Enhancing Biomass Production of Chlorella vulgaris in Anaerobically Digested Swine Wastewater Using Carbon Supplementation and Simultaneous Lipid Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Wastewater Collection, Pretreatment, and Analysis
2.2. Strain and Cultivation Conditions
2.3. Experimental Setup
2.4. Analytical Procedures
2.4.1. Measurement of Microalgal Growth
2.4.2. Nutrient Removal Analysis
2.4.3. Determination of Pigments
2.4.4. Determination of Carbohydrate and Protein Content
2.4.5. Lipid and Fatty Acid Methyl Ester (FAME) Analysis
2.4.6. Statistical Analysis
3. Results and Discussion
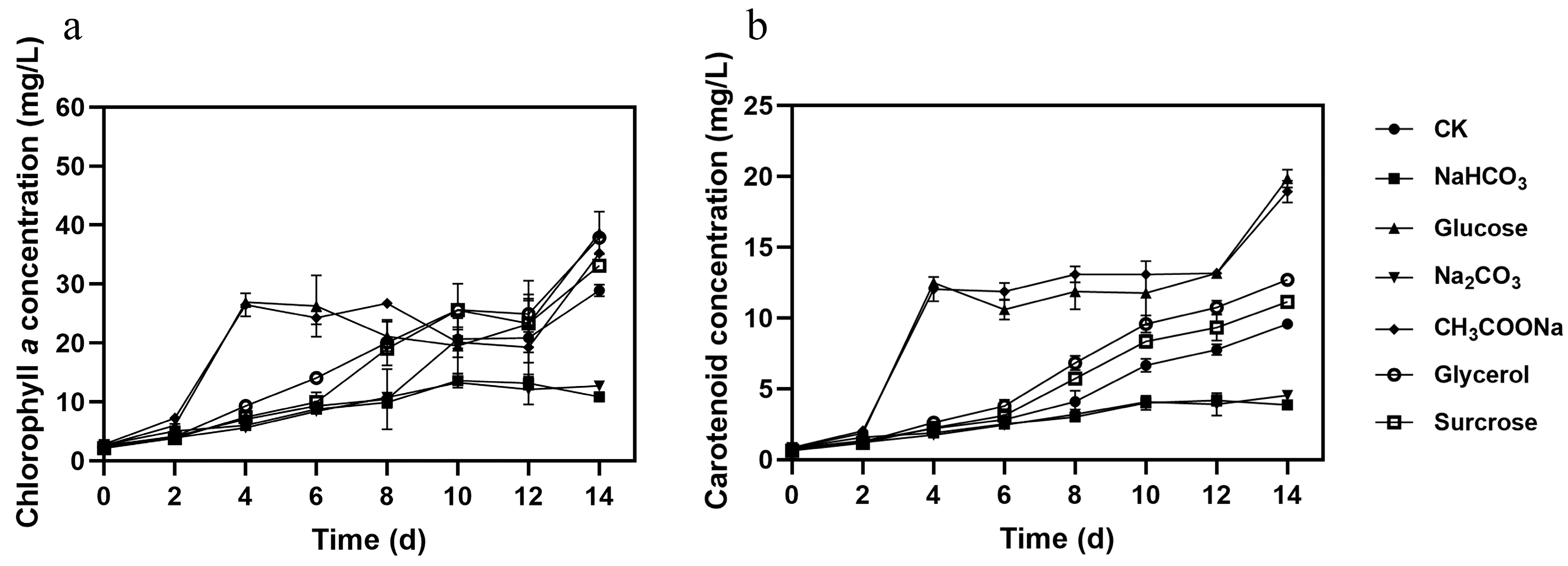
3.1. Effect of Different Carbon Sources on Microalgal Growth, Pigments, and Nutrient Removal
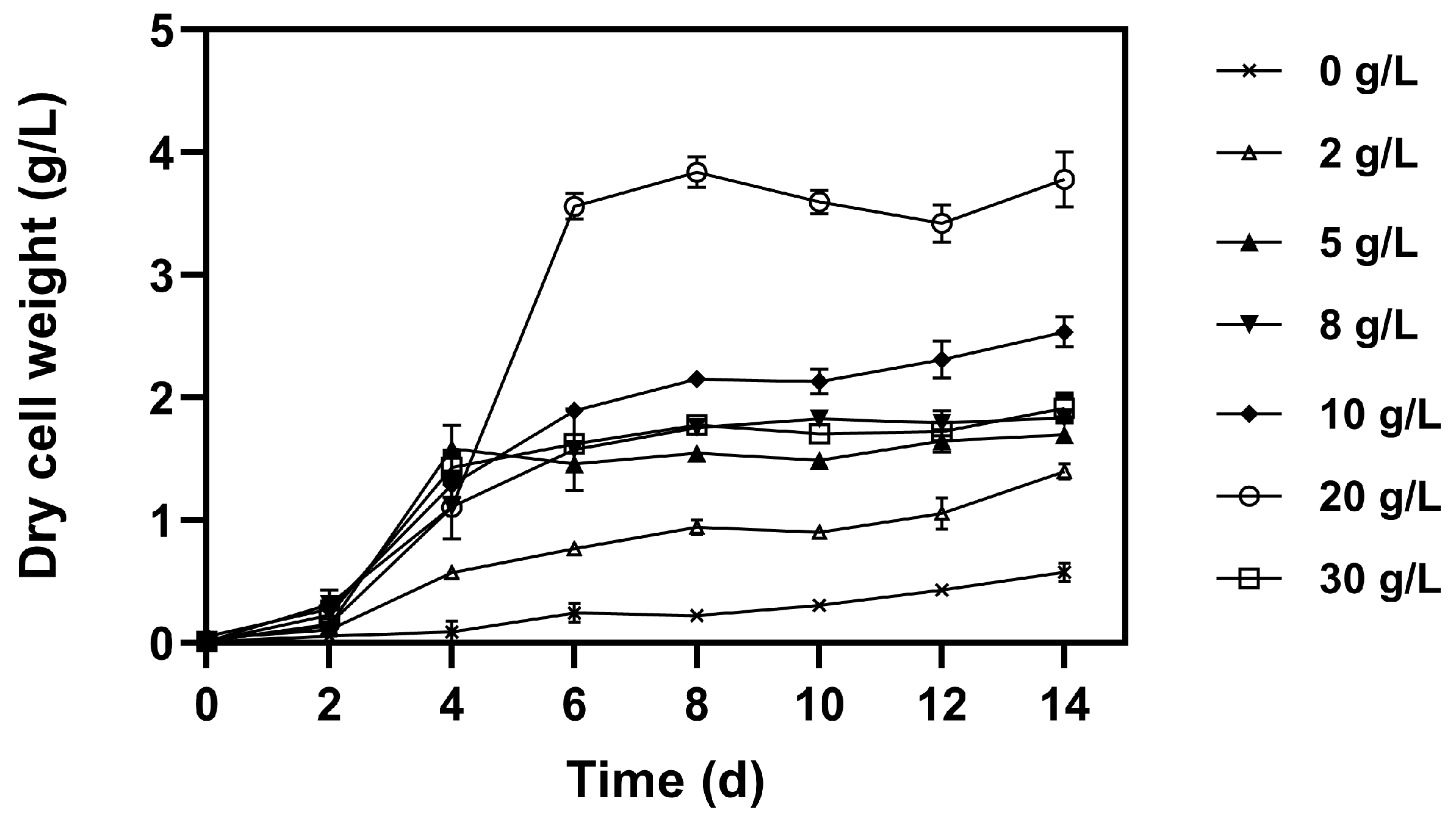
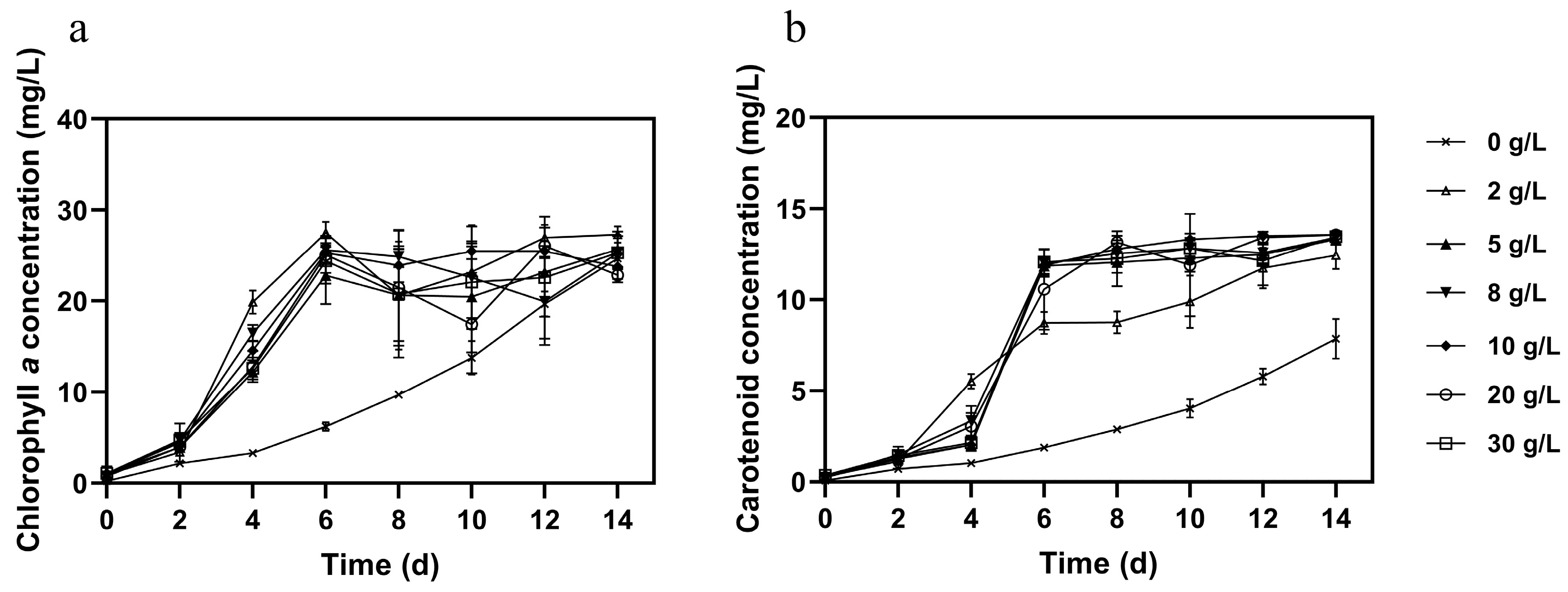
3.2. Effect of Different Glucose Concentration on Microalgal Growth, Pigments, and Nutrient Removal
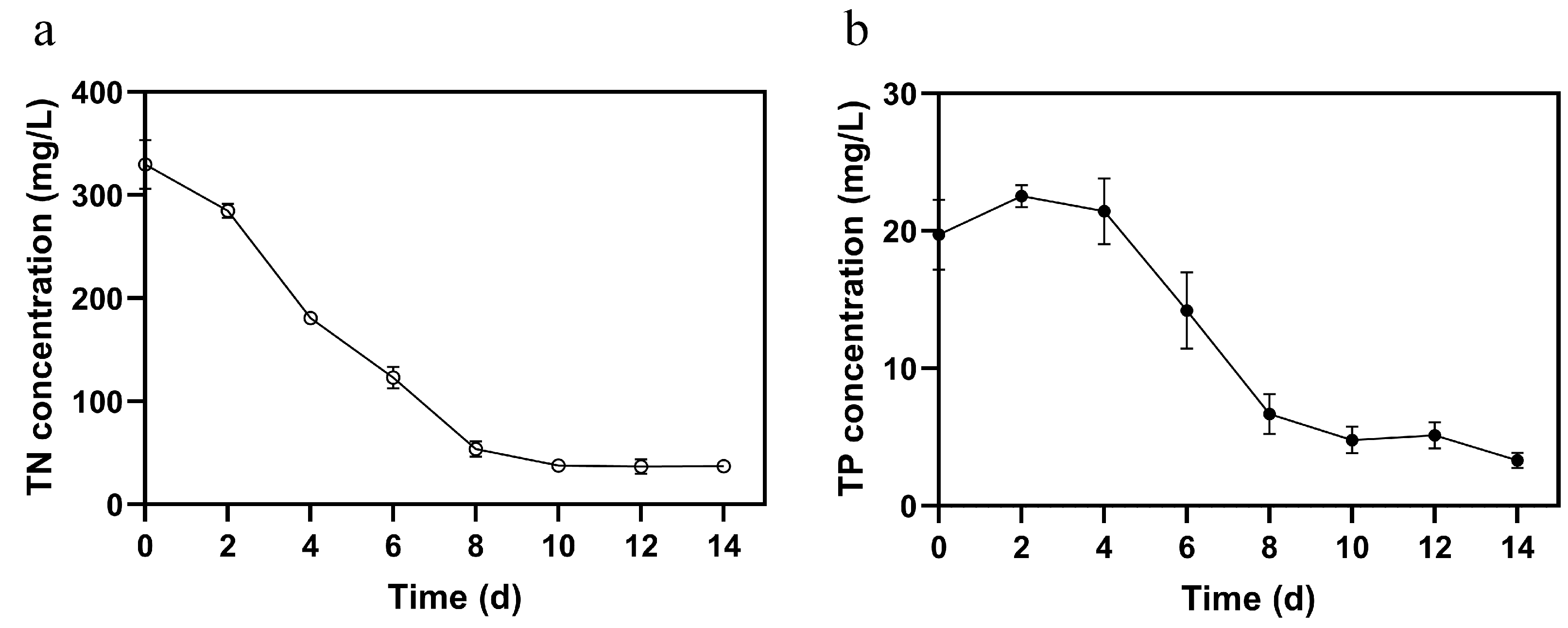
3.3. Biomass Growth and Nutrient Removal Capacity of FACHB-8 in ADSW with Optimized Glucose Supplementation
3.4. Lipid Production, Biomass, and FAME Composition in ADSW with Optimized Glucose Supplementation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Islam, M.A.; Heimann, K.; Brown, R.J. Microalgae biodiesel: Current status and future needs for engine performance and emissions. Renew. Sustain. Energy Rev. 2017, 79, 1160–1170. [Google Scholar] [CrossRef]
- Akpan, J.; Olanrewaju, O. Sustainable energy development: History and recent advances. Energies 2023, 16, 7049. [Google Scholar] [CrossRef]
- Owusu, P.A.; Asumadu-Sarkodie, S. A review of renewable energy sources, sustainability issues and climate change mitigation. Cogent Eng. 2016, 3, 1167990. [Google Scholar] [CrossRef]
- Ganesan, R.; Manigandan, S.; Samuel, M.S.; Shanmuganathan, R.; Brindhadevi, K.; Chi, N.T.L.; Duc, P.A.; Pugazhendhi, A. A review on prospective production of biofuel from microalgae. Biotechnol. Rep. 2020, 27, e00509. [Google Scholar] [CrossRef] [PubMed]
- Ozdalgic, B.; Ustun, M.; Dabbagh, S.R.; Haznedaroglu, B.Z.; Kiraz, A.; Tasoglu, S. Microfluidics for microalgal biotechnology. Biotechnol. Bioeng. 2021, 118, 1716–1734. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Peng, L.; Fu, D.; Chu, H.; Wang, Z.; Qi, H. Biofuel production from microalgae: A review. Environ. Chem. Lett. 2020, 18, 285–297. [Google Scholar] [CrossRef]
