A Review on Bioactive Compounds and Pharmacological Activities of Citrus unshiu
Abstract
1. Introduction
2. Methods
3. Botanical Description and Bioactive Compounds of C. unshiu Marc.
4. Pharmacological Activities of Satsuma Mandarin
4.1. Anticancer
4.2. Anti-Obesity and Anti-Diabetic Activities
4.3. Hepatoprotective and Lipid-Lowering Activities
4.4. Gastroprotective Activity
4.5. Neuroprotective Activities
4.6. Skin-Protective Activity
4.7. Cardioprotective Activity
4.8. Nasal Airway-Protective Activity
4.9. Antioxidant Activity
4.10. Anti-Inflammatory Activity
4.11. Anti-Microbial Activity
4.12. Summary of Pharmacological Activities
5. Conclusions
6. Limitations and Future Perspectives
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 8-oxodG | 8-oxo-7,8-dihydro-2′-deoxyguanosine |
| Abca1 | ATP-binding cassette subfamily A member 1 |
| ABTS+ | Diammonium salt |
| ACC | Acetyl-CoA carboxylase |
| Akt | Threonine kinase |
| ALT | Alanine aminotransferase |
| AMPK | AMP-activated protein kinase |
| ApoA | Apolipoprotein A |
| ApoC | Apolipoprotein C |
| ASAT | Aspartate aminotransferase |
| Bad | Bcl-2 antagonist of cell death |
| Bax | Bcl-2-associated X protein |
| BC | Bladder cancer |
| BRC | Breast cancer |
| Bcl-2 | B-cell lymphoma-2 |
| Bcl-xl | B-cell lymphoma-extra large |
| BDNF | Brain-derived neurotrophic factor |
| Bid | BH3-interacting death domain |
| C/EBPα | CCAAT/enhancer binding protein alpha |
| CC | Cervical cancer |
| CCl | CC chemokine ligands |
| Ccna2 | Cyclin A2 |
| Ccnb1 | Cyclin B1 |
| Ccnb2 | Cyclin B2 |
| Cdk1 | Cyclin-dependent kinase |
| c-Fos | Oncogene c-Fos |
| cIAP | Cellular inhibitor of apoptosis |
| COX-2 | Cyclooxygenase-2 |
| CPT-1 | Carnitine palmitoyltransferase-1 |
| CRC | Colorectal cancer |
| CREB | Cyclic AMP-response element-binding protein |
| CXCL | CXC motif chemokine ligand |
| Cyp51 | Lanosterol 14-α-demethylase |
| DNCB | Dinitrochlorobenzene |
| DPPH | 1,1-diphenyl-2-picryl-hydrazyl |
| Elovl6 | Elongation of very-long-chain fatty acids protein 6 |
| ER | Endoplasmic reticulum |
| ERK | Extracellular signal-regulated kinase |
| FAK | Focal adhesion kinase |
| FAS | Fas cell surface death receptor |
| FasL | Fas ligand |
| Fasn | Fatty acid synthase |
| Fga | Fibrinogen A alpha |
| GSH | Glutathione |
| GSH-Px | Glutathione peroxidase activity |
| GSSG | Oxidized glutathione |
| H2O2 | Hydrogen peroxide |
| Hdlbp | High-density lipoprotein binding protein |
| HMF | 3,5,6,7,8,3′,4′-heptamethoxyflavone |
| Hmgcs1 | 3-hydroxy-3-methylglutaryl-CoA synthase 1 |
| HMGR | 3-hydroxy-3-methyl-glutaryl-CoA reductase |
| HO-1 | Heme oxygenase-1 |
| IC50 | Half-maximal inhibitory concentration |
| Idi1 | Isopentenyl-diphosphate δ isomerase 1 |
| IFN-γ | Interferon-gamma |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| IκB | IkappaB kinase |
| JAK | Janus kinase |
| JNK | c-jun NH2-terminal kinase |
| Kng | Kininogen |
| LDH | Lactate dehydrogenase |
| LPS | Lipopolysaccharide |
| MAFbx | Muscle atrophy F-box |
| MAPK | Mitogen-activated protein kinase |
| MCM | Mini-chromosome maintenance |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MDA | Malondialdehyde |
| MDC | Macrophage-derived chemokine |
| ME | Malic enzyme |
| MKK | Mitogen-activated protein kinase kinase |
| MMP | Metalloproteinase |
| MSC | Melanoma skin cancer |
| mTORC1 | Mammalian target of rapamycin complex 1 |
| MuRF1 | Muscle RING finger 1 |
| Myh | β-myosin heavy chain |
| NF-κB | Nuclear factor kappa B |
| NO | Nitric oxide |
| NOX2 | NADPH oxidase 2 |
| ORAC | Oxygen radical absorbance capacity |
| p21 | Protein 21 |
| p38 | Protein 38 |
| p50 | Protein 50 |
| p53 | Protein 53 |
| PaC | Pancreatic cancer |
| PAP | Phosphatidate phosphohydrolase |
| PARP | Poly(ADP-ribose) Polymerase |
| PCNA | Proliferating cell nuclear antigen |
| PGE2 | Prostaglandin 2 |
| PI3K | Phosphatidylinositol 3-kinase |
| PKC | Protein kinase C |
| PPARα | Peroxisome proliferator-activated receptor gamma |
| PPARγ | Peroxisome proliferator-activated receptor alpha |
| FRAP | Ferric-reducing antioxidant power |
| RCC | Renal cell carcinoma |
| ROS | Reactive oxygen species |
| SA-β-gal | β-galactosidase |
| Scd1 | Stearoyl-CoA desaturase |
| SCD-1 | Stearoyl-CoA desaturase-1 |
| SCFAs | Short chain fatty acids |
| Serpinc1 | Serpin family C member 1 |
| SOD | Superoxide dismutase |
| SPT | Serine palmitoyltransferase |
