Abstract
Anterior cruciate ligament reconstruction with concurrent lateral extra-articular tenodesis enhances rotational stability. However, not many studies describe radiological features following the abovementioned procedure. The purpose of this study was to evaluate the visibility and describe the sonographic morphology of the iliotibial band strip harvested during the modified Lemaire technique and to establish a correlation between these findings and clinical outcomes. Thirty-two consecutive patients underwent primary or revision anterior cruciate ligament reconstruction with the addition of lateral extra-articular tenodesis by the mini-open modified Lemaire technique. All individuals completed the following preoperative and postoperative questionnaires: the KOOS-pain, KOOS-symptoms, KOOS-ADL, KOOS-sport, KOOS-quality, IKDC, Lysholm, and WOMAC. Each patient underwent a postoperative ultrasound examination to evaluate the lateral extra-articular procedure, especially the harvested iliotibial band strip. The most common ultrasound findings of the iliotibial band strip were its hyperechoic appearance (87.1%), location at the level of the femur (58.1%), no surrounding effusion (83.9%), and no power Doppler signal (100%). Effusion seen in sonographic images was correlated with KOOS-symptoms postoperative scores (p = 0.0115). However, there were no correlations between other iliotibial band strip sonographic features and clinical outcomes. The functional score value increased in each patient compared to preoperative measurements. This is the first study that evaluated the sonographic features of the iliotibial band strip after the lateral extra-articular procedure by the mini-open modified Lemaire technique. Our study indicates that ultrasonography is a useful tool in identifying the iliotibial band strip after the mentioned procedure. Effusion observed around the strip on ultrasound was significantly associated with worse KOOS-symptoms scores, suggesting potential clinical relevance.
1. Introduction
The knee is the second most frequently injured body site among high school athletes, and anterior cruciate ligament (ACL) tears constitute over 50% of all knee injuries [1,2]. ACL tears affect 68.6 per 100,000 individuals annually, and 125,000–200,000 ACL reconstructions (ACLRs) are performed yearly in the United States [3]. Although the annual cost of ACLR in the U.S. is approximately one billion dollars, it appears to be a cost-effective procedure compared to conservative treatment [1,4]. ACLR restores the knee’s native biomechanics and increases both anteroposterior and rotational stability. Moreover, this procedure also addresses concomitant intra-articular lesions, such as meniscal tears and chondral lesions [5,6,7]. Although the long-term clinical outcomes of standard intra-articular ACLR techniques have been proven to reliably reduce knee instability, these techniques do not fully restore the anterolateral rotational stability of the knee joint [8,9,10]. The reason behind rotatory knee laxity is that ACL injury often results in damage to the anterolateral knee structures (including the anterolateral ligament [ALL]) [11]. The integration of lateral extra-articular tenodesis (LET) with ACLR enhances the anterolateral complex of the knee, prevents persistent postoperative rotational instability, and reduces the risk of graft failure [12,13]. Several studies have reported that LET biomechanically aids in shifting the load from the ACL graft, which subsequently decreases excess anterior tibial translation and rotation [12,13,14,15,16]. Despite the abundant body of work on ACL identification in magnetic resonance imaging (MRI) and ultrasonography, the literature does not provide much information on postoperative ultrasound findings of the iliotibial band (ITB) strip that is harvested during the modified Lemaire technique [17]. Ultrasonography is a non-invasive and cost-effective tool that allows assessment of soft tissue structures, making it particularly suitable for evaluating the ITB strip. The primary objective of this study was to determine the detectability of the ITB strip using ultrasound imaging and to describe its postoperative sonographic appearance in patients undergoing combined ACLR and LET by the modified Lemaire technique. Secondary aims included identifying any correlation between sonographic findings of the ITB strip and clinical outcomes. A better understanding of this subject creates potential opportunities to improve currently used therapeutic algorithms.
2. Materials and Methods
2.1. General Data
This study received approval from the local bioethics committee and all participants gave informed consent before undergoing any study procedures (KB 347/2023). Clinical participants consisted of 32 patients who underwent either primary or revision ACLR with concomitant LET using the mini-open modified Lemaire technique. All patients presented with chronic knee instability and pain. Preoperative confirmation of instability was based on physical examination, as well as the identification of ACL tears or retears on non-contrast knee MRIs.
