Abstract
With the continuous expansion of urban areas, the treatment of urban sewage is facing significant challenges. Tens of thousands of tons of municipal sludge (MS) are produced annually, which not only occupies substantial land resources but also poses potential environmental threats, thereby complicating wastewater treatment processes. Proper management of MS has thus become a critical issue requiring urgent attention. Meanwhile, water pollution continues to worsen, endangering both ecological systems and human health. MS contains a variety of organic compounds with active functional groups capable of forming strong coordination interactions with various waterborne pollutants. Building on this foundation, we successfully develop a multifunctional adsorbent using MS as the raw material through biomineralization. The synthesized adsorbent shows outstanding performance, exhibiting high adsorption capacity for fluoride (F−) and hexavalent uranium (U(VI)) in high-fluorine uranium-containing wastewater, effectively reducing the concentrations of these harmful substances. Additionally, the adsorbent shows strong affinity for the cationic dye methylene blue, making it highly suitable for the treatment of wastewater from the printing and dyeing industries. Notably, the adsorbent also possesses antibacterial properties, demonstrating significant bactericidal activity against Gram-negative E. coli in wastewater. The multifunctional adsorbent not only offers a novel solution to enhancing water quality and safety, but also represents a promising strategy for sustainable wastewater treatment.
1. Introduction
Water is known as the source of life for all living beings on Earth, and it is the foundation of life and a driving force behind the economic development of human society. However, with the rapid expansion of human activities and the global economy, the production of sewage has experienced explosive growth, placing immense pressure on water supply resources and creating a significant crisis. When untreated sewage is disposed of into water bodies, harmful substances in sewage are often difficult to degrade and accumulate over time, which not only disrupts the balance of aquatic ecosystems, causing the death of numerous aquatic organisms, but also poses potential threats to human health. Furthermore, the worsening pollution exacerbates the scarcity of freshwater resources, presenting major challenges to human health and environmental sustainability [1,2,3]. Common water pollutants include dyes [4], heavy metal ions [5], organic pollutants, nutrients, fluoride, bacteria, and radioactive elements. The discharge of wastewater poses a serious threat to both aquatic ecosystems and human health. To meet the discharge standards of sewage, these pollutants must be effectively removed. Common treatment methods for wastewater include adsorption, biofilm filtration, and precipitation. However, due to the typically complex composition of wastewater, treatment methods must be tailored to the specific pollutants involved.
With the ongoing depletion of global fossil fuel reserves, nuclear energy has increasingly emerged as a primary alternative energy source. Uranium, as the main nuclear fuel, is widely utilized in nuclear power generation [6,7]. However, the development and utilization of nuclear energy—such as uranium enrichment, uranium conversion, and nuclear fuel element manufacturing—involve processes that convert uranium dioxide (UO22+) into gaseous uranium hexafluoride (UF6). The evaporation and hydrolysis of UF6 can generate large volumes of wastewater containing high concentrations of U(VI) and fluoride [8,9]. In industrial settings, ammonium diuranate and ammonium uranyl carbonate are commonly used to treat fluoride-rich nuclear wastewater [8,10]. However, these methods generate considerable amounts of radioactive wastewater containing fluoride ions (F−). In high-fluorine uranium-containing wastewater, the concentration of uranyl ions typically ranges from tens to hundreds of ppm, while the F− concentration can reach 5 to 10 g/L. The strong chemical affinity between F− and uranium leads to the formation of various uranyl fluoride coordination complexes, such as UO2F+ and UO2F2. These complexes significantly hinder the separation of uranium and fluoride, complicating uranium recovery and reducing the efficiency of traditional treatment methods such as adsorption and ion exchange [11]. Therefore, the extraction of uranium in the presence of high F− concentrations remains a critical challenge for both environmental protection and the sustainable recovery of uranium resources [12,13,14,15].
As important synthetic compounds, dyes are chemically designed to adhere to various materials, such as textiles, fur, and plastics, imparting a wide range of colors [16,17,18]. Due to their versatility, dyes are widely used in industries such as papermaking and food production. Among these dyes, methylene blue (MB), a cationic organic dye, is particularly notable. It is water-soluble and imparts a blue coloration to the water, which interferes with the photosynthesis of aquatic plants, leading to oxygen depletion and posing a threat to the survival of aquatic organisms. Moreover, when MB enters the human body through contaminated drinking water it can cause adverse health effects and may even contribute to the development of certain diseases [19,20]. MB exhibits redox properties and can react with biological organisms, organic compounds, and metal ions in water, thereby altering key water quality parameters such as color, odor, and biochemical oxygen demand (BOD). If discharged without proper treatment, MB poses serious risks to both environmental and human health.
Due to increasing water pollution, E. coli has become one of the primary contaminants in water bodies [21]. It can enter the environment through fecal discharge and other pathways. However, it is often accompanied by various other pathogens, posing significant risks to both human health and the ecological environment. Globally, E. coli contamination in water bodies is a widespread concern. According to a report by the World Health Organization (WHO), approximately 80% of diseases in developing countries are associated with unsafe drinking water and poor sanitation, with E. coli contamination being a major contributing factor. In many underdeveloped regions of Africa and Asia, the lack of adequate sewage treatment facilities and clean water supplies has resulted in frequent cases of E. coli exceeding safe levels in rivers, lakes, groundwater, and other water sources [22]. Even in countries with relatively advanced sewage treatment systems, E. coli contamination in water bodies remains a recurring issue. Common sources include agricultural non-point source pollution and the discharge of livestock and poultry waste. Therefore, addressing E. coli contamination is a critical aspect of wastewater treatment, essential for ensuring water safety and public health.
Municipal sludge (MS) is a solid waste generated during the sewage treatment process. The inorganic substances in MS are mainly oxides of silicon, iron, aluminum, and calcium. Its composition is relatively complex, containing a variety of inorganic substances, organic substances, and microorganisms. The organic components in MS primarily include carbohydrates, proteins, fats, and humic acids, which originate from plant matter in sewage, microbial biomass, and organic waste generated by human activities. These organic substances are rich in functional groups such as -OH, -COOH, -NH2, and C=O, which exhibit strong coordination capabilities. As a result, they can readily form complexes with heavy metal ions, making MS highly effective for the removal of heavy metal ions from wastewater [23]. Liu et al. prepared alkaline sludge biochar from septic tank sludge as a sustainable adsorbent for phosphorus recovery in agriculture [24]. First, the septic tank sludge was pyrolyzed at 500 °C to produce the original biochar, and then the basic biochar was prepared by the mixed pyrolysis of KOH and the original biochar at 800 °C. This kind of biochar preparation process is simple, but requires a higher temperature to pyrolyze the sludge, increasing the production cost. Therefore, we prepare a multifunctional adsorbent, by making full use of MS’s rich functional groups, without pyrolysis, which can remove a variety of pollutants in the water body (radioactive elements, fluoride, dyes, and bacteria, etc.), so as to cope with different pollutants in the water body and realize the process of wastewater purification. The biggest advantage of the adsorbent is that it not only realizes the resource utilization of MS, but also the prepared multifunctional adsorbent can better cope with the complex sewage environment.
