A Multiple Regression Model Analysing Additional Sources of Dietary Fibre as a Factor Affecting the Development of the Gastrointestinal Tract in Broiler Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Chemical Analysis
2.3. Length and Weight Measurements
2.4. Statistical Analysis
3. Results
3.1. Performance of Broiler Chickens
3.2. Weight of Individual Organs
3.3. Length of Intestines
3.4. Correlation Between Dietary Fibre Types and Measurements of Intestines and Organs
3.5. Model of the Development of the Gastrointestinal Tract of Broiler Chickens at Day 35
4. Discussion
4.1. Animal Models in Human Studies
4.2. Diet Differentiation and Dietary Fibre
4.3. Kind of Fibre and Its Effect on Performance of Broiler Chickens
4.4. Morphometric Analysis of the Selected Organs and Their Correlation with Food Fibre in the Diet
4.5. Morphometric Analysis of Intestinal Sections and Their Correlation with Dietary Fibre in the Diet
4.6. Experimental Model Based on Morphometric Measurements and Dietary Fibre Content
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arcuri, S.; Pennarossa, G.; Pasquariello, R.; Prasadani, M.; Gandolfi, F.; Brevini, T.A.L. Generation of porcine and rainbow trout 3D intestinal models and their use to investigate astaxanthin effects in vitro. Int. J. Mol. Sci. 2024, 25, 5966. [Google Scholar] [CrossRef]
- Jia, H.J.; Chang, Y.; Song, J. The pig as an optimal animal for cardiovascular research. Lab Anim. 2024, 53, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Sun, Y.; Shujaat, S.; Braem, A.; Politis, C.; Jacobs, R. 3D-printed porous Ti6Al4V scaffolds for long bone repair in animal models: A systematic review. J. Orthop. Surg. Res. 2022, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Roy, S.; Ghosh, D.; Nandi, S.K. Role of animal models in biomedical research: A review. Lab Anim. Res. 2022, 38, 18. [Google Scholar] [CrossRef]
- Flores-Santin, J.; Burggren, W.W. Beyond the chicken: Alternative avian models for developmental physiological research. Front. Physiol. 2021, 12, 712633. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Zhang, M.; Feng, J. Gut microbiota alleviates intestinal injury induced by extended exposure to light inhibiting the activation of NLRP3 inflammasome in broiler chickens. Int. J. Mol. Sci. 2024, 25, 6695. [Google Scholar] [CrossRef]
- Tregaskes, C.A.; Kaufman, J. Chickens as a simple system for scientific discovery: The example of the MHC. Mol. Immunol. 2021, 135, 12–20. [Google Scholar] [CrossRef]
- De Paula Reis, M.; Sakomura, N.K.; Teixeira, I.A.M.A.; Da Silva, E.P.; Kebreab, E. Partitioning the efficiency of utilization of amino acids in growing broilers: Multiple linear regression and multivariate approaches. PLoS ONE 2018, 13, e0208488. [Google Scholar] [CrossRef]
- Wen, J.-J.; Li, M.-Z.; Hu, J.-L.; Wang, J.; Wang, Z.-Q.; Chen, C.-H.; Yang, J.-R.; Huang, X.-J.; Xie, M.-Y.; Nie, S.-P. Different dietary fibers unequally remodel gut microbiota and charge up anti-obesity effects. Food. Hydrocoll. 2023, 140, 108617. [Google Scholar] [CrossRef]
- Adam, C.L.; Williams, P.A.; Dalby, M.J.; Garden, K.; Thomson, L.M.; Richardson, A.J.; Gratz, S.W.; Ross, A.W. Different types of soluble dietary fibre decrease food intake, body weight gain and adiposity in young adult male rats. Nutr. Metab. 2014, 11, 36. [Google Scholar] [CrossRef]
- Baky, M.H.; Salah, M.; Ezzelarab, N.; Shao, P.; Elshahed, M.S.; Farag, M.A. Insoluble dietary fibers: Structure, metabolism, interactions with human microbiome, and role in gut homeostasis. Crit. Rev. Food Sci. Nutr. 2022, 64, 1954–1968. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, B.; Wen, L.; Wang, F.; Yu, H.; Chen, D.; Su, X.; Zhang, C. Effects of dietary fiber on human health. Food Sci. Hum. Wellness 2022, 11, 1–10. [Google Scholar] [CrossRef]
- Zhang, C.; Hao, E.; Chen, X.; Huang, C.; Lin, G.; Chen, H.; Wang, D.; Shi, L.; Xuan, F.; Cheng, D.; et al. Dietary fiber level improve growth performance, nutrient digestibility, immune and intestinal morphology of broilers from day 22 to 42. Animals 2023, 13, 1227. [Google Scholar] [CrossRef]