Inclusion criteria were as follows: a complete novel ACL injury or ACL graft injury with symptomatic instability, age 18–64 years, and patients’ expectations of retuting to their pre-injury level of physical activity is a seperate criteria; it should be “patients’ expectations of returning to their pre-injury level of physical activity, general health condition according to the American Society of Anesthesiologists (ASA)” Physical Status Classification System ≤ III. Exclusion criteria were as follows: an ACL tear without symptomatic instability, significant malalignment, prior surgery to the contralateral knee, contralateral knee injury, an ASA classification ≥ IV, and age < 18 years or ≥65 years.
2.2. Operative Technique
All patients underwent ACLR with concomitant LET using the mini-open modified Lemaire technique. Arthroscopy was performed to evaluate additional injuries and confirm ACL tears. The standard ACLR technique was performed after arthroscopic evaluation of the knee joint. An autologous 4-strand hamstring graft (semitendinosus and gracilis tendons) was performed in primary ACLR, while a bone–patellar tendon–bone graft (BTB) was performed in revision ACLR. Both grafts were harvested using standard techniques. LET, based on the mini-open modified Lemaire technique, was performed with a 4–5 cm skin incision between the lateral femoral epicondyle and Gerdy tubercle. The ITB was identified and a 1 cm wide × 8 cm long strip was harvested. Distal insertion of the ITB was preserved, while the proximal part of the graft was slipped under the LCL. Subsequently, the ITB strip was fixed slightly proximally and posteriorly to the lateral epicondyle of the femur using a self-punching anchor under moderate tension.
All patients participated in the same standardized postoperative rehabilitation protocol and were provided with external hinge braces for 6 weeks.
2.3. Follow-Up Examination
Patients were asked preoperatively to complete demographic information and the following questionnaires: the Knee Injury and Osteoarthritis Outcome Score pain subscale (KOOS-pain), symptoms subscale (KOOS-symptoms), function in daily living subscale (KOOS-ADL), function in sport and recreation subscale (KOOS-sport), and knee-related Quality of Life subscale (KOOS-quality); the International Knee Documentation Committee subjective knee evaluation form (IKDC); the Lysholm knee scoring scale (Lysholm); and the Western Ontario and McMaster Universities Arthritis Index (WOMAC). With a minimum follow-up of 55 days, all patients came back to our department and postoperative KOOS-pain, KOOS-symptoms, KOOS-ADL, KOOS-sport, KOOS-quality, IKDC, Lysholm, and WOMAC questionnaires were administered. Each KOOS subscale and the IKDC and Lysholm scales calculate and grade an overall score from 0 to 100 based on the answered questions. Higher scores indicate better function and fewer symptoms. Conversely, higher scores (from 0 to 96) on the WOMAC indicate functional limitations, worse pain, and stiffness. Subsequently, all patients underwent ultrasound examination performed by 2 orthopaedic surgeons experienced in musculoskeletal sonography (Esaote My Lab X5) using a 4–15 MHz linear transducer. The examiners were blinded to any previous imaging data. The protocol of the sonographic examination was as follows: a supine position, the knee in 90° of flexion, a linear transducer placed over the lateral knee in a coronal oblique plane, the location of the lateral collateral ligament (LCL) extending from the femur to the fibula in its long axis, and identification of the ITB strip in its short axis as a rounded structure below the LCL. The visible scar on the skin after lateral extra-articular tenodesis and characteristic bone landmarks, such as the lateral condyle of the femur, Gerdy’s tubercle, and the fibular head, assisted in ITB identification. During examination, the following sonographic parameters were documented: measurements of width and height, colour Doppler assessment of the ITB strip, ITB strip echogenicity, the presence of effusion around the ITB strip, and the location of the ITB strip according to designated zones. Throughout the follow-up, intraoperative and postoperative complications and reinjury were documented.
2.4. Statistical Analysis
Statistical calculations were performed in GraphPad, Prism software (GraphPad 8.0.1 Software, Dotmatics, UK). The normality of distribution was verified using the Shapiro–Wilk test. Effusion and echogenicity were analysed using the Mann–Whitney test, LET strip location by the Kruskal–Wallis test, and width and height using Spearman’s correlation analysis (two-tailed). Qualitative variables were characterized by the number of observations and their structures. For numerical variables, i.e., mean and standard deviation, median and range were calculated depending on the type of distribution. The level of statistical significance was less than 0.05.
