Construction of a Chemical Kinetic Mechanism of Five-Component Surrogate Fuel for RP-3 Kerosene
Abstract
1. Introduction
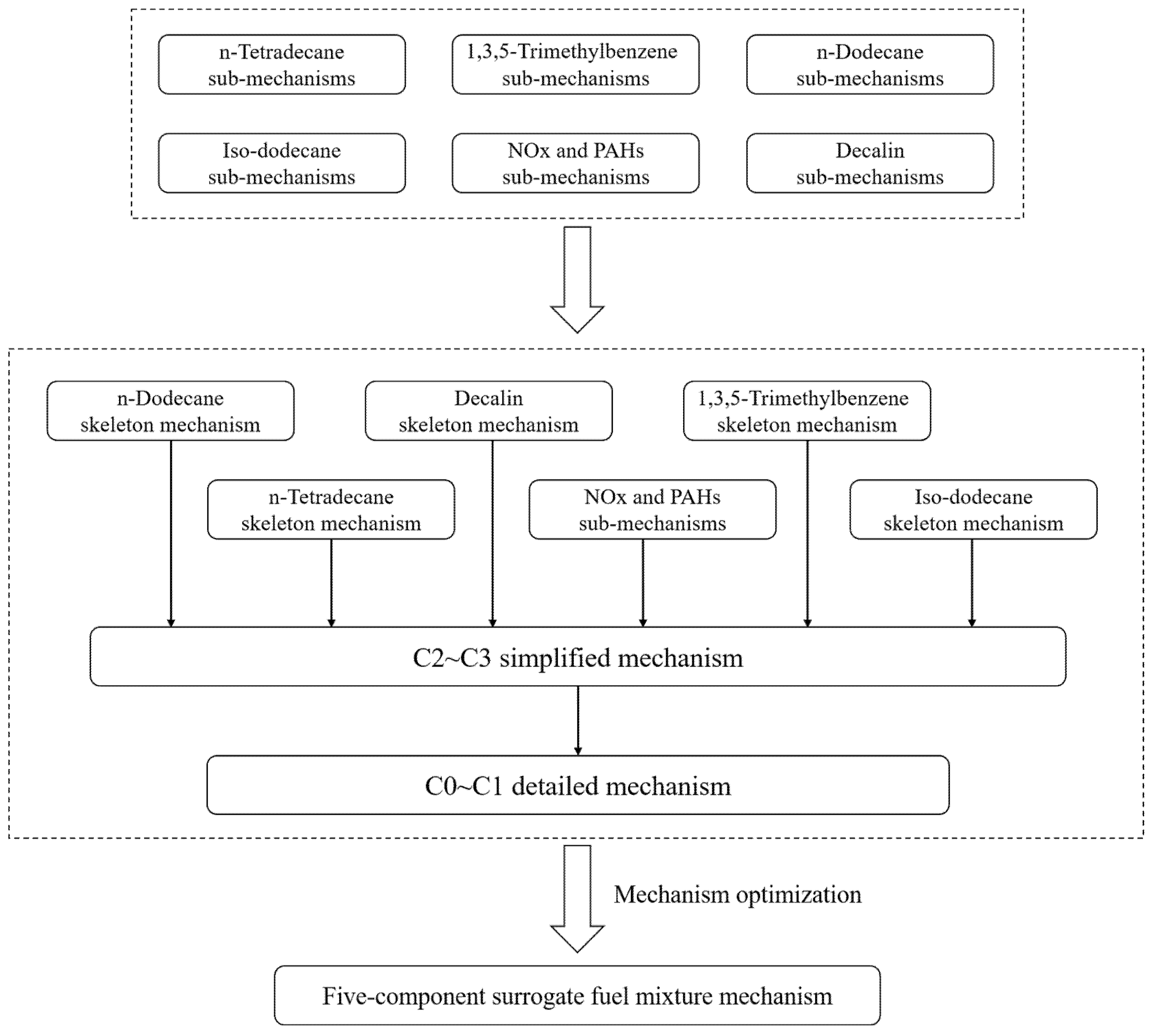
2. Development of Chemical Kinetic Mechanisms
3. Modification of Kinetic Parameters for Mechanism of Surrogate Fuel Mixture
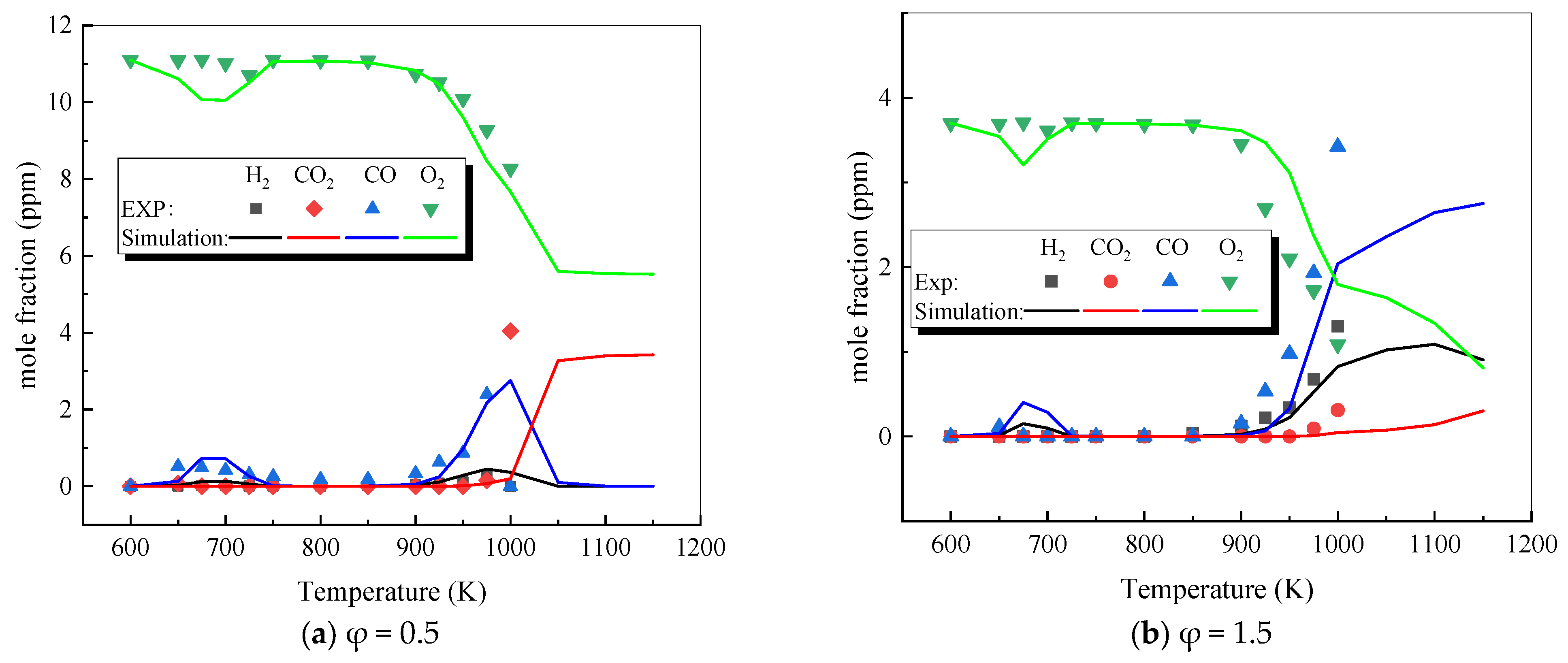
3.1. Verification of Multi-Component Mixture Mechanism
