Abstract
The environmental hazards caused by the massive generation and improper disposal of industrial solid wastes (e.g., high calcium desulphurization ash, HCDA) and the growing safety risks posed by the increasing number of underground mine goafs generated by mining activities have become serious environmental and geotechnical challenges. To address the dual issues, this study develops a novel desulfurization ash–slag-based paste backfill (DSPB) material using HCDA and granulated blast furnace slag (GBFS) as primary constituents. The effects of cementitious material ratios, polycarboxylate superplasticizer (PCE), and sodium silicate (SS) on rheological properties of DSPB were investigated through a shear rheology experiment and fitting rheological model to assess the flow conditions in pipeline transportation. In addition, the mechanism was investigated through microanalysis. The results showed that with the decrease in desulfurization ash-to-slag ratio, the initial yield stress and plastic viscosity decreased by up to 88% and 34.9%, respectively; PCE via “card house” structural effects made the rheological parameters increase and then decrease, and a dosage of more than 1.2% significantly improved the rheological properties; and SS initially reduced the rheological parameters, but excessive doping (greater than 1.0%) led to an increase. These findings establish the relationship between DSPB composition and rheological properties, provide a practical solution for waste resource utilization and surface stabilization, and provide a scientific basis for the microstructure–rheology relationship of cementitious systems.
1. Introduction
With the rapid development of industry and urbanization in China, the problem of massive generation of solid waste has become increasingly prominent [,,]. Desulfurization ash is a solid waste produced by energy companies such as thermal power plants in order to reduce the air pollution caused by the emission of sulfur oxides and solidify harmful gases using flue gas desulfurization (FGD) technology [,]. China is the largest producer of desulfurization ash in the world, with an annual increment of about 2 million tons [], and its massive accumulation not only occupies land resources but also pollutes groundwater, soil, and the atmosphere, thus threatening human health [,]. Therefore, exploring methods of resource utilization of solid wastes such as desulfurization ash is of great significance to alleviate the environmental pressure and achieve sustainable development.
Desulfurization ash usually contains CaSO4 and CaSO3, as well as free calcium oxide (f-CaO) and partially unreacted Ca(OH)2, with a high calcium content and high alkalinity. Many experimental studies have been devoted to the development of desulfurization ash products for diverse applications in different fields [,,,,]. For example, based on the characteristics of wide sources of FGD ash, low energy consumption, and no waste residue or waste water, FGD ash is used as auxiliary admixtures for the production of building materials, cement blending materials, composite wall materials, and road base materials [,,,]. Li et al. [] and Wei et al. [] developed a highly efficient method for the co-disposal of multiple hazardous solid wastes by applying FGD ash in combination with red mud. Feng et al. [] used desulfurization ash to prepare highly efficient and environmentally friendly cement-based grouting fireproofing materials. In summary, the application of desulfurization ash in cementitious materials has good energy-saving and environmental protection properties. However, desulfurization ash has a very low content of reactive components such as SiO2 and Al2O3 and lacks gelling activity, which can hinder the generation of C-S-H gel and ettringite [,]. In addition, when desulfurization ash reacts with water, Ca(OH)2 is generated from f-CaO, CaSO3 is generated from CaSO3·0.5H2O, and ettringite is generated, all of which lead to volume expansion and fracture of the material, reducing stability and durability, and thus limiting the use in engineering applications [,]. Therefore, previous studies usually focus on the use of FGD ash as an additive to cementitious materials, but the utilization rate is limited and not sufficient to absorb the large amount of solid waste. Fewer existing studies have addressed the large-scale utilization of desulfurization ash, such as mine backfilling [].
Mine backfilling can not only dispose of a large amount of solid waste but also effectively control surface subsidence, reduce damage to the ecological environment, and take into account resource utilization and economic benefits [,,,,]. The selection of cementitious materials in backfill materials is a decisive factor affecting the backfill effect and cost. Currently, mines usually prepare backfill materials by mixing solid wastes as aggregates with binders such as ordinary Portland cement (OPC) [] or by using reactive solid wastes as admixtures to minimize the amount of binder [,]. The use of OPC leads to an increase in filling costs, and the production process is energy intensive, with high CO2 emissions []. Therefore, the development of a low-cost, high-performance, and green low-carbon all-solid waste mine backfill cementitious material to replace OPC has become a key focus of current researchers []. In this study, high calcium desulfurization ash (HCDA) is used as the calcium raw material in the cementitious material and contains unreacted calcium hydroxide, which can be partially used as an alkali activator to composite stimulate the potential activity of the substrate. Therefore, the utilization of HCDA with granulated blast furnace slag (GBFS) to develop desulfurization ash–slag-based paste backfill (DSPB) materials can effectively reduce costs and lead to the resource utilization of solid waste.
Fresh paste backfill (PB) is usually prepared in a backfill plant at the surface and then transported by gravity or pumping through pipelines into underground excavated goaf areas [,]. The strength of backfill material is an important indicator of its ability to effectively support the structure of the goaf area, while the flow characteristics are a key factor in determining whether the backfill material can be successfully pumped to the working face through pipeline transportation and be constructed properly []. The flowability of PB is mainly related to its yield stress and viscosity (i.e., rheological properties) []. As a non-Newtonian fluid, the complex rheological properties of freshly mixed DSPB slurry directly affect its transportation in the pipeline. Therefore, it is extremely important to study the rheological properties of DSPB, which is of great significance for the safe and effective implementation of backfill operations [].
In response to the above problems, many scholars have made remarkable progress in the study of rheological properties of PB materials. M. Fall et al. [] used a slump test to determine the rheological properties of cement paste backfill (CPB) and to study the effect of their fine particle content on the rheological and mechanical properties of the paste. Z. Guo et al. [] investigated the effect of superplasticizer on rheological properties of superfine-tailing CPB by shear rheology tests. The above studies have shown that slump and rheological tests are important methods for studying the rheological properties of slurries. Common methods for controlling the rheological properties of slurries include adjusting the water–solid ratio, the proportion of material components, and the external environment (e.g., temperature, humidity, and pH, etc.), and adding admixtures []. DSPB, as a high-solid-content-dispersed solid–liquid flow system, is susceptible to particle precipitation, and the bias seepage phenomenon when the content of water is too high, on the contrary, will lead to a lack of fluidity. Therefore, in order to achieve better backfill results, the most effective means of improving the rheological properties was tested by rheological characterization methods.
While existing studies have used solid wastes such as desulfurization ash as mine backfill cementitious materials, their application in paste backfill is still limited due to insufficient understanding of the effect of multi-mixture coupling on rheological properties and their changes. Current backfill rheology studies mainly focus on the rheological model and parameters of slurry fitting and macroscopic fluidity, and lack an analysis of the mechanism of system rheological property–microstructure changes.
Therefore, unlike traditional methods for rheological characterization of backfill pastes, this study investigated the effects of HCDA, polycarboxylate superplasticizer (PCE), and sodium silicate (SS) content on the rheological properties of DSPB. The flow curves were fitted by a shear rheology test, and its characteristics were analyzed by rheological parameters. In addition, the workability of DSPB under different influencing factors was analyzed by measuring the setting time and conducting mini-slump tests. The effect of free water transition within the slurry system on the rheological properties of DSPB was investigated by low-field nuclear magnetic resonance (LF-NMR). Finally, the effect mechanisms of the differences were revealed by microanalysis.
2. Materials and Methods
2.1. Raw Materials
In this study, DSPB was prepared using GBFS as the cementitious material, HCDA as the activator and cured component, SS as the activator, and tap water as the mixing water. The HCDA used in the experiment was provided by Hunan Zhuzhou Glass Factory, with the appearance of yellowish-white powder and a BET specific surface area of 4.830 m2/g. The GBFS used was from Hunan Xiangtan Iron and Steel Group, with the appearance of grayish-white powder and a BET specific surface area of 0.958 m2/g. The activators were Na2O⋅9SiO2 particles (AR) with a purity of >99% and an SiO2/Na2O molar ratio of 3.37. PCE powder was used as a water-reducing agent, with a water reduction of 25%. The chemical composition of the raw material was determined by X-ray fluorescence spectroscopy (XRF) analysis, as shown in Table 1. Figure 1 shows the analytical results of the X-ray diffraction (XRD) test of the raw material.

Table 1.
Chemical composition of raw materials.
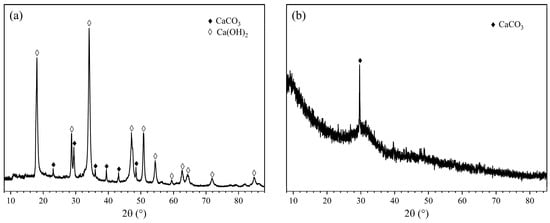
Figure 1.