- Katam, G.B.; K, M.M.; Warkhade, G.S. Review on algae for biodiesel fuel production, its characteristics comparison with other and their impact on performance, combustion and emissions of diesel engine. World J. Eng. 2020, 14, 127–138. [Google Scholar] [CrossRef]
- Rashid, N.; Rehman, M.S.U.; Sadiq, M.; Mahmood, T.; Han, J.I. Current status, issues and developments in microalgae derived biodiesel production. Renew. Sustain. Energy Rev. 2014, 40, 760–778. [Google Scholar] [CrossRef]
- Suparmaniam, U.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Lee, K.T.; Shuit, S.H. Insights into the microalgae cultivation technology and harvesting process for biofuel production: A review. Renew. Sustain. Energy Rev. 2019, 115, 109361. [Google Scholar] [CrossRef]
- Saratale, R.G.; Ponnusamy, V.K.; Jeyakumar, R.B.; Sirohi, R.; Piechota, G.; Shobana, S.; Dharmaraja, J.; Lay, C.; Saratale, G.D.; Shin, H.S.; et al. Microalgae cultivation strategies using cost–effective nutrient sources: Recent updates and progress towards biofuel production. Bioresour. Technol. 2022, 361, 127691. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Ali, S.S.; Ramadan, H.; El-Aswar, E.I.; Eltawab, R.; Ho, S.H.; Elsamahy, T.; Li, S.; El-Sheekh, M.M.; Schagerl, M.; et al. Microalgae-based wastewater treatment: Mechanisms, challenges, recent advances, and future prospects. Environ. Sci. Ecotechnol. 2023, 13, 100205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ho, S.H.; Cheng, C.L.; Guo, W.Q.; Nagarajan, D.; Ren, N.Q.; Lee, D.-J.; Chang, J.-S. Perspectives on the feasibility of using microalgae for industrial wastewater treatment. Bioresour. Technol. 2016, 222, 485–497. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Pires, J.C.; Simões, M. A review on the use of microalgal consortia for wastewater treatment. Algal Res. 2017, 24, 403–415. [Google Scholar] [CrossRef]
- Sátiro, J.; Cunha, A.; Gomes, A.P.; Simões, R.; Albuquerque, A. Optimization of microalgae–bacteria consortium in the treatment of paper pulp wastewater. Appl. Sci. 2022, 12, 5799. [Google Scholar] [CrossRef]
- Satiro, J.; Gomes, A.; Florencio, L.; Simões, R.; Albuquerque, A. Effect of microalgae and bacteria inoculation on the startup of bioreactors for paper pulp wastewater and biofuel production. J. Environ. Manag. 2024, 362, 121305. [Google Scholar] [CrossRef] [PubMed]
- Dragone, G. Challenges and opportunities to increase economic feasibility and sustainability of mixotrophic cultivation of green microalgae of the genus Chlorella. Renew. Sustain. Energy Rev. 2022, 160, 112284. [Google Scholar] [CrossRef]