3. Results
A total of 32 patients were included in this study; 28 individuals (87.5%) were males and 4 were females (12.5%). The mean age in the studied group was 35.7 ± 12.1 years (range 20–63). A total of 27 patients (84.4%) underwent primary ACLR and LET surgery, while 5 patients (15.6%) had revision ACL and LET. Ultrasound examination was performed an average 234.5 ± 136.3 days (range 55–478) after surgery.
The ITB strip was seen in 31 individuals (96.9%). In one patient it was impossible to localize the graft using ultrasounds. On ultrasonography, effusion around the LET strip was present in five patients (16.1%). Regarding echogenicity, the hyperechoic LET strip was present in 27 (87.1%) patients, and the hypoechoic ITB strip in 4 (12.9%). Measurements in ultrasounds revealed that the average width of ITB strips was 5.0 ± 1.6 mm, while their height was 4.6 mm ± 1.2 mm. Localization of ITB strips was as follows: 18 LET strips (58.1%) were localized at the level of the femur, 11 (35.5%) were between the femur and the tibia, and 2 (6.5%) were at the level of the tibia. The vascularization ITB strip was not present using power Doppler sonography; the signal was negative. A schematic illustration depicting the ITB strip is presented in Figure 1, while particular sonographic features are presented in Figure 2. Ultrasound images of ITB strips in Figure 2 were obtained from different patients showing their normal sonographic appearance and potential pathologies. It is important to note that the sonographic appearance of the ITB strip may differ depending on the level and orientation of the scan and the device used. These factors may influence the ITB strip echotexture and the visibility of surrounding structures. Demographic data and sonographic features are shown in Table 1.
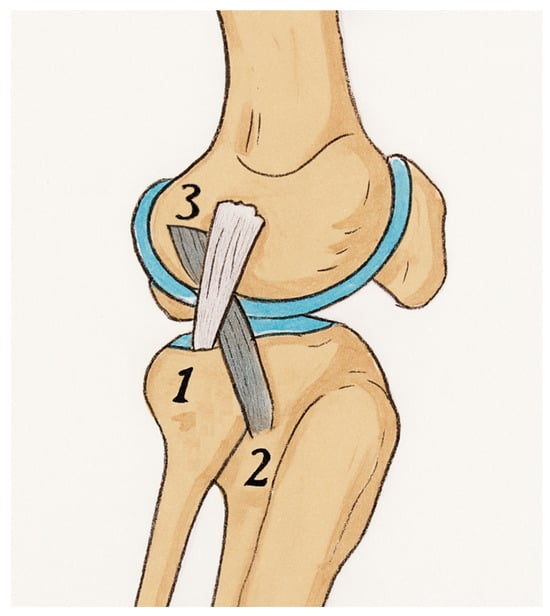
Figure 1.
Illustration showing the harvested ITB strip (grey strip) passed under the LCL (white strip) following the modified Lemaire technique. 1—lateral collateral ligament, 2—Gerdy’s tubercule (the point of insertion for the ITB), 3—attachment site of the proximal fragment of the harvested graft that should be located proximally and posteriorly to the lateral epicondyle of the femur.
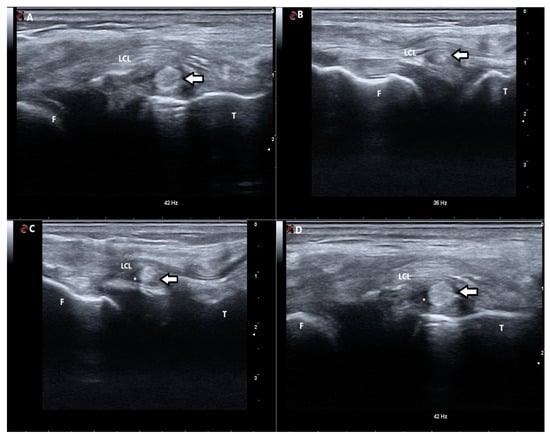
Figure 2.