XRD patterns of raw materials. (a) HCDA; (b) GBFS.
Based on the activity analysis, the sum of active CaO and MgO content in the GBFS was 51.930%, and the alkalinity coefficient was 1.58, whose value is greater than 1, indicating that the GBFS belonged to alkaline slag, and the cementing performance was better than that of acidic slag. Its quality coefficient was 2.25, with the value reflecting the ratio between the active components and the low active and inactive components in the slag. The larger the value, the higher the activity, so GBFS had high hydration activity []. The HCDA contained 85.231% CaO and had a high pH value, which is alkaline.
2.2. Specimen Preparation and Mixing Procedure
The mix proportions of the prepared DSPB are listed in Table 2. The flowchart of the sample preparation and test method is shown in Figure 2. The mix design focuses on the effects of the proportions of HCDA and GBFS, and the dosage of PCE and the alkali activator SS. Samples with an HCDA and GBFS mass ratio of 90:10, PCE doping of 0.6%, and SS doping of 1.0% were selected as the reference group (Control). The selection of these experimental parameters was based on the results of the original pre-tests and the needs of practical applications in mining enterprises. All the excitants required for the experimental design were dissolved in water in advance to prepare a homogeneous alkaline solution. Before preparing the slurry, the mixing pot and blades were wiped with a damp cloth. First, the prepared SS solution, PCE, and water were poured into the mixing pot, then desulfurization ash and slag were added together and mixed well. The liquid–solid ratio of all the mixes was studied at a design value of 0.5. The paste was first stirred at low speed for 120 s, then the stirring was stopped and the paste was scraped from the paddles and walls into the pot during a rest period of 30 s. Finally, the paste was stirred at high speed for 120 s and at low speed for 60 s. All the samples were prepared under the same conditions of room temperature (about 23 °C).

Table 2.
Component ratios of DSPB mixture preparation.
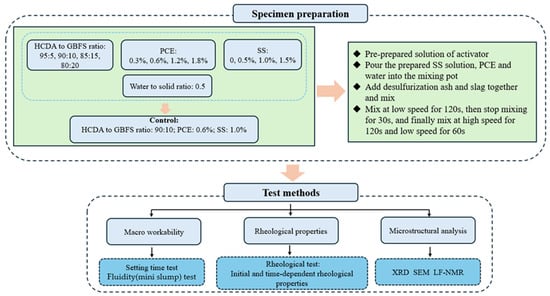
Figure 2.
Experimental flow chart.
2.3. Test Methods
2.3.1. Setting Time Test
Knowing the setting time is very important for the efficiency and economic benefits of backfilling operations. Therefore, the initial and final setting times of the DSPB samples were determined using a Vicat apparatus according to the Chinese standard GB/T 1346-2011 []. The prepared fresh slurries were placed in truncated-top conical molds, and all samples were cured in a curing apparatus at a certain temperature (23 °C) and humidity (RH ≥ 90%) to test the initial and final setting times.
2.3.2. Rheological Test
The test equipment was an Anton Paar MCR 301 rheometer. During the tests, the temperature was fixed at 23 °C to limit the number of variables in the experiment. The rheometer was used to characterize the rheological behavior of DSPB by determining the yield stress, plastic viscosity, and flow index. A series of upward and downward segments were applied to obtain the flow curve of the slurry, as shown in Figure 3 []. The test program consisted of a pre-shear of 90 s at 100 s−1 to ensure repeatability of the test. This was followed by a string of shear cycles implemented by increasing from 0 to 10 s−1 in 45 s and from 10 to 100 s−1 in another 45 s and then down ramps in the same time. This cycle was repeated 6 times within 30 min, with each test repeated 3 times [].
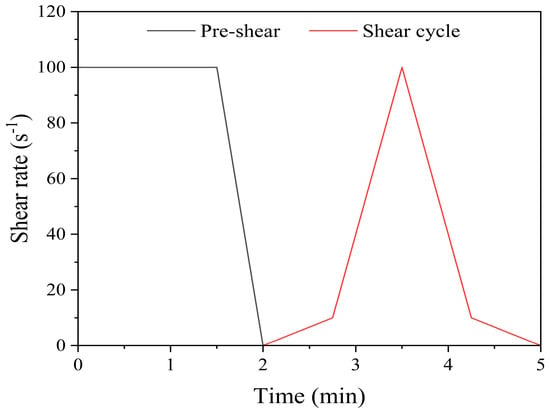
Figure 3.
Dynamic rheology test program.
2.3.3. Fluidity Test (Mini Slump)
According to the Chinese standard GB/T 8077-2012 [], the fluidity of DSPB slurry can be determined by a mini slump test. The test was conducted using a truncated-top conical mold with a height of 60 mm and upper and lower diameters of 36 and 60 mm, respectively. The prepared slurry was poured into the container, then lifted vertically and emptied to allow the slurry to flow freely on the table. After 30 s, the maximum diameters of the two vertical directions on the flow table were measured, and the average value was taken as the fluidity of the slurry. The fluidity of the DSPB samples was determined after 5 min, 10 min, 15 min, and 30 min of hydration, corresponding to the 1st, 2nd, 3rd, and 6th shear rheology cycles, and 60 min, respectively.
2.3.4. Microstructure Experiment
- XRD measurement
The hydration was stopped by placing about 5 g of sample into 100 mL of anhydrous ethanol and replaced with an equal amount of ethanol after 10 min and 24 h. The samples were removed after 2 d and dried in a vacuum oven at 40 °C to avoid further hydration. The dried samples were ground into powder and passed through a 300-mesh sieve. The XRD spectra of the samples were measured using a Rigaku Smart Lab SE diffractometer. The XRD scanning range was set from 5° to 90°, with a scanning step width of 0.02° and a scanning speed of 2°/min.
- 2.
- Scanning electronic microscopy(SEM) measurement
The hydrated samples were dried as described above. SEM images of the dried samples were visualized by ultra-deep-field 3D microscopy (TESCAN MIRA LMS, Brno, Czech Republic) with an accelerating voltage of 20 kV.
- 3.
- LF-NMR experiment
LF-NMR relaxometry is a promising powerful and effective nondestructive method for measuring microstructural features of cement-based materials []. NMR spectra of fresh DSPB slurry were measured at different times using a Niumag Low-Frequency NMR Analyzer to determine the transverse relaxation time (T2) for the samples. In the LF-NMR experiment, 20 ± 0.01 g of the paste was sealed in a sample vial, and the weight of the sealed vial was measured before and after the experiment to confirm that no water had evaporated from the paste sample []. The Carr–Purcell–Meiboom–Gill (CPMG) pulse sequence was used for the experiments, with a resonance frequency of 21 MHz and a sampling frequency of 100 kHz, and the temperature of the inner bore of the magnet was set to 25 °C. For each CPMG sequence, 2000 echoes were acquired, with an echo time of 0.105 ms and a total relaxation delay of 210 ms []. The NMR signals were converted to T2 distributions by inverse Laplace transform (ILT). The distributions of T2 at 15, 30, 45, and 60 min after mixing were measured by using different DSPB samples according to the set program.
3. Results and Discussion
3.1. Setting Time
Figure 4 shows the measurements of initial and final setting times for different DSPB slurries. Figure 4a shows that both the initial and the final setting times of the slurries have a tendency to increase with a decreasing HCDA ratio. This may be attributed to high levels of f-CaO and SO3 in HCDA, leading to changes in condensation time. The f-CaO reacted with free water to produce Ca(OH)2, which made the pH value increase, which also favored the solubilization of reactive silica and aluminum in the GBFS and accelerated the generation of AFt and C-S-H gels, thereby shortening the coagulation time []. On the contrary, Ca2+ dissolved to quickly form Ca(OH)2 micelles and attract negatively charged HCDA particles, thus shortening the coagulation time. The high water demand of HCDA was another factor contributing to the shortening of the setting time []. The formation of hydration products and the consumption of free water were the main reasons for the shortening of the condensation time.
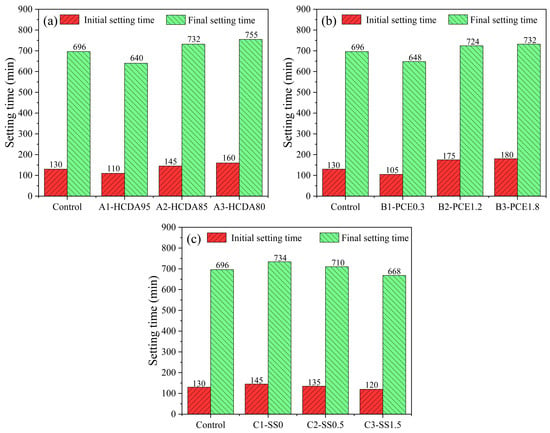
Figure 4.