- Gupta, P.L.; Choi, H.J.; Pawar, R.R.; Jung, S.P.; Lee, S.M. Enhanced biomass production through optimization of carbon source and utilization of wastewater as a nutrient source. J. Environ. Manag. 2016, 184, 585–595. [Google Scholar] [CrossRef]
- Mondal, M.; Ghosh, A.; Tiwari, O.N.; Gayen, K.; Das, P.; Mandal, M.K.; Halder, G. Influence of carbon sources and light intensity on biomass and lipid production of Chlorella sorokiniana BTA 9031 isolated from coalfield under various nutritional modes. Energy Convers. Manag. 2017, 145, 247–254. [Google Scholar] [CrossRef]
- Abreu, A.P.; Morais, R.C.; Teixeira, J.A.; Nunes, J. A comparison between microalgal autotrophic growth and metabolite accumulation with heterotrophic, mixotrophic and photoheterotrophic cultivation modes. Renew. Sustain. Energy Rev. 2022, 159, 112247. [Google Scholar] [CrossRef]
- Ma, S.; Huang, Y.; Zhu, X.; Xia, A.; Zhu, X.; Liao, Q. Growth-based dynamic light transmission modeling and optimization in microalgal photobioreactors for high efficiency CO2 fixation. Renew. Sustain. Energy Rev. 2024, 197, 114414. [Google Scholar] [CrossRef]
- Hu, J.; Nagarajan, D.; Zhang, Q.; Chang, J.S.; Lee, D.J. Heterotrophic cultivation of microalgae for pigment production: A review. Biotechnol. Adv. 2018, 36, 54–67. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Chu, H.; Zhou, X.; Zhang, Y. Advances in microalgae-based livestock wastewater treatment: Mechanisms of pollutants removal, effects of inhibitory components and enhancement strategies. Chem. Eng. J. 2024, 483, 149222. [Google Scholar] [CrossRef]
- Plöhn, M.; Spain, O.; Sirin, S.; Silva, M.; Escudero-Oñate, C.; Ferrando-Climent, L.; Allahverdiyeva, Y.; Funk, C. Wastewater treatment by microalgae. Physiol. Plant. 2021, 173, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, Y.; Chen, Z.; Chen, Y.; Wen, Y.; Chen, B. Removal of nutrients from undiluted anaerobically treated piggery wastewater by improved microalgae. Bioresour. Technol. 2016, 222, 130–138. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Bashan, Y.; Esther Puente, M. Organic carbon supplementation of sterilized municipal wastewater is essential for heterotrophic growth and removing ammonium by the microalga Chlorella Vulgaris 1. J. Phycol. 2011, 47, 190–199. [Google Scholar] [CrossRef]
- Salama, E.S.; Kurade, M.B.; Abou-Shanab, R.A.; El-Dalatony, M.M.; Yang, I.S.; Min, B.; Jeon, B.H. Recent progress in microalgal biomass production coupled with wastewater treatment for biofuel generation. Renew. Sustain. Energy Rev. 2017, 79, 1189–1211. [Google Scholar] [CrossRef]