In response to the escalating issue of water pollution, we utilize the abundant functional groups present in the organic matter on the surface of MS to synthesize an adsorbent which is capable of effectively treating high-fluorine uranium-containing wastewater. In this study, cinnamaldehyde (CID) is incorporated to enhance the antibacterial properties of the composite against E. coli in water. Additionally, zirconium dioxide (ZrO2) nanoparticles are loaded onto the material through a biomineralization process (Figure 1). The resulting adsorbent (MS/CID/ZrO2) demonstrates high efficiency in removing fluoride and uranyl ions, while also effectively eliminating dyes (such as MB) and exhibiting bactericidal activity against E. coli in water.
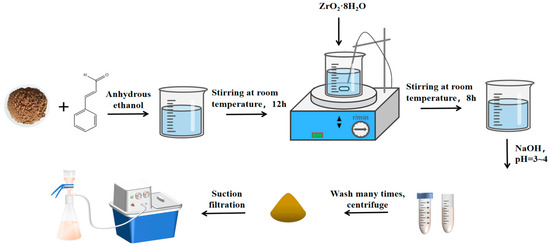
Figure 1.
Synthesis process of the adsorbent of MS/CID/ZrO2 via biomineralization.
2. Materials and Methods
2.1. Chemicals and Materials
MS (powder, activated sludge from Maidao sewage treatment plant) and uranyl acetate (C4H10O8U, 99%). The uranyl acetate (C4H10O8U, 99%) was dissolved in deionized water to prepare a U(VI) stock solution (400 mg/L), which was stored in the dark until further use. Before the adsorption experiments, the U(VI) stock solution was diluted with distilled water to obtain working solutions of varying concentrations.
Arsenazo III (C22H18As2N4O14S2, 95%) was purchased from the Shanghai MACLIN Biochemical Technology Co., Ltd. (Shanghai, China). Disodium ethylenediaminetetraacetic acid and dihydrate (C10H14N2Na2O8·2H2O, 98%) were purchased from the Shandong Keyuan Biochemical Co., Ltd. (Laizhou, China). ZrOCl2·8H2O (98%), NaF (98%), CID (C6H5CHCHCHO), NaOH (96%), HCl (36–38%), and NaCl (99%) were bought from the Sinopharm Chemical Reagent Co., Ltd. (Beijing, China).
All chemicals used in this study were of analytical reagent grade and were used without further purification. Deionized water was produced using a Millipore Milli-Q water system (Merck, Darmstadt, Germany) by a laboratory ultrapure water machine (resistivity of 18 Ω·m).
2.2. Preparation of MS Powder
After drying the MS in an oven at 110 °C, it was ground using a crusher. The resulting material was then sieved through a 200-mesh screen to obtain the MS powder sample.
2.3. Preparation of MS/CID/ZrO2 Through Biomineralization
A mass of 0.5 g of 200-mesh MS powder was accurately weighed and placed in a beaker. Then, 15 mL of absolute ethanol and 10 μL of CID solution were added, and the mixture was stirred at room temperature for 12 h. Subsequently, 0.3, 0.6, and 0.9 g of ZrOCl2·8H2O powder were weighed and respectively added into the mixed solution under stirring for 12 h to create three mixtures. Each mixture was then stirred at room temperature for an additional 8 h. After the reaction, the pH of the mixed solution was adjusted to 3.5–4.0 using 0.1 M NaOH solution. The mixed solution was centrifuged at 12,000 rpm for 5 min to take the lower precipitate, which was then dried at 60 °C for 12 h. The samples MS/CID/ZrO2-0.3, MS/CID/ZrO2-0.6, and MS/CID/ZrO2-0.9 were obtained, respectively.
2.4. Characterization Techniques
The microstructure of the MS/CID/ZrO2 samples was observed using a scanning electron microscope (SEM, Regulus 8100, Hitachi, Tokyo, Japan). The functional groups of MS/CID/ZrO2 were analyzed using a Fourier transform infrared spectrometer (FT-IR, Nicolet iS 50, Nicolet Instrument, Madison, WI, USA). The samples before and after adsorption of U were analyzed by X-ray photoelectron spectroscopy (XPS) to study its adsorption mechanism. X-ray diffraction (XRD) analysis of MS/CID/ZrO2 was carried out using an X-ray diffractometer. The XRD patterns were identified by power XRD using a D/max 2500 PC (Rigaku, Tokyo, Japan) with Cu kα radiation (λ = 0.15406 nm).
3. Results and Discussion
3.1. Characterizations of MS/CID/ZrO2 Composites
To better observe the microstructure of the MS/CID/ZrO2 composites, we analyzed MS and MS/CID/ZrO2 using SEM, and the results are shown in Figure 2. Figure 2a,b display the SEM images of MS, revealing a rough surface structure characterized by layered and stacked granular features. In contrast, Figure 2c,d present the SEM images of MS/CID/ZrO2, which exhibits a smoother surface structure. Adjusting the pH to between 3.5 and 4.0 during the solution preparation process is conducive to the formation of very small ZrO2 nanoparticles (approximately 5–20 nm) [25,26]. We suggest that this smoother surface resulted from the addition of ZrOCl2·8H2O, where the in situ-generated biomimetic ZrO2 nanoparticles filled the original pores in MS.
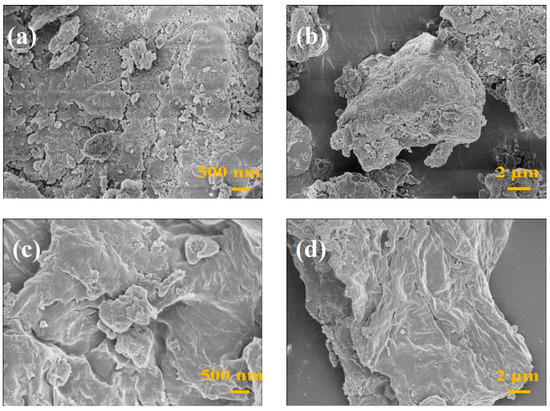
Figure 2.