3.2. Modification of Dynamic Parameters
4. Result and Discussion
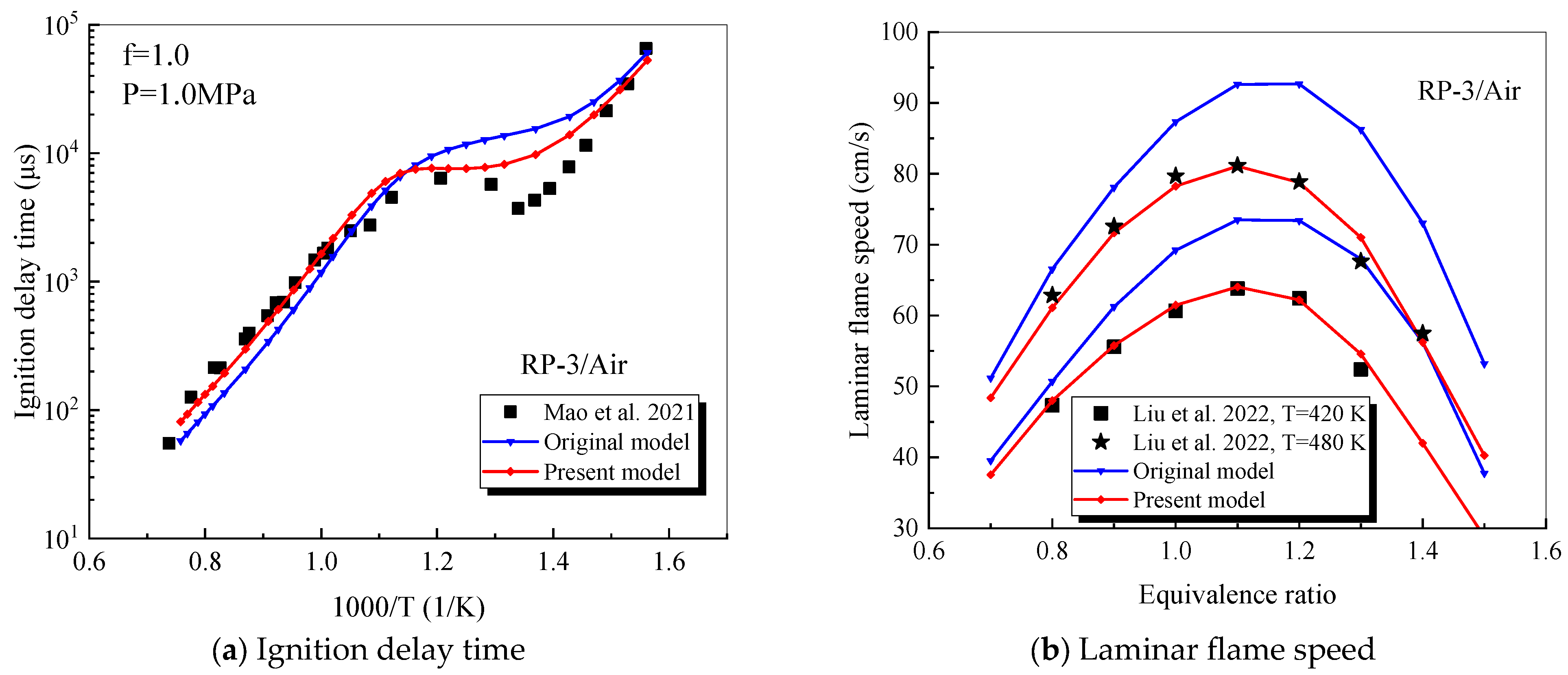
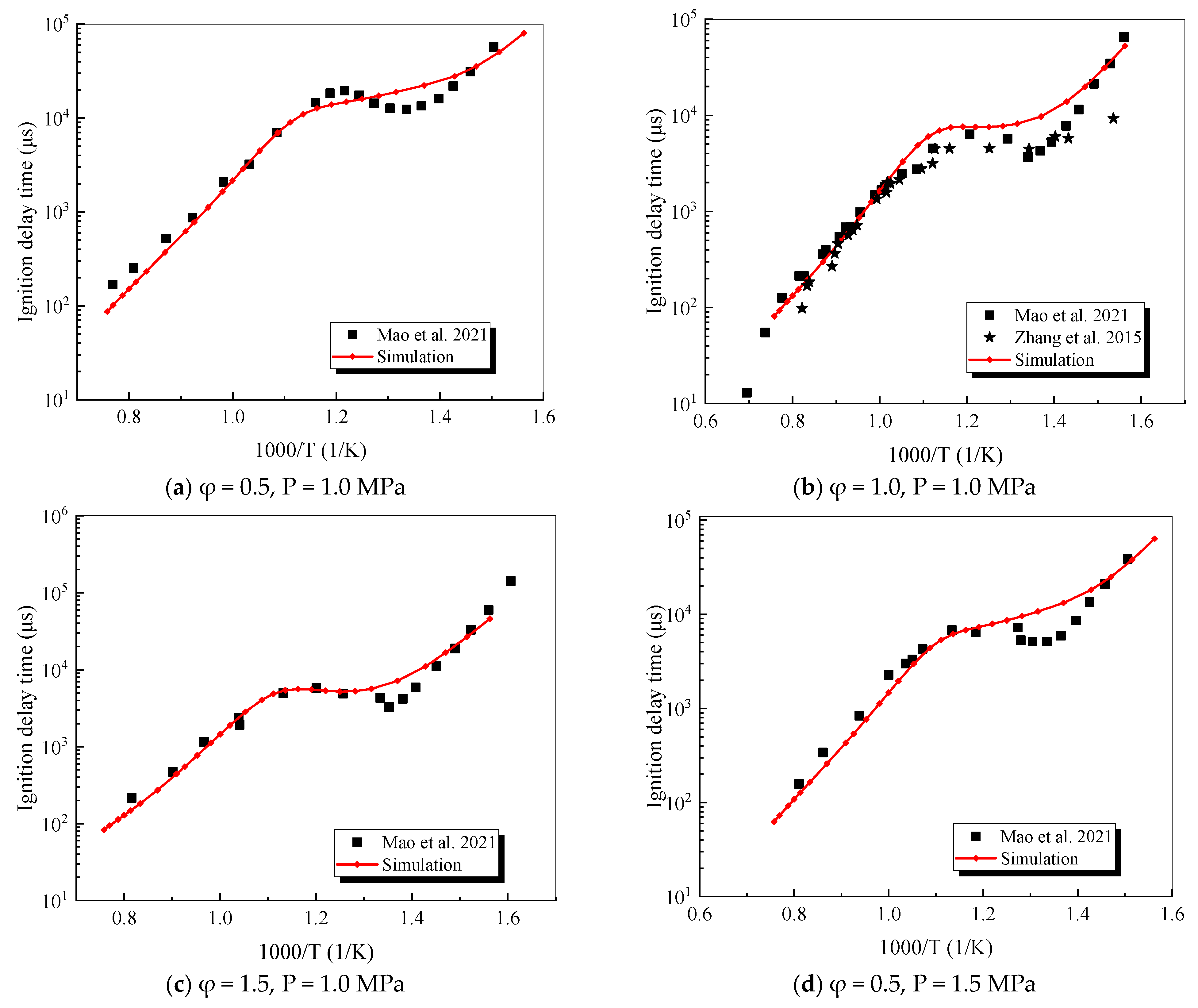
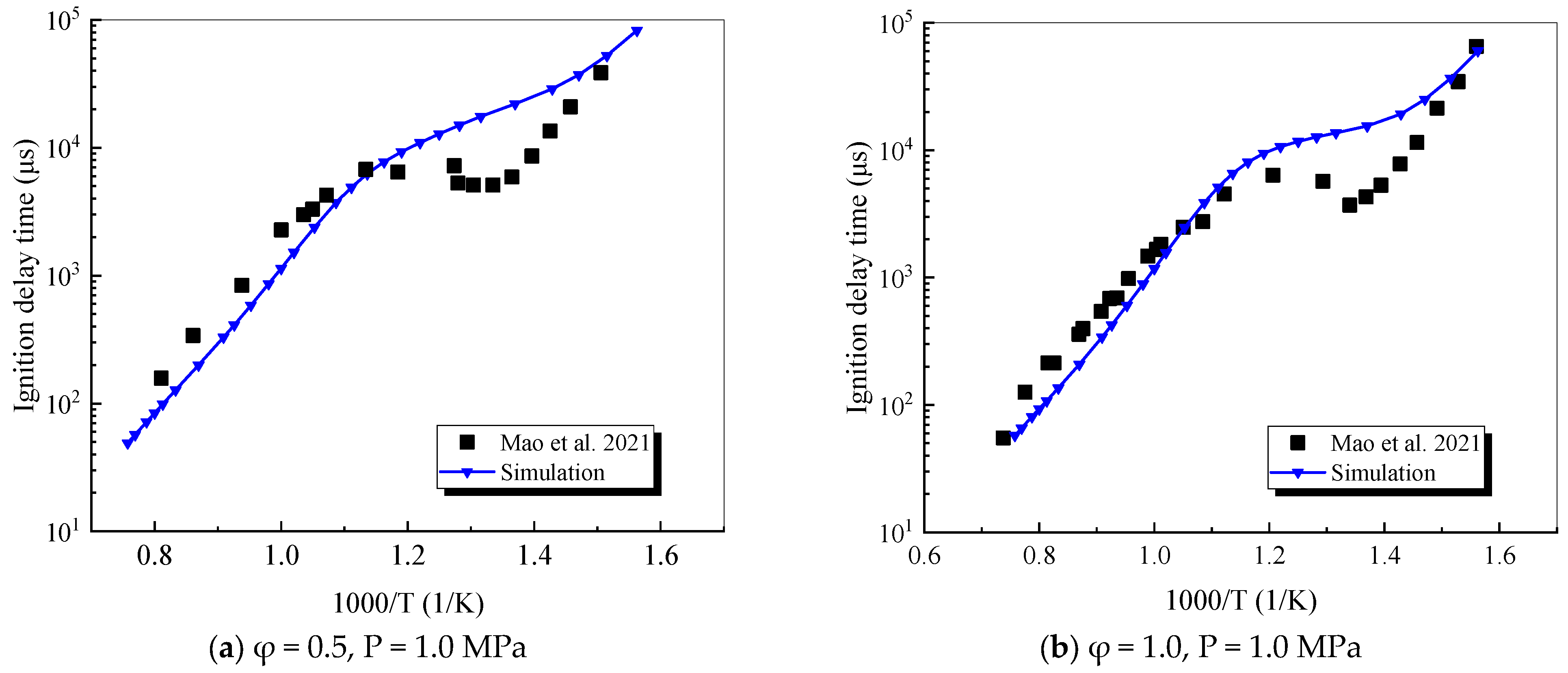
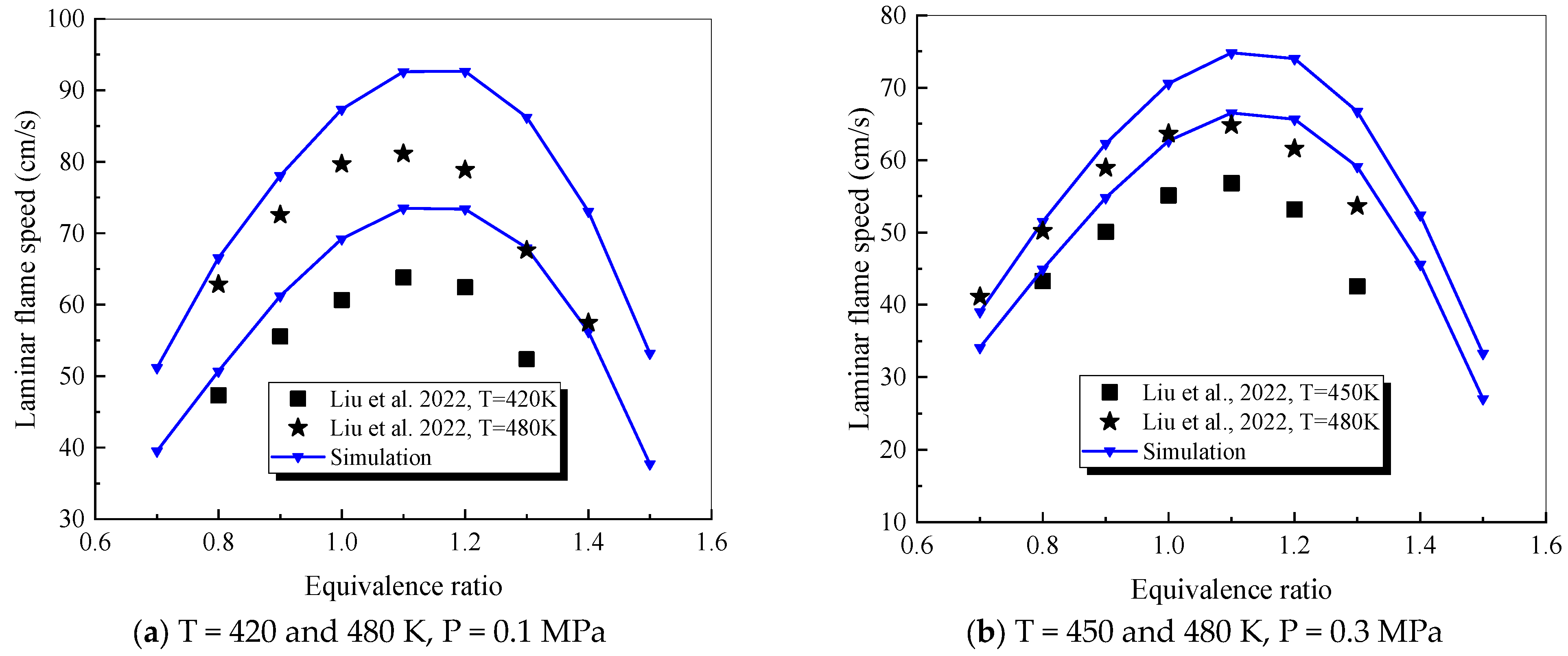
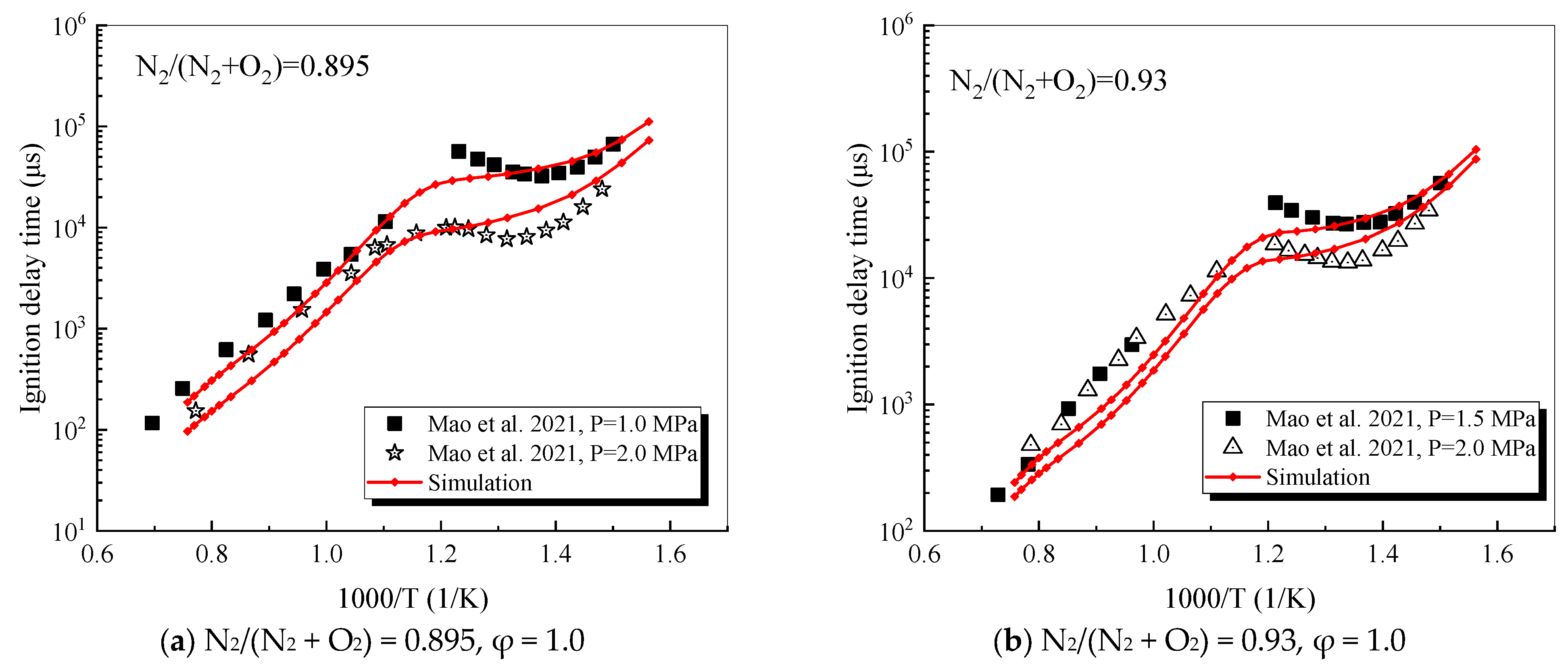
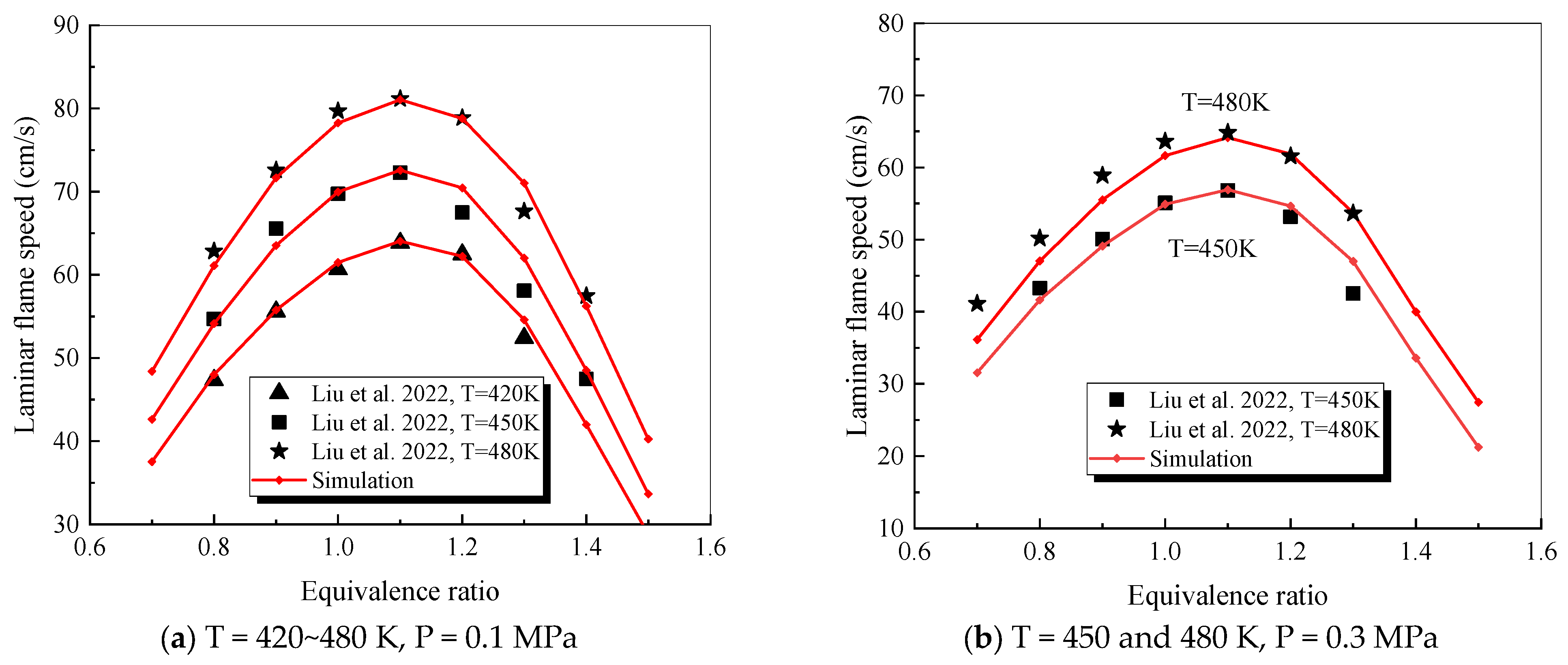
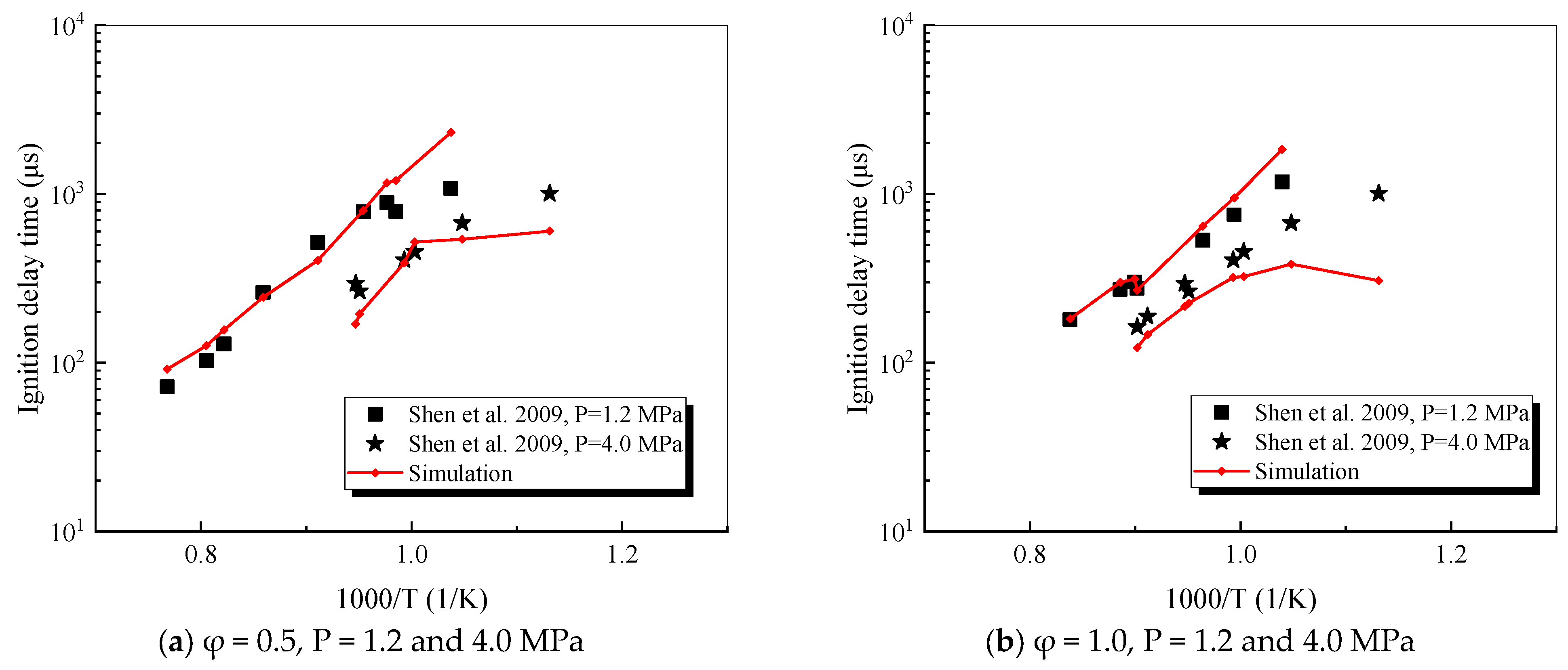
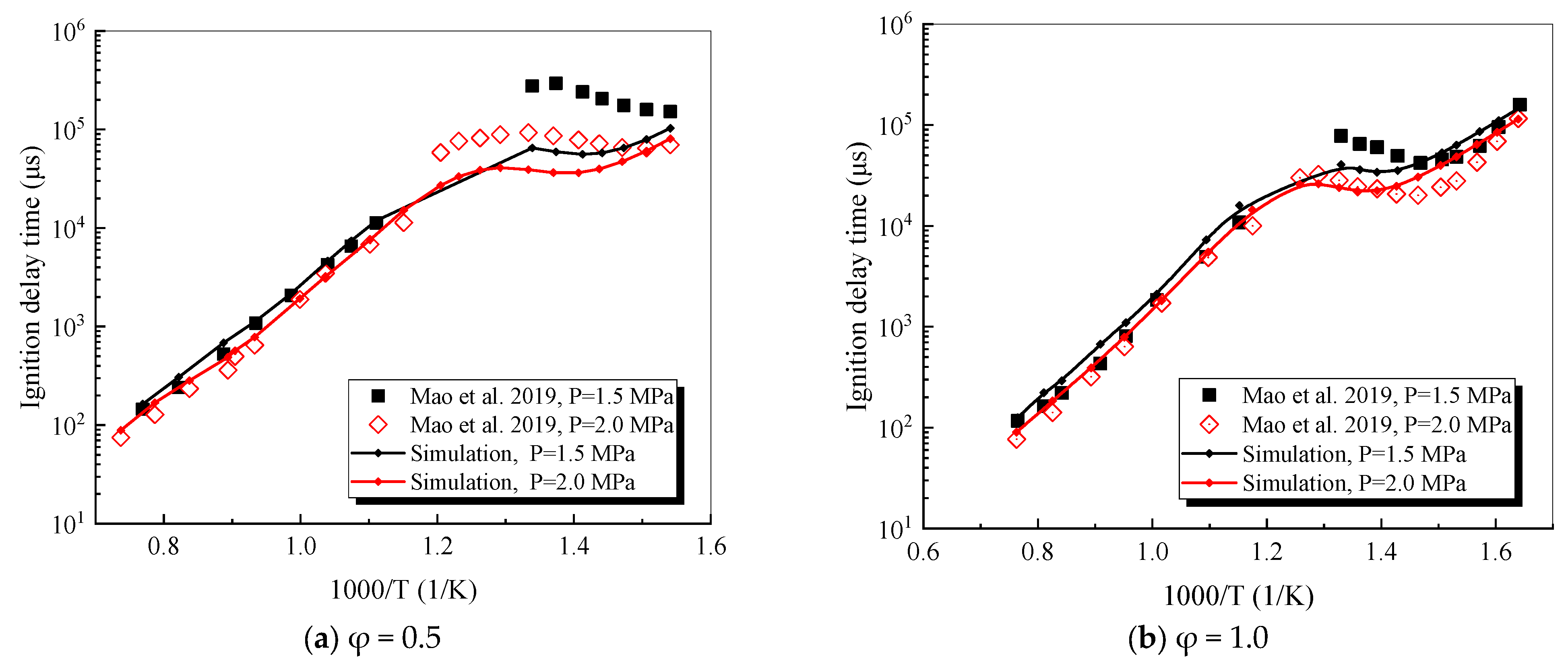