- Evans, L.; Hennige, S.J.; Willoughby, N.; Adeloye, A.J.; Skroblin, M.; Gutierrez, T. Effect of organic carbon enrichment on the treatment efficiency of primary settled wastewater by Chlorella vulgaris. Algal Res. 2017, 24, 368–377. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Escalante, F.M.; De-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef]
- Moreno-Garcia, L.; Adjallé, K.; Barnabé, S.; Raghavan, G.S.V. Microalgae biomass production for a biorefinery system: Recent advances and the way towards sustainability. Renew. Sustain. Energy Rev. 2017, 76, 493–506. [Google Scholar] [CrossRef]
- Chen, Z.; Xiao, Y.; Liu, T.; Yuan, M.; Liu, G.; Fang, J.; Yang, B. Exploration of microalgal species for nutrient removal from anaerobically digested swine wastewater and potential lipids production. Microorganisms 2021, 9, 2469. [Google Scholar] [CrossRef] [PubMed]
- Al-Hammadi, M.; Güngörmüşler, M. New insights into Chlorella vulgaris applications. Biotechnol. Bioeng. 2024, 121, 1486–1502. [Google Scholar] [CrossRef] [PubMed]
- Tale, M.; Ghosh, S.; Kapadnis, B.; Kale, S. Isolation and characterization of microalgae for biodiesel production from Nisargruna biogas plant effluent. Bioresour. Technol. 2014, 169, 328–335. [Google Scholar] [CrossRef]
- Deshmukh, S.; Kumar, R.; Bala, K. Microalgae biodiesel: A review on oil extraction, fatty acid composition, properties and effect on engine performance and emissions. Fuel Process. Technol. 2019, 191, 232–247. [Google Scholar] [CrossRef]
- Lin, T.S.; Wu, J.Y. Effect of carbon sources on growth and lipid accumulation of newly isolated microalgae cultured under mixotrophic condition. Bioresour. Technol. 2015, 184, 100–107. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Ji, M.K.; Yun, H.S.; Park, Y.T.; Kabra, A.N.; Oh, I.H.; Choi, J. Mixotrophic cultivation of a microalga Scenedesmus obliquus in municipal wastewater supplemented with food wastewater and flue gas CO2 for biomass production. J. Environ. Manag. 2015, 159, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Cheah, W.Y.; Show, P.L.; Juan, J.C.; Chang, J.S.; Ling, T.C. Enhancing biomass and lipid productions of microalgae in palm oil mill effluent using carbon and nutrient supplementation. Energy Convers. Manag. 2018, 164, 188–197. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Rodrigues, C.M.; Pires, J.C.; Simões, M. The effect of increasing CO2 concentrations on its capture, biomass production and wastewater bioremediation by microalgae and cyanobacteria. Algal Res. 2016, 14, 127–136. [Google Scholar] [CrossRef]