- Mulla, N.A.; Desai, D.N.; Avari, P.E.; Ranade, A.S. Use of natural insoluble fiber in oat hulls (Avena sativa) as non-antibiotic growth promoter in broilers. Int. J. Livest. Res. 2020, 10, 156–164. [Google Scholar] [CrossRef]
- Babatunde, O.O.; Park, C.S.; Adeola, O. Nutritional potentials of atypical feed ingredients for broiler chickens and pigs. Animals 2021, 11, 1196. [Google Scholar] [CrossRef]
- Jamroz, D.; Jakobsen, K.; Bach Knudsen, K.E.; Wiliczkiewicz, A.; Orda, J. Digestibility and Energy value of non-starch polysaccharides in young chickens, ducks and geese, fed diets containing high amounts of barley. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 131, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Fourie, A.B.; Wandrag, D.B.R. Effect of different dietary fibre raw material sources on production and gut development in fast-growing broilers. S. Afr. J. Anim. Sci. 2024, 54, 166–175. [Google Scholar] [CrossRef]
- Wróblewska, P.; Hikawczuk, T.; Szuba-Trznadel, A.; Wiliczkiewicz, A.; Zinchuk, A.; Rusiecka, A.; Laszki-Szcząchor, K. Effect of triticale grain in diets on performance, development of gastrointestinal tract and microflora in crop and ileum of broiler chickens. Microorganisms 2024, 12, 1239. [Google Scholar] [CrossRef]
- Berrocoso, J.D.; García-Ruiz, A.; Page, G.; Jaworski, N.W. The effect of added oat hulls or sugar beet pulp to diets containing rapidly or slowly digestible protein sources on broiler growth performance from 0 to 36 days of age. Poult. Sci. 2020, 99, 6859–6866. [Google Scholar] [CrossRef]
- Jiménez-Moreno, E.; Frikha, M.; de Coca-Sinova, A.; Lázaro, R.P.; Mateos, G.G. Oat hulls and sugar beet pulp in diets for broilers. 2. Effects on the development of the gastrointestinal tract and on the structure of the jejunal mucosa. Anim. Feed Sci. Technol. 2013, 182, 44–52. [Google Scholar] [CrossRef]
- Ahmmad, G.S.; Lim, C.B.; Kim, I.H. Effect of dietary almond hull on growth performance, nutrient digestibility, organ weight, caecum microbial counts, and noxious gas emission in broilers. Braz. J. Poult. Sci. 2024, 26, 1–8. [Google Scholar] [CrossRef]
- Wróblewska, P.; Hikawczuk, T.; Sierżant, K.; Wiliczkiewicz, A.; Szuba-Trznadel, A. Effect of oat hull as a source of insoluble dietary fibre on changes in the microbial status of gastrointestinal tract in broiler chickens. Animals 2022, 12, 2721. [Google Scholar] [CrossRef] [PubMed]
- Aziz-Aliabadi, F.; Hassanabadi, A.; Golian, A.; Zerehdaran, S. Optimisation of broilers performance to different dietary levels of fibre and different levels and sources of fat from 0 to 14 days of age. Ital. J. Anim. Sci. 2021, 20, 395–405. [Google Scholar] [CrossRef]
- Asp, N.-G. Dietary fibre-definition, chemistry and analytical determination. Mol. Asp. Med. 1987, 9, 17–29. [Google Scholar] [CrossRef]
- Sadeghi, A.; Toghyani, M.; Gheisari, A. Effect of various fiber types and choice feeding of fiber on performance, gut development, humoral immunity, and fiber preference in broiler chicks. Poult. Sci. 2015, 94, 2734–2743. [Google Scholar] [CrossRef]
- Taylor, J.; Sakkas, P.; Kyriazakis, I. What are the limits to feed intake of broilers on bulky feeds? Poult. Sci. 2021, 100, 100825. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, S.; Itani, K.; Kaczmarek, S.; Smith, A.; Svihus, B. Influence of particle size and inclusion level of oat hulls on retention and passage in the anterior digestive tract of broilers. Brit. Poult. Sci. 2024, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Adewole, D.I.; Oladokun, S.; Santin, E. Effect of organic acids-essential oils blend and oat fiber combination on broiler chicken growth performance, blood parameters, and intestinal health. Anim. Nutr. 2021, 7, 1039–1051. [Google Scholar] [CrossRef]