Initial and final setting times of DSPB samples with different (a) HCDA-to-GBFS ratio; (b) PCE additions; (c) SS additions.
From Figure 4b, it can be seen that the setting time was prolonged with the increase in PCE dosage. PCE molecules adsorbed on the surface of HCDA and GBFS particles prevented f-CaO from reacting with water, thus preventing Ca(OH)2 from further reacting with other reactive substances, which had a certain inhibiting effect on the hydration of slag, which prolonged the setting time macroscopically. The results of other studies have also shown that the setting time of pastes always increases as the PCE dosage increases, due to the fact that PCE affects the dissolution of slag particles [,].
The results in Figure 4c show that the setting time gradually decreased as the SS content increased from 0. This was due to the fact that the increase in alkali activator accelerated the solution of Si and Al, releasing large quantities of AlO4 and SiO4 tetrahedras, which promoted the generation of gel and thus shortened the setting time. The findings of other scholars are similar to those of the present study and could support this interpretation well [,].
The initial setting time of DSPB samples was basically more than 2 h, and the final setting time was about 12 h, which indicates that the slurry would not be set too early during the transportation of the pipeline, leading to the phenomenon of a blocking pipe, and could also be set as soon as possible after the pouring to obtain early strength so that the construction can be continued.
3.2. Rheological Property Analysis
Rheological properties are one of the fundamental characteristics of materials, which largely influence the transportability and pourability of fresh slurries and can reflect the intrinsic connection between the macro-fluidity of a slurry and its microstructure. The operating features of fresh slurries can be characterized by rheological parameters like yield stress, plastic viscosity, and flow index [,]. Shear rate versus shear stress curves fitted according to a mathematical rheological model can represent the rheological behavior of the slurry.
3.2.1. Rheology Model Fitting and Initial Rheological Parameters
Figure 5a shows the flow curves of the shear stress versus shear rate for Control group samples. The shear stress of the sample increased with the increase in shear rate in a range of 0 to 100 s−1 under different conditions, and the curve had a clear trend of an increasing slope at a high shear rate from 80 to 100 s−1, which indicates that the slurry was a typical shear-thickening fluid. Moreover, the relationship curves between shear stress and shear rate in the descending slope section of the DSPB slurry fit the Herschel–Bulkley model well, indicating that the DSPB was a non-Newtonian fluid with yield stress. Because the DSPB exhibited distinct shear-thickening behavior with measurable yield stress, this aligns with the H–B model’s capacity to describe both yield stress and power law behavior simultaneously. The correlation coefficients (R2) of all the fitted results were >0.99, with an R2 of 0.996 and 0.998.
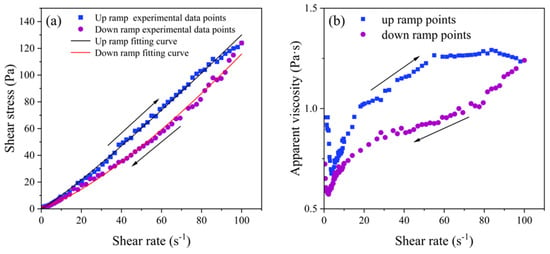
Figure 5.
(a) Shear cycle of the Control group fitted with the Herschel–Bulkley model; (b) viscosity profile of the Control group.
The dependence of viscosity on shear rate is also another rheological property of non-Newtonian fluids. Apparent viscosity is the ratio of shear stress and shear rate acting on the fluid, reflecting the internal structure of the sample to resist deformation and impede the flow characteristics. The smaller the value, the easier the flow. The shear-thickening characteristics of the DSPB slurry were more significantly characterized under the viscosity curve.
Figure 5b shows the relationship between the apparent viscosity of the Control and the shear rate, from which it can be seen that there was a period of rapid decrease in the apparent viscosity of the sample in the shear rate from 0 to 5 s−1, while after the minimum viscosity, the slope of the viscosity curve increased continuously with further increase in the shear rate and then gradually leveled off, thus showing the shear-thickening behavior. This was due to the high solid content of the slurry and the tendency of the HCDA particles to aggregate into clusters, forming relatively large and compact polymer particles. The limited free water in the slurry could not completely wrap around the larger polymer particles, and its surface did not form a complete bonded water film, so at high shear rates, there was no water film lubrication of polymer particles colliding with each other more frequently, resulting in greater frictional resistance.
The Herschel–Bulkley model is shown in Equation (1):
where τy is the yield stress (Pa), K is the consistency coefficient (Pa·sn), and n is the flow index, which is for characterizing the non-linear relationship between shear stress and shear rate []. When n = 1 for the Bingham model, it is a Newtonian fluid; when n > 1, it is a shear-thickening fluid; and when n < 1, it is a shear-thinning fluid.
However, the H–B model cannot provide a direct formula for another important rheological parameter, plastic viscosity μp. de Larrard F. derived empirical formulas such as Equation (2) through experimental studies [].
where is the maximum shear rate during rheological testing.
Parameters such as the yield stress, consistency coefficient, and flow index of the slurry were determined from the descent curves of each hysteresis cycle and obtained by fitting the Herschel–Bulkley model, and then the plastic viscosity was calculated according to Equation (2). The results are shown in Table 3 and Figure 6.

Table 3.
Fitting results of initial rheological parameters of DSPB.
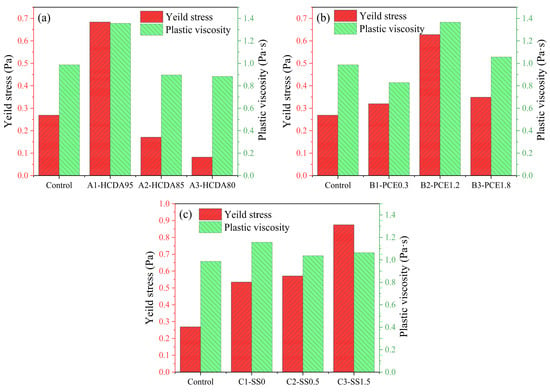
Figure 6.
Initial rheological parameters of DSPB slurry with different dosages of (a) HCDA, (b) PCE, and (c) SS.
Table 3 and Figure 6a show that when the content of HDCA decreased, yield stress and plastic viscosity gradually decreased by up to 88% and 34.9%, respectively. This indicates that HCDA increased the yield stress of fresh DSPB. Therefore, the ability of the slurry to resist deformation gradually increased when the shear stress was lower than a certain value [,]. This may be because, in the case of higher HCDA mass concentration, the particles absorb water to form clusters and connect with each other to form a network structure, which leads to difficult mixing and the mixture not flowing easily, and as the proportion of GBFS increases, GBFS particles will destroy the flocculation structure formed by the desulfurization ash [].
Figure 6b shows that as the PCE dosage increased from 0.3% to 1.8%, both the yield stress and the plastic viscosity increased gradually at first, reaching a maximum at the B2-PCE1.2 group, and then decreased. Some studies also show that at low dosages of PCE, the yield stress of the paste could be even higher than that of the unadded samples [,]. When PCE dosage is small, part of the “card house” structure formed by HCDA in the slurry is linked by PCE molecules, which leads to a more stable structure, manifested by a higher plastic viscosity and yield stress. Meanwhile, after the destruction of the “card house” structure, the solid particles are susceptible to the adsorption of PCE linkage, resulting in its plastic viscosity and yield stress with the increase in dosage. When the dosage is high enough, the linking effect of PCE molecules is weakened and adsorbed on the particle surface to produce repulsive force, exacerbating the Brownian diffusion motion of solid particles, which disperses particles to release more free water, which greatly enhances the rheological properties [].
By observing Figure 6c, it can be seen that when no activator was added, the yield stress and plastic viscosity were higher at that time. On the other hand, when SS doping was 0.5%, the yield stress was significantly reduced. This was due to the plasticizing effect: oligomers adsorbed on the HCDA and GBFS particle surface, thus leading to inadequately dissolved Ca2+ in the solution and higher repulsive forces in the bilayer, while yield stress gradually increased with the increase in SS doping and reached the maximum value. The reason is that as the doping increases, the silicate oligomer content increases, as well as the pH value, which means that it is able to dissolve the slag quickly, so the mixture has a higher content of both Ca2+ and silicate oligomers, enhancing attraction and flocculation []. The amount of the initial C-(A)-S-H gel generated also increases, which enhances the interactions between particles, thus greatly increasing the yield stress []. When SS was added, the plastic viscosity appeared to be significantly lower than that of the no-addition group, which is also due to the increase in silicate oligomers leading to an increase in electrostatic repulsion on the particle surface, so the plastic viscosity was reduced. The Na2O⋅nSiO2 solution itself had a high viscosity, so the plastic viscosity increased slightly with the increase in dosage [].