- Swarnalatha, G.V.; Hegde, N.S.; Chauhan, V.S.; Sarada, R. The effect of carbon dioxide rich environment on carbonic anhydrase activity, growth and metabolite production in indigenous freshwater microalgae. Algal Res. 2015, 9, 151–159. [Google Scholar] [CrossRef]
- Kong, W.; Yang, S.; Wang, H.; Huo, H.; Guo, B.; Liu, N.; Zhang, A.; Niu, S. Regulation of biomass, pigments, and lipid production by Chlorella vulgaris 31 through controlling trophic modes and carbon sources. J. Appl. Phycol. 2020, 32, 1569–1579. [Google Scholar] [CrossRef]
- Lu, W.; Liu, S.; Lin, Z.; Lin, M. Enhanced microalgae growth for biodiesel production and nutrients removal in raw swine wastewater by carbon sources supplementation. Waste Biomass Valorization 2021, 12, 1991–1999. [Google Scholar] [CrossRef]
- Najafabadi, H.A.; Malekzadeh, M.; Jalilian, F.; Vossoughi, M.; Pazuki, G. Effect of various carbon sources on biomass and lipid production of Chlorella vulgaris during nutrient sufficient and nitrogen starvation conditions. Bioresour. Technol. 2015, 180, 311–317. [Google Scholar] [CrossRef]
- Chiranjeevi, P.; Mohan, S.V. Critical parametric influence on microalgae cultivation towards maximizing biomass growth with simultaneous lipid productivity. Renew. Energy 2016, 98, 64–71. [Google Scholar] [CrossRef]
- Begum, H.; Yusoff, F.M.; Banerjee, S.; Khatoon, H.; Shariff, M. Availability and utilization of pigments from microalgae. Crit. Rev. Food Sci. Nutr. 2016, 56, 2209–2222. [Google Scholar] [CrossRef]
- Ambati, R.R.; Gogisetty, D.; Aswathanarayana, R.G.; Ravi, S.; Bikkina, P.N.; Bo, L.; Yuepeng, S. Industrial potential of carotenoid pigments from microalgae: Current trends and future prospects. Crit. Rev. Food Sci. Nutr. 2019, 59, 1880–1902. [Google Scholar] [CrossRef] [PubMed]
- Martin-Jézéquel, V.; Hildebrand, M.; Brzezinski, M.A. Silicon metabolism in diatoms: Implications for growth. J. Phycol. 2000, 36, 821–840. [Google Scholar] [CrossRef]
- Van Den Hende, S.; Vervaeren, H.; Boon, N. Flue gas compounds and microalgae:(Bio-) chemical interactions leading to biotechnological opportunities. Biotechnol. Adv. 2012, 30, 1405–1424. [Google Scholar] [CrossRef]
- Liang, Y.; Sarkany, N.; Cui, Y. Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol. Lett. 2009, 31, 1043–1049. [Google Scholar] [CrossRef]
- ICIS Chemical Pricing. Available online: https://www.icis.com/explore/commodities/chemicals/ (accessed on 26 April 2025).