- Röhe, I.; Zentek, J. Lignocellulose as an insoluble fiber source in poultry nutrition: A review. J. Anim. Sci. Biotechnol. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Ahsan, T.; Tahir, M.; Naz, S.; Khan, R.U.; Alhidary, I.A.; Abdelrahman, S.H.; Selvaggi, M. Effect of soy hulls as alternative ingredient on growth performance, carcase quality, nutrients digestibility and intestinal histological features in broilers. Ital. J. Anim. Sci. 2024, 23, 1336–1347. [Google Scholar] [CrossRef]
- Bamedi, A.; Salari, S.; Baghban, F. Changes in performance, cecal microflora counts and intestinal histology of Japanese quails fed diets containing different fibre sources. Vet. Anim. Sci. 2024, 25, 100386. [Google Scholar] [CrossRef]
- Jha, R.; Mishra, P. Dietary fiber in poultry nutrition and their effects on nutrient utilization, performance, gut health, and on the environment: A review. J. Anim. Sci. Biotechnol. 2021, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Adewole, D.; MacIsaac, J.; Fraser, G.; Rathgeber, B. Effect of oat hulls incorporated in the diet of fed as free choice on growth performance, carcass yield, gut morphology and digesta short chain fatty acids of broiler chickens. Suatainability 2020, 12, 3744. [Google Scholar] [CrossRef]
- Itani, K.; Apajalahti, J.; Smith, A.; Ghimire, S.; Svihus, B. The effect of increasing the level of oat hulls, extent of grinding and their interaction on the performance, gizzard characteristics and gut health of broiler chickens fed oat-based pelleted diets. Anim. Feed Sci. Technol. 2024, 308, 115858. [Google Scholar] [CrossRef]
- Rawash, M.A.; Farkas, V.; Such, N.; Mezőlaki, Á.; Menyhárt, L.; Pál, L.; Csitári, G.; Dublecz, K. Effects of barley- and oat-based diets on some gut parameters and microbiota composition of the small intestine and ceca of broiler chicken. Agriculture 2023, 13, 169. [Google Scholar] [CrossRef]
- Aziz-Aliabadi, F.; Hassanabadi, A.; Zerehdaran, S.; Noruzi, H. Evaluation of the effect of different levels of fiber and fat on young broiler’s performance, pH, and viscosity of digesta using response surface methodology. Iran. J. Appl. Anim. Sci. 2023, 13, 333–343. [Google Scholar]
- Adewole, D. Effect of dietary supplementation with coarse or extruded oat hulls on growth performance, blood biochemical parameters, ceca microbiota and short chain fatty acids in broiler chickens. Animals 2020, 10, 1429. [Google Scholar] [CrossRef]
- Naeem, M.; Burton, E.; Scholey, D.; Alkhtib, A.; Broadberry, S. Efficacy of oat hulls varying in particle size in mitigating performance deterioration in broilers fed low-density crude protein diets. Poult. Sci. 2023, 102, 102979. [Google Scholar] [CrossRef]
- Garçon, C.J.J.; Ellis, J.L.; Powell, C.D.; Navarro Villa, A.; Garcia Ruiz, A.I.; France, J.; de Vries, S. A dynamic model to measure retention of solid and liquid digesta fractions in chickens fed diets with differing fibre sources. Animal 2023, 17, 100867. [Google Scholar] [CrossRef]
- Bila, L.; Tyasi, T.L.; Tongwane, T.W.N.; Mulaudzi, A.P. Correlation and path analysis of body weight and biometric traits of Ross 308 breed of broiler chickens. J. World Poult. Res. 2021, 11, 344–351. [Google Scholar] [CrossRef]
- Ebong, U.N.; Sam, I.M.; Essien, C.A.; Okon, L.S. Estimation of carcass yield from morphometric traits of ROSS 308 strain of broiler chickens raised in humid zone of Nigeria. AJAFS 2023, 7, 52–61. [Google Scholar] [CrossRef]
- Dong, J.Q.; Zhang, X.Y.; Wang, S.Z.; Jiang, X.F.; Zhang, K.; Ma, G.W.; Wu, M.Q.; Li, H.; Zhang, H. Construction of multiple linear regression models using blood biomarkers for selecting against abdominal fat traits in broilers. Poult. Sci. 2018, 97, 17–23. [Google Scholar] [CrossRef] [PubMed]
- World’s Poultry Science Association; Nutrition of the European Federation of Branches Subcommittee Energy of the Working Group (Beekbergen). European Tables of Energy Values of Feeds for Poultry, 3rd ed.; WPSA: Wageningen, The Netherlands, 1989; pp. 11–28. [Google Scholar]