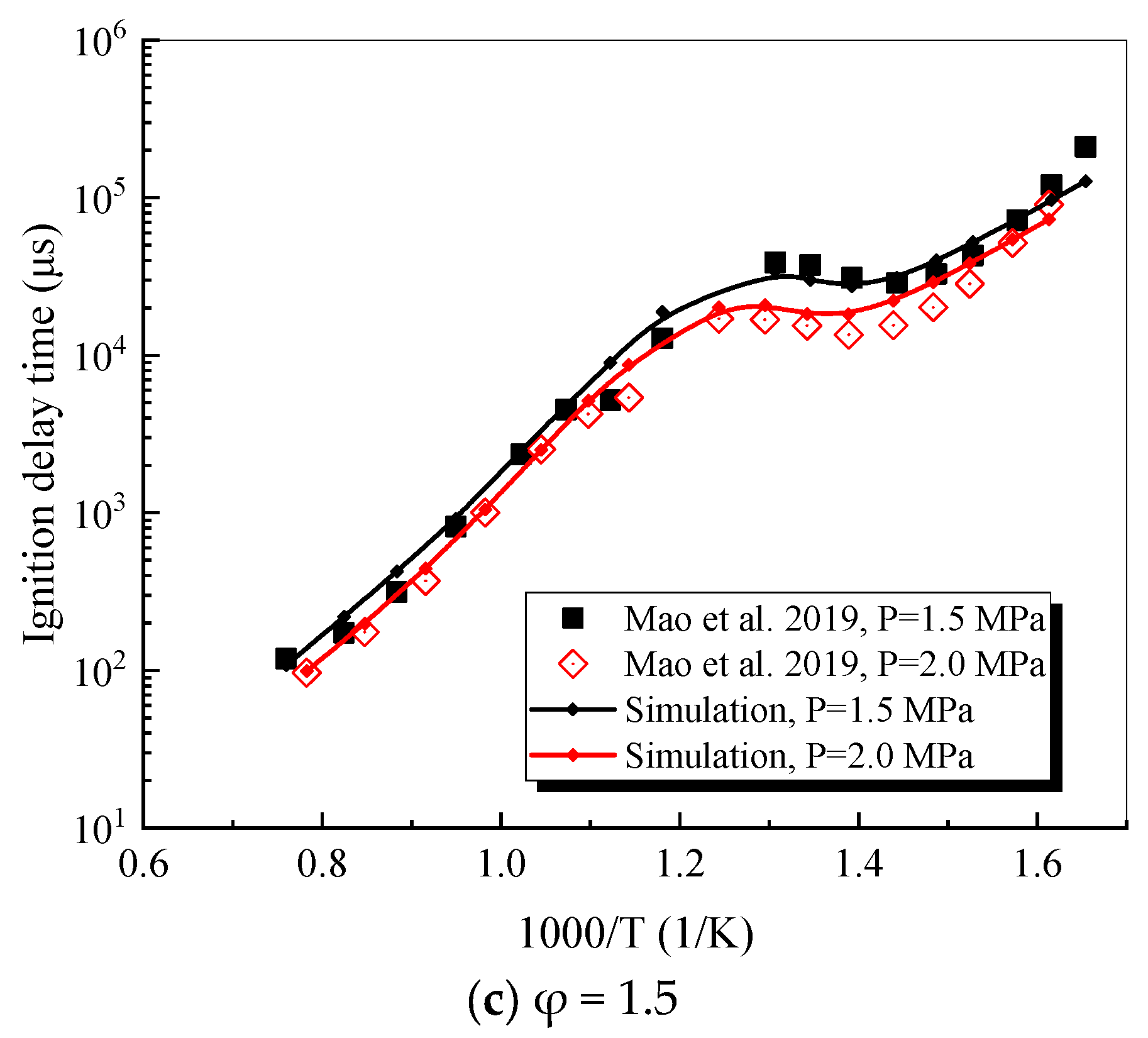
4.1. Verification of RP-3 Combustion Parameters
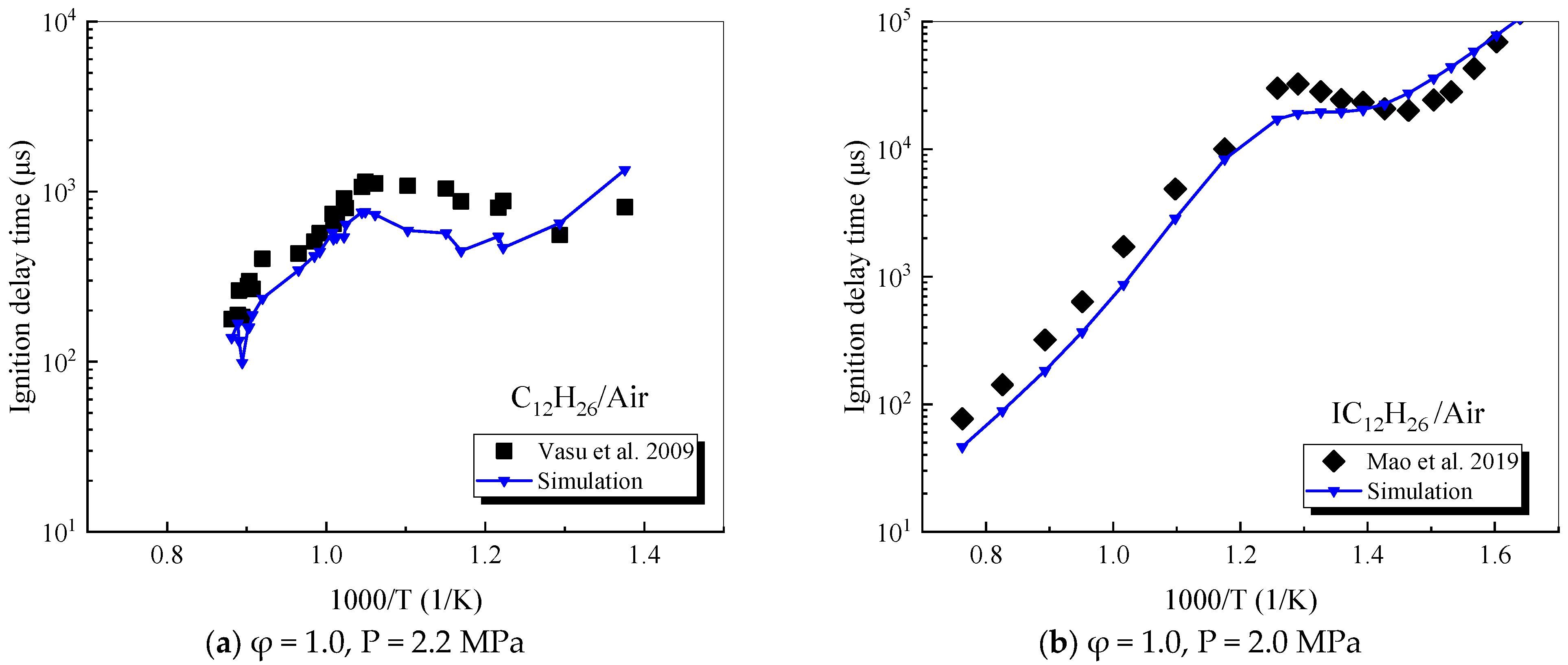
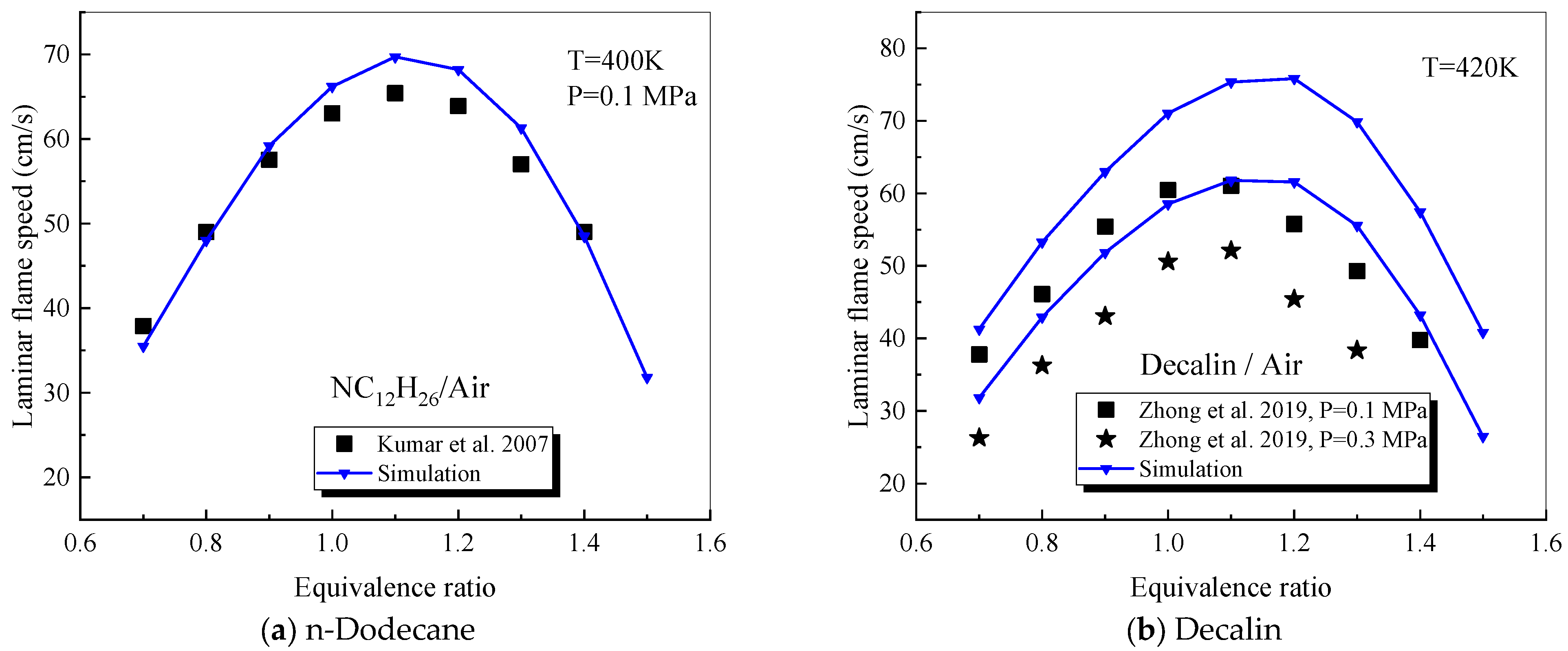
4.2. Verification of Single-Component Combustion Parameters
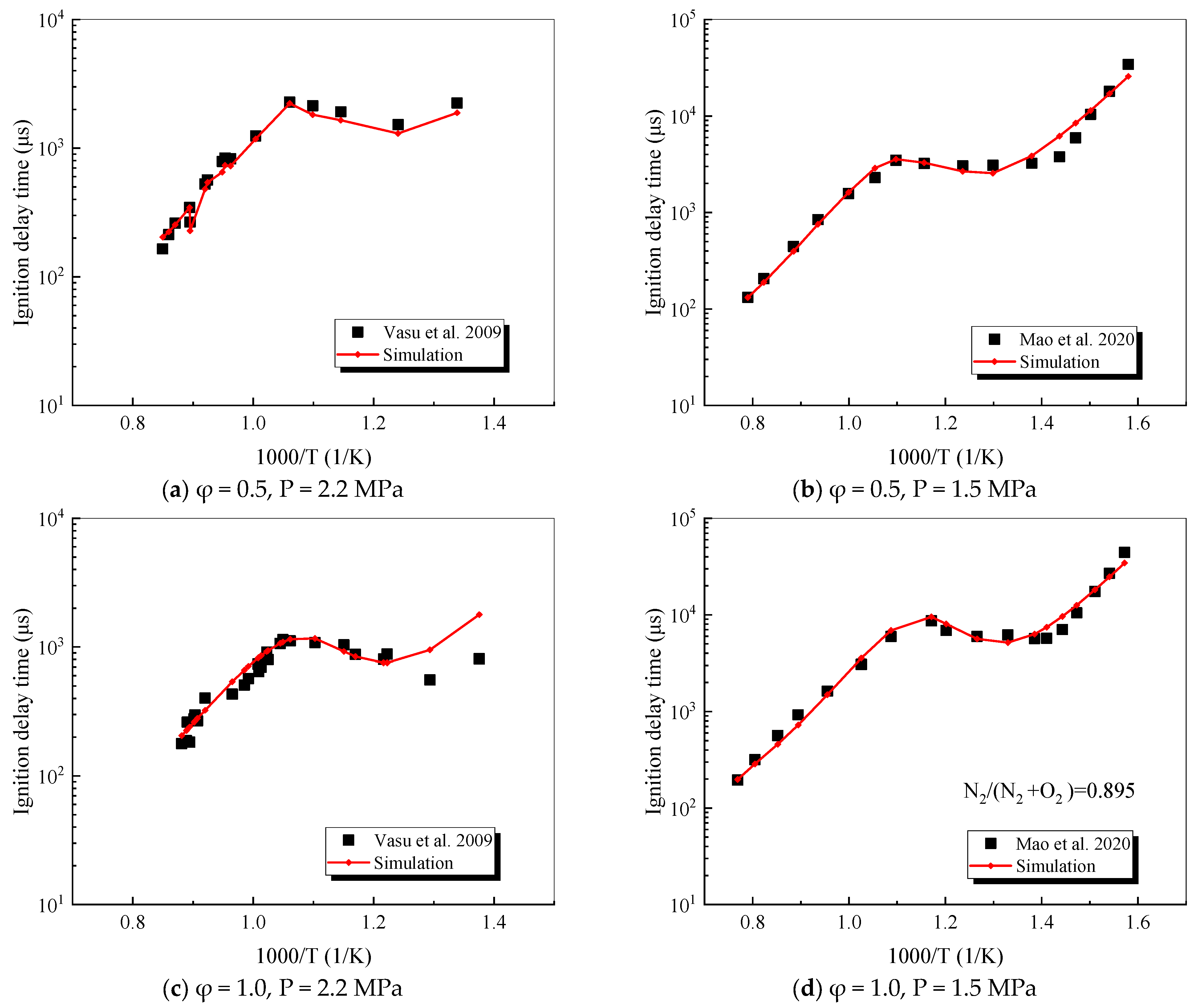
4.2.1. n-Dodecane
4.2.2. n-Tetradecane
4.2.3. Iso-Dodecane
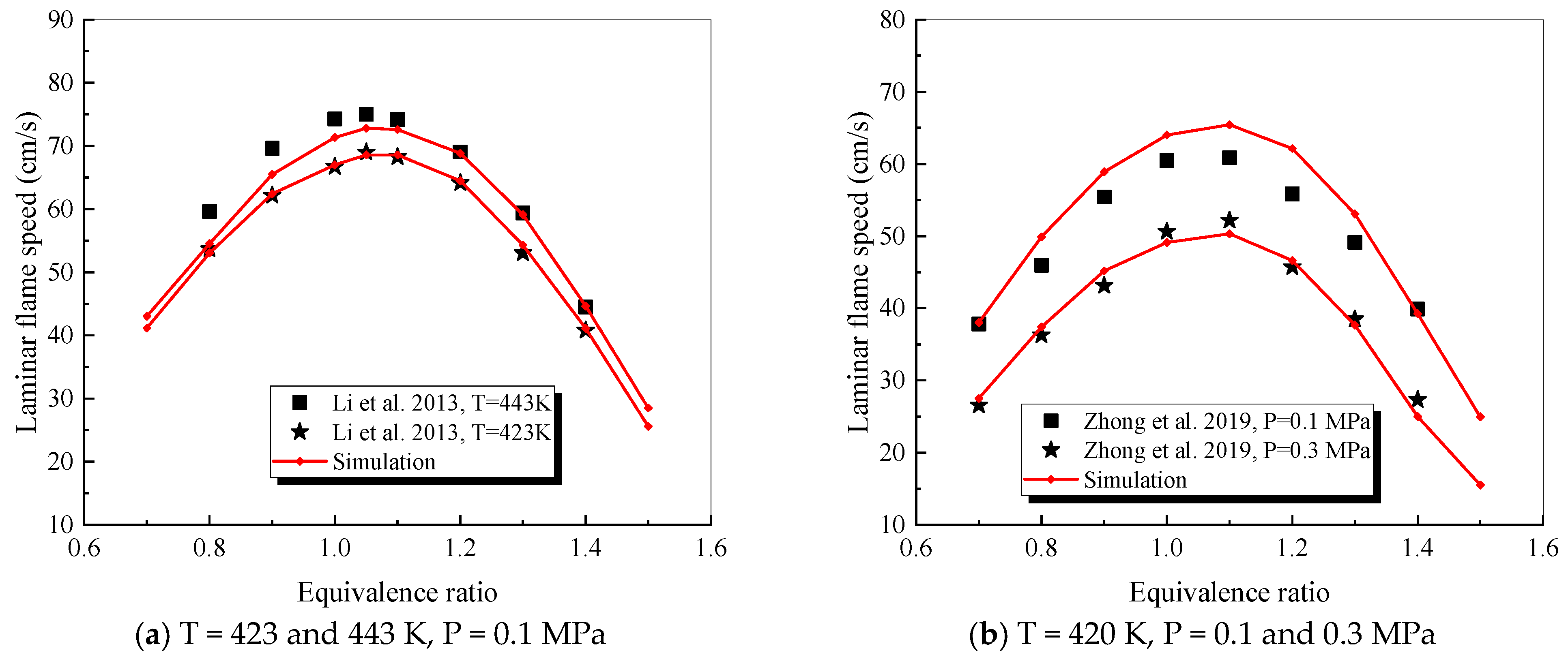
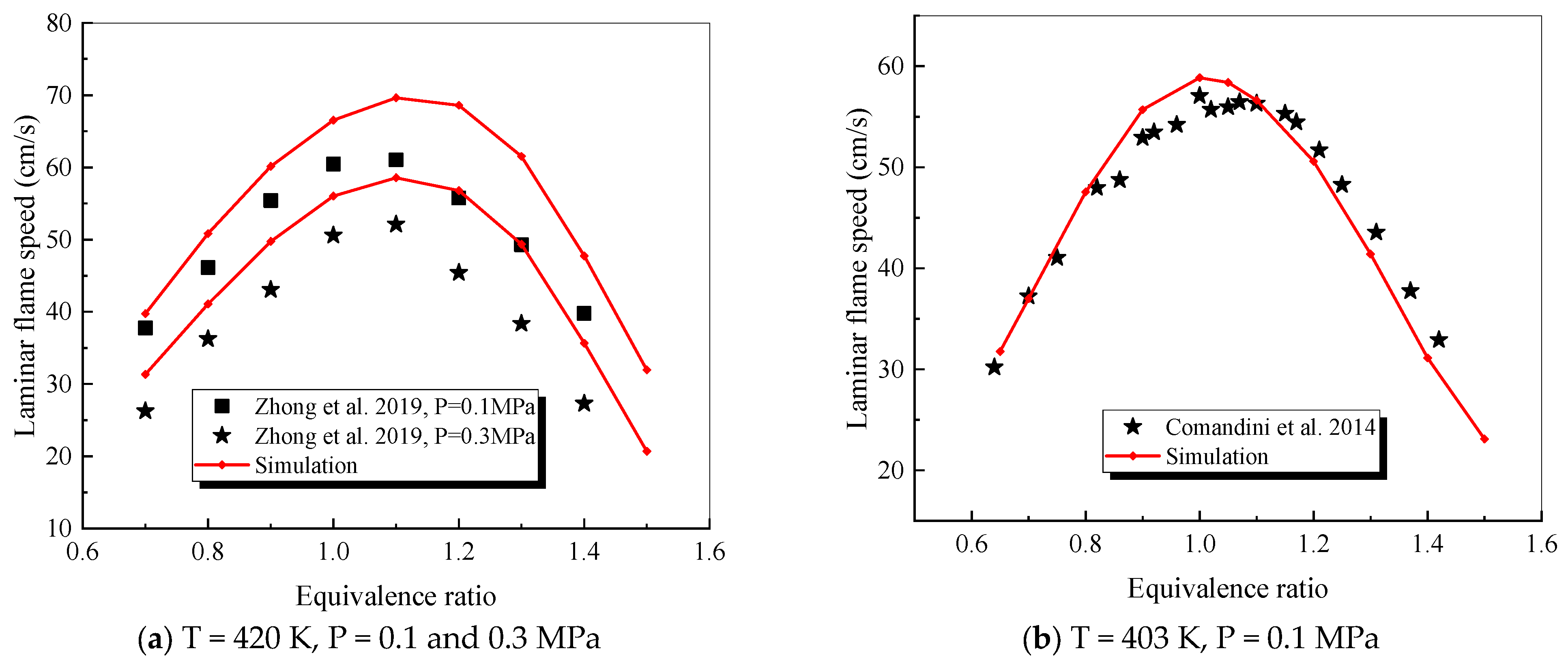
