 10.76 | 294.71 | 107.43 | 2.73 | 7.62 | 22.91 | |
| B4 | 29.46 | 10.14 | 205.84 | 91.91 | 2.70 | 7.18 | 40.99 | |
| B5 | 30.14 | 7.38 | 182.30 | 89.58 | 2.69 | 5.22 | 42.29 | |
| B6 | 21.69 | 6.27 | 208.63 | 100.85 | 2.65 | 4.44 | 30.25 | |
| B7 | 24.94 | 9.91 | 325.07 | 108.24 | 2.63 | 7.02 | 34.48 | |
| B8 | 32.71 | 13.45 | 372.85 | 156.08 | 2.41 | 9.52 | 44.66 | |
| B9 | 23.95 | 10.72 | 262.34 | 106.09 | 2.44 | 7.59 | 32.80 | |
| B10 | 25.93 | 10.69 | 261.51 | 114.70 | 2.42 | 7.57 | 35.86 | |
| B11 | 27.45 | 11.29 | 319.60 | 113.29 | 2.43 | 8.00 | 37.95 | |
| B12 | 16.15 | 7.29 | 299.50 | 96.27 | 2.44 | 5.17 | 22.17 | |
| B13 | 29.60 | 8.77 | 235.22 | 118.28 | 2.41 | 6.21 | 41.25 | |
| B14 | 14.84 | 6.31 | 176.90 | 100.85 | 2.40 | 4.47 | 20.46 | |
| Mean ± SD | 24.84 ± 5.61 | 9.60 ± 2.15 | 259.32 ± 58.86 | 107.34 ± 16.57 | 2.54 ± 0.13 | 6.80 ± 1.52 | 34.25 ± 7.88 | |
| Normality Test * Shapiro-Wilk ** Levene’s Test | * p = 0.001 | ** p < 0.001 | * p < 0.004 | * p < 0.001 | * p < 0.001 | ** p < 0.001 | * p < 0.001 | |
| Significance | *1 p < 0.001 | *1 p = 0.001 | *1 p = 0.002 | *1 p < 0.001 | *1 p < 0.001 | *1 p < 0.001 | *1 p < 0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega-Martínez, G.; Ramos-Becerril, F.J.; Gutiérrez-Martínez, J.; Vera-Hernández, A.; Alvarado-Serrano, C.; Leija-Salas, L. Dynamic Heart Rate Variability Vector and Premature Ventricular Contractions Patterns in Adult Hemodialysis Patients: A 48 h Risk Exploration. Appl. Sci. 2025, 15, 5122. https://doi.org/10.3390/app15095122
Vega-Martínez G, Ramos-Becerril FJ, Gutiérrez-Martínez J, Vera-Hernández A, Alvarado-Serrano C, Leija-Salas L. Dynamic Heart Rate Variability Vector and Premature Ventricular Contractions Patterns in Adult Hemodialysis Patients: A 48 h Risk Exploration. Applied Sciences. 2025; 15(9):5122. https://doi.org/10.3390/app15095122
Chicago/Turabian StyleVega-Martínez, Gabriel, Francisco José Ramos-Becerril, Josefina Gutiérrez-Martínez, Arturo Vera-Hernández, Carlos Alvarado-Serrano, and Lorenzo Leija-Salas. 2025. "Dynamic Heart Rate Variability Vector and Premature Ventricular Contractions Patterns in Adult Hemodialysis Patients: A 48 h Risk Exploration" Applied Sciences 15, no. 9: 5122. https://doi.org/10.3390/app15095122
APA StyleVega-Martínez, G., Ramos-Becerril, F. J., Gutiérrez-Martínez, J., Vera-Hernández, A., Alvarado-Serrano, C., & Leija-Salas, L. (2025). Dynamic Heart Rate Variability Vector and Premature Ventricular Contractions Patterns in Adult Hemodialysis Patients: A 48 h Risk Exploration. Applied Sciences, 15(9), 5122. https://doi.org/10.3390/app15095122