The initial rheological parameter yield stress of DSPB was less than 1 Pa under the condition of HCDA doping of higher than 80%, the highest value was only 0.875 Pa, and the highest value of plastic viscosity calculated by the consistency index was only 1.36 Pa·s. Especially in the sample of the Control group, the high additive amount of HCDA was kept at 90% level, and the doping amount of PCE and SS was only at the middle level, but its rheological properties in the groups had excellent performance, which reduces the cost of additives and, at the same time, can eliminate a large amount of solid-waste HCDA, and all the performance meets the requirements of mine backfill pipeline pumping and construction work performance.
Cemented paste backfill (CPB) was compared with the conventional mine filling binder OPC and prepared by replacing part of the OPC with solid wastes such as slag or fly ash (FA). The yield stress of DSPB was significantly lower than that of the general CPB samples, and most of the plastic viscosity values were the same as that of CPB, which was slightly higher than that of high HCDA or SS dosage, but all of them showed good rheological properties. The significant advantage of DSPB is that it can be used in conjunction with GBFS to completely replace OPC in the preparation of PB, which significantly reduces the cost of backfill materials in mines while maintaining performance, and reduces energy consumption and carbon emissions in the OPC production process []. Compared with other alternative binder slag or FA, HCDA is less expensive and more hazardous to the environment and needs to be disposed of urgently. Reasonable disposal of a certain scale can be rewarded by the government, which is conducive to reducing the cost for enterprises [].
3.2.2. Time-Dependent Rheological Parameters
Figure 7 shows the yield stress and plastic viscosity of different DSPB samples over time under six consecutive shear cycles. Figure 7a,b shows that both the yield stress and the plastic viscosity of the slurry decreased with time when the HCDA dosage was 95%. When the slag dosage was small, most of the slurry was still in HCDA particle suspension and the initial flocculation structure was more complete, and with the increase in shear time, the damage to the fragile flocculation structure increased. With the decrease in HCDA ratio, the trends of yield stress and plastic viscosity were decreasing and then increasing. This is because after shear disruption of the flocculating structure, more silica–oxygen and alumina–oxygen tetrahedra are released from slag dissolution as the reaction time increases, thus inducing the generation of initial hydration products around the particles and an increase in colloidal cohesion of the slurry.
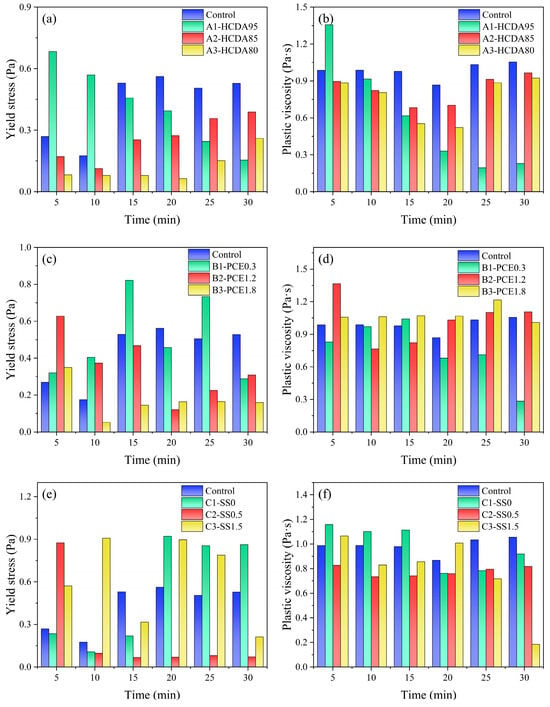
Figure 7.
Yield stress of DSPB with (a) different HCDA ratios, (c) PCE, and (e) SS dosages and plastic viscosity of DSPB with (b) different HCDA ratios, (d) PCE, and (f) SS dosages for the first 30 min.
Figure 7c,d shows that the yield stress increased with reaction time for PCE dosages of 0.3% and 0.6%, while the opposite trend was observed for 1.2% and 1.8%. This indicates that a larger PCE dosage is beneficial in maintaining the flow properties of the slurry. This may be due to the fact that the PCE molecules attached to the slurry particles generate electron-layer repulsion on the surface, which reduces the attraction between the particles and acts as a lubricant. There was no significant difference in the plastic viscosity values with time and no obvious pattern in the trend of change, except for PCE0.3, which reached a minimum value of 0.28 Pa·s at 30 min.
The yield stress increased significantly after 15 min of reaction for both no SS addition and SS doping of 1.0%, while the yield stress decreased significantly with reaction time for SS doping of 0.5% (Figure 7e). When no activator was added, the flocculated agglomeration structure of solid particles within the DSPB developed faster with time, affecting the rheological properties of the slurry. When the SS doping was higher, it was conducive to promoting the release of SiO4 and AlO4, as well as providing more silicon sources for the system, which promoted the hydration reaction rate and accelerated the gel generation []. From Figure 7f, it can be seen that the plastic viscosity of the slurry over time decreased more as the SS dosing increased. This may have been due to the change in the effect of plasticization of silicate oligomers on the viscosity [].
Although the trend of the rheological properties of each group of DSPB samples after 30 min of hydration reaction was different, the maximum values of the rheological parameters did not exceed 0.9 Pa and 1.36 Pa·s after the change, which still showed good rheological performance indexes. Combined with the setting time data of DSPB, it can be seen that it maintained good fluidity in the first 2 h in pipeline transportation, and it was not easy for accidents such as pipe plugging to occur.
Compared with the CPB prepared by using OPC or other alternatives such as slag and FA, DSPB did not show an obvious trend of rising yield stress and plastic viscosity at the beginning of the reaction, but it did tend to be stable with better fluidity, which may be closely related to the lower hydration activity of HCDA and the slower hydration reaction rate [].
3.3. Mini-Slump
As shown in Figure 8a, when the proportion of HCDA was 85%, its initial fluidity (252 mm) was the largest in the group. As the proportion of HCDA gradually decreased from 95% to 85%, the initial fluidity of the slurry slowly increased until it reached the maximum value, while it started to decrease when it continued to decrease to 80%. With the increase in reaction time, the results of the fluidity at 30 min and 60 min show that the ratio of HCDA to GBFS was correlated with the fluidity of the slurry, and the larger the proportion of GBFS, the better the fluidity of the slurry. The initial fluidity of DSPB decreased with increasing HCDA content. This trend is closely related to the water requirement of HCDA [].
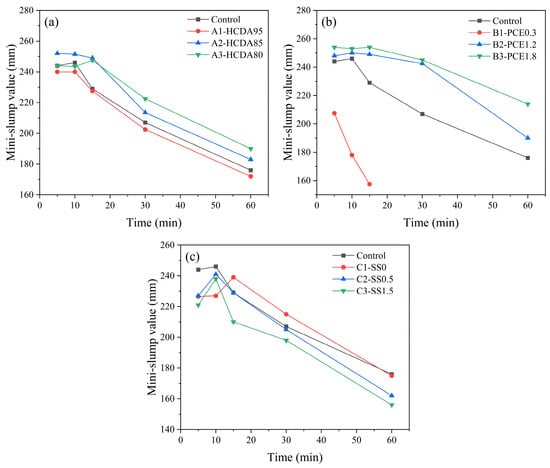
Figure 8.
Effects of (a) HCDA ratio, (b) PCE, and (c) SS dosing on the mini-slump value of the DSPB slurry.
Figure 8b shows the effect of PCE, with a significant increase in the initial and subsequent fluidity of the slurry with an increase in dosage. When the PCE dosing was 3%, the initial fluidity was the lowest, the loss of fluidity was also very fast, and the fluidity was lost after 15 min. With the increase in PCE dosing, the fluidity increased and the loss of fluidity with time decreased. This shows that by adding PCE, polycarboxylic acid molecules can be effectively adsorbed on the surface of HCDA particles due to the surface repulsion of molecules, thus dispersing the agglomerated particles, and free water can be lubricated in the interstices of the particles, which effectively improves the macroscopic mobility and performance of the slurry [,].
From Figure 8c, it can be seen that the initial fluidity was less than that of the Control group when no activator was doped, as well as when SS was doped at 1% and 1.5%. This may be due to the repulsive bilayer force generated by the adsorption of silicate oligomers on the surface of slag particles in the system, and thus the initial fluidity without activator was lower. On the other hand, when the SS doping was greater than 0.5%, the decrease in the initial fluidity may have been due to the higher viscosity of the SS solution itself, as well as due to more free water being consumed for the dissolution process. As the reaction time grew, the fluidity decreased with the increase in SS doping. The C1-SS0 and Control groups had less loss of fluidity with time than the groups with higher activator dosing due to the incorporation of SS. SS provides the system with a high source of alkalinity and silica, which is a necessary condition for the generation of the C-S-H gel by the hydration reaction [].