- Li, X.; Xu, H.; Wu, Q. Large-scale biodiesel production from microalga Chlorella protothecoides through heterotrophic cultivation in bioreactors. Biotechnol. Bioeng. 2007, 98, 764–771. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Wei, D.; Chen, G. High yields of fatty acid and neutral lipid production from cassava bagasse hydrolysate (CBH) by heterotrophic Chlorella protothecoides. Bioresour. Technol. 2015, 191, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Pawar, S.B.; Pandey, R.A. Current practices and challenges in using microalgae for treatment of nutrient rich wastewater from agro-based industries. Sci. Total Environ. 2019, 687, 1107–1126. [Google Scholar] [CrossRef]
- Yan, D.; Lu, Y.; Chen, Y.F.; Wu, Q. Waste molasses alone displaces glucose-based medium for microalgal fermentation towards cost-saving biodiesel production. Bioresour. Technol. 2011, 102, 6487–6493. [Google Scholar] [CrossRef]
- Abomohra, A.; Li, M.; Faisal, S.; Li, L.; Elsayed, M. Maximizing nitrogen removal and lipid production by microalgae under mixotrophic growth using response surface methodology: Towards enhanced biodiesel production. Fermentation 2022, 8, 682. [Google Scholar] [CrossRef]
- Li, T.; Liu, Y.H.; Lu, F.P.; Jiang, Y. Effects of glucose assimilation on lutein and chlorophyll biosyntheses in the green alga Chlorella pyrenoidosa. In Proceedings of the 2012 International Conference on Applied Biotechnology (ICAB 2012), Tianjin, China, 18–19 October 2012; Springer: Berlin/Heidelberg, Germany, 2014; Volume 2, pp. 943–954. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.; Lu, Q.; Zhou, W.; Huo, S.; Cheng, P.; Liu, J.; Addy, M.; et al. Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef] [PubMed]
- Powell, N.; Shilton, A.N.; Pratt, S.; Chisti, Y. Factors influencing luxury uptake of phosphorus by microalgae in waste stabilization ponds. Environ. Sci. Technol. 2008, 42, 5958–5962. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Guo, L.; Li, X.; Wang, Y. Effect of phosphorus concentration and light/dark condition on phosphorus uptake and distribution with microalgae. Bioresour. Technol. 2021, 340, 125745. [Google Scholar] [CrossRef]
- Standardization Administration of China. GB 18596–2001; Standard for Hazardous Waste Storage. China Standards Press: Beijing, China, 2001.
- Kumar, A.; Ergas, S.; Yuan, X.; Sahu, A.; Zhang, Q.; Dewulf, J.; Malcata, F.X.; van Langenhove, H. Enhanced CO2 fixation and biofuel production via microalgae: Recent developments and future directions. Trends Biotechnol. 2010, 28, 371–380. [Google Scholar] [CrossRef]
- Yen, H.W.; Hu, I.C.; Chen, C.Y.; Nagarajan, D.; Chang, J.S. Design of photobioreactors for algal cultivation. In Biofuels from Algae; Elsevier: Amsterdam, The Netherlands, 2019; pp. 225–256. [Google Scholar] [CrossRef]
- Ma, X.; Mi, Y.; Zhao, C.; Wei, Q. A comprehensive review on carbon source effect of microalgae lipid accumulation for biofuel production. Sci. Total Environ. 2022, 806, 151387. [Google Scholar] [CrossRef]
- Markou, G.; Vandamme, D.; Muylaert, K. Microalgal and cyanobacterial cultivation: The supply of nutrients. Water Res. 2014, 65, 186–202. [Google Scholar] [CrossRef]
- Qi, F.; Pei, H.; Ma, G.; Zhang, S.; Mu, R. Improving productivity and quality of biodiesel from Chlorella vulgaris SDEC-3M through customized process designs. Energy Convers. Manag. 2016, 129, 100–107. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Z.; Xiao, Y.; Yuan, M.; Zhou, C.; Liu, G.; Fang, J.; Yang, B. Biochemical and morphological changes triggered by nitrogen stress in the oleaginous microalga Chlorella vulgaris. Microorganisms 2022, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Zheng, P.; Ding, A.; Zhang, M.; Abbas, G.; Li, W. Source analysis of organic matter in swine wastewater after anaerobic digestion with EEM-PARAFAC. Environ. Sci. Pollut. Res. 2017, 24, 6770–6778. [Google Scholar] [CrossRef]