SEM images of (a,b) MS and (c,d) MS/CID/ZrO2.
As an essential analytical technique, FTIR spectroscopy was used to identify various functional groups on the surface of the adsorbent. When infrared light interacts with a solid material, the chemical bonds undergo changes in shape, whether through stretching, contracting, or bending. Consequently, regardless of the molecular structure, each functional group tends to absorb infrared light within a specific wavenumber range. Figure 3a presents the FTIR spectra of MS and MS/CID/ZrO2. A broad peak band between 3650 and 3000 cm−1 is observed, indicating the formation and alteration of hydrogen bonds. We suggest hydrogen bonding interactions between MS and MS/CID/ZrO2 are responsible for that [27,28,29]. Additionally, the absorption peaks at 3300 cm−1 and 3383 cm−1 are attributed to the stretching vibrations of -OH groups. Both MS and ZrO2 nanoparticles contain abundant -OH groups, and the peak intensity increases after the in situ formation of ZrO2 nanoparticles [30]. The absorption peaks at 1640 cm−1 for MS and 1659 cm−1 for MS/CID/ZrO2 are associated with the C=O stretching vibrations of carboxyl/carbonyl groups [31], and the peaks at 1530 cm−1 for both MS and MS/CID/ZrO2 may correspond to -C=N bonds [32], resulting from the reaction between the amino groups in MS and the aldehyde groups in CID. The absorption peak at 1030 cm−1 is likely related to C-O bonds [33]. It should be noted that the FTIR spectrum of MS/CID/ZrO2 reveals a new absorption peak at 573 cm−1, corresponding to the Zr-O functional group, and confirming the successful biomineralization formation of ZrO2 nanoparticles on MS [34]. To further confirm the formation of ZrO2 nanoparticles, XRD analysis was performed on the MS/CID/ZrO2 composites. As shown in Figure 3b, the XRD pattern exhibits characteristic peaks consistent with the standard XRD pattern of ZrO2, providing clear evidence for the formation of ZrO2 nanoparticles within the MS/CID/ZrO2 composites.
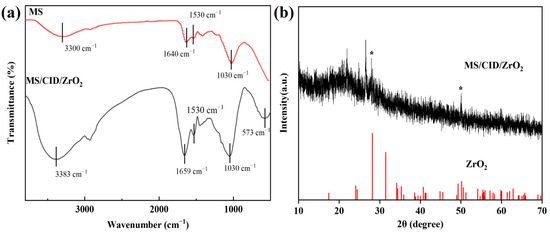
Figure 3.
(a) FTIR spectra of MS and MS/CID/ZrO2 composites. (b) XRD pattern of MS/CID/ZrO2 composites (☆ the marked peak is characteristic of ZrO2, the red line represents the ZrO2 standard card).
Considering that chemisorption involves changes in the chemical states of the adsorbed atoms, corresponding shifts in their specific binding energies are observed in the XPS spectra. To study the adsorption mechanism of U(VI) by MS/CID/ZrO2, we used XPS to analyze the samples before and after the uranium adsorption. The high-resolution spectra of N and O atoms were analyzed using two-component Gaussian–Lorentzian sum functions to confirm the changes in their chemical states (Figure 4a). As shown in Figure 4b, after treating high-fluorine uranium-containing wastewater with MS/CID/ZrO2, the peaks corresponding to U 4f7/2 and U 4f5/2 appear at 382.03 and 391.92 eV [35], respectively, with a spin-orbit splitting of 9.89 eV [36]. This confirms the successful binding of U(VI) onto MS/CID/ZrO2.
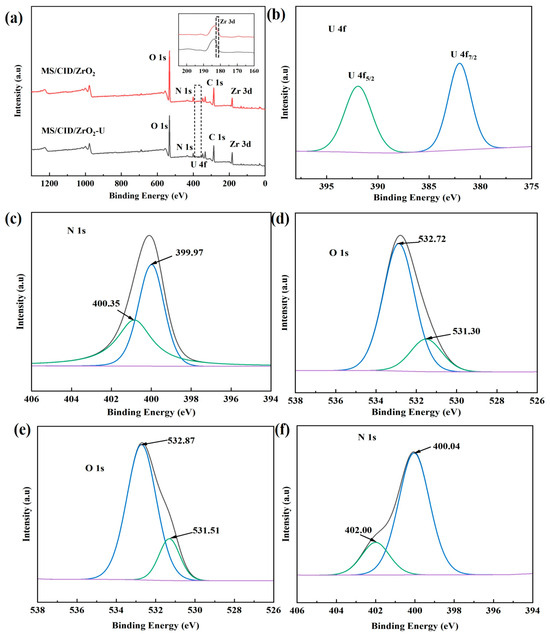
Figure 4.
(a) XPS spectra of MS/CID/ZrO2 before and after uranium adsorption (illustration is a larger view of the Zr 3d peak), (b) high-resolution XPS spectra of MS/CID/ZrO2 in U 4f region, (c,e) high-resolution N 1s XPS spectra before and after uranium adsorption by MS/CID/ZrO2, and (d,f) high-resolution O 1s XPS spectra before and after uranium adsorption by MS/CID/ZrO2.
The O 1s and N 1s peaks of the MS/CID/ZrO2 composites before and after the U(VI) adsorption were analyzed, and the results are shown in Figure 4c,e and Figure 4d,f, respectively. The high-resolution N 1s spectrum of the sample before the uranium adsorption was deconvoluted into two peaks at 399.97 and 400.35 eV, corresponding to C-NH2 and C=N-OH [37], respectively. After the U(VI) adsorption, the N 1s spectrum shifted to higher binding energies (400.04 and 402.00 eV). This shift can be attributed to the formation of coordination bonds between the lone pair electrons of nitrogen atoms and uranium, which reduced the electron density around the N atoms and increased their binding energy [38,39]. Similarly, as shown in Figure 4d, the high-resolution O 1s spectrum of the sample before the uranium adsorption was deconvoluted into two peaks at 531.30 and 532.72 eV, corresponding to -COOH and -OH groups in MS/CID/ZrO2, respectively. After the adsorption, the positions of these peaks shifted to 531.51 and 532.87 eV, respectively. This shift, similar to the case for N atoms, indicates that O atoms also participated in the coordination with uranium. From the inset of Figure 4a, it can be seen that after adsorption, the Zr 3d peak of MS/CID/ZrO2 shifted to the direction of higher binding energy, which may be due to the participation of F−- and Zr(IV)-bonded hydroxyl groups in the adsorption reaction, resulting in the formation of new zirconium species (Zr oxyfluoride or ZrF4) [40,41].