- Smulikowska, S.; Rutkowski, A. Polish Requirements of Poultry Nutrition, 4th ed.; Instytut Fizjologii i Żywienia Zwierząt, PAN: Jabłonna, Poland, 2005. (In Polish) [Google Scholar]
- AOAC. Official Methods of Analysis, 19th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2012. [Google Scholar]
- Boazar, E.; Salari, S.; Erfanimejd, N.; Fakhur, K.M. Effect of mash and pellet diets containing different sources of fiber on growth performance and cecal microbial population of broiler chickens. J. Livest. Sci. Technol. 2021, 9, 9–22. [Google Scholar] [CrossRef]
- Hikawczuk, T. Effect of Non-Starch Polysaccharides of Cereal Grains on Physiological Parameters of Crop and Ileum, and Digestibility of Nutrients in Broiler Chicken. Ph.D. Thesis, Uniwersytet Przyrodniczy we Wrocławiu, Wroclaw, Poland, 2013. Available online: https://www.dbc.wroc.pl/dlibra/publication/24396/edition/21350#description (accessed on 17 May 2013). (In Polish).
- Bednarczyk, M.; Dunislawska, A.; Stadnicka, K.; Grochowska, E. Chicken embryo as a model in epigenetic research. Poult. Sci. 2021, 100, 101164. [Google Scholar] [CrossRef]
- Wachholz, G.E.; Rengel, B.D.; Vargesson, N.; Fraga, L.R. From the farm to the lab: How chicken embryos contribute to the field of teratology. Front. Genet. 2021, 12, 666726. [Google Scholar] [CrossRef]
- Beacon, T.H.; Davie, J.R. The chicken model organism for epigenomic research. Genome 2021, 64, 476–489. [Google Scholar] [CrossRef]
- Fu, Y.; Cheng, H.-W. The influence of cecal microbiota transplantation on chicken injurious behavior: Perspective in human neuropsychiatric research. Biomolecules 2024, 14, 1017. [Google Scholar] [CrossRef]
- Fu, J.; Zheng, Y.; Gao, Y.; Xu, W. Dietary fiber intake and gut microbiota in human health. Microorganisms 2022, 10, 2507. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.; Feng, Q.-Q.; Ojo, O.O.; Wang, X.-H. The role of dietary fibre in modulating gut microbiota dysbiosis in patients with type 2 diabetes: A systematic review and meta-analysis of randomised controlled trials. Nutrients 2020, 12, 3239. [Google Scholar] [CrossRef]
- Niekamp, P.; Kim, C.H. Microbial metabolite dysbiosis and colorectal cancer. Gut Liver 2023, 17, 190–203. [Google Scholar] [CrossRef]
- Wickramasuriya, S.S.; Park, I.; Lee, K.; Lee, Y.; Kim, W.H.; Nam, H.; Lillehoj, H.S. Role of physiology, immunity, microbiota and infectious diseases in the gut health of poultry. Vaccines 2022, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Shehata, A.A.; Yalçin, S.; Latorre, J.D.; Basiouni, S.; Attia, Y.A.; El-Wahab, A.A.; Visscher, C.; El-Seedi, H.R.; Huber, C.; Hafez, H.M.; et al. Probiotics, prebiotics, and phytogenic substances for optimizing gut health in poultry. Microorganisms 2022, 10, 395. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, L.; Lin, H.; Shen, J.; Zhang, Y.; Lu, L.; Zhang, X.; Ma, X. Bacillus subtilis M6 improves intestinal barrier, antioxidant capacity and gut microbial composition in AA broiler. Front. Nutr. 2022, 9, 965310. [Google Scholar] [CrossRef] [PubMed]
- Denbow, D.M. Chapter 14—Gastrointestinal Anatomy and Physiology. In Sturkie’s Avian Physiology, 6th ed.; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar] [CrossRef]
- Lawal, R.A.; Hanotte, O. Domestic chicken diversity: Origin, distribution, and adaptation. Anim. Genet. 2021, 52, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Tejeda, O.J.; Kim, W.K. Role of dietary fiber in poultry nutrition. Animals 2021, 11, 461. [Google Scholar] [CrossRef]
- Van Emous, R.A.; Mens, A.J.W.; Winkel, A. Effects of diet density and feeding frequency during the rearing period on broiler breeder performance. Br. Poult. Sci. 2021, 62, 686–694. [Google Scholar] [CrossRef]
- Röhe, I.; Vahjen, W.; Metzger, F.; Zentek, J. Effect of a “diluted” diet containing 10% lignocellulose on the gastrointestinal tract, intestinal microbiota, and excreta characteristics of dual purpose laying hens. Poult. Sci. 2020, 99, 310–319. [Google Scholar] [CrossRef]