Sonographic pictures of ITB strips obtained from different patients showing their normal sonographic appearance and potential pathologies. (A) Ultrasonographic short-axis scan of the iliotibial band (ITB) strip (arrow) located at the level of the tibia. The ITB strip is hyperechoic. (B) Ultrasonographic short-axis scan of the iliotibial band (ITB) strip (arrow) located between the femur and the tibia. The echogenicity of the ITB strip is slightly altered with centres of significantly decreased echogenicity. (C) Ultrasonographic short-axis scan of the iliotibial band (ITB) strip (arrow) located between the femur and the tibia. The ITB strip is hyperechoic with the surrounding hypoechogenic area, suggesting the presence of fluid (*). (D) Ultrasonographic short-axis scan of the iliotibial band (ITB) strip (arrow) located at the level of the tibia. The ITB strip is hyperechoic with the surrounding hypoechogenic area, suggesting the presence of fluid (*). F—femur; T—tibia; LCL—lateral collateral ligament; *—presence of fluid.

Table 1.
Patient demographics and sonographic features.
Functional score values increased in each case compared to pre-operative measurements. There were statistically significant increases in KOOS-pain, KOOS-symptoms, KOOS-ADL, KOOS-sport, KOOS-quality, IKDC, and Lysholm scores. WOMAC scores decreased postoperatively and were also statistically significant, which indicates improved clinical results. Outcome scores are presented in Table 2. The negative correlation between the postoperative KOOS-symptoms score and effusion around the ITB strip was noticed and is presented in Figure 3. However, KOOS-pain, KOOS-ADL, KOOS-sport, KOOS-quality, IKDC, Lysholm, and WOMAC scores were not correlated.

Table 2.
Preoperative and postoperative clinical outcomes comparison.
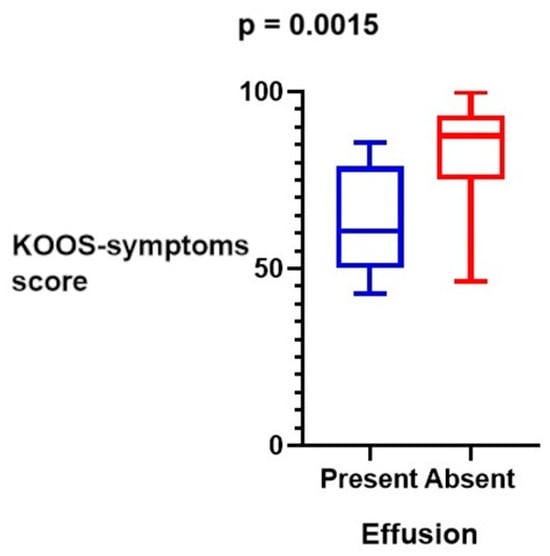
Figure 3.
The negative correlation between postoperative KOOS-symptoms scores and effusion around ITB strips.
Correlations of ultrasound features of ITB strips (such as width and height) and clinical outcomes assessed using scales are summarized in Table 3, Table 4 and Table 5. There were no correlations between clinical outcomes and the ITB strips’ width, height, echogenicity, or location.

Table 3.
Significance levels (p-values) of correlations between ultrasound features (width and height) and clinical scores.

Table 4.
Significance levels (p-values) of correlations between ultrasound features (echogenicity and effusion) and clinical scores using the Mann–Whitney test. *—statistical significance.

Table 5.
Significance levels (p-values) of correlations between ultrasound features (location of the ITB strip) and clinical scores using the Kruskal–Wallis test.
4. Discussion
To our knowledge, this was the first investigation that evaluated the visibility of the ITB strip, examined its morphology using ultrasound, and tried to find any links between ultrasound features and clinical outcomes. The detectability of the ITB strip using ultrasound imaging was presented and its sonographic appearance was described. This study also demonstrated that effusion around the ITB strip in the ultrasound was negatively correlated with the KOOS-symptoms subscale that includes questions regarding knee joint stiffness in various positions, swelling, sensations of clicking and locking, as well as the ability to achieve a full range of motion. This relationship may be considered a negative symptom after a LET procedure indicating irritation or inflammation of the ITB strip and potentially contributing to mechanical symptoms mentioned above [18]. However, other functional scores, such as KOOS-pain, KOOS-ADL, KOOS-sport, IKDC, Lysholm, and WOMAC scores, did not support this indication. Moreover, other correlations between ultrasound features and clinical scores were not provided.