3.2. Adsorption Experiment of High-Fluorine Uranium-Containing Wastewater
In the adsorption experiment, 10 mL of simulated high-fluoride uranium-containing wastewater solution (U(VI) concentrations of 10, 100, and 200 mg/L, F− concentration of 100 mg/L) was added to the prepared MS/CID/ZrO2 sample, and stirred with a glass rod at room temperature for about 15 s in a beaker. Subsequently, the solution mixed with the sample was subjected to rapid vacuum filtration using a vacuum filtration machine at an operating pressure of −0.1 MPa. After filtration, the filtrate was taken and its absorbance measured at wavelengths of 620 and 652 nm using a UV spectrophotometer. The concentrations of F− and U(VI) were calculated based on the absorbance. The uranium concentration was determined by Arsenazo III spectrophotometry [42], and the test for the fluorine concentration is specifically described in the Supplementary File.
The adsorption efficiency R (%) of MS/CID/ZrO2 for U(VI), F−, and MB was calculated by the following Formula (1):
in which C0 (mg/L) is the initial concentrations of U(VI), F−, and MB, and Ce (mg/L) is the equilibrium concentrations of U(VI), F−, and MB.
The removal efficiencies of the composites of MS/CID/ZrO2-0.3, MS/CID/ZrO2-0.6, and MS/CID/ZrO2-0.9 towards U(VI) and F− were tested using a simulated high-fluorine uranium-containing wastewater with 10 mg/L U(VI) and 100 mg/L F−. As shown in Figure 5a, the results indicate that the removal efficiencies of all composites towards both U(VI) and F- exceeded 99%. Given the similar removal performance across all the samples, we selected the 0.3 g ZrOCl2·8H2O precursor sample (MS/CID/ZrO2-0.3, approximately 0.65 g) for subsequent dye adsorption and antibacterial experiments, considering the economic cost.
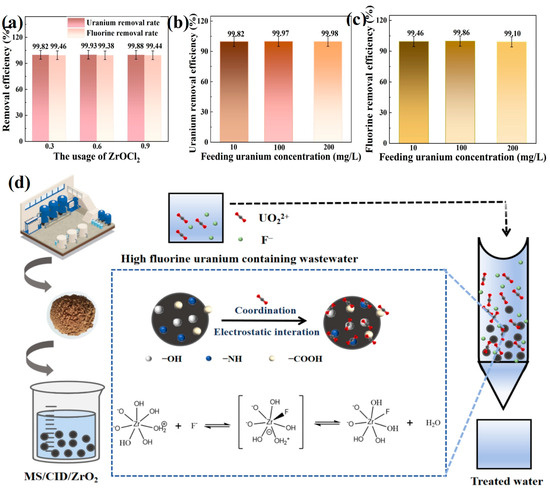
Figure 5.
(a) Removal efficiency of F− and U(VI) in high-fluoride uranium-containing wastewater by MS/CID/ZrO2 with different Zr contents. (b) The removal efficiency of U(VI) and F− (c) in high-fluoride uranium-containing wastewater with different uranium concentrations by MS/CID/ZrO2. (d) Adsorption mechanism diagram of MS/CID/ZrO2.
To further investigate the removal efficiency of the MS/CID/ZrO2 composites in high-fluoride uranium-containing solutions, we tested simulated wastewater solutions with varying uranium concentrations (10, 100, and 200 mg/L) and a constant F− concentration of 100 mg/L. As shown in Figure 5b, when the U(VI) concentration was 10 mg/L, the removal efficiency of MS/CID/ZrO2-0.3 for U(VI) reached 99.82%. At the U(VI) concentrations of 100 and 200 mg/L, the removal efficiency increased to 99.97% and 99.98%, respectively. Similarly, the F− removal efficiency of the MS/CID/ZrO2-0.3 exceeded 99% for all tested uranium concentrations (Figure 5c). The removal efficiency of MS for F− in high-fluorine uranium-containing wastewater was poor, about 70% (Figure S1). In order to evaluate the saturated adsorption performance of the MS/CID/ZrO2, the MS/CID/ZrO2 sample was dried, and 0.1 g of MS/CID/ZrO2 was mixed with 500 mL of high-fluorine uranium-containing wastewater (U(VI) and F− concentrations: 100 mg/L). The results showed that the adsorption capacity of the MS/CID/ZrO2 for U(VI) was 310.60 mg/g, and the saturated capacity for F− was 107.98 mg/L. The adsorption of U(VI) and F− by the MS/CID/ZrO2 can be attributed to the interactions between surface functional groups, such as hydroxyl (-OH), Zr-OH, -NH, and -NH2, and U(VI). These groups exhibited electrostatic attraction and complexation with U(VI), and their active sites demonstrated high adsorption capacity for various uranium hydrolysis products, based on previous reports [43,44].
The adsorption of F− by MS/CID/ZrO2 is primarily due to the in situ biomineralization formation of ZrO2 nanoparticles. In acidic solutions, the positively charged surface sites attract negatively charged F− through electrostatic interactions. Previous studies have shown that one ZrO2 molecule can bind with up to five F−, significantly enhancing the adsorbent capacity of materials for fluoride removal [45,46].
3.3. Dye Adsorption Performance
For the dye adsorption experiments, the MS/CID/ZrO2 composites were added into 10 mL of simulated MB wastewater solution with different MB concentrations. By rapid vacuum filtration with an operating pressure of −0.1 MPa, the filtrate was collected and the remaining MB concentration was measured using an enzyme-linked immunosorbent assay (ELISA) reader. The principle of measuring the MB concentration with an enzyme-linked immunosorbent assay (ELISA) reader is the same as that of using an ultraviolet spectrophotometer to measure MB absorbance at a specific wavelength. The absorbance of an MB solution with a known concentration was measured at 660 nm, and the absorbance was compared with the standard curve of methylene blue according to previous reports [47,48] in order to estimate the solubility of MB in the sample solutions, and finally the removal rate of MB was calculated.