- Hikawczuk, T.; Szuba-Trznadel, A.; Wróblewska, P.; Wiliczkiewicz, A. Oat hull as a source of lignin-cellulose complex in diets containing wheat or barley and its effect on performance and morphometric measurements of gastrointestinal tract in broiler chickens. Agriculture 2023, 13, 896. [Google Scholar] [CrossRef]
- Giraldo, G.A.G.; Mantovan, J.; Marim, B.M.; Kishimea, J.O.F.; Mali, S. Surface modification of cellulose from oat hull with citric acid using ultrasonication and reactive extrusion assisted processes. Polysaccharides 2021, 2, 218–233. [Google Scholar] [CrossRef]
- Martínez-Gómez, S.; Yáñez, R.; Alonso, J.L. A new strategy for a separate manufacture arabinooligosaccharides and oligogalacturonides by hydrothermal treatment of sugar beet pulp. Food Bioprocess. Technol. 2024, 17, 4711–4723. [Google Scholar] [CrossRef]
- Beukema, M.; Faas, M.M.; de Vos, P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: Impact via gut microbiota and direct effects on immune cells. Exp. Mol. Med. 2020, 52, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Morgan, N.; Bhuiyan, M.M.; Hopcraft, R. Non-starch polysaccharide degradation in the gastrointestinal tract of broiler chickens fed commercial-type diets supplemented with either a single dose of xylanase, a double dose of xylanase, or a cocktail of non-starch polysaccharide-degrading enzymes. Poult. Sci. 2022, 101, 101846. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.T. Sources and levels of copper affect liver copper profile, intestinal morphology and cecal microbiota population of broiler chickens fed wheat-soybean meal diets. Sci. Rep. 2022, 12, 2249. [Google Scholar] [CrossRef]
- Juanchich, A.; Urvoix, S.; Hennequet-Antier, C.; Narcy, A.; Mignon-Grasteau, S. Phenotypic timeline of gastrointestinal tract development in broilers divergently selected for digestive efficiency. Poult. Sci. 2021, 100, 1205–1212. [Google Scholar] [CrossRef]
- Rasool, A.; Qaisrani, S.N.; Khalique, A.; Hussain, J. Insoluble fiber source influences performance, nutrients digestibility, gut development and carcass traits of broilers. Pak. J. Agric. Sci. 2023, 60, 355–365. [Google Scholar]
- González-Alvarado, J.M.; Jiménez-Moreno, E.; González-Sánchez, D.; Lázaro, R.; Mateos, G.G. Effect of inclusion of oat hulls and sugar beet pulp in the diet on productive performance and digestive traits of broilers from 1 to 42 days of age. Anim. Feed Sci. Technol. 2010, 162, 37–46. [Google Scholar] [CrossRef]
- Kimiaeitalab, M.V.; Cámara, L.; Mirzaie-Goudarzi, S.; Jiménez-Moreno, E.; Mateos, G.G. Effects of the inclusion of sunflower hulls in the diet on growth performance and digestive tract traits of broilers and pullets fed a broiler diet from zero to 21 d of age. A comparative study. Poult. Sci. 2017, 96, 581–592. [Google Scholar] [CrossRef]
- Kakhki, R.A.M.; Navarro-Villa, A.; de los Mozos, J.; de Vries, S.; García-Ruiz, A.I. Evaluation of fibrous feed ingredients alternatives to oat hulls as a source of feed structure in broiler diets. Poult. Sci. 2024, 103, 104297. [Google Scholar] [CrossRef]
- Shang, Q.; Wu, D.; Liu, H.; Manfuz, S.; Piao, X. The impact of wheat bran on the morphology and physiology of the gastrointestinal tract in broiler chickens. Animals 2020, 10, 1831. [Google Scholar] [CrossRef]
- Hetland, H.; Svihus, B.; Krogdahl, Å. Effects of oat hulls and wood shavings on digestion in broilers and layers fed diets based on whole or ground wheat. Br. Poult. Sci. 2003, 44, 275–282. [Google Scholar] [CrossRef]
- Abdel-Daim, A.S.A.; Tawfeek, S.S.; El-Nahas, E.S.; Hassan, A.H.A.; Youssef, I.M.I. Effect of feeding potato peels and sugar beet pulp with or without enzyme on nutrient digestibility, intestinal morphology, and meat quality of broiler chickens. Poult. Sci. J. 2020, 8, 189–199. [Google Scholar] [CrossRef]