3.4. XRD Analysis
Figure 9 shows the hydration product analysis of DSPB after 60 min of reaction. The main hydration products were C-S-H gel, ettringite, gypsum, Ca(OH)2, and CaCO3, as well as the incompletely reacted CaO and CaSO3. It can be seen from the XRD pattern that the effects of different dosages of HCDA, PCE, and SS on the early hydration products were relatively small, which indicates that these factors have no obvious influence on the mechanism of hydration but only contribute to the hydration rate. The most diffraction peaks in the system were those of Ca(OH)2 and CaCO3 due to HCDA’s high f-CaO content, which reacted with water to generate Ca(OH)2. Only a portion reacted with GBFS, and most unreacted Ca(OH)2 remained as flocculent colloids, which subsequently underwent carbonation with atmospheric CO2 to form CaCO3. The flocculating structure of Ca(OH)2 colloids and CaCO3 precipitation contributed to the decrease in slurry fluidity.
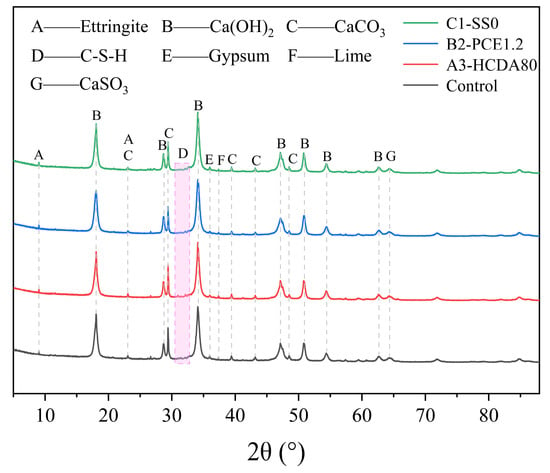
Figure 9.
XRD analysis of DSPB after 60 min of reaction.
Ca(OH)2 involved in the reaction can accelerate the dissolution of silica–oxygen tetrahedra and aluminum–oxygen tetrahedra in GBFS and react with them as well as with SO42− in solution to generate C-S-H gel and ettringite, and the generation of hydration products promotes the establishment of the microstructure, which is the main factor leading to the change in the rheological properties [].
3.5. SEM Analysis
Figure 10 shows the SEM images of DSPB after 60 min of reaction. From the figure, it can be seen that as the hydration reaction proceeded, different products were formed on the surface of the particles, but the main hydration products were all the same, and the hydration mechanisms of the different groups were all basically the same. The degree of reaction of the C1 group was the lowest, the slag particle surface was slightly etched, only a small amount of gel and Aft was generated, and many flaky Ca(OH)2 crystals were precipitated (Figure 10d). This may have been due to the absence of SS and the low pH, which resulted in slow dissolution of the slag. In contrast, the surfaces of the Control group and A3 particles were eroded to a greater extent, resulting in the formation of more flocculent precipitates (Figure 10a,b). High pH and abundant silicate oligomers accelerated the reaction.
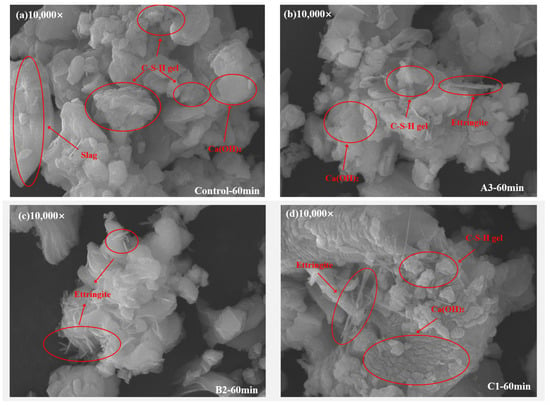
Figure 10.
SEM images of DSPB after 60 min of reaction. (a) Control; (b) A3-HCDA80; (c) B2-PCE1.2; (d) C1-SS0.
Numerous needle-like AFt crystals were observed as adhering to C-S-H gel surfaces, forming a highly polymerized, intertwined floc structure, which impacts rheological properties. Additionally, hydration products and precipitated Ca(OH)2 crystals create irregular particle surfaces, leading to mutual hindrance during flow and increased flow resistance.
3.6. LF-NMR Analysis
Water, as the main dispersion medium of backfill slurry, provides an efficient medium for particle-to-particle contact through changes in its existence. LF-NMR was utilized to determine the transverse relaxation time T2 of 1H protons of water molecules in different DSPB at 15, 30, 45, and 60 min. The higher the T2 peak, the more free water molecules in the slurry. Because the water in the slurry is related to the agglomeration structure of the solid particles and the hydration reaction products, understanding the rate of diffusion and exchange of water molecules between the free and bound water can help to study the rheological properties and the solidification process.
The T2 profile can be categorized into four peaks, with T24 usually appearing after 15 min of reaction, of which T21 (0.1–0.5 ms), centered at 0.2 ms, and T22 (1–2 ms), centered at 1.5 ms, represent the water in slurry capillaries (bound water); T23 (10–60 ms) represents a large amount of free water in the inter-particles; and T24 (100–700 ms) represents a small amount of free water within large pores in the slurry [].
Figure 11 shows that the T21 and T22 peaks of HCDA80 were the highest, and the peaks decreased with the increase in HCDA amount, reaching the lowest value at HCDA95 and remaining consistent throughout the 60 min reaction process, which indicates that the proportion of capillary water was higher when the HCDA doping amount was 80%. The two peaks of T23 and T24 can be defined as the oozing water and unreacted water in the slurries []. T23Control > T23HCDA85 > T23HCDA80 > T23HCDA95 throughout the 60 min, and its peak area decreased with reaction time, with the greatest reduction at 15–30 min. This indicates that when the HCDA doping was 90%, the more unexplored pores there were in the system, the higher the free water content was, and the more and larger the generated drain channels were, the larger the particle spacing was. With the increase in reaction time, the free water content decreased more at 30 min, when the fluidity became worse, which is also consistent with the mini-slump value decreasing more obviously at 30 min. Therefore, high free water content in slurries is a key element in improving and preserving the rheological properties. At the same time, the peak of T24 began to appear. This is because the hydration products C-S-H gel and ettringite began to be generated, the hydration products were agglomerated and adsorbed to each other, and the early microstructure of the slurry began to be generated, with the appearance of a small amount of free water that existed in the large pores [].
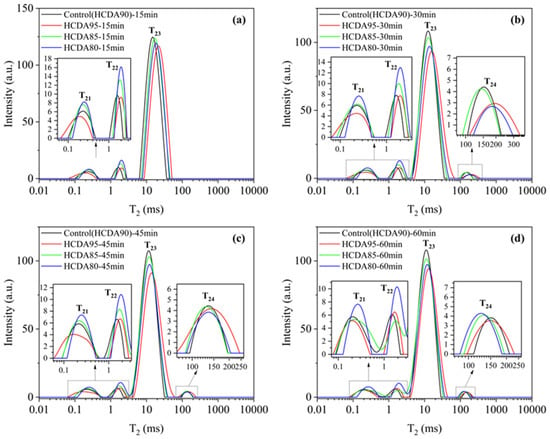
Figure 11.
T2 distribution of DSPB slurries with different HCDA ratios. Different reaction times: (a) 15 min; (b) 30 min; (c) 45min; (d) 60min.
Figure 12 shows that T21, T22, and T23 of PCE0.3 were the highest in all the samples, and the peak of T23 of PCE1.2 was the maximum at 30 min. As the reaction time continued to be extended, the highest value was still PCE0.3. Throughout the reaction process of 60 min, the peak area of T23 did not change much with time, which indicates that the addition of PCE had a significant effect on the retention of free water content in the system, which was conducive to maintaining the good fluidity of the slurry.
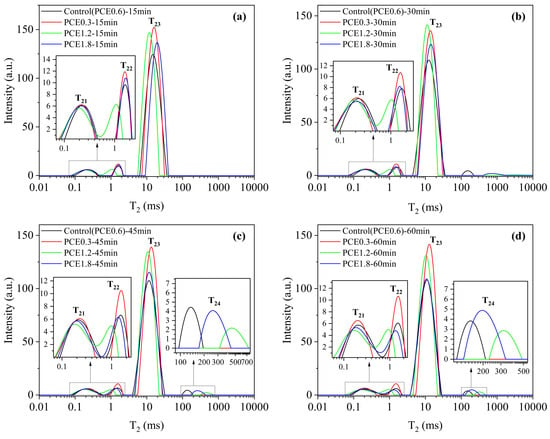
Figure 12.