Sonographic measurements showed that the average width of ITB strips was 5.0 ± 1.6 mm, while their height was 4.6 mm ± 1.2 mm and their shape in a short axis was rounded. Essentially, in the Lemaire technique or its modification, it is recommended to obtain a flat graft with a width of 1 to 1.5 cm [19]. The discrepancy between the sonographic measurement and the intraoperative dimensions of the ITB graft harvested according to the surgical technique may result from the fact that the ITB strip undergoes moderate tension during fixation to the lateral femoral condyle. This tension may lead to a reduction in its width and a more rounded shape. Moreover, changes in its size and shape may also be attributed to spontaneous ligamentization processes similar to those observed during the maturation of ACL grafts [20]. In other LET techniques, the following ITB strip widths are advised: the MacIntosh technique and its modification (Arnold and Coker), 2 cm; and the Ellison technique, 1.5 cm [21]. However, if a thinner or wider LET strip led to better clinical results, then surgical techniques recommending a thinner or wider strip would be preferred, but the literature shows that different LET techniques provide similar functional outcomes and rotational stability [22]. This may partially explain why no correlation between the ITB size and clinical outcomes was demonstrated in this study. Regarding the echogenicity of the ITB strip, our results suggest that a hyperechogenic appearance is physiological, as the majority of cases (87.1%) demonstrated this pattern. In contrast, hypoechogenicity may indicate an ongoing pathological process. However, this ultrasound feature was not associated with clinical outcome scores. The absence of a power Doppler signal in the ITB strip may be interpreted as a lack of hyperemia, which typically correlates with the absence of active inflammation or neovascularization. This is consistent with studies showing that mature tendon or graft tissues exhibit no Doppler activity under normal physiological conditions. In contrast, an increased Doppler signal is often associated with tendinopathy or inflammatory changes [23].
There are a few studies that evaluated patients after ACLR and LET procedures, mainly using MRI rather than other imaging techniques. For instance, Lobo et al. presented the ITB strip’s appearance after a modified Lemaire technique on the coronal and axial planes. On the coronal plane, the ITB strip coursed obliquely, and on both planes a gap in the central portion of the ITB was observed that was secondary to graft harvesting. They also included imaging findings of the attachment of the ITB strip using the interference screw in the lateral femoral epicondyle [17]. However, the number of studies evaluating ITB strip morphology assessed using MRI or other imaging techniques is limited. Researchers primarily focus on radiological assessment of results of ACLR and LET procedures. For instance, some authors presented postoperative complications of the LET procedure, such as LET graft failure, hematoma, infection, tunnel convergence, etc., seen in X-ray, computed tomography, and MRI [24]. Other scientists who investigated the LET graft using MRI suggested that it absorbs a portion of the forces acting on the knee, thereby reducing the load on the ACL graft, which may hasten its regeneration [25]. For instance, Cavaignac et al. presented that the addition of LET to ACLR improves the maturation of the ACL graft and its incorporation as assessed using MRI at the 12-month follow-up. However, there were no differences in clinical outcomes between ACLR alone and ACLR+LET groups [26]. On the other hand, Rojas et al. showed that the application of the LET procedure during primary ACLR may slow the healing hamstring ACL graft at 10 months postoperatively, as observed via MRI [27]. There were also studies using quantitative MRI aimed at assessing accelerated osteoarthritis after the ACLR + LET procedure, but it was not proven [28].
As there are cadaveric studies that investigated biomechanical properties of LET depending on the location of its attachment to the lateral femoral epicondyle, we decided to divide localization of the ITB strip under LCL as seen in ultrasound into three zones: at the level of femur, between the femur and the tibia, and at the level of the tibia. For instance, biomechanical studies show that LET insertion proximal to the LCF may present a steady length change pattern and lower the total strain range compared to situations when its insertion is localized anteriorly and distally to the LCF [29]. Moreover, apart from localization of the LET attachment, graft behaviour may be influenced by its course, which finally may affect knee joint kinematics and cause overconstraint in internal rotation [30]. Therefore, we designated the aforementioned three zones, as they could reflect the fibre route and the location of the ITB strip attachment. However, we did not find any relationship between the ITB strip area and clinical outcomes. This may suggest that our zones do not accurately reflect the location of LET attachment; that the postoperative healing process of knee tissues is complex and ultimately affects knee biomechanics that cannot be predicted on cadavers; or that different factors during surgery, such as graft size, graft tension, the fixation method, and knee angle during ITB strip attachment, affect the final result.