- Dixon, L.M.; Brocklehurst, S.; Hills, J.; Foister, S.; Wilson, P.W.; Reid, A.M.A.; Caughey, S.; Sandilands, V.; Boswell, T.; Dunn, I.C.; et al. Dilution of broiler breeder diets with oat hulls prolongs feeding but does not affect central control of appetite. Poult. Sci. 2024, 103, 104262. [Google Scholar] [CrossRef] [PubMed]
- Yokhana, J.S.; Parkinson, G.; Frankel, T.L. Effect of insoluble fiber supplementation applied of different ages on digestive organ weight and digestive enzymes of layer-strain poultry. Poult. Sci. 2016, 95, 550–559. [Google Scholar] [CrossRef]
- Gonzalez-Ortiz, G.; Sola-Oriol, D.; Martinez-Mora, M.; Perez, J.F.; Bedford, M.R. Response of broiler chickens fed wheat-based diets to xylanase supplementation. Poult. Sci. 2017, 96, 2776–2785. [Google Scholar] [CrossRef]
- Wang, A.Y.-M.; Sea, M.M.-M.; Ng, K.; Wang, M.; Chan, I.H.-S.; Lam, C.W.-K.; Sanderson, J.E.; Woo, J. Dietary fiber intake, myocardial injury, and major adverse cardiovascular events among end-stage kidney disease patients: A prospective cohort study. Kidney Int. Rep. 2019, 4, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Saadatmand, N.; Toghyani, M.; Gheisari, A. Effects of dietary fiber and threonine on performance, intestinal morphology and immune responses in broiler chickens. Anim. Nutr. 2019, 5, 248–255. [Google Scholar] [CrossRef]
- Scholey, D.V.; Marshall, A.; Cowan, A.A. Evaluation of oats with varying hull inclusion in broiler diets up to 35 days. Poult. Sci. 2020, 99, 2566–2572. [Google Scholar] [CrossRef] [PubMed]
- Jangiaghdam, S.; Mirzaie Goudarzi, S.; Saki, A.A.; Zamani, P. Growth performance, nutrient digestibility, gastrointestinal tract traits in response to dietary fiber sources in broiler chickens. Poult. Sci. J. 2022, 10, 185–196. [Google Scholar] [CrossRef]
- De Souza Leite, B.G.; Granghelli, C.A.; de Arruda Roque, F.; Bueno Carvalho, R.S.; Scapin Lopes, M.H.; Pelissari, P.H.; Tuckmantel Dias, M.; da Silva Araújo, C.S.; Araújo, L.F. Evaluation of dietary lignin on broiler performance, nutrient digestibility, cholesterol and triglycerides concentrations, gut morphometry, and lipid oxidation. Poult. Sci. 2024, 103, 103518. [Google Scholar] [CrossRef]
- Ginindza, M.; Mbatha, K.R.; Ng’ambi, J. Dietary crude fiber levels for optimal productivity of male Ross 308 broiler and Venda chickens aged 1 to 42 days. Animals 2022, 12, 1333. [Google Scholar] [CrossRef]
- Rybicka, A.; del Pozo, R.; Carro, D.; García, J. Effect of type of fiber and its physiochemical properties on performance, digestive transit time and cecal fermentation in broilers from 1 to 23d of age. Poult. Sci. 2024, 103, 103192. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, N.R.; Santos, F.R.; Sousa Silva, M.R.; Rissato, I.S.; Roque, G.C.; Silva, C.M.; Barros, H.S.S.; Silva, N.F.; Minafra, C.S.; Araújn Neto, F.R. Dietary levels of soluble and insoluble fibre sources for young slow-growing broilers. Czech J. Anim. Sci. 2024, 69, 139–154. [Google Scholar] [CrossRef]
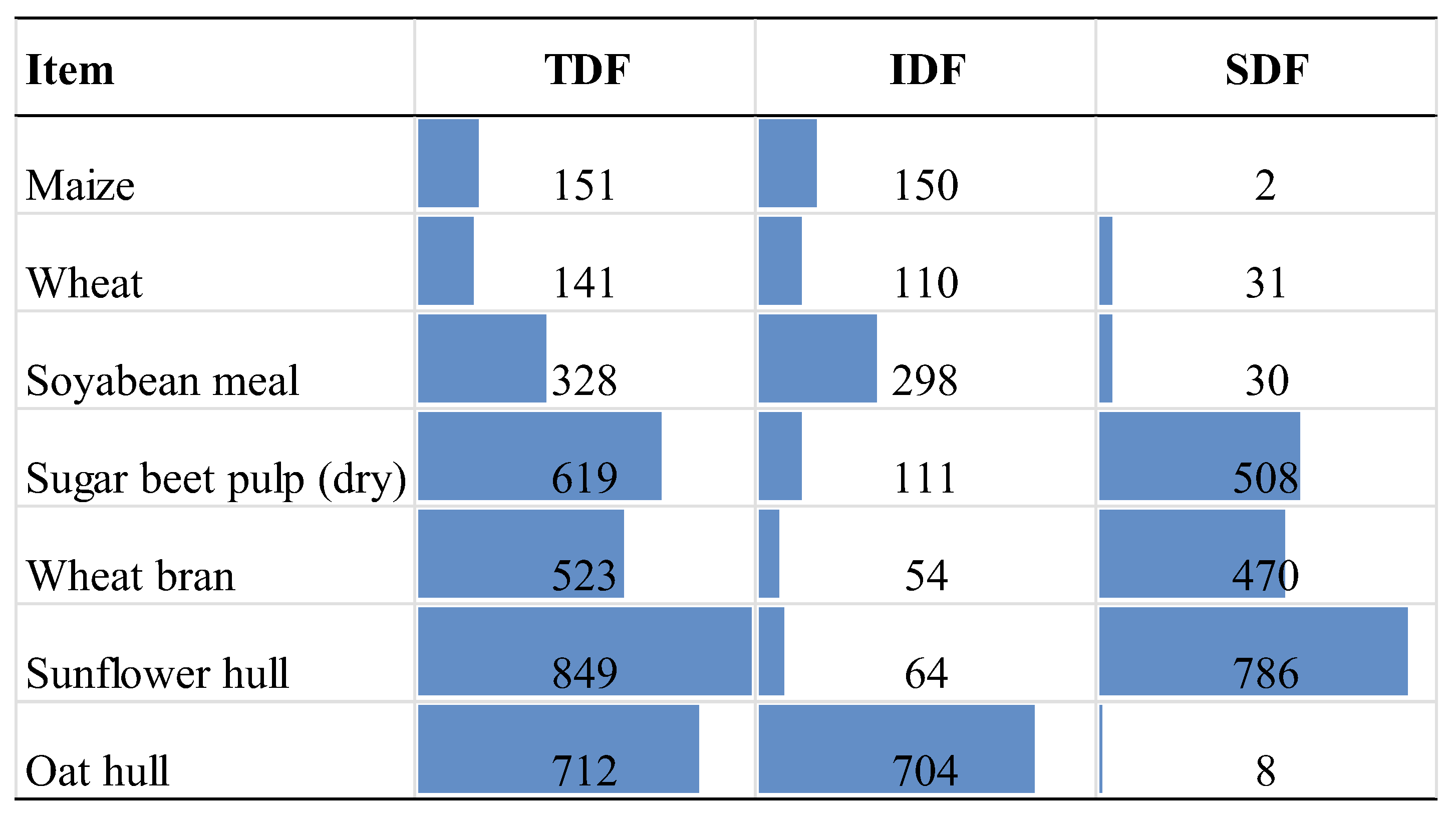
| Item | Control | 3% OH | 3% SH | 3% SBP | 3% WB |
|---|---|---|---|---|---|
| Maize | 50.2 | 11.9 | 7.9 | 11.1 | 10.3 |
| Wheat | 536.6 | 529.5 | 534.2 | 537.0 | 540.9 |
| Soybean meal | 320 | 325 | 327 | 322 | 318 |
| Soy oil | 53 | 63 | 61 | 61 | 61 |
| Oat hull | 30 | ||||
| Sunflower hull | 30 | ||||
| Dry sugar beet pulp | 30 | ||||
| Wheat bran | 30 | ||||
| NaCl | 2.2 | 2.2 | 2.2 | 2.2 | 2.2 |
| Sodium bicarbonate | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Monocalcium phosphate | 12.5 | 12.6 | 12.6 | 12.5 | 12.6 |
| Chalk | 14.2 | 14.2 | 14.2 | 13.5 | 14.2 |
| DL-methionine (98%) | 2.39 | 2.47 | 2.49 | 2.47 | 2.43 |
| HCl-L-lysine (78%) | 1.86 | 1.86 | 1.86 | 1.86 | 1.86 |
| Premix DKA starter 0.5% * | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Chemical composition (g/kg) | |||||
| Metabolisable energy, MJ | 12.51 | 12.51 | 12.50 | 12.51 | 12.50 |
| Crude protein, g | 220.2 | 220.1 | 220.5 | 219.8 | 220.2 |
| Crude fibre | 30.2 | 38.1 | 44.0 | 35.8 | 33.1 |