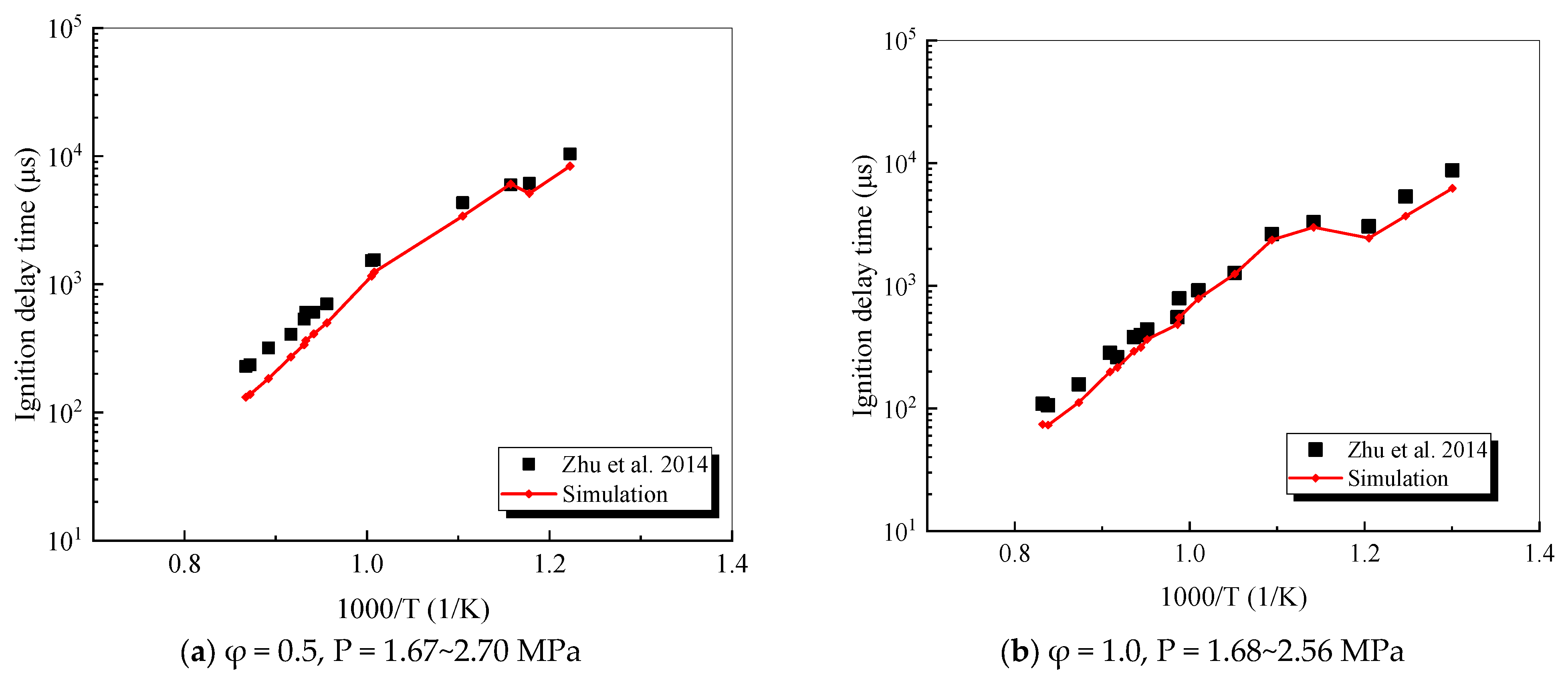
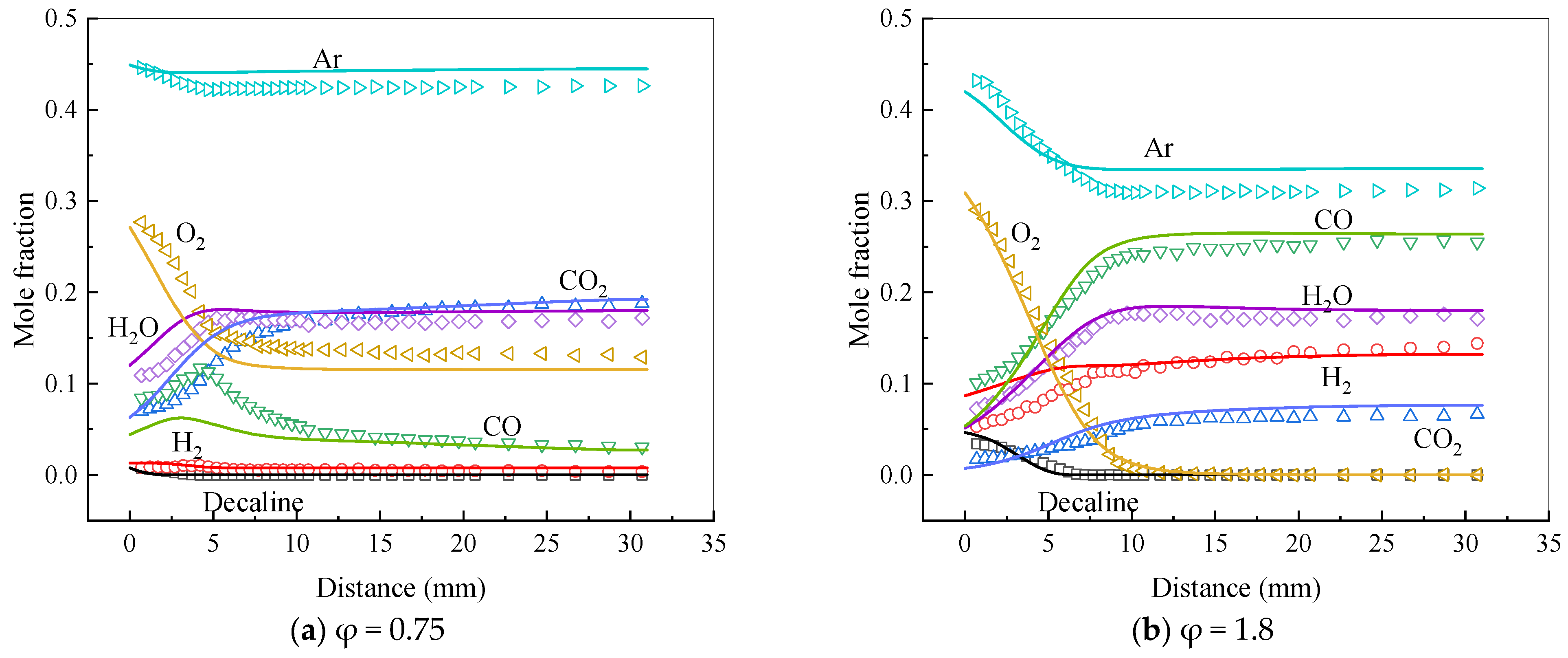
4.2.4. Decalin
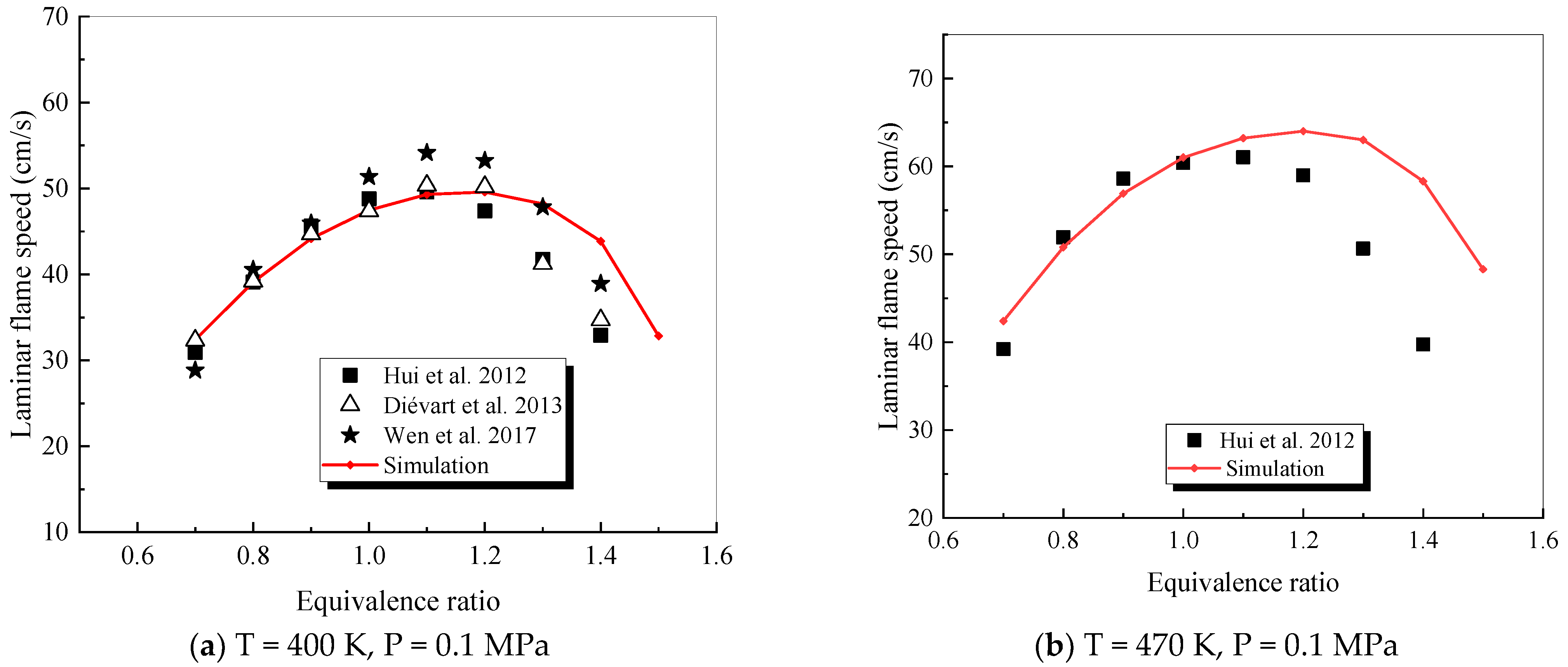
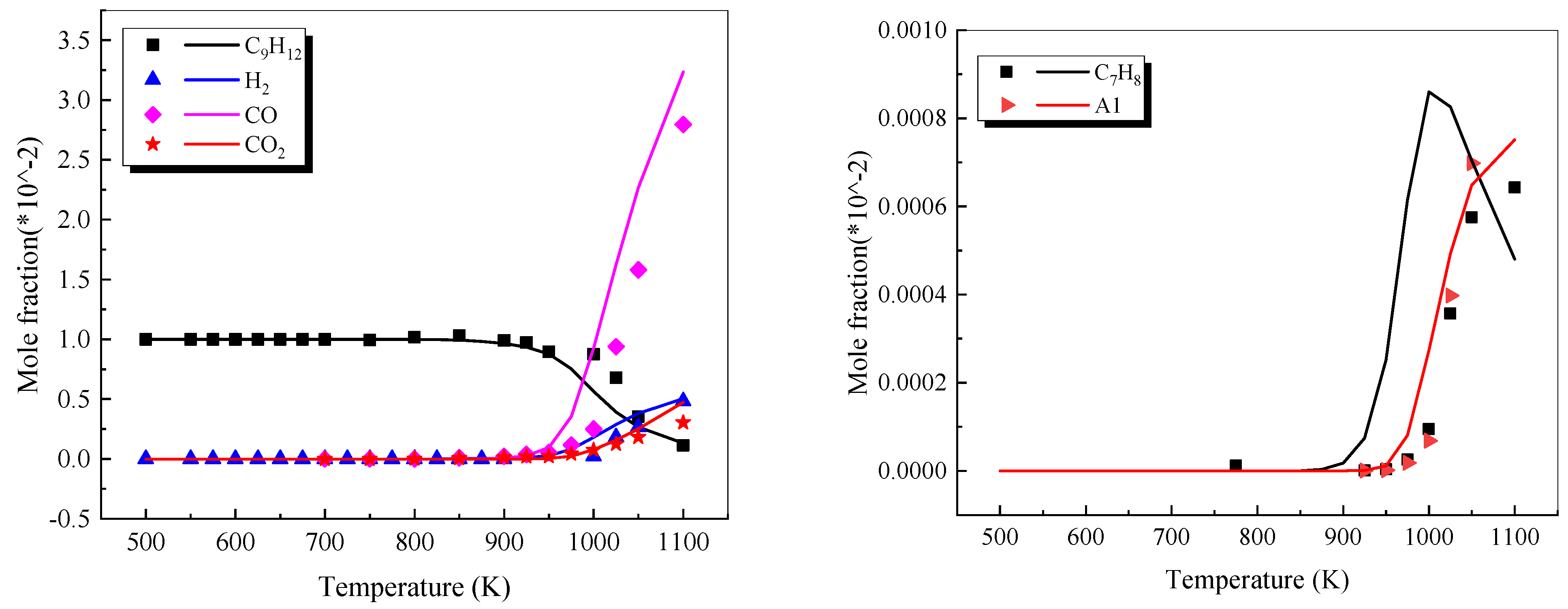
4.2.5. 1,3,5-trimethylbenzene
5. Conclusions
- (1)
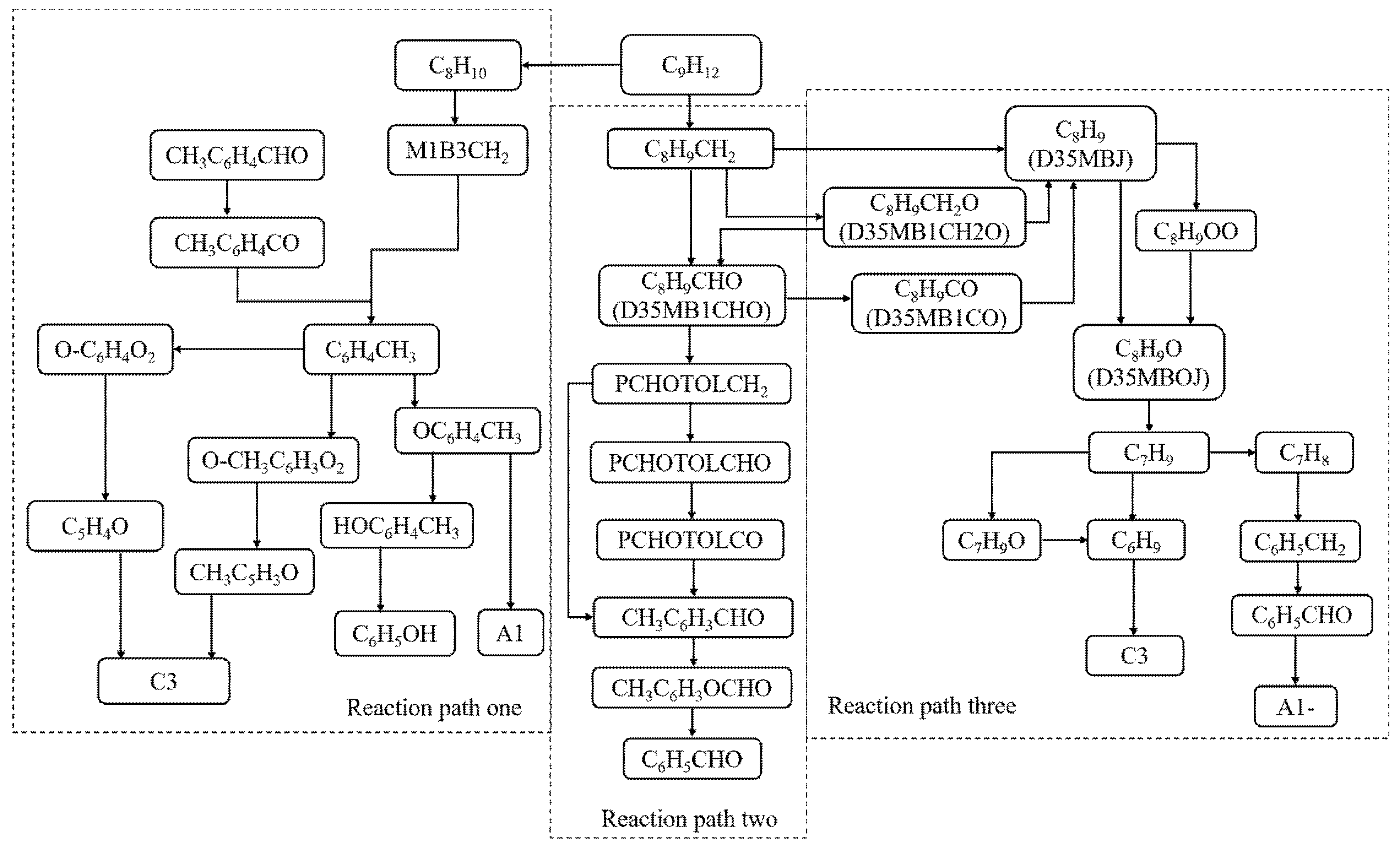
- The reaction pathways of 1,3,5-trimethylbenzene were analyzed using the generation rate analysis method, and three main low-temperature reaction pathways of 1,3,5-trimethylbenzene were determined. A simplified mechanism consisting of 22 species and 69 elementary reactions was obtained.
- (2)
- Based on six selection criteria, including MW, H/C, CN, LHV, TSI and density, five-component surrogate fuels consisting of n-dodecane, n-tetradecane, isododecane, decalin, and 1,3,5-trimethylbenzene (12.1%, 13.5%, 31.8%, 32.6%, and 10% by mole) were constructed. The single-component mechanisms of each fuel and the simplified mechanism of 1,3,5-trimethylbenzene were coupled with the transitional C2–C3 mechanism and the detailed C0-C1 mechanism using the decoupling method, resulting in a five-component surrogate fuel mechanism containing 142 species and 502 elementary reactions.