This pilot study provides initial insight into the utility of ultrasonography for the detection and evaluation of the ITB strip after the modified Lemaire technique. Based on these preliminary findings, future research should focus on larger, multicentre cohorts to validate the observed results and further expand the prognostic value of ultrasound-detected effusion. Additionally, long-term studies may enhance understanding of graft integration and postoperative knee function. This study had several limitations. Firstly, the sample size was relatively small and consisted of the local population with complex knee injuries. However, this sample size was considered sufficient for the primary aim of the study—namely, to assess the feasibility of identifying the ITB strip using ultrasound, which has not been described in the literature to date. Secondly, clinical outcomes could be affected by applying two types of treatment—ACLR and LET. However, separation of these two methods is not practiced anymore due to poor clinical results after treating ACL rupture sole with LET. Thirdly, the follow-up period was different among patients and the range of days between surgery and examination was wide. Lastly, sonographic examination highly depends on the device used and the examiner’s experience.
5. Conclusions
This is the first study that focused on sonography of the ITB strip after a LET procedure by the modified Lemaire technique in conjunction with ACLR. Ultrasound seems to be useful in detecting the ITB strip, which may allow the verification of a successful LET procedure. Even though ultrasound examination is limited due to its subjective nature, certain sonographic findings regarding ITB strips were presented. Further research on the effusion around harvested ITB strips is needed to establish whether its effusion may influence clinical outcomes in patients.
Author Contributions
Conceptualization, J.E. and J.Z.; methodology, J.E.; validation, J.Z. and P.P.; formal analysis, J.E.; investigation, J.E.; resources, J.Z.; writing—original draft preparation, J.E.; writing—review and editing, J.E.; supervision, P.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Komisja Bioetyczna Uniwersytetu Mikołaja Kopernika w Toruniu, Collegium Medicum im. Ludwika Rydygiera w Bydgoszczy (KB 347/2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author, the data are not publicly available due to privacy and ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sayampanathan, A.A.; Howe, B.K.T.; Bin Abd Razak, H.R.; Chi, C.H.; Tan, A.H.C. Epidemiology of Surgically Managed Anterior Cruciate Ligament Ruptures in a Sports Surgery Practice. J. Orthop. Surg. 2017, 25, 2309499016684289. [Google Scholar] [CrossRef] [PubMed]
- Swenson, D.M.; Collins, C.L.; Best, T.M.; Flanigan, D.C.; Fields, S.K.; Comstock, R.D. Epidemiology of Knee Injuries among Us High School Athletes, 2005/06–2010/11. Med. Sci. Sports Exerc. 2013, 45, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Biały, M.; Kublin, K.; Brzuszkiewicz-Kuźmicka, G.; Gnat, R. Myofascial and Movement Tests after Anterior Cruciate Ligament Reconstruction. J. Hum. Kinet. 2022, 83, 67–75. [Google Scholar] [CrossRef]
- Afzali, T.; Fangel, M.V.; Vestergaard, A.S.; Rathleff, M.S.; Ehlers, L.H.; Jensen, M.B. Cost-Effectiveness of Treatments for Non-Osteoarthritic Knee Pain Conditions: A Systematic Review. PLoS ONE 2018, 13, e0209240. [Google Scholar] [CrossRef]
- Montalvo, A.M.; Schneider, D.K.; Webster, K.E.; Yut, L.; Galloway, M.T.; Heidt, R.S.; Kaeding, C.C.; Kremcheck, T.E.; Magnussen, R.A.; Parikh, S.N.; et al. Anterior Cruciate Ligament Injury Risk in Sport: A Systematic Review and Meta-Analysis of Injury Incidence by Sex and Sport Classification. J. Athl. Train. 2019, 54, 472–482. [Google Scholar] [CrossRef]