| Total dietary fibre | 188.4 | 204.6 | 209.2 | 201.6 | 197.8 |
| Insoluble dietary fibre | 162.1 | 178.2 | 159.3 | 160.1 | 157.4 |
| Soluble dietary fibre | 26.3 | 26.4 | 49.9 | 41.5 | 40.4 |
| Calcium | 9.4 | 9.4 | 9.4 | 9.4 | 9.4 |
| Pavailable | 4.3 | 4.3 | 4.3 | 4.3 | 4.4 |
| Sodium | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 |
| L-lysine | 12.00 | 12.02 | 12.06 | 11.95 | 12.02 |
| DL-methionine | 5.51 | 5.53 | 5.56 | 5.52 | 5.52 |
| Item | Control | 3% OH | 3% SH | 3% SBP | 3% WB |
|---|---|---|---|---|---|
| Maize | 76.4 | 55.6 | 54.8 | 58.1 | 62.4 |
| Wheat | 550 | 527 | 527 | 527 | 527 |
| Soybean meal | 267 | 273 | 275 | 272 | 268 |
| Soy oil | 66.0 | 73.5 | 72.5 | 73.0 | 72.5 |
| Oat hull | 30 | ||||
| Sunflower hull | 30 | ||||
| Dry sugar beet pulp | 30 | ||||
| Wheat bran | 30 | ||||
| NaCl | 2.2 | 2.2 | 2.2 | 2.2 | 2.2 |
| Sodium bicarbonate | 2.4 | 2.4 | 2.4 | 2.4 | 2.4 |
| Monocalcium phosphate | 11.6 | 11.9 | 11.8 | 11.6 | 11.3 |
| Chalk | 14.2 | 14.6 | 14.6 | 14.0 | 14.9 |
| DL-methionine (98%) | 2.39 | 2.39 | 2.39 | 2.41 | 2.38 |
| HCl-L-lysine (78%) | 2.82 | 2.95 | 2.81 | 2.89 | 2.82 |
| Premix DKA grower 0.5% | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Chemical composition (g/kg) | |||||
| Metabolisable energy, MJ | 13.03 | 12.96 | 13.00 | 13.02 | 12.99 |
| Crude protein, g | 200.0 | 199.9 | 200.2 | 200.0 | 200.3 |
| Crude fibre | 28.9 | 36.8 | 42.7 | 34.4 | 31.8 |
| Total dietary fibre | 176.82 | 193.71 | 198.34 | 190.93 | 187.30 |
| Insoluble dietary fibre | 151.65 | 168.87 | 150.14 | 151.14 | 148.78 |
| Soluble dietary fibre | 25.12 | 24.79 | 48.17 | 39.74 | 38.49 |
| Calcium | 9.2 | 9.2 | 9.2 | 9.2 | 9.2 |
| Pavailable | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| Sodium | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 |
| L-lysine | 11.44 | 11.57 | 11.51 | 11.50 | 11.51 |
| DL-methionine | 5.24 | 5.20 | 5.20 | 5.21 | 5.23 |
| Item | Body Weight, kg | Feed Intake, kg | FCR, kg/kg | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 7 | 21 | 35 | 7–21 | 21–35 | 7–35 | 7–21 | 21–35 | 7–35 | |
| ASC * | |||||||||
| Control | 0.132 | 0.65 A | 1.77 Aa | 0.96 bc | 1.78 ab | 2.75 | 1.87 A | 1.59 AB | 1.68 Aa |
| Oat hull | 0.134 | 0.56 C | 1.73 a | 1.01 ab | 1.71 b | 2.78 | 2.35 C | 1.53 A | 1.72 Aab |
| Sunflower hull | 0.132 | 0.64 AB | 1.73 a | 0.99 abc | 1.84 a | 2.80 | 1.95 A | 1.69 BC | 1.75 ab |
| Sugar beet pulp | 0.132 | 0.61 B | 1.66 B | 1.03 Aa | 1.77 ab | 2.74 | 2.16 B | 1.69 BC | 1.80 bc |
| Wheat bran | 0.134 | 0.61 B | 1.64 Bb | 0.94 Bc | 1.81 a | 2.78 | 2.00 AB | 1.77 C | 1.86 Bc |
| SEM | 0.001 | 0.007 | 0.017 | 0.012 | 0.021 | 0.022 | 0.042 | 0.025 | 0.023 |
| p-value | 0.235 | 0.000 | 0.033 | 0.026 | 0.040 | 0.586 | 0.000 | 0.000 | 0.007 |
| Item | Weight of | |||
|---|---|---|---|---|
| Proventriculus | Gizzard | Heart | Liver | |
| ASC * | ||||
| Control | 6.8 Bb | 26.1 C | 11.2 ab | 39.0 |
| Oat hull | 6.7 Bb | 35.4 A | 12.3 Aa | 40.1 |
| Sunflower hull | 7.5 a | 33.8 AB | 9.9 B | 40.0 |
| Dry sugar beet pulp | 7.6 a | 29.2 BC | 11.1 ab | 37.1 |
| Wheat bran | 8.0 A | 29.8 BC | 10.1 b | 37.4 |
| SEM | 0.153 | 0.830 | 0.274 | 0.607 |
| p-value | 0.004 | 0.002 | 0.017 | 0.313 |
| Item | Duodenum | Jejunum | Ileum | Ceca (Mean) | Large Intestine | Total Length of Intestines |
|---|---|---|---|---|---|---|
| ASC * | ||||||
| Control | 28.0 a | 76.0 | 77.6 A | 19.0 | 9.7 A | 210.3 Aa |
| Oat hull | 25.6 Bb | 77.2 | 77.3 A | 18.0 | 9.7 A | 207.8 a |
| Sunflower hull | 28.3 A | 73.7 | 70.3 B | 17.5 | 8.9 AB | 198.7 ab |
| Dry sugar beet pulp | 26.5 ab | 71.7 | 70.2 B | 16.7 | 8.2 B | 193.3 Bb |
| Wheat bran | 27.6 a | 75.3 | 72.9 AB | 17.7 | 8.8 AB | 202.4 ab |
| SEM | 0.318 | 0.682 | 0.908 | 0.257 | 0.155 | 1.729 |
| p-value | 0.027 | 0.090 | 0.022 | 0.475 | 0.010 | 0.007 |
| TDF | IDF | SDF | PW | GW | DL | JL | IL | CL | LL | HW | LW | |||
| TDF | 0.09 | 0.64 | 0.25 | 0.69 | −0.06 | −0.01 | −0.23 | −0.20 | −0.13 | −0.17 | 0.17 | |||