T2 distribution of DSPB slurries with different PCE dosages. Different reaction times: (a) 15 min; (b) 30 min; (c) 45min; (d) 60min.
As shown in Figure 13, when no activator SS was added, the T23 peak was the highest and significantly higher than that of the other samples with added activators, while the T21 and T22 peaks were smaller than those of the other groups and the peaks were left-shifted. This indicates that the hydration degree of the sample without activator was much smaller than that with the addition, at which time there was less generation of small void structures in the slurry, a lower proportion of capillary water, a slower rate of hydration reaction, and less free water consumed. With the prolongation of the reaction time, the change in the T23 peak was also smaller, indicating that the addition of SS had an obvious promotion effect on the hydration reaction in the system, which led to a loss of rheological properties in the slurry.
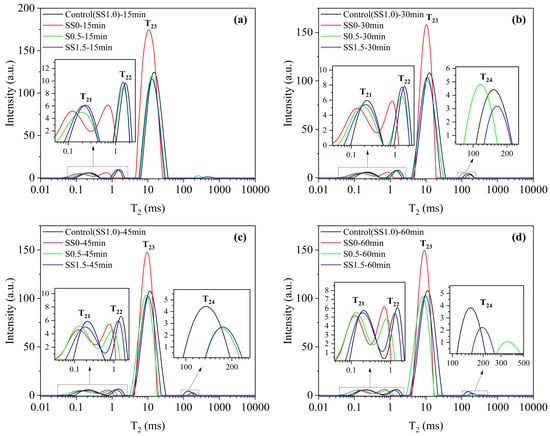
Figure 13.
T2 distribution of DSPB slurries with different SS dosages. Different reaction times: (a) 15 min; (b) 30 min; (c) 45min; (d) 60min.
4. Conclusions
The novel solid waste-based backfill material developed in this study can treat and utilize desulfurization ash in large quantities. The effect of different HCDA, PCE, and SS additions on the rheological properties of DSPB was investigated. The conclusions to be drawn are listed below.
(1) The setting time of DSPB slurry increased with decreasing HCDA content, increasing PCE dosing prolonged the setting time, and increasing SS content shortened it, but regardless of the changing influencing factors, the DSPB samples demonstrated suitable setting characteristics to meet the construction requirements.
(2) The initial yield stress and plastic viscosity decreased by up to 88% and 34.9% with decreasing HCDA doping. At high HCDA dosing, the rheological parameters of the slurry decreased with time, attributed to the disruption of the flocculated structure of the slurry. With the decrease in HCDA dosage, the rheological parameters decreased and then increased, which was related to the increased release of reactive Si and Al due to the increase in slag dosage. When the PCE dosage increased, the “card house” structure led to an increase and then a decrease in the initial yield stress and plastic viscosity of the DSPB, and a high dosage of PCE improved the rheological properties by dispersing the particles. PCE showed an increase in yield stress with time at low dosage. The opposite was true at high dosage, and there was no obvious pattern to the change in plastic viscosity with time. SS significantly reduced the yield stress initially, but increased it with increasing dosage due to the C-S-H gel generated. Meanwhile, SS reduced the initial plastic viscosity, but at high dosage it resulted in a slight increase due to the viscosity of the sodium silicate solution itself. The role of SS on time-dependent rheology is reflected in that it could accelerate the hydration reaction and gel generation and reduce the rheological parameters; the high dosage of SS could further reduce the plastic viscosity, which may be related to the plasticizing effect of silicate oligomers.
(3) The proportion of HCDA and GBFS affected the fluidity of DSPB. The initial fluidity was the largest in 85% HCDA, and with the reduction in the proportion of HCDA, the fluidity was significantly improved. The increase in PCE doping significantly improved the fluidity and reduced the loss. Activator SS doping on the slurry flow complex and a moderate amount of silicate oligomer plasticization reduced the initial fluidity. Excessive addition due to its own viscosity was higher, and the hydration reaction and C-S-H gel generation were promoted, so the fluidity was reduced.
The findings in this study indicate the huge potential of using novel DSPB material for mine backfilling. The current study was carried out mainly in a laboratory, as well as in the field for preliminary practical checks. The study mainly investigated the rheological properties of DSPB and lacked the assessment of durability properties such as wet and dry cycling and carbonation resistance, as well as environmental safety assessment such as heavy metal ion leaching capability. In the future, the engineering practice of using DSPB in combination with mine waste rock and tailing to prepare full tailing backfill paste is needed to expand its application and enhance its utilization.
Author Contributions
Writing—original draft, project administration, supervision, W.L.; methodology, investigation, J.C.; writing—review and editing, funding acquisition, data curation, W.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Provincial Special Project for the Construction of National Sustainable Development Agenda Innovation Demonstration Zone in Chenzhou City (2023sfq50).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mao, Y.; Wu, H.; Wang, W.; Jia, M.; Che, X. Pretreatment of municipal solid waste incineration fly ash and preparation of solid waste source sulphoaluminate cementitious material. J. Hazard. Mater. 2020, 385, 121580. [Google Scholar] [CrossRef]
- Wu, C.; Li, J.; Lu, Y.; Zhu, D. The influence of industrial solid waste in conjunction with lepidolite tailings on the mechanical properties and microstructure of cemented backfill materials. Constr. Build. Mater. 2024, 419, 135422. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, R.; Zhang, B.; Han, B. Research on mechanical properties and mix proportion design of solid waste-based cemented paste backfill. Case Stud. Constr. Mater. 2024, 21, e03618. [Google Scholar] [CrossRef]
- Navarrete, I.; Vargas, F.; Martinez, P.; Paul, A.; Lopez, M. Flue gas desulfurization (FGD) fly ash as a sustainable, safe alternative for cement-based materials. J. Clean. Prod. 2021, 283, 124646. [Google Scholar] [CrossRef]
- de Castro, R.d.P.V.; de Medeiros, J.L.; Araújo, O.d.Q.F.; de Andrade Cruz, M.; Ribeiro, G.T.; de Oliveira, V.R. Fluidized bed treatment of residues of semi-dry flue gas desulfurization units of coal-fired power plants for conversion of sulfites to sulfates. Energy Convers. Manag. 2017, 143, 173–187. [Google Scholar] [CrossRef]
- Li, X.; Han, J.; Liu, Y.; Dou, Z.; Zhang, T. Summary of research progress on industrial flue gas desulfurization technology. Sep. Purif. Technol. 2022, 281, 119849. [Google Scholar] [CrossRef]
- Mendes, B.C.; Pedroti, L.G.; Fontes, M.P.F.; Ribeiro, J.C.L.; Vieira, C.M.F.; Pacheco, A.A.; Azevedo, A.R.G.D. Technical and environmental assessment of the incorporation of iron ore tailings in construction clay bricks. Constr. Build. Mater. 2019, 227, 116669. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, K.; He, X.; Wei, Z.; Zhang, J. Study on proportioning experiment and performance of solid waste for underground backfilling. Mater. Today Commun. 2022, 32, 103863. [Google Scholar] [CrossRef]
- Koukouzas, N.; Ketikidis, C.; Itskos, G.; Spiliotis, X.; Karayannis, V.; Papapolymerou, G. Synthesis of CFB-Coal Fly Ash Clay Bricks and Their Characterisation. Waste Biomass Valorization 2011, 2, 87–94. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Zhang, Z.; Wang, Y.; Xue, Y.; Hao, X.; Lu, Y. Circulating Fluidized Bed Fly Ash Mixed Functional Cementitious Materials: Shrinkage Compensation of f-CaO, Autoclaved Hydration Characteristics and Environmental Performance. Materials 2021, 14, 6004. [Google Scholar] [CrossRef]
- Jia, G.; Wang, Y.; Yang, F. A Review on the Application of Circulating Fluidized Bed Fly Ash in Building Materials. Adv. Mater. Sci. Eng. 2022, 2022, 1–19. [Google Scholar] [CrossRef]
- Lee, J.; Lee, T. Effects of High CaO Fly Ash and Sulfate Activator as a Finer Binder for Cementless Grouting Material. Materials 2019, 12, 3664. [Google Scholar] [CrossRef]