To further investigate the removal efficiency of MS/CID/ZrO2 towards MB, we prepared simulated wastewater solutions with varying MB concentrations (1, 10, 50, 100, and 300 mg/L). After the adsorption by MS/CID/ZrO2, a significant color change was observed, with the treated water becoming nearly transparent (Figure 6a). Then, we calculated the removal efficiency of the hybrid adsorbent. When the concentrations of the MB solutions were 1, 10, 50, 100, and 300 mg/L, the removal efficiency was 99.90%, 99.99%, 99.99%, 99.79%, and 99.74%, respectively (Figure 6b), which proves the excellent removal efficiency of the MS/CID/ZrO2 towards MB. The removal efficiency of MB by MS is relatively poor, especially at low concentrations (Figure S2). In order to evaluate the saturated adsorption performance of MS/CID/ZrO2 on MB, the MS/CID/ZrO2 sample was dried, and 0.1 g of MS/CID/ZrO2 was mixed with 250 mL of MB solution with a concentration of 50 mg/L. The results showed that the adsorption capacity of MS/CID/ZrO2 for MB was 100.55 mg/g.
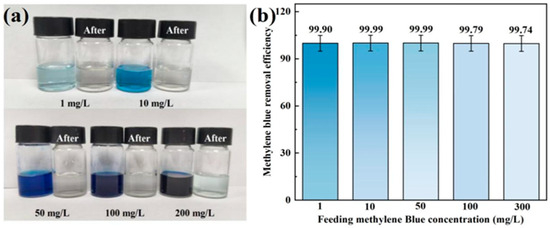
Figure 6.
Color change (a) and removal rate (b) of the MS/CID/ZrO2 composites towards MB.
3.4. Antibacterial Performance
To further investigate the antibacterial properties of MS/CID/ZrO2, we evaluated the antibacterial activities of both MS and MS/CID/ZrO2 using the plate counting method. E. coli was inoculated into the LB liquid medium and cultured at 37 °C and 120 r/min for 12 h. After the culture, the bacterial suspension was diluted with sterile water to achieve a concentration of 1.5 × 105 CFU/mL. Under sterile conditions, 5 mL of E. coli solution was mixed with the MS/CID/ZrO2 composites under continuous stirring for 10 min. Then, the mixed solution was treated by rapid vacuum filtration at a pressure of −0.1 MPa to collect the filtrate.
Next, 100 μL of the filtrate and 100 μL of the 1.5 × 105 CFU/mL E. coli suspension were transferred and coated onto plates. Each sample is coated with the plate three times. The coated plates were placed in a 37 °C air bath constant-temperature incubator and inverted for 12 h of culture. After the culture was completed, the growth of E. coli on each plate was observed. Compared with the plates of the control group coated with 1.5 × 105 CFU/mL E. coli bacterial solution, there was a small amount of bacterial growth on the MS sample plates, indicating that MS has a certain antibacterial ability. There was almost no growth of E. coli on the MS/CID/ZrO2 sample plate, indicating that there were no live bacterial residues in the filtrate after the mixture of MS/CID/ZrO2 and the bacterial liquid and suction filtration. This proved that the MS/CID/ZrO2 material has a significant antibacterial effect on E. coli (Figure 7). We suggest that both CID and ZrO2 nanoparticles in the MS/CID/ZrO2 composites had a synergistic antibacterial effect. CID is a broad-spectrum antibacterial agent that has strong inhibitory effects on both Gram-positive and Gram-negative bacteria [49]. It acts on the cell wall of E. coli, causing morphological changes on the cell surface and disrupting the cell membrane, which leads to the leakage of intracellular contents. This disruption affects the normal physiological activities of the bacteria, ultimately resulting in cell death [50]. ZrO2 nanoparticles can alter the permeability of E. coli membranes, leading to the deposition of nanoparticles in bacterial membranes and cytoplasmic regions of cells. The surface of ZrO2 nanoparticles carries a positive charge, while E. coli carries a negative charge. Therefore, the electromagnetic attraction between ZrO2 nanoparticles and E. coli causes oxidation and bacterial death [51,52].
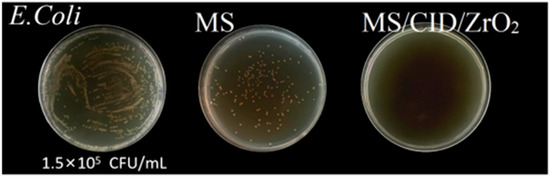
Figure 7.
Plate counting method was used to test the antibacterial effect of MS and MS/CID/ZrO2 against Gram-negative E. coli.
4. Sustainability Analysis
To evaluate the sustainability of the material, we conducted a comprehensive multi-factor evaluation of MS/CID/ZrO2 using the Sustainability Footprint (OSF) method [53], which has been applied to analyze the sustainability of various water purification materials. Based on the three core principles of ecological sustainability, economic sustainability, and social sustainability, we selected eight specific factors to evaluate the sustainability of MS/CID/ZrO2. These factors included environmental friendliness, degradability, waste utilization, sources, ease of preparation, cost, removal efficiency, and recyclability. We evaluated each factor based on three levels, scoring them according to the criteria of low (L, i = 1), medium (M, i = 2), and high (H, i = 3). Unlike the other factors, due to the negative impact of “cost” on the sustainability of materials, the effects generated by “cost” are opposite to other factors, corresponding to low (L, i = 3), medium (M, i = 2), and high (H, i = 1), respectively. The overall sustainability of the material was calculated using the OSF Formula (2):
in which j represents sustainability and i represents the corresponding ranking score.
We chose four previously published materials, including biochar, zeolite, diatomaceous earth, and metal–organic frameworks, to compare with MS/CID/ZrO2 to study their sustainability. According to Formula (2) above, they correspond to sustainability values of 62.5%, 66.6%, 70.8%, 70.8%, and 75%, respectively, as shown in Figure 8. It can be found that the MS/CID/ZrO2 reveals relative advantages in terms of cost, source, and ease of preparation. The production of MS increases dramatically every year, providing a continuous supply of raw materials for the preparation of MS/CID/ZrO2. Due to its simple preparation process, it offers distinct advantages as an adsorbent for water treatment applications.