- LaBella, C.R.; Hennrikus, W.; Hewett, T.E.; Council on Sports Medicine and Fitness, and Section on Orthopaedics; Brenner, J.S.; Brookes, M.A.; Demorest, R.A.; Halstead, M.E.; Kelly, A.K.W.; Koutures, C.G.; et al. Anterior Cruciate Ligament Injuries: Diagnosis, Treatment, and Prevention. Pediatrics 2014, 133, e1437–e1450. [Google Scholar] [CrossRef]
- Tuca, M.; Valderrama, I.; Eriksson, K.; Tapasvi, S. Current Trends in Anterior Cruciate Ligament Surgery. A Worldwide Benchmark Study. J. ISAKOS 2023, 8, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, R.A.; Reinke, E.K.; Huston, L.J.; Hewett, T.E.; Spindler, K.P.; Andrish, J.T.; Jones, M.H.; Parker, R.D.; McCarty, E.C.; Marx, R.G.; et al. Effect of High-Grade Preoperative Knee Laxity on Anterior Cruciate Ligament Reconstruction Outcomes. Am. J. Sports Med. 2016, 44, 3077–3082. [Google Scholar] [CrossRef]
- Na, B.-R.; Kwak, W.-K.; Seo, H.-Y.; Seon, J.-K. Clinical Outcomes of Anterolateral Ligament Reconstruction or Lateral Extra-Articular Tenodesis Combined with Primary ACL Reconstruction: A Systematic Review with Meta-Analysis. Orthop. J. Sports Med. 2021, 9, 23259671211023099. [Google Scholar] [CrossRef]
- Grassi, A.; Pizza, N.; Al-zu’bi, B.B.H.; Fabbro, G.D.; Lucidi, G.A.; Zaffagnini, S. Clinical Outcomes and Osteoarthritis at Very Long-Term Follow-up After ACL Reconstruction: A Systematic Review and Meta-Analysis. Orthop. J. Sports Med. 2022, 10, 23259671211062238. [Google Scholar] [CrossRef]
- Musahl, V.; Getgood, A.; Neyret, P.; Claes, S.; Burnham, J.M.; Batailler, C.; Sonnery-Cottet, B.; Williams, A.; Amis, A.; Zaffagnini, S.; et al. Contributions of the Anterolateral Complex and the Anterolateral Ligament to Rotatory Knee Stability in the Setting of ACL Injury: A Roundtable Discussion. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Devitt, B.M.; Bell, S.W.; Ardern, C.L.; Hartwig, T.; Porter, T.J.; Feller, J.A.; Webster, K.E. The Role of Lateral Extra-Articular Tenodesis in Primary Anterior Cruciate Ligament Reconstruction: A Systematic Review with Meta-Analysis and Best-Evidence Synthesis. Orthop. J. Sports Med. 2017, 5, 2325967117731767. [Google Scholar] [CrossRef] [PubMed]
- Temperato, J.; Ewing, M.; Nuelle, C.W. Lateral Extra-Articular Tenodesis with Iliotibial Band Using Knotless All-Suture Anchor Femoral Fixation. Arthrosc. Tech. 2023, 12, e677–e682. [Google Scholar] [CrossRef]
- Feller, J.A.; Devitt, B.M.; Webster, K.E.; Klemm, H.J. Augmentation of Primary ACL Reconstruction with a Modified Ellison Lateral Extra-Articular Tenodesis in High-Risk Patients: A Pilot Study. Orthop. J. Sports Med. 2021, 9, 23259671211021351. [Google Scholar] [CrossRef]
- Rowan, F.E.; Huq, S.S.; Haddad, F.S. Lateral Extra-Articular Tenodesis with ACL Reconstruction Demonstrates Better Patient-Reported Outcomes Compared to ACL Reconstruction Alone at 2 Years Minimum Follow-Up. Arch. Orthop. Trauma. Surg. 2019, 139, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Joseph, L.; Demey, G.; Chamu, T.; Schmidt, A.; Germain, A.; van Rooij, F.; Saffarini, M.; Dejour, D. Adding a Modified Lemaire Procedure to ACLR in Knees with Severe Rotational Knee Instability Does Not Compromise Isokinetic Muscle Recovery at the Time of Return-to-Play. J. Exp. Ortop. 2020, 7, 84. [Google Scholar] [CrossRef]
- Lôbo, C.F.T.; Helito, P.V.P.; Bordalo-Rodrigues, M.; Helito, C.P. Computed Tomography (CT), X-Ray, and MRI Evaluation of Two Anterolateral Knee Reconstruction Techniques: Lateral Extra-Articular Tenodesis (LET) and the Anterolateral Ligament (ALL) Reconstruction. Skelet. Radiol. 2020, 49, 1037–1049. [Google Scholar] [CrossRef]