- Han, L.; Pei, H.; Hu, W.; Han, F.; Song, M.; Zhang, S. Nutrient removal and lipid accumulation properties of newly isolated microalgal strains. Bioresour. Technol. 2014, 165, 38–41. [Google Scholar] [CrossRef]
- Sun, Z.L.; Sun, L.Q.; Chen, G.Z. Microalgal cultivation and nutrient removal from digested piggery wastewater in a thin-film flat plate photobioreactor. Appl. Biochem. Biotechnol. 2019, 187, 1488–1501. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.B.; Zhao, X.C.; Yang, L.B.; Liao, J.Y.; Zhou, Y.Y. Enhanced biomass and lipid production for cultivating Chlorella pyrenoidosa in anaerobically digested starch wastewater using various carbon sources and up-scaling culture outdoors. Biochem. Eng. J. 2018, 135, 105–114. [Google Scholar] [CrossRef]
- Wu, P.F.; Teng, J.C.; Lin, Y.H.; Hwang, S.C.J. Increasing algal biofuel production using Nannocholropsis oculata cultivated with anaerobically and aerobically treated swine wastewater. Bioresour. Technol. 2013, 133, 102–108. [Google Scholar] [CrossRef]



| Parameters | Non-Autoclaved ADSW | Autoclaved ADSW |
|---|---|---|
| TN (mg/L) | 370.67 ± 26.42 | 332.47 ± 41.37 |
| TP (mg/L) | 16.68 ± 0.81 | 12.400 ± 0.91 |
| NH4+-N (mg/L) | 221.60 ± 3.60 | 100.73 ± 12.59 |
| COD (mg/L) | 1050.19 ± 18.83 | 702.25 ± 12.18 |
| TOC (mg/L) | 356.45 ± 10.72 | 168.01 ± 5.74 |
| IC (mg/L) | 301.32 ± 10.67 | 157.98 ± 6.65 |
| pH | 7.45 ± 0.62 | 8.47 ± 0.53 |
| Carbon Source | Biomass Production (g/L) | Biomass Productivity (mg/L/d) | Maximum Biomass Productivity (mg/L/d) | Specific Growth Rate (/d) | Maximum Specific Growth Rate (/d) |
|---|---|---|---|---|---|
| CK | 0.92 ± 0.09 e | 43.81 ± 9.67 e | 166.67 ± 16.27 e | 0.08 ± 0.02 c | 0.31 ± 0.02 b |
| NaHCO3 | 0.99 ± 0.17 de | 50.95 ± 15.68 de | 261.25 ± 51.27 cd | 0.09 ± 0.02 c | 0.27 ± 0.11 b |
| Glucose | 3.90 ± 0.15 a | 258.10 ± 15.24 a | 830.00 ± 45.89 a | 0.19 ± 0.01 a | 0.86 ± 0.04 a |
| Na2CO3 | 0.88 ± 0.12 e | 42.26 ± 12.00 e | 185.00 ± 45.21 ed | 0.08 ± 0.02 c | 0.28 ± 0.08 b |
| CH3COONa | 3.33 ± 0.23 b | 216.31 ± 18.63 b | 542.50 ± 4.33 b | 0.17 ± 0.01 a | 0.70 ± 0.01 a |
| Glycerol | 1.62 ± 0.17 c | 94.52 ± 10.82 c | 301.25 ± 37.12 c | 0.12 ± 0.01 b | 0.29 ± 0.04 b |
| Sucrose | 1.31 ± 0.10 cd | 70.83 ± 3.93 cd | 235.83 ± 46.46 cde | 0.10 ± 0.01 bc | 0.40 ± 0.19 b |
| Carbon Source | TN | TP | ||||
|---|---|---|---|---|---|---|
| Initial Concentration (mg/L) | Final Concentration (mg/L) | Removal Efficiency (%) | Initial Concentration (mg/L) | Final Concentration (mg/L) | Removal Efficiency (%) | |
| CK | 332.47 ± 41.37 | 37.77 ± 5.86 | 88.58 ± 2.26 b | 12.40 ± 0.91 | 6.93 ± 0.41 | 44.16 ± 1.13 b |
| NaHCO3 | 110.20 ± 6.00 | 35.40 ± 2.69 | 67.84 ± 3.00 c | 21.47 ± 1.60 | 6.23 ± 0.89 | 43.76 ± 4.38 b |
| Glucose | 453.13 ± 11.35 | 42.43 ± 3.84 | 90.62 ± 1.19 a | 17.53 ± 0.34 | 5.78 ± 1.00 | 76.14 ± 0.76 a |
| Na2CO3 | 189.80 ± 18.37 | 24.95 ± 3.60 | 86.56 ± 3.89 ab | 20.33 ± 1.00 | 4.18 ± 0.13 | 23.06 ± 6.78 c |
| CH3COONa | 189.20 ± 22.84 | 23.48 ± 3.69 | 87.65 ± 0.62 a | 24.53 ± 3.87 | 12.44 ± 0.90 | 48.32 ± 9.76 b |
| Glycerol | 233.87 ± 17.50 | 45.37 ± 6.15 | 80.32 ± 5.02 b | 12.60 ± 0.71 | 12.03 ± 0.24 | 50.63 ± 7.82 b |
| Sucrose | 281.00 ± 37.54 | 31.367 ± 1.479 | 85.25 ± 5.29 ab | 19.00 ± 1.88 | 15.81 ± 0.77 | 69.93 ± 3.78 a |