- (3)
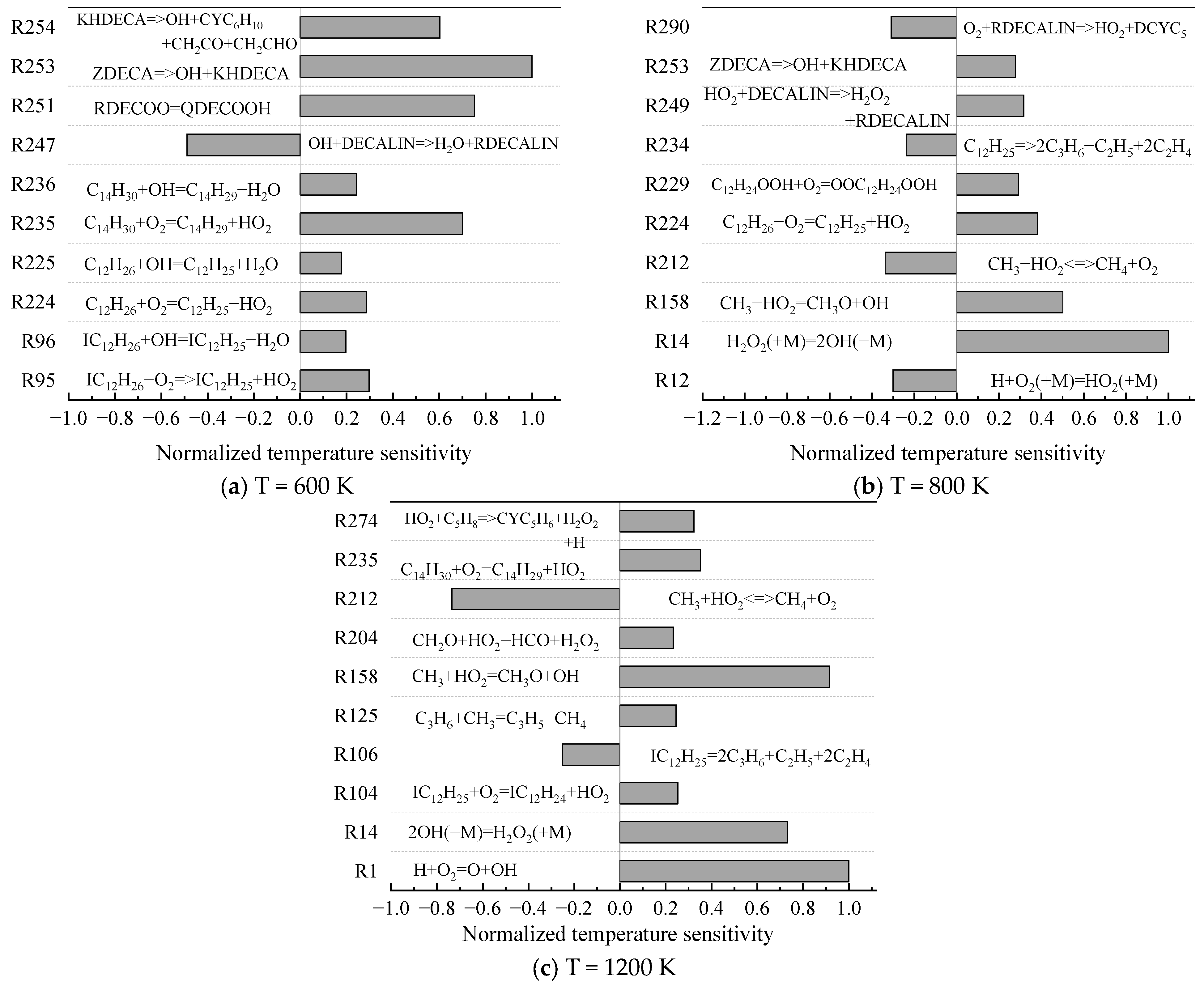
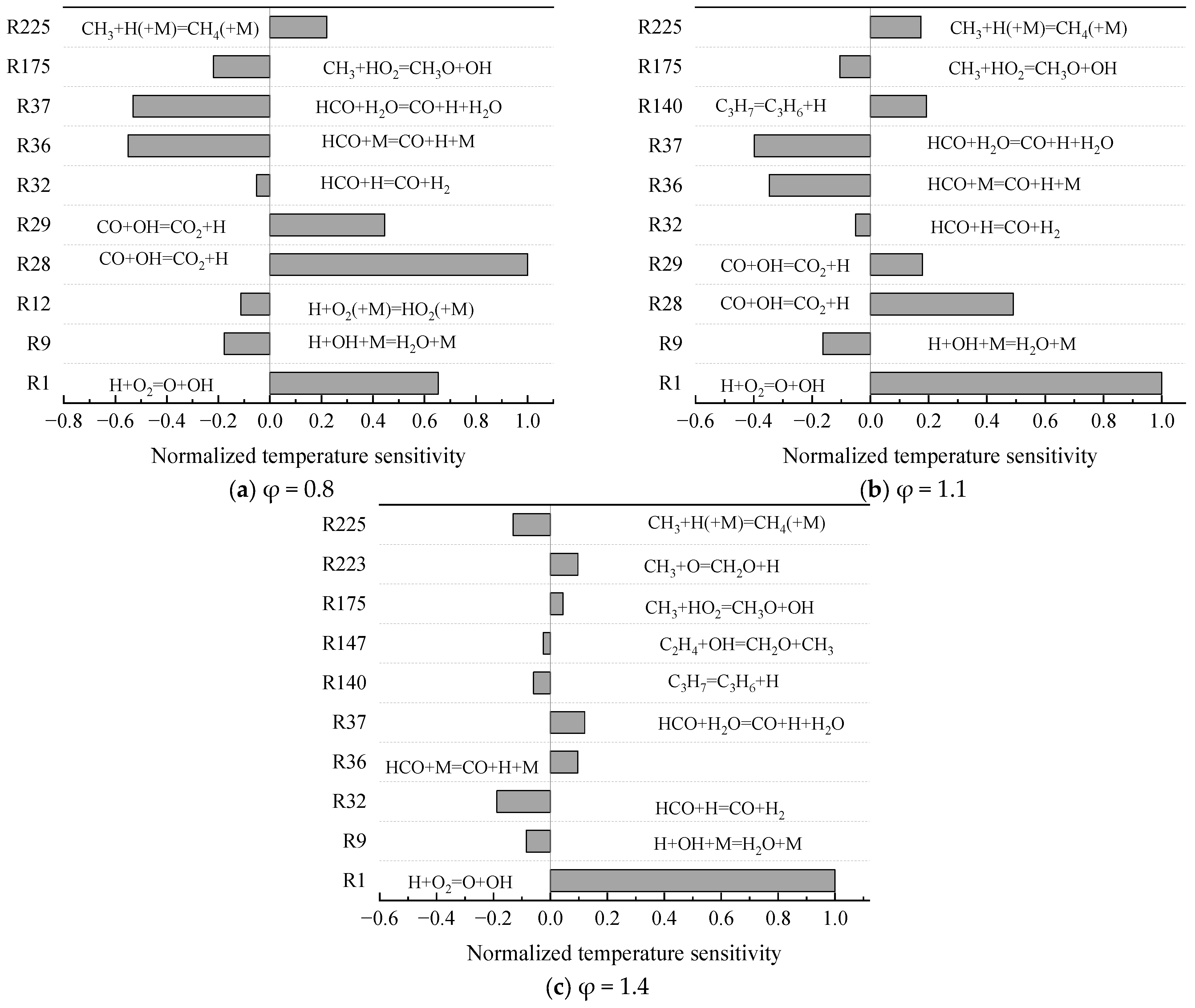
- The mechanism of the surrogate fuel mixture was analyzed using the temperature sensitivity analysis method. Based on the comparison between the predicted values and the experimental values of the mechanism, the kinetic parameters of the key reactions involving small molecules such as R1, R36, and R37 containing H radicals and OH radicals, as well as the reactions generating small-molecule radicals through the endothermic reaction of HCO radicals, were corrected. The reliability of the corrected mechanism was verified by comparing the ignition delay time, laminar flame speed, and combustion products of some components with the experimental data. The comparison between the simulation results and the experimental data shows that in the high-temperature region at 1 MPa and above 1000 K, the mechanism can well predict the ignition delay time of the fuel, especially at 1.0 and 1.5, where the NTC phenomenon of the simulation values is more obvious.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, Y.; Liu, Y.-B.; Cao, B.-Y. C4+ Surrogate Models for Thermophysical Properties of Aviation Kerosene RP-3 at Supercritical Pressures. Energy Fuels 2021, 35, 7858–7865. [Google Scholar] [CrossRef]
- Yu, B.; Jiang, X.; He, D.; Wang, C.; Wang, Z.; Cai, Y.; Yu, J.; Yu, J.J. Development of a Chemical-Kinetic Mechanism of a Four-Component Surrogate Fuel for RP-3 Kerosene. ACS Omega 2021, 6, 23485–23494. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Gong, X.; Deng, T.; Yu, J. Study on a Novel Methodology for Developing the Skeletal Mechanism of RP-3 Aviation Kerosene. ACS Omega 2023, 8, 37282–37292. [Google Scholar] [CrossRef]
- Dooley, S.; Won, S.H.; Chaos, M.; Heyne, J.; Ju, Y.; Dryer, F.L.; Kumar, K.; Sung, C.-J.; Wang, H.; Oehlschlaeger, M.A.; et al. A jet fuel surrogate formulated by real fuel properties. Combust. Flame 2010, 157, 2333–2339. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Richter, S.; Naumann, C.; Braun-Unkhoff, M.; Tian, Z.-Y. Combustion study of a surrogate jet fuel. Combust. Flame 2019, 202, 252–261. [Google Scholar] [CrossRef]
- Kim, D.; Martz, J.; Violi, A. A surrogate for emulating the physical and chemical properties of conventional jet fuel. Combust. Flame 2014, 161, 1489–1498. [Google Scholar] [CrossRef]
- Luning Prak, D.J.; Simms, G.R.; Dickerson, T.; McDaniel, A.; Cowart, J.S. Formulation of 7-Component Surrogate Mixtures for Military Jet Fuel and Testing in Diesel Engine. ACS Omega 2022, 7, 2275–2285. [Google Scholar] [CrossRef]
- Xu, Z.; Han, H.; Li, Y.; Zhu, M. Molecular dynamics simulation study on thermophysical properties of a three-component RP-3 surrogate fuel at sub/supercritical pressure. Int. Commun. Heat Mass Transf. 2025, 161, 108170. [Google Scholar] [CrossRef]
- Fu, Y.; Zhi, H.; Wang, J.; Sun, J.; Wen, J.; Xu, G. Numerical research on heat transfer and thermal oxidation coking characteristics of aviation kerosene RP-3 under supercritical pressure. Int. Commun. Heat Mass Transf. 2024, 159, 108109. [Google Scholar] [CrossRef]
- Zheng, D.; Yu, W.M.; Zhong, B.J. RP-3 Aviation Kerosene Surrogate Fuel and the Chemical Reaction Kinetic Model. Acta Phys.-Chim. Sin. 2015, 31, 636–642. [Google Scholar] [CrossRef]
- Xu, K.; Meng, H. Analyses of surrogate models for calculating thermophysical properties of aviation kerosene RP-3 at supercritical pressures. Sci. China Technol. Sci. 2015, 58, 510–518. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, Y.; Fang, W.; Liu, Y.; Li, J. A simplified chemical reaction mechanism for two-component RP-3 kerosene surrogate fuel and its verification. Fuel 2018, 227, 127–134. [Google Scholar] [CrossRef]
- Yi, R.; Chen, X.; Chen, C.P. Surrogate for Emulating Physicochemical and Kinetics Characteristics of RP-3 Aviation Fuel. Energy Fuels 2019, 33, 2872–2879. [Google Scholar] [CrossRef]
- Liu, J.; Hu, E.; Yin, G.; Huang, Z.; Zeng, W. An experimental and kinetic modeling study on the low-temperature oxidation, ignition delay time, and laminar flame speed of a surrogate fuel for RP-3 kerosene. Combust. Flame 2022, 237, 111821. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Gu, G.; Yang, T. Simplification and verification of chemical reaction mechanism of RP-3 aviation kerosene. Process Saf. Environ. Prot. 2024, 190, 288–297. [Google Scholar] [CrossRef]
- Du, L.-J.; Liu, Y.-X.; Tian, Z.-Y. An experimental and modeling study of oxidation of real RP-3 aviation kerosene. Fuel 2021, 305, 121476. [Google Scholar] [CrossRef]
- Kim, D.; Violi, A. Hydrocarbons for the next generation of jet fuel surrogates. Fuel 2018, 228, 438–444. [Google Scholar] [CrossRef]
- Won, S.H.; Haas, F.M.; Tekawade, A.; Kosiba, G.; Oehlschlaeger, M.A.; Dooley, S.; Dryer, F.L. Combustion characteristics of C4 iso-alkane oligomers: Experimental characterization of iso-dodecane as a jet fuel surrogate component. Combust. Flame 2016, 165, 137–143. [Google Scholar] [CrossRef]
- Mao, Y.; Feng, Y.; Wu, Z.; Wang, S.; Yu, L.; Raza, M.; Qian, Y.; Lu, X. The autoignition of iso-dodecane in low to high temperature range: An experimental and modeling study. Combust. Flame 2019, 210, 222–235. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Y.; Wang, X.; Zhou, Q.; Duan, Q. Development of physical-chemical surrogate models and skeletal mechanism for the spray and combustion simulation of RP-3 kerosene fuels. Energy 2021, 215, 119090. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Bai, Y.; Zhou, Q.; Yang, W. Development and verification of a physical–chemical surrogate model of RP-3 kerosene with skeletal mechanism for aircraft SI engine. Fuel 2022, 311, 122626. [Google Scholar] [CrossRef]
- Mao, Y.; Yu, L.; Qian, Y.; Wang, S.; Wu, Z.; Raza, M.; Zhu, L.; Hu, X.; Lu, X. Development and validation of a detailed kinetic model for RP-3 aviation fuel based on a surrogate formulated by emulating macroscopic properties and microscopic structure. Combust. Flame 2021, 229, 111401. [Google Scholar] [CrossRef]
- Zhong, B.-J.; Peng, H.-S. Development of a Skeletal Mechanism for Aviation Kerosene Surrogate Fuel. J. Propuls. Power 2019, 35, 645–651. [Google Scholar] [CrossRef]
- Abdalla, A.O.G.; Ying, Y.; Jiang, B.; He, X.; Liu, D. Comparative study on characteristics of soot from n-decane and RP-3 kerosene normal/inverse diffusion flames. J. Energy Inst. 2020, 93, 62–75. [Google Scholar] [CrossRef]
- Yu, W.; Yang, W.; Tay, K.; Zhao, F. An optimization method for formulating model-based jet fuel surrogate by emulating physical, gas phase chemical properties and threshold sooting index (TSI) of real jet fuel under engine relevant conditions. Combust. Flame 2018, 193, 192–217. [Google Scholar] [CrossRef]
- Liu, J.; Hu, E.; Zeng, W.; Zheng, W. A new surrogate fuel for emulating the physical and chemical properties of RP-3 kerosene. Fuel 2020, 259, 116210. [Google Scholar] [CrossRef]
- Mao, Y.; Yu, L.; Wu, Z.; Tao, W.; Wang, S.; Ruan, C.; Zhu, L.; Lu, X. Experimental and kinetic modeling study of ignition characteristics of RP-3 kerosene over low-to-high temperature ranges in a heated rapid compression machine and a heated shock tube. Combust. Flame 2019, 203, 157–169. [Google Scholar] [CrossRef]
- Yu, Z.; Wei, S.; Wu, C.; Wu, L.; Sun, L.; Zhang, Z. Development and verification of RP-3 aviation kerosene surrogate fuel models using a genetic algorithm. Fuel 2022, 312, 122853. [Google Scholar] [CrossRef]
- Chang, Y.; Jia, M.; Liu, Y.; Li, Y.; Xie, M.; Yin, H. Application of a Decoupling Methodology for Development of Skeletal Oxidation Mechanisms for Heavy n-Alkanes from n-Octane to n-Hexadecane. Energy Fuels 2013, 27, 3467–3479. [Google Scholar] [CrossRef]
- Fang, X.; Huang, X.; Chen, W.; Qiao, X.; Ju, D. Development of a skeletal surrogate mechanism for emulating combustion characteristics of diesel from direct coal liquefaction. Combust. Flame 2020, 218, 84–97. [Google Scholar] [CrossRef]
- Li, G.; Yang, W.; Tay, K.L.; Yu, W.; Chen, L. A reduced and robust reaction mechanism for toluene and decalin oxidation with polycyclic aromatic hydrocarbon predictions. Fuel 2020, 259, 116233. [Google Scholar] [CrossRef]
- Diévart, P.; Kim, H.H.; Won, S.H.; Ju, Y.; Dryer, F.L.; Dooley, S.; Wang, W.; Oehlschlaeger, M.A. The combustion properties of 1,3,5-trimethylbenzene and a kinetic model. Fuel 2013, 109, 125–136. [Google Scholar] [CrossRef]
- Slavinskaya, N.A.; Frank, P. A modelling study of aromatic soot precursors formation in laminar methane and ethene flames. Combust. Flame 2009, 156, 1705–1722. [Google Scholar] [CrossRef]