- Chiang, Y.-P.; Wang, T.-G.; Lew, H.L. Application of High Resolution Ultrasound for Examination of the Knee Joint. J. Med. Ultrasound 2007, 15, 203–212. [Google Scholar] [CrossRef]
- McAleese, T.; Murgier, J.; Cavaignac, E.; Devitt, B.M. A Review of Marcel Lemaire’s Original Work on Lateral Extra-Articular Tenodesis. J. ISAKOS 2024, 9, 431–437. [Google Scholar] [CrossRef]
- Sánchez, M.; Anitua, E.; Azofra, J.; Prado, R.; Muruzabal, F.; Andia, I. Ligamentization of Tendon Grafts Treated with an Endogenous Preparation Rich in Growth Factors: Gross Morphology and Histology. Arthrosc. J. Arthrosc. Relat. Surg. 2010, 26, 470–480. [Google Scholar] [CrossRef]
- Slette, E.L.; Mikula, J.D.; Schon, J.M.; Marchetti, D.C.; Kheir, M.M.; Turnbull, T.L.; LaPrade, R.F. Biomechanical Results of Lateral Extra-Articular Tenodesis Procedures of the Knee: A Systematic Review. Arthrosc. J. Arthrosc. Relat. Surg. 2016, 32, 2592–2611. [Google Scholar] [CrossRef]
- Laksana, I.K.M.; Aryana, I.W.; Pratistha, I.R.T. Functional Outcomes of ACL Reconstruction with Lateral Extra-Articular Tenodesis Using Modified Lemaire Procedure Versus Modified MacIntosh Procedure: A Systematic Review. Orthop. J. Sports Med. 2023, 11, 2325967121S00870. [Google Scholar] [CrossRef]
- Suzuki, T. Power Doppler Ultrasonographic Assessment of the Ankle in Patients with Inflammatory Rheumatic Diseases. World J. Orthop. 2014, 5, 574–584. [Google Scholar] [CrossRef][Green Version]
- Marshall, D.C.; Silva, F.D.; Goldenberg, B.T.; Quintero, D.; Baraga, M.G.; Jose, J. Imaging Findings of Complications After Lateral Extra-Articular Tenodesis of the Knee: A Current Concepts Review. Orthop. J. Sports Med. 2022, 10, 23259671221114820. [Google Scholar] [CrossRef] [PubMed]
- Retzky, J.S.; Chipman, D.E.; Mintz, D.N.; Cordasco, F.A.; Green, D.W. Association of Lateral Extra-Articular Tenodesis with Improved Graft Maturity on MRI 2 Years After ACL Reconstruction with Quadriceps Tendon Autograft in Skeletally Immature Athletes. Orthop. J. Sports Med. 2024, 12, 23259671231211885. [Google Scholar] [CrossRef] [PubMed]
- Cavaignac, E.; Mesnier, T.; Marot, V.; Fernandez, A.; Faruch, M.; Berard, E.; Sonnery-Cottet, B. Effect of Lateral Extra-Articular Tenodesis on Anterior Cruciate Ligament Graft Incorporation. Orthop. J. Sports Med. 2020, 8, 2325967120960097. [Google Scholar] [CrossRef]
- Rojas, G.; Perelli, S.; Ibanez, M.; Formagnana, M.; Ormazabal, I.; Monllau, J.C. Effect of Modified Lemaire Anterolateral Extra-Articular Tenodesis on the Magnetic Resonance Imaging Maturity Signal of Anterior Cruciate Ligament Hamstring Graft. Am. J. Sports Med. 2021, 49, 2379–2386. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Hegarty, P.; Vivacqua, T.; Firth, A.; Milner, J.S.; Pritchett, S.; Willits, K.; Litchfield, R.; Bryant, D.; Getgood, A.M.J. Quantitative MRI Analysis of Patellofemoral Joint Cartilage Health 2 Years After Anterior Cruciate Ligament Reconstruction and Lateral Extra-Articular Tenodesis. Am. J. Sports Med. 2024, 52, 1773–1783. [Google Scholar] [CrossRef]
- Jaecker, V.; Naendrup, J.-H.; Pfeiffer, T.R.; Bouillon, B.; Shafizadeh, S. Radiographic Landmarks for Femoral Tunnel Positioning in Lateral Extra-Articular Tenodesis Procedures. Am. J. Sports Med. 2019, 47, 2572–2576. [Google Scholar] [CrossRef]
- Kittl, C.; Halewood, C.; Stephen, J.M.; Gupte, C.M.; Weiler, A.; Williams, A.; Amis, A.A. Length Change Patterns in the Lateral Extra-Articular Structures of the Knee and Related Reconstructions. Am. J. Sports Med. 2015, 43, 354–362. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).