| Glucose Concentration (g/L) | Biomass Production (g/L) | Biomass Productivity (mg/L/d) | Maximum Biomass Productivity (mg/L/d) | Specific Growth Rate (/d) | Maximum Specific Growth Rate (/d) |
|---|---|---|---|---|---|
| 0 | 0.58 ± 0.07 e | 40.36 ± 6.84 e | 75.00 ± 14.14 f | 0.31 ± 0.07 a | 0.88 ± 0.24 b |
| 2 | 1.40 ± 0.06 d | 98.45 ± 4.17 d | 240.00 ± 13.23 e | 0.35 ± 0.09 a | 0.85 ± 0.01 b |
| 5 | 1.70 ± 0.02 c | 119.17 ± 1.09 c | 724.17 ± 92.38 b | 0.30 ± 0.06 a | 1.24 ± 0.15 ab |
| 8 | 1.84 ± 0.03 c | 129.29 ± 1.29 c | 383.75 ± 90.16 d | 0.33 ± 0.08 a | 1.62 ± 0.29 a |
| 10 | 3.04 ± 0.12 b | 213.21 ± 13.27 b | 506.67 ± 62.77 cd | 0.31 ± 0.07 a | 0.99 ± 0.47 ab |
| 20 | 3.81 ± 0.21 a | 271.31 ± 18.02 a | 1350.00 ± 91.92 a | 0.42 ± 0.05 a | 1.00 ± 0.17 ab |
| 30 | 1.92 ± 0.10 c | 135.71 ± 8.92 c | 601.67 ± 25.66 bc | 0.37 ± 0.07 a | 1.45 ± 0.56 ab |
| Glucose Concentration (g/L) | TN | TP | ||||
|---|---|---|---|---|---|---|
| Initial Concentration (mg/L) | Final Concentration (mg/L) | Removal Efficiency (%) | Initial Concentration (mg/L) | Final Concentration (mg/L) | Removal Efficiency (%) | |
| 0 | 329.50 ± 50.77 | 27.03 ± 0.96 | 91.60 ± 1.00 ab | 19.21 ± 0.86 | 13.87 ± 3.36 | 37.38 ± 9.56 e |
| 2 | 349.90 ± 13.71 | 27.90 ± 2.41 | 92.15 ± 0.63 a | 21.53 ± 1.97 | 12.82 ± 1.99 | 40.58 ± 5.73 de |
| 5 | 347.93 ± 14.01 | 41.95 ± 4.85 | 87.97 ± 0.93 c | 18.80 ± 1.00 | 4.13 ± 0.60 | 78.03 ± 2.86 a |
| 8 | 312.33 ±12.99 | 49.25 ± 2.17 | 84.76 ± 0.62 d | 19.95 ± 0.93 | 9.32 ± 0.49 | 53.21 ± 3.63 cd |
| 10 | 347.47 ± 29.22 | 47.42 ± 3.89 | 86.22 ± 2.34 cd | 22.18 ± 0.88 | 8.95 ± 1.04 | 59.65 ± 4.52 bc |
| 20 | 329.80 ± 23.55 | 37.23 ± 4.44 | 88.70 ± 1.17 bc | 19.73 ± 2.54 | 3.32 ± 0.56 | 82.93 ± 4.18 a |
| 30 | 297.40 ± 34.47 | 46.33 ± 0.74 | 84.27 ± 1.96 d | 22.25 ± 1.26 | 6.55 ± 1.28 | 70.70 ± 4.23 ab |
| Fatty Acid | Percentage (%) |
|---|---|
| C14:0 | 4.51 ± 0.29 |
| C14:1 | 2.96 ± 0.16 |
| C16:0 | 26.99 ± 0.72 |
| C16:1 | 4.16 ± 0.63 |
| C18:0 | 6.26 ± 0.15 |
| C18:1 n-9 | 15.97 ± 1.11 |
| C18:2 n-6 | 25.51 ± 1.09 |
| C20:0 | 13.00 ± 0.42 |
| C20:5 n-3 | 0.64 ± 0.11 |
| SFA a | 50.77 ± 0.97 |
| UFA b | 49.23 ± 0.97 |
| MUFA c | 23.09 ± 1.63 |
| PUFA d | 26.14 ± 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Yuan, M.; Huang, C.; Chen, Q.; Wang, J.; Chen, X.; Yang, H.; Fang, J.; Yang, B. Enhancing Biomass Production of Chlorella vulgaris in Anaerobically Digested Swine Wastewater Using Carbon Supplementation and Simultaneous Lipid Production. Appl. Sci. 2025, 15, 5103. https://doi.org/10.3390/app15095103
Zhou C, Yuan M, Huang C, Chen Q, Wang J, Chen X, Yang H, Fang J, Yang B. Enhancing Biomass Production of Chlorella vulgaris in Anaerobically Digested Swine Wastewater Using Carbon Supplementation and Simultaneous Lipid Production. Applied Sciences. 2025; 15(9):5103. https://doi.org/10.3390/app15095103
Chicago/Turabian StyleZhou, Chenkai, Mingmin Yuan, Cuifeng Huang, Qiqi Chen, Jiamin Wang, Xinting Chen, Hua Yang, Jun Fang, and Bo Yang. 2025. "Enhancing Biomass Production of Chlorella vulgaris in Anaerobically Digested Swine Wastewater Using Carbon Supplementation and Simultaneous Lipid Production" Applied Sciences 15, no. 9: 5103. https://doi.org/10.3390/app15095103
APA StyleZhou, C., Yuan, M., Huang, C., Chen, Q., Wang, J., Chen, X., Yang, H., Fang, J., & Yang, B. (2025). Enhancing Biomass Production of Chlorella vulgaris in Anaerobically Digested Swine Wastewater Using Carbon Supplementation and Simultaneous Lipid Production. Applied Sciences, 15(9), 5103. https://doi.org/10.3390/app15095103