- Slavinskaya, N.A.; Riedel, U.; Dworkin, S.B.; Thomson, M.J. Detailed numerical modeling of PAH formation and growth in non-premixed ethylene and ethane flames. Combust. Flame 2012, 159, 979–995. [Google Scholar] [CrossRef]
- Wang, H.; Deneys Reitz, R.; Yao, M.; Yang, B.; Jiao, Q.; Qiu, L. Development of an n-heptane-n-butanol-PAH mechanism and its application for combustion and soot prediction. Combust. Flame 2013, 160, 504–519. [Google Scholar] [CrossRef]
- Wang, H.; Yao, M.; Yue, Z.; Jia, M.; Reitz, R.D. A reduced toluene reference fuel chemical kinetic mechanism for combustion and polycyclic-aromatic hydrocarbon predictions. Combust. Flame 2015, 162, 2390–2404. [Google Scholar] [CrossRef]
- Zhang, K.; Xin, Q.; Mu, Z.; Niu, Z.; Wang, Z. Numerical simulation of diesel combustion based on n-heptane and toluene. Propuls. Power Res. 2019, 8, 121–127. [Google Scholar] [CrossRef]
- Sun, X.; Liang, X.; Shu, G.; Wang, Y.; Chen, Y. Effect of toluene content on the combustion and emissions of large two-stroke marine diesel engine. Appl. Therm. Eng. 2019, 159, 113909. [Google Scholar] [CrossRef]
- Patel, A.; Kong, S.-C.; Reitz, R.D. Development and Validation of a Reduced Reaction Mechanism for HCCI Engine Simulations. In Proceedings of the SAE 2004 World Congress & Exhibition, Detroit, MI, USA, 11 March 2004; SAE Technical Paper. SAE Mobilus: Warrendale, PA, USA, 2004. [Google Scholar] [CrossRef]
- Vasu, S.S.; Davidson, D.F.; Hong, Z.; Vasudevan, V.; Hanson, R.K. n-Dodecane oxidation at high-pressures: Measurements of ignition delay times and OH concentration time-histories. Proc. Combust. Inst. 2009, 32, 173–180. [Google Scholar] [CrossRef]
- Kumar, K.; Sung, C.-J. Laminar flame speeds and extinction limits of preheated n-decane/O2/N2 and n-dodecane/O2/N2 mixtures. Combust. Flame 2007, 151, 209–224. [Google Scholar] [CrossRef]
- Bugler, J.; Somers, K.P.; Silke, E.J.; Curran, H.J. Revisiting the Kinetics and Thermodynamics of the Low-Temperature Oxidation Pathways of Alkanes: A Case Study of the Three Pentane Isomers. J. Phys. Chem. A 2015, 119, 7510–7527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Banyon, C.; Bugler, J.; Curran, H.J.; Rodriguez, A.; Herbinet, O.; Battin-Leclerc, F.; B’Chir, C.; Heufer, K.A. An updated experimental and kinetic modeling study of n-heptane oxidation. Combust. Flame 2016, 172, 116–135. [Google Scholar] [CrossRef]
- Lin, S.; Sun, W.; Guo, L.; Cheng, P.; Sun, Y.; Zhang, H. Development of a reduced mechanism of a three components surrogate fuel for the coal-to-liquid and diesel combustion simulation. Fuel 2021, 294, 120370. [Google Scholar] [CrossRef]
- Wu, Z.; Mao, Y.; Raza, M.; Zhu, J.; Feng, Y.; Wang, S.; Qian, Y.; Yu, L.; Lu, X. Surrogate fuels for RP-3 kerosene formulated by emulating molecular structures, functional groups, physical and chemical properties. Combust. Flame 2019, 208, 388–401. [Google Scholar] [CrossRef]
- Klippenstein, S.J.; Harding, L.B.; Davis, M.J.; Tomlin, A.S.; Skodje, R.T. Uncertainty driven theoretical kinetics studies for CH3OH ignition: HO2 + CH3OH and O2 + CH3OH. Proc. Combust. Inst. 2011, 33, 351–357. [Google Scholar] [CrossRef]
- Sun, X.; Liang, X.; Shu, G.; Wang, Y.; Wang, Y.; Yu, H. Development of a Reduced n-Tetradecane–Polycyclic Aromatic Hydrocarbon Mechanism for Application to Two-Stroke Marine Diesel Engines. Energy Fuels 2017, 31, 941–952. [Google Scholar] [CrossRef]
- Smith, G.P.; Golden, D.M.; Frenklach, M.; Moriarty, N.W.; Eiteneer, B.; Goldenberg, M.; Bowman, C.T.; Hanson, R.K.; Song, S.; Gardiner, W.C., Jr.; et al. GRI-MECH 3.0. Available online: http://www.me.berkeley.edu/gri_mech/ (accessed on 12 December 2003).
- Zhang, C.; Li, B.; Rao, F.; Li, P.; Li, X. A shock tube study of the autoignition characteristics of RP-3 jet fuel. Proc. Combust. Inst. 2015, 35, 3151–3158. [Google Scholar] [CrossRef]
- Mao, Y.; Raza, M.; Wu, Z.; Zhu, J.; Yu, L.; Wang, S.; Zhu, L.; Lu, X. An experimental study of n-dodecane and the development of an improved kinetic model. Combust. Flame 2020, 212, 388–402. [Google Scholar] [CrossRef]
- Hui, X.; Sung, C.-J. Laminar flame speeds of transportation-relevant hydrocarbons and jet fuels at elevated temperatures and pressures. Fuel 2013, 109, 191–200. [Google Scholar] [CrossRef]
- Shen, H.-P.S.; Steinberg, J.; Vanderover, J.; Oehlschlaeger, M.A. A Shock Tube Study of the Ignition of n-Heptane, n-Decane, n-Dodecane, and n-Tetradecane at Elevated Pressures. Energy Fuels 2009, 23, 2482–2489. [Google Scholar] [CrossRef]
- Li, B.; Liu, N.; Zhao, R.; Zhang, H.; Egolfopoulos, F.N. Flame propagation of mixtures of air with high molecular weight neat hydrocarbons and practical jet and diesel fuels. Proc. Combust. Inst. 2013, 34, 727–733. [Google Scholar] [CrossRef]
- Zhu, Y.; Davidson, D.F.; Hanson, R.K. Pyrolysis and oxidation of decalin at elevated pressures: A shock-tube study. Combust. Flame 2014, 161, 371–383. [Google Scholar] [CrossRef]
- Comandini, A.; Dubois, T.; Abid, S.; Chaumeix, N. Comparative Study on Cyclohexane and Decalin Oxidation. Energy Fuels 2014, 28, 714–724. [Google Scholar] [CrossRef]
- Zeng, M.; Li, Y.; Yuan, W.; Li, T.; Wang, Y.; Zhou, Z.; Zhang, L.; Qi, F. Experimental and kinetic modeling study of laminar premixed decalin flames. Proc. Combust. Inst. 2017, 36, 1193–1202. [Google Scholar] [CrossRef]
- Hui, X.; Das, A.K.; Kumar, K.; Sung, C.-J.; Dooley, S.; Dryer, F.L. Laminar flame speeds and extinction stretch rates of selected aromatic hydrocarbons. Fuel 2012, 97, 695–702. [Google Scholar] [CrossRef]
- Wenming, X.; Qingyao, X.; Nanlei, L.; Diankai, W. Experiment of laminar combustion characteristics of 1,3,5-trimethylbenzene. J. Aerosp. Power 2017, 32, 2941–2946. [Google Scholar] [CrossRef]
- Wang, B.-Y.; Liu, Y.-X.; Weng, J.-J.; Pan, G.-F.; Tian, Z.-Y. An experimental and modeling study on the low temperature oxidation of surrogate for JP-8 part II: Comparison between neat 1,3,5-trimethylbenzene and its mixture with n-decane. Combust. Flame 2018, 192, 517–529. [Google Scholar] [CrossRef]

| Item | Octane Number | H/C | Lower Heating Value (MJ/L) | Molecular Weight | Density (20 °C) (g/cm3) | Smoke Point |
|---|---|---|---|---|---|---|
| RP-3 [27] | 43.3 | 1.963 | 42.8 | 150 | 0.778 | 20 |
| Simulation | 43.278 | 1.9608 | 43.48 | 157.02 | 0.787 | 20.177 |
| NO. | Reactions | Kinetic Parameters | ||
|---|---|---|---|---|
| A | b | Ea (J/mol) | ||
| 1 | C7H8 + O2 = C6H5CH2 + HO2 | 2.18 × 107 | 2.5 | 47,300.0 |
| 2 | C7H8 + OH = C6H5CH2 + H2O | 1.77 × 105 | 2.4 | −602.0 |
| 3 | C6H5CH2 + O2 = C6H5CHO + OH | 3.00 × 1015 | −1.6 | 47.0 |
| 4 | C6H5CHO +OH = A1− + CO + H2O | 2.89 × 108 | 1.3 | −1573.0 |
| Reactions | Modification | Kinetic Parameters | Ref. | ||
|---|---|---|---|---|---|
| A | b | E (J/mol) | |||
| R1.:H + O2 = O + OH | Before | 2.644 × 1016 | −0.671 | 1.7041 × 104 | |
| After | 1.04 × 1014 | 0.0 | 1.5286 × 104 | [31] | |
| R12: H + O2(+M) = HO2(+M) | Before | 5.116 × 1012 | 0.440 | 0.0 | |
| After | 1.475 × 1012 | 0.6 | 0.0 | [46] | |
| R14: 2OH(+M) = H2O2(+M) H2O2(+M) = 2OH(+M) | Before | 1.110 × 1014 | −0.370 | 0.0 | |
| After | 2.0 × 1012 | 0.9 | 4.875 × 104 | [31] | |
| R212: CH3 + HO2 <=> CH4 + O2 | Before | 3.1600 × 1012 | 0.00 | 0.00 | |
| After | 1.160 × 105 | 2.230 | −3022.0 | [29] | |
| R28: CO + OH = CO2 + H | Before | 8.0 × 1011 | 0.1 | 7352.0 | |
| R29: CO + OH = CO2 + H | Before | 8.78 × 1010 | 0.0 | −16.0 | |
| After | 1.04 × 1014 | 0.0 | 1.5286 × 104 | [47] | |
| R36: HCO + M = CO + H + M | Before | 1.87 × 1017 | −1.0 | 1.70 × 104 | |
| After | 5.7 × 1011 | 0.66 | 1.487 × 104 | [31] | |
| R37: HCO + H2O = CO + H + H2O | Before | 2.2440 × 1018 | −1.0 | 1.70 × 104 | |
| After | 1.5 × 1018 | −1.0 | 1.70 × 104 | [48] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, C.; Zheng, Z.; Chen, Q. Construction of a Chemical Kinetic Mechanism of Five-Component Surrogate Fuel for RP-3 Kerosene. Appl. Sci. 2025, 15, 4971. https://doi.org/10.3390/app15094971
Dai C, Zheng Z, Chen Q. Construction of a Chemical Kinetic Mechanism of Five-Component Surrogate Fuel for RP-3 Kerosene. Applied Sciences. 2025; 15(9):4971. https://doi.org/10.3390/app15094971
Chicago/Turabian StyleDai, Changxuan, Zhaolei Zheng, and Qin Chen. 2025. "Construction of a Chemical Kinetic Mechanism of Five-Component Surrogate Fuel for RP-3 Kerosene" Applied Sciences 15, no. 9: 4971. https://doi.org/10.3390/app15094971
APA StyleDai, C., Zheng, Z., & Chen, Q. (2025). Construction of a Chemical Kinetic Mechanism of Five-Component Surrogate Fuel for RP-3 Kerosene. Applied Sciences, 15(9), 4971. https://doi.org/10.3390/app15094971