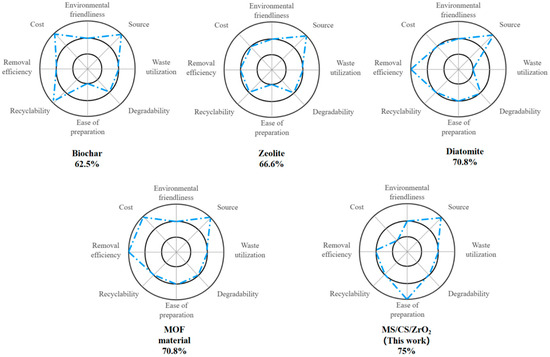
Figure 8.
Sustainability analysis of MS/CID/ZrO2 through a comparison with four other U(VI) adsorbents.
5. Conclusions
In summary, we used MS to prepare multifunctional MS/CID/ZrO2 adsorbents via biomineralization with excellent removal efficiency for different pollutants in water. The aldehyde group of CID reacted with the amino group on the surface of MS and grafted the CID to the surface of MS. Then ZrOCl2·8H2O was added and ZrO2 nanoparticles were formed in the original position of the material. CID and ZrO2 nanoparticles exhibited a synergistic antibacterial effect, and revealed excellent removal effects on E. coli in water. In high-fluoride uranium-containing wastewater with three different uranium concentrations, the removal efficiency of U(VI) and F- by the MS/CID/ZrO2 composites reached over 99%, and the removal rate of MB by MS/CID/ZrO2 also reached over 99%. Additionally, the MS/CID/ZrO2 demonstrated a high bactericidal effect on E. coli. The obtained results indicated that both CID and ZrO2 nanoparticles contributed to the high adsorption performance of the synthesized MS/CID/ZrO2. It is evident that MS/CID/ZrO2 performs effectively under various wastewater conditions. Its low cost, simple fabrication process, and high adsorption efficiency highlight its significant potential in the field of water purification.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15094794/s1, Figure S1: (a) Removal efficiency of uranium in high-fluorine uranium-containing wastewater by MS. (b) Removal efficiency of fluorine in high-fluorine uranium-containing wastewater by MS. Figure S2: Removal efficiency of MB by MS.
Author Contributions
Conceptualization, G.W. and L.G.; data curation, W.Y. and X.F.; formal analysis, W.Y., X.F. and W.L.; funding acquisition, G.W. and L.G.; investigation, W.Y. and W.L.; methodology, W.Y. and X.F.; project administration, G.W. and L.G.; software, X.F.; supervision, G.W. and L.G.; validation, W.L.; writing—original draft, W.Y.; writing—review and editing, G.W. and L.G. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the financial support of the Taishan Scholars Program of Shandong Province (No. tsqn201909104) and the High-Grade Talents Plan of Qingdao University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
We appreciate Hao Wan, who works in the Institute of Advanced Cross-field Science, College of Life Sciences, Qingdao University, for her help in some experiments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mekonnen, M.M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Sun, S.; Fu, G.; Hall, J.W.; Ni, Y.; He, L.; Yi, J.; Zhao, N.; Du, Y.; Pei, T.; et al. Pollution exacerbates China’s water scarcity and its regional inequality. Nat. Commun. 2020, 11, 650. [Google Scholar] [CrossRef]
- Yang, W.; Li, W.; Lei, Y.; He, P.; Wei, G.; Guo, L. Functionalization of cellulose-based sponges: Design, modification, environmental applications, and sustainability analysis. Carbohydr. Polym. 2024, 348, 121175. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A.; Inamuddin, n.; Asiri, A.M. Exploring the Reusability of Synthetically Contaminated Wastewater Containing Crystal Violet Dye using Tectona grandis Sawdust as a Very Low-Cost Adsorbent. Sci. Rep. 2018, 8, 8314. [Google Scholar] [CrossRef] [PubMed]
- Al-Yaari, M.; Saleh, T.A. Mercury Removal from Water Using a Novel Composite of Polyacrylate-Modified Carbon. ACS Omega 2022, 7, 14820–14831. [Google Scholar] [CrossRef]
- Wang, P.; Dong, F.; He, D.; Liu, S.; Chen, N.; Huo, T. Organic acid mediated photoelectrochemical reduction of U(VI) to U(IV) in waste water: Electrochemical parameters and spectroscopy. RSC Adv. 2021, 11, 23241–23248. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Shi, X.; Yang, M.; Tan, Q.; Xu, Z.; Ma, B.; Pan, D.; Wu, W. Phosphate and illite colloid pose a synergistic risk of enhanced uranium transport in groundwater: A challenge for phosphate immobilization remediation of uranium contaminated environmental water. Water Res. 2024, 255, 121514. [Google Scholar] [CrossRef]
- Badawy, S.M.; Sokker, H.H.; Othman, S.H.; Hashem, A. Cloth filter for recovery of uranium from radioactive waste. Radiat. Phys. Chem. 2005, 7, 125–130. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, J.; Liu, Z.; Wu, W. Environmentally Friendlier Approach to Nuclear Industry: Recovery of Uranium from Carbonate Solutions Using Ionic Liquids. Ind. Eng. Chem. Res. 2015, 54, 8624–8628. [Google Scholar] [CrossRef]
- Nizinski, C.A.; Olson, J.; Chalifoux, A.M.; Kurtyka, N.; Athon, M.T.; Tenner, T.; McDonald, L.W. Identification and Elemental Impurity Analysis of Heterogeneous Morphologies in Uranium Oxides Synthesized from Uranyl Fluoride Precursors. ACS Omega 2023, 54, 8624–8628. [Google Scholar] [CrossRef]
- Lin, T.; Chen, T.; Jiao, C.; Zhang, H.; Hou, K.; Jin, H.; Liu, Y.; Zhu, W.; He, R. Ion pair sites for efficient electrochemical extraction of uranium in real nuclear wastewater. Nat. Commun. 2024, 15, 4149. [Google Scholar] [CrossRef] [PubMed]