| IDF | 0.09 | −0.51 | −0.47 | 0.16 | −0.24 | 0.51 | 0.57 | 0.46 | 0.45 | 0.27 | 0.36 | Correlation coefficient [+/−] | ||
| SDF | 0.64 | −0.51 | 0.43 | 0.26 | 0.16 | −0.26 | −0.56 | −0.23 | −0.51 | −0.37 | 0.07 | no | 0.00–0.35 | |
| PW | 0.25 | −0.47 | 0.43 | 0.14 | 0.31 | −0.06 | −0.10 | −0.22 | −0.28 | −0.03 | 0.15 | weak | 0.36–0.57 | |
| GW | 0.69 | 0.16 | 0.26 | 0.14 | −0.16 | 0.24 | 0.12 | −0.14 | 0.09 | 0.14 | 0.25 | medium | 0.58–0.80 | |
| DL | −0.06 | −0.24 | 0.16 | 0.31 | −0.16 | −0.10 | −0.08 | 0.28 | −0.01 | −0.23 | 0.22 | strong | 0.81–1.00 | |
| JL | −0.01 | 0.51 | −0.26 | −0.06 | 0.24 | −0.10 | 0.66 | 0.51 | 0.50 | 0.21 | 0.56 | |||
| IL | −0.23 | 0.57 | −0.56 | −0.10 | 0.12 | −0.08 | 0.66 | 0.52 | 0.47 | 0.49 | 0.52 | |||
| CL | −0.20 | 0.46 | −0.23 | −0.22 | −0.14 | 0.28 | 0.51 | 0.52 | 0.34 | 0.06 | 0.55 | |||
| LL | −0.13 | 0.45 | −0.51 | −0.28 | 0.09 | −0.01 | 0.50 | 0.47 | 0.34 | 0.24 | 0.10 | |||
| HW | −0.17 | 0.27 | −0.37 | −0.03 | 0.14 | −0.23 | 0.21 | 0.49 | 0.06 | 0.24 | 0.35 | |||
| LW | 0.17 | 0.36 | 0.07 | 0.15 | 0.25 | 0.22 | 0.56 | 0.52 | 0.55 | 0.10 | 0.35 | |||
| DV | Intercept | Coefficients for Independent Variables as Constituents of Equation with Sign | p-Value | R2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TDF | IDF | SDF | PW | GW | DW | JL | IL | CL | LL | HW | LW | ||||
| PW | 3.855 | 0.000 | −0.043 | 0.025 | - | 0.029 | 0.160 | 0.068 | 0.054 | −0.173 | −0.229 | 0.130 | −0.033 | 0.125 | 0.24 |
| GW | −45.687 | 0.436 | −0.101 | −0.186 | 0.787 | - | −0.399 | −0.065 | 0.000 | −0.044 | 1.398 | −0.134 | 0.357 | 0.073 | 0.30 |
| DW | 26.527 | 0.016 | −0.045 | 0.000 | 0.732 | −0.068 | - | −0.267 | 0.002 | 0.503 | 0.818 | −0.270 | 0.202 | 0.144 | 0.22 |
| JL | 31.861 | −0.061 | 0.072 | 0.082 | 0.748 | −0.026 | −0.640 | - | 0.299 | 0.236 | 1.533 * | −0.578 | 0.500 * | 0.002 | 0.59 |
| IL | 8.718 | −0.084 | 0.138 | −0.127 | 1.065 | 0.000 | 0.010 | 0.539 | - | 0.585 | −0.643 | 0.638 | 0.108 | 0.002 | 0.58 |
| CL | 3.550 | −0.097 | 0.082 | 0.079 | −0.378 | −0.004 | 0.239 | 0.047 | 0.064 | - | 0.066 | −0.108 | 0.159 | 0.002 | 0.43 |
| LL | −0.409 | −0.016 | 0.014 | −0.018 | −0.232 | 0.053 | 0.181 | 0.141 * | −0.033 | 0.030 | - | 0.094 | −0.093 | 0.099 | 0.27 |
| HW | 11.913 | 0.045 | −0.032 | −0.104 | 0.443 | −0.017 | −0.200 | −0.179 | 0.109 | −0.169 | 0.316 | - | 0.186 | 0.177 | 0.19 |
| LW | −32.160 | 0.157 | −0.128 | −0.077 | −0.335 | 0.135 | 0.448 | 0.461 * | 0.056 | 0.739 | −0.930 | 0.555 | - | 0.007 | 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hikawczuk, T.; Wróblewska, P.; Szuba-Trznadel, A.; Rusiecka, A.; Zinchuk, A.; Laszki-Szcząchor, K. A Multiple Regression Model Analysing Additional Sources of Dietary Fibre as a Factor Affecting the Development of the Gastrointestinal Tract in Broiler Chickens. Appl. Sci. 2025, 15, 4994. https://doi.org/10.3390/app15094994
Hikawczuk T, Wróblewska P, Szuba-Trznadel A, Rusiecka A, Zinchuk A, Laszki-Szcząchor K. A Multiple Regression Model Analysing Additional Sources of Dietary Fibre as a Factor Affecting the Development of the Gastrointestinal Tract in Broiler Chickens. Applied Sciences. 2025; 15(9):4994. https://doi.org/10.3390/app15094994
Chicago/Turabian StyleHikawczuk, Tomasz, Patrycja Wróblewska, Anna Szuba-Trznadel, Agnieszka Rusiecka, Andrii Zinchuk, and Krystyna Laszki-Szcząchor. 2025. "A Multiple Regression Model Analysing Additional Sources of Dietary Fibre as a Factor Affecting the Development of the Gastrointestinal Tract in Broiler Chickens" Applied Sciences 15, no. 9: 4994. https://doi.org/10.3390/app15094994
APA StyleHikawczuk, T., Wróblewska, P., Szuba-Trznadel, A., Rusiecka, A., Zinchuk, A., & Laszki-Szcząchor, K. (2025). A Multiple Regression Model Analysing Additional Sources of Dietary Fibre as a Factor Affecting the Development of the Gastrointestinal Tract in Broiler Chickens. Applied Sciences, 15(9), 4994. https://doi.org/10.3390/app15094994