- Ren, K.; Ma, S.; Feng, Y.; Xu, N.; Bai, S. Study on the composite gravel preparation and the synergistic absorption of CO2 by fly ash of CFB boiler. Fuel 2023, 342, 127843. [Google Scholar] [CrossRef]
- Li, X.; Chen, Q.; Ma, B.; Huang, J.; Jian, S.; Wu, B. Utilization of modified CFBC desulfurization ash as an admixture in blended cements: Physico-mechanical and hydration characteristics. Fuel 2012, 102, 674–680. [Google Scholar] [CrossRef]
- Yang, L.; Jing, M.; Lu, L.; Zhu, X.; Zhao, P.; Chen, M.; Li, L.; Liu, J. Effects of modified materials prepared from wastes on the performance of flue gas desulfurization gypsum-based composite wall materials. Constr. Build. Mater. 2020, 257, 119519. [Google Scholar] [CrossRef]
- Wang, C.-Q.; Cheng, L.-X.; Wang, Z.-Y.; Qi, C.-J.; Huang, D.-M.; Wei, S. Preparation and properties of high blending phosphogypsum-desulfurization ash-waste soil based functional prefabricated autoclaved aerated concrete slabs. Constr. Build. Mater. 2024, 423, 135879. [Google Scholar] [CrossRef]
- Li, K.; Zhou, Z.; Cao, W.; Zhang, Y. Effect of dry desulfurization ash as a filler on asphalt pavement performance. Constr. Build. Mater. 2024, 412, 134692. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Li, Z.; Ren, Y.; Wang, Y.; Zhang, W. Preparation, characterization and application of red mud, fly ash and desulfurized gypsum based eco-friendly road base materials. J. Clean. Prod. 2021, 284, 124777. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, Q.; Sun, Z.; Huang, X.; Gan, M.; Ji, Z.; Chen, X.; Fan, X. Co-disposal of semi-dry desulfurization residue and red mud into high performance alkali activated material. Constr. Build. Mater. 2022, 350, 128776. [Google Scholar] [CrossRef]
- Feng, X.; Wang, C.; Ding, S. Performance of desulfurization ash for the preparation of grouting fire prevention material. Environ. Sci. Pollut. Res. 2019, 26, 19228–19240. [Google Scholar] [CrossRef]
- Kovler, K. Setting and Hardening of Gypsum-Portland Cement-Silica Fume Blends, Part 2: Early Strength, DTA, XRD, and SEM Observations. Cem. Concr. Res. 1998, 28, 523–531. [Google Scholar] [CrossRef]
- Rathnayake, M.; Julnipitawong, P.; Tangtermsirikul, S.; Toochinda, P. Utilization of coal fly ash and bottom ash as solid sorbents for sulfur dioxide reduction from coal fired power plant: Life cycle assessment and applications. J. Clean. Prod. 2018, 202, 934–945. [Google Scholar] [CrossRef]
- Blanco, F.; Garcia, M.P.; Ayala, J.; Mayoral, G.; Garcia, M.A. The effect of mechanically and chemically activated fly ashes on mortar properties. Fuel 2006, 85, 2018–2026. [Google Scholar] [CrossRef]
- Liu, S.; Liu, W.; Jiao, F.; Qin, W.; Yang, C. Production and resource utilization of flue gas desulfurized gypsum in China—A review. Environ. Pollut. 2021, 288, 117799. [Google Scholar] [CrossRef]
- Ma, Y.; Nie, Q.; Xiao, R.; Hu, W.; Han, B.; Polaczyk, P.A.; Huang, B. Experimental investigation of utilizing waste flue gas desulfurized gypsum as backfill materials. Constr. Build. Mater. 2020, 245, 118393. [Google Scholar] [CrossRef]
- Ren, J.; Liu, B.; Guo, J.; Liu, J.; Xing, F.; Zhu, H.; Zhao, L.; Mi, T. Bio-treatment of municipal solid waste incineration fly ash: A sustainable path for recyclability. J. Clean. Prod. 2024, 434, 139869. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Wu, A.; Shi, D.; Yang, S.; Ruan, Z.; Song, X.; Zhang, M. Early mechanical strength, hydration mechanism and leaching behavior of alkali-activated slag/fly ash paste filling materials. J. Build. Eng. 2024, 84, 108481. [Google Scholar] [CrossRef]
- Song, X.; Huang, Y.; Wang, S.; Yu, H.; Hao, Y. Macro-mesoscopic mechanical properties and damage progression of cemented tailings backfill under cyclic static load disturbance. Compos. Struct. 2023, 322, 117433. [Google Scholar] [CrossRef]
- Zhang, S.; Du, W.; Jin, Y.; Li, Y. Performance and hydration mechanism of fly ash coal-based solid waste backfill material affected by multiple factors. Mater. Today Commun. 2024, 41, 110639. [Google Scholar] [CrossRef]
- Wu, M.; Wang, C.; Zuo, Y.; Yang, S.; Zhang, J.; Luo, Y. Study on strength prediction and strength change of Phosphogypsum-based composite cementitious backfill based on BP neural network. Mater. Today Commun. 2024, 41, 110331. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Li, B.; Zhou, N.; Xiao, X.; Li, M.; Zhu, C. Environmental behavior of construction and demolition waste as recycled aggregates for backfilling in mines: Leaching toxicity and surface subsidence studies. J. Hazard. Mater. 2020, 389, 121870. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Liu, L.; Xu, L.; Wang, J.; Yang, P.; Qu, H. A preliminary study of aeolian sand-cement-modified gasification slag-paste backfill: Fluidity, microstructure, and leaching risks. Sci. Total Environ. 2022, 830, 154766. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qiu, J.; Wu, P.; Guo, Z.; Zhang, S.; Sun, X. Preparing a binder for cemented paste backfill using low-aluminum slag and hazardous oil shale residue and the heavy metals immobilization effects. Powder Technol. 2022, 399, 117167. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, H.; Tang, X.; Wang, Y.; An, H.; Liu, J. Historical trend and decarbonization pathway of China’s cement industry: A literature review. Sci. Total Environ. 2023, 891, 164580. [Google Scholar] [CrossRef]
- Clavier, K.A.; Watts, B.; Liu, Y.; Ferraro, C.C.; Townsend, T.G. Risk and performance assessment of cement made using municipal solid waste incinerator bottom ash as a cement kiln feed. Resour. Conserv. Recycl. 2019, 146, 270–279. [Google Scholar] [CrossRef]
- Liu, S.; Fall, M. Fresh and hardened properties of cemented paste backfill: Links to mixing time. Constr. Build. Mater. 2022, 324, 126688. [Google Scholar] [CrossRef]
- Haiqiang, J.; Fall, M.; Cui, L. Yield stress of cemented paste backfill in sub-zero environments: Experimental results. Miner. Eng. 2016, 92, 141–150. [Google Scholar] [CrossRef]
- Yin, S.; Yan, Z.; Chen, X.; Wang, L. Effect of fly-ash as fine aggregate on the workability and mechanical properties of cemented paste backfill. Case Stud. Constr. Mater. 2022, 16, e01039. [Google Scholar] [CrossRef]
- Guo, Z.; Qiu, J.; Pel, L.; Zhao, Y.; Zhu, Q.; Kwek, J.W.; Zhang, L.; Jiang, H.; Yang, J.; Qu, Z. A contribution to understanding the rheological measurement, yielding mechanism and structural evolution of fresh cemented paste backfill. Cem. Concr. Compos. 2023, 143, 105221. [Google Scholar] [CrossRef]
- Sagade, A.; Fall, M. Study of fresh properties of cemented paste backfill material with ternary cement blends. Constr. Build. Mater. 2024, 411, 134287. [Google Scholar] [CrossRef]
- Fall, M.; Benzaazoua, M.; Saa, E.G. Mix proportioning of underground cemented tailings backfill. Tunn. Undergr. Space Technol. 2008, 23, 80–90. [Google Scholar] [CrossRef]
- Guo, Z.; Sun, X.; Zhang, X.; Qiu, J.; Jiang, H.; Zhao, Y.; Wu, P.; Zhang, Q. Effect of superplasticizer on rheology and thixotropy of superfine-tailings cemented paste backfill: Experiment and modelling. Constr. Build. Mater. 2022, 316, 125693. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, K.; Chen, Y.; Wu, H.; Li, C. Rheological performance regulation of solidified soil slurry for mine reclamation: Superplasticizer selection and structural characterization based on in-situ techniques. Constr. Build. Mater. 2024, 445, 137954. [Google Scholar] [CrossRef]
- GB/T 203—2008; Granulated Blast Furnace Slag Used for Cement Production. China National Standardization Administration Committee: Beijing, China, 2008.
- GB/T 1346-2011; Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of The Portland Cement. China National Standardization Administration Committee: Beijing, China, 2011.