- Busquim e Silva, R.; Kazimi, M.S.; Hejzlar, P. Nuclear fuel recycling: National and regional options for the US nuclear energy system. Energy Environ. Sci. 2010, 3, 996–1010. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Zhu, X.; Meng, C.; Dong, Z.; Xiao, S.; Wang, Y.; Wang, Y.; Cao, X.; Liu, Y. Exciton dissociation and transfer behavior and surface reaction mechanism in Donor–Acceptor organic semiconductor photocatalytic separation of uranium. Appl. Catal. B Environ. Energy 2023, 332, 122751. [Google Scholar] [CrossRef]
- Wang, C.; Helal, A.S.; Wang, Z.; Zhou, J.; Yao, X.; Shi, Z.; Ren, Y.; Lee, J.; Chang, J.K.; Fugetsu, B.; et al. Uranium In Situ Electrolytic Deposition with a Reusable Functional Graphene-Foam Electrode. Adv. Mater. 2021, 33, 2102633. [Google Scholar] [CrossRef]
- Liu, M.; Wang, K.; Wang, H.; Lu, J.; Xu, S.; Zhao, L.; Wang, X.; Du, J. Simple and sensitive colorimetric sensors for the selective detection of Cu(ii). RSC Adv. 2021, 11, 11732–11738. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, S.; Gong, Y.; Wang, L. Potential trade-off between water consumption and water quality: Life cycle assessment of nonaqueous solvent dyeing. Water Res. 2022, 215, 118222. [Google Scholar] [CrossRef]
- Routoula, E.; Patwardhan, S.V. Degradation of Anthraquinone Dyes from Effluents: A Review Focusing on Enzymatic Dye Degradation with Industrial Potential. Environ. Sci. Technol. 2020, 54, 647–664. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Geng, Q.; Yang, J.; Liu, Y.; Liu, C. Hybrid System of Flocculation–Photocatalysis for the Decolorization of Crystal Violet, Reactive Red X-3B, and Acid Orange II Dye. ACS Omega 2020, 5, 31131–31145. [Google Scholar] [CrossRef]
- Kiani, A.; Haratipour, P.; Ahmadi, M.; Zare-Dorabei, R.; Mahmoodi, A. Efficient removal of some anionic dyes from aqueous solution using a polymer-coated magnetic nano-adsorbent. J. Water Supply Res. Technol. AQUA 2017, 66, 239–248. [Google Scholar] [CrossRef]
- Ghafourian, N.; Hosseini, S.N.; Mahmoodi, Z.; Masnabadi, N.; Thalji, M.R.; Abhari, A.R.; Al Zoubi, W.; Chong, K.F.; Ali, G.A.M.; Bakr, Z.H. TiO2-Mica 450 composite for photocatalytic degradation of methylene blue using UV irradiation. Emergent Mater. 2023, 6, 1527–1536. [Google Scholar] [CrossRef]
- Gunda, N.S.K.; Chavali, R.; Mitra, S.K. A hydrogel based rapid test method for detection of Escherichia coli (E. coli) in contaminated water samples†. Analyst 2016, 141, 2920–2929. [Google Scholar] [CrossRef] [PubMed]
- Aijuka, M.; Santiago, A.E.; Girón, J.A.; Nataro, J.P.; Buys, E.M. Enteroaggregative Escherichia coli is the predominant diarrheagenic E. coli pathotype among irrigation water and food sources in South Africa. Int. J. Food Microbiol. 2018, 278, 44–51. [Google Scholar] [CrossRef]
- Li, L.; Iqbal, J.; Zhu, Y.; Zhang, P.; Chen, W.; Bhatnagar, A.; Du, Y. Chitosan/Ag-hydroxyapatite nanocomposite beads as a potential adsorbent for the efficient removal of toxic aquatic pollutants. Int. J. Biol. Macromol. 2018, 120, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, H.; Zhang, Y.; Lichtfouse, E. Efficient phosphate recycling by adsorption on alkaline sludge biochar. Environ. Chem. Lett. 2022, 21, 21–30. [Google Scholar] [CrossRef]
- Zhang, Q.; Bolisetty, S.; Cao, Y.; Handschin, S.; Adamcik, J.; Peng, Q.; Mezzenga, R. Selective and Efficient Removal of Fluoride from Water: In Situ Engineered Amyloid Fibril/ZrO2 Hybrid Membranes. Angew. Chem. Int. Ed. 2019, 58, 6012–6016. [Google Scholar] [CrossRef]
- Yang, D.; Li, Y.; Wang, Y.; Jiang, Z. Bioinspired synthesis of mesoporous ZrO2 nanomaterials with elevated defluoridation performance in agarose gels. RSC Adv. 2014, 4, 49811–49818. [Google Scholar] [CrossRef]
- Varshney, N.; Sahi, A.K.; Poddar, S.; Vishwakarma, N.K.; Kavimandan, G.; Prakash, A.; Mahto, S.K. Freeze–Thaw-Induced Physically Cross-linked Superabsorbent Polyvinyl Alcohol/Soy Protein Isolate Hydrogels for Skin Wound Dressing: In Vitro and In Vivo Characterization. ACS Appl. Mater. Interfaces 2022, 14, 14033–14048. [Google Scholar] [CrossRef]
- Yan, W.; Han, Y.; Hou, Y.; Wang, D.; Yu, M. Effects of polyvinyl alcohol incorporation on the physical and antioxidant properties of soy protein isolate/Xanthoceras sorbifolia husk extract active films. Food Biosci. 2023, 55, 102962. [Google Scholar] [CrossRef]
- Xuyan, D.; Yanlong, L.; Guoqing, H.; Junxia, X.; Liping, G.; Liang, L. Preparation and characterization of soybean Protein isolate/chitosan/sodium alginate ternary complex coacervate phase. LWT Food Sci. Technol. 2021, 150, 112081. [Google Scholar]
- Peng, L.; Bai, H.; Rong, L.; Liu, J.; Wang, G.; Wang, J.; Xian, H. Efficient Separation of Uranium in Solution by ZnFe2O4 Doped with ZrO2: Adsorption Behaviors and Mechanism Study. Water Air Soil Pollut. 2024, 235, 228. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Z.-Y.; Wang, X.; Ding, C.; Cheng, W.; Yu, S.-H.; Wang, X. Macroscopic and Microscopic Investigation of U(VI) and Eu(III) Adsorption on Carbonaceous Nanofibers. Environ. Sci. Technol. 2016, 50, 4459–4467. [Google Scholar] [CrossRef]
- Monier, M.; Elsayed, N.H. Selective extraction of uranyl ions using ion-imprinted chelating microspheres. J. Colloid Interface Sci. 2014, 423, 113–122. [Google Scholar] [CrossRef]