- Yang, T.; Zhu, H.; Zhang, Z.; Gao, X.; Zhang, C.; Wu, Q. Effect of fly ash microsphere on the rheology and microstructure of alkali-activated fly ash/slag pastes. Cem. Concr. Res. 2018, 109, 198–207. [Google Scholar] [CrossRef]
- Puertas, F.; Varga, C.; Alonso, M.M. Rheology of alkali-activated slag pastes. Effect of the nature and concentration of the activating solution. Cem. Concr. Compos. 2014, 53, 279–288. [Google Scholar] [CrossRef]
- GB/T 8077-2012; Methods for Testing Uniformity of Concrete Admixture. China National Standardization Administration Committee: Beijing, China, 2012.
- Huang, T.; Yuan, Q.; Zuo, S.; Shi, C. Evolution of elastic behavior of alite paste at early hydration stages. J. Am. Ceram. Soc. 2020, 103, 6490–6504. [Google Scholar] [CrossRef]
- Huang, T.; Yuan, Q.; He, F.; Xie, Y. Understanding the mechanisms behind the time-dependent viscoelasticity of fresh C3A–gypsum paste. Cem. Concr. Res. 2020, 133, 106084. [Google Scholar] [CrossRef]
- Bligh, M.W.; d’Eurydice, M.N.; Lloyd, R.R.; Arns, C.H.; Waite, T.D. Investigation of early hydration dynamics and microstructural development in ordinary Portland cement using 1H NMR relaxometry and isothermal calorimetry. Cem. Concr. Res. 2016, 83, 131–139. [Google Scholar] [CrossRef]
- Nguyen, H.-A.; Chang, T.-P.; Chen, C.-T.; Huang, T.-Y. Engineering and creep performances of green super-sulfated cement concretes using circulating fluidized bed combustion fly ash. Constr. Build. Mater. 2022, 346, 128274. [Google Scholar] [CrossRef]
- Liu, W.; Liu, X.; Zhang, L.; Wan, Y.; Li, H.; Jiao, X. Rheology, mechanics, microstructure and durability of low-carbon cementitious materials based on circulating fluidized bed fly ash: A comprehensive review. Constr. Build. Mater. 2024, 411, 134688. [Google Scholar] [CrossRef]
- Li, P.P.; Yu, Q.L.; Brouwers, H.J.H. Effect of PCE-type superplasticizer on early-age behaviour of ultra-high performance concrete (UHPC). Constr. Build. Mater. 2017, 153, 740–750. [Google Scholar] [CrossRef]
- Chen, X.; Yang, X.; Wu, K.; Chen, Q.; Yang, Z.; Xu, L.; Li, H. Understanding the role of C–S–H seed/PCE nanocomposites, triethanolamine, sodium nitrate and PCE on hydration and performance at early age. Cem. Concr. Compos. 2023, 139, 105002. [Google Scholar] [CrossRef]
- Chang, J.J. A study on the setting characteristics of sodium silicate-activated slag pastes. Cem. Concr. Res. 2003, 33, 1005–1011. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, Y.; Wang, K.; Bu, Y.; Wang, C.; Ma, C.; Liu, H. Delaying the hydration of portland cement by sodium silicate: Setting time and retarding mechanism. Constr. Build. Mater. 2019, 205, 543–548. [Google Scholar] [CrossRef]
- Yang, P.; Liu, L.; Suo, Y.; Qu, H.; Xie, G.; Zhang, C.; Deng, S.; Lv, Y. Basic characteristics of magnesium-coal slag solid waste backfill material: Part I. preliminary study on flow, mechanics, hydration and leaching characteristics. J. Environ. Manag. 2023, 329, 117016. [Google Scholar] [CrossRef]
- Dai, X.; Aydin, S.; Yardimci, M.Y.; Lesage, K.; De Schutter, G. Early age reaction, rheological properties and pore solution chemistry of NaOH-activated slag mixtures. Cem. Concr. Compos. 2022, 133, 104715. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, C.J.; Jiao, D.W.; An, X.P. Review on Rheological Properties, Models and Measurements for Fresh Cementitious Materials. J. Chin. Ceram. Soc. 2017, 45, 700–709. [Google Scholar] [CrossRef]
- de Larrard, F.; Ferraris, C.F.; Sedran, T. Fresh concrete: A Herschel-Bulkley material. Mater. Struct. 1998, 31, 494–498. [Google Scholar] [CrossRef]
- Lopez Gonzalez, P.L.; Novais, R.M.; Labrincha, J.A.; Blanpain, B.; Pontikes, Y. Modifications of basic-oxygen-furnace slag microstructure and their effect on the rheology and the strength of alkali-activated binders. Cem. Concr. Compos. 2019, 97, 143–153. [Google Scholar] [CrossRef]
- Sun, R.; Wang, D. The property, structure, and phase evolution of a binary cementitious material derived from sintering flue gas desulphurization ash and steel slag. J. Build. Eng. 2024, 86, 108908. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, T.; Ni, W.; Li, K.; Gao, W.; Wang, K.; Zhang, Y. The mechanism of hydrating and solidifying green mine fill materials using circulating fluidized bed fly ash-slag-based agent. J. Hazard. Mater. 2021, 415, 125625. [Google Scholar] [CrossRef]
- Feneuil, B.; Pitois, O.; Roussel, N. Effect of surfactants on the yield stress of cement paste. Cem. Concr. Res. 2017, 100, 32–39. [Google Scholar] [CrossRef]
- Qian, Y.; Lesage, K.; El Cheikh, K.; De Schutter, G. Effect of polycarboxylate ether superplasticizer (PCE) on dynamic yield stress, thixotropy and flocculation state of fresh cement pastes in consideration of the critical micelle concentration (CMC). Cem. Concr. Res. 2018, 107, 75–84. [Google Scholar] [CrossRef]
- Tadros, T.F. Rheology of Dispersions: Principles and Applications; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Kashani, A.; Provis, J.L.; Qiao, G.G.; van Deventer, J.S.J. The interrelationship between surface chemistry and rheology in alkali activated slag paste. Constr. Build. Mater. 2014, 65, 583–591. [Google Scholar] [CrossRef]
- Palacios, M.; Gismera, S.; Alonso, M.M.; d’Espinose de Lacaillerie, J.B.; Lothenbach, B.; Favier, A.; Brumaud, C.; Puertas, F. Early reactivity of sodium silicate-activated slag pastes and its impact on rheological properties. Cem. Concr. Res. 2021, 140, 106302. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Wu, A.; Jiang, H.; Peng, Q.; Zhang, X. Evaluation of time-dependent rheological properties of cemented paste backfill incorporating superplasticizer with special focus on thixotropy and static yield stress. J. Cent. South Univ. 2022, 29, 1239–1249. [Google Scholar] [CrossRef]
- Jiang, H.; Fall, M.; Yilmaz, E.; Li, Y.; Yang, L. Effect of mineral admixtures on flow properties of fresh cemented paste backfill: Assessment of time dependency and thixotropy. Powder Technol. 2020, 372, 258–266. [Google Scholar] [CrossRef]
- Puertas, F.; Fernández-Jiménez, A.; Blanco-Varela, M.T. Pore solution in alkali-activated slag cement pastes. Relation to the composition and structure of calcium silicate hydrate. Cem. Concr. Res. 2004, 34, 139–148. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, Z.; Hu, J.; Yu, Q.; Shi, C. Effects of anionic species of activators on the rheological properties and early gel characteristics of alkali-activated slag paste. Cem. Concr. Res. 2022, 162, 106968. [Google Scholar] [CrossRef]
- He, Y.; Zhang, X.; Shui, L.; Wang, Y.; Gu, M.; Wang, X.; Wang, H.; Peng, L. Effects of PCEs with various carboxylic densities and functional groups on the fluidity and hydration performances of cement paste. Constr. Build. Mater. 2019, 202, 656–668. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, B.; Tang, Y.; Zhao, G.; Pang, Z.; Shi, C. Innovative strategies for time-release PCE design and cement paste flowability control. Cem. Concr. Compos. 2024, 154, 105785. [Google Scholar] [CrossRef]
- Jiao, D.; Shi, C.; Yuan, Q. Time-dependent rheological behavior of cementitious paste under continuous shear mixing. Constr. Build. Mater. 2019, 226, 591–600. [Google Scholar] [CrossRef]
- Zhou, K.; Ai, K.; Zhang, J.; Li, J. Nuclear Magnetic Rresonance Characteristics in Fresh Filling Slurry. Sci. Technol. Rev. 2013, 31, 50–53. [Google Scholar] [CrossRef]
- Ji, Y.; Sun, Z.; Yang, X.; Li, C.; Tang, X. Assessment and mechanism study of bleeding process in cement paste by 1H low-field NMR. Constr. Build. Mater. 2015, 100, 255–261. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).