- Majeed, M.D.; Roushani, M. Synthesis and Characterization of Novel Chitosan/Graphene Oxide/Poly (Vinyl Alcohol) Aerogel Nanocomposite for High Efficiency Uranium (VI) Removal from Wastewaters. J. Cluster Sci. 2023, 35, 903–914. [Google Scholar] [CrossRef]
- Kumari, S.; Sharma, E.; Verma, J.; Dalal, J.; Kumar, A. Structural and photoluminescence properties of Dy-doped nanocrystalline ZrO2 for optoelectronics application. Ceram. Int. 2023, 49, 20185–20192. [Google Scholar] [CrossRef]
- Li, W.P.; Han, X.Y.; Wang, X.Y.; Wang, Y.Q.; Wang, W.X.; Xu, H.; Tan, T.S.; Wu, W.S.; Zhang, H.X. Recovery of uranyl from aqueous solutions using amidoximated polyacrylonitrile/exfoliated Na-montmorillonite composite. Chem. Eng. J. 2015, 279, 735–746. [Google Scholar] [CrossRef]
- Heisbourg, G.; Hubert, S.; Dacheux, N.; Purans, J. Kinetic and thermodynamic studies of the dissolution of thoria-urania solid solutions. J. Nucl. Mater. 2004, 335, 5–13. [Google Scholar] [CrossRef]
- Wei, X.; Liu, Q.; Zhang, H.; Lu, Z.; Liu, J.; Chen, R.; Li, R.; Li, Z.; Liu, P.; Wang, J. Efficient removal of uranium(VI) from simulated seawater using amidoximated polyacrylonitrile/FeOOH composites. Dalton Trans. 2017, 46, 15746–15756. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, Y.; Li, L.; Wang, Y. Preparation of amidoxime-functionalized mesoporous silica nanospheres (ami-MSN) from coal fly ash for the removal of U(VI). Sci. Total Environ. 2018, 626, 219–227. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Wang, X.; Liu, D.; Liu, D. Preparation of amidoxime functionalized titanate nanosheets for efficient extraction of uranium from aqueous solution. J. Solid State Chem. 2020, 290, 121562. [Google Scholar] [CrossRef]
- Dou, X.; Mohan, D.; Pittman, C.U.; Yang, S. Remediating fluoride from water using hydrous zirconium oxide. Chem. Eng. J. 2012, 198, 236–245. [Google Scholar] [CrossRef]
- Parashar, K.; Ballav, N.; Debnath, S.; Pillay, K.; Maity, A. Hydrous ZrO2 decorated polyaniline nanofibres: Synthesis, characterization and application as an efficient adsorbent for water defluoridation. J. Colloid Interface Sci. 2017, 508, 342–358. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.; Zhang, Y.; Liu, X.; Sun, S.; Qin, S.; Huang, J.; Chen, B. Construction of amidoxime-functionalized magnetic hydroxyapatite with enhanced uranium extraction performance from aqueous solution and seawater. Chemosphere 2023, 346, 140257. [Google Scholar] [CrossRef]
- Negm, S.H.; Abd El-Magied, M.O.; El Maadawy, W.M.; Abdel Aal, M.M.; Abd El Dayem, S.M.; Taher, M.A.; Abd El-Rahem, K.A.; Rashed, M.N.; Cheira, M.F. Appreciatively Efficient Sorption Achievement to U(VI) from the El Sela Area by ZrO2/Chitosan. Separations 2022, 9, 311. [Google Scholar] [CrossRef]
- Lei, Y.; Li, W.; Han, Y.; Wang, L.; Wu, H.; He, P.; Wei, G.; Guo, L. Biomimetic ZrO2-modified seaweed residue with excellent fluorine/ bacteria removal and uranium extraction properties for wastewater purification. Water Res. 2024, 252, 121219. [Google Scholar] [CrossRef]
- Mohan, S.; Singh, D.K.; Kumar, V.; Hasan, S.H. Effective removal of Fluoride ions by rGO/ZrO2 nanocomposite from aqueous solution: Fixed bed column adsorption modelling and its adsorption mechanism. J. Fluor. Chem. 2016, 194, 40–50. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, G.; Liu, B.; Kong, H.; Xiong, Z.; Guo, L.; Wei, G. Biomineralization of ZrO2 nanoparticles on graphene oxide-supported peptide/cellulose binary nanofibrous membranes for high-performance removal of fluoride ions. Chem. Eng. J. 2021, 430, 132721. [Google Scholar] [CrossRef]
- Bulut, Y.; Karaer, H. Removal of Methylene Blue from Aqueous Solution by Crosslinked Chitosan-g-Poly(Acrylic Acid)/Bentonite Composite. Chem. Eng. Commun. 2014, 202, 1635–1644. [Google Scholar] [CrossRef]
- Manna, S.; Roy, D.; Saha, P.; Gopakumar, D.; Thomas, S. Rapid methylene blue adsorption using modified lignocellulosic materials. Process Saf. Environ. Prot. 2017, 107, 346–356. [Google Scholar] [CrossRef]
- Cheng, D.M.; Kuhn, P.; Poulev, A.; Rojo, L.E.; Lila, M.A.; Raskin, I. In vivo and in vitro antidiabetic effects of aqueous cinnamon extract and cinnamon polyphenol-enhanced food matrix. Food Chem. 2012, 135, 2994–3002. [Google Scholar] [CrossRef]
- Duan, X.; Qin, D.; Li, H.; Zhang, T.; Han, Y.; Huang, Y.Q.; He, D.; Wu, K.; Chai, X.; Chen, C. Study of antimicrobial activity and mechanism of vapor-phase cinnamaldehyde for killing Escherichia coli based on fumigation method. Front. Nutr. 2022, 9, 1040152. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Mat Yajid, M.A.; Kumar Gupta, R.; Mohd Yusof, N.; Bakhsheshi-Rad, H.R.; Ghandvar, H.; Ghasemi, E. Antibacterial activities and corrosion behavior of novel PEO/nanostructured ZrO2 coating on Mg alloy. Trans. Nonferrous Met. Soc. China 2018, 28, 1571–1581. [Google Scholar] [CrossRef]
- Tabassum, N.; Kumar, D.; Verma, D.; Bohara, R.A.; Singh, M.P. Zirconium oxide (ZrO2) nanoparticles from antibacterial activity to cytotoxicity: A next-generation of multifunctional nanoparticles. Mater. Today Commun. 2021, 26, 102156. [Google Scholar] [CrossRef]
- Peydayesh, M.; Mezzenga, R. Protein nanofibrils for next generation sustainable water purification. Nat. Commun. 2021, 12, 3248. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).