| SREBP1c | Sterol regulatory element-binding protein-1c |
| STAT1 | Signal transducer and activator of transcription 1 |
| TARC | Thymus and activation-regulated chemokine |
| TEAC | Trolox equivalent antioxidant capacity |
| Th | T-helper type |
| Th17 | T-helper 17 |
| TIMP | Tissue inhibitor of matrix metalloproteinases |
| TNF-α | Tumor necrosis factor-alpha |
| Tnnc1 | Troponin C 1 |
| Tpm3 | Tropomyosin 3 |
| TRAIL | TNF-related apoptosis-inducing ligand |
| TrkB | Tropomyosin receptor kinase B |
| UCP2 | Uncoupling protein 2 |
| UCP3 | Uncoupling protein 3 |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| XIAP | X-linked inhibitor of apoptosis protein |
| βCX | β-cryptoxanthin |
| γ-GTP | Gamma glutamyl transferase |
References
- Liu, Y.; Heying, E.; Tanumihardjo, S.A. History, global distribution, and nutritional importance of Citrus fruits. Compr. Rev. Food Sci. Food Saf. 2012, 11, 530–545. [Google Scholar] [CrossRef]
- Lv, X.; Zhao, S.; Ning, Z.; Zeng, H.; Shu, Y.; Tao, O.; Xiao, C.; Lu, C.; Liu, Y. Citrus fruits as a treasure trove of active natural metabolites that potentially provide benefits for human health. Chem. Cent. J. 2015, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.-S. Bioactive compounds of Citrus fruits: A review of composition and health benefits of carotenoids, flavonoids, limonoids, and terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Addi, M.; Elbouzidi, A.; Abid, M.; Tungmunnithum, D.; Elamrani, A.; Hano, C. An overview of bioactive flavonoids from Citrus fruits. Appl. Sci. 2022, 12, 29. [Google Scholar] [CrossRef]
- Pasdaran, A.; Hamedi, A.; Shiehzadeh, S.; Hamedi, A. A review of Citrus plants as functional foods and dietary supplements for human health, with an emphasis on meta-analyses, clinical trials, and their chemical composition. Clin. Nutr. ESPEN 2023, 54, 311–336. [Google Scholar] [CrossRef]
- Andrade, M.A.; Barbosa, C.H.; Shah, M.A.; Ahmad, N.; Vilarinho, F.; Khwaldia, K.; Silva, A.S.; Ramos, F. Citrus by-products: Valuable source of bioactive compounds for food applications. Antioxidants 2022, 12, 38. [Google Scholar] [CrossRef]
- Yazici, K.; Balijagic, J.; Goksu, B.; Bilgin, O.F.; Ercisli, S. Comparison of some fruit quality parameters of selected 12 mandarin genotypes from black sea region in Turkey. ACS Omega 2023, 8, 19719–19727. [Google Scholar] [CrossRef]
- Panwar, D.; Saini, A.; Panesar, P.S.; Chopra, H.K. Unraveling the scientific perspectives of Citrus by-products utilization: Progress towards circular economy. Trends Food Sci. Technol. 2021, 111, 549–562. [Google Scholar] [CrossRef]
- Hsouna, A.B.; Sadaka, C.; Mekinić, I.G.; Garzoli, S.; Švarc-Gajić, J.; Rodrigues, F.; Morais, S.; Moreira, M.M.; Ferreira, E.; Spigno, G.; et al. The chemical variability, nutraceutical value, and food-industry and cosmetic applications of Citrus plants: A critical review. Antioxidants 2023, 12, 481. [Google Scholar] [CrossRef]
- Šafranko, S.; Šubarić, D.; Jerković, I.; Jokić, S. Citrus by-products as a valuable source of biologically active compounds with promising pharmaceutical, biological and biomedical potential. Pharmaceuticals 2023, 16, 1081. [Google Scholar] [CrossRef]
- Munir, H.; Yaqoob, S.; Awan, K.A.; Imtiaz, A.; Naveed, H.; Ahmad, N.; Naeem, M.; Sultan, W.; Ma, Y. Unveiling the chemistry of Citrus peel: Insights into nutraceutical potential and therapeutic spplications. Foods 2024, 13, 1681. [Google Scholar] [CrossRef]
- Nieto, G.; Fernández-López, J.; Pérez-Álvarez, J.A.; Peñalver, R.; Ros-Berruezo, G.; Viuda-Martos, M. Valorization of Citrus co-products: Recovery of bioactive compounds and application in meat and meat products. Plants 2021, 10, 1069. [Google Scholar] [CrossRef]
- Russo, C.; Maugeri, A.; Lombardo, G.E.; Musumeci, L.; Barreca, D.; Rapisarda, A.; Cirmi, S.; Navarra, M. The second life of Citrus fruit waste: A valuable source of bioactive compounds. Molecules 2021, 26, 5991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhu, S.; Yang, W.; Huang, Q.; Ho, C.-T. The biological fate and bioefficacy of Citrus flavonoids: Bioavailability, biotransformation, and delivery systems. Food Funct. 2021, 12, 3307–3323. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Ogawa, K.; Yano, M. Absorption, storage and distribution of β-cryptoxanthin in rat after chronic administration of Satsuma mandarin (Citrus unshiu MARC.) juice. Biol. Pharm. Bull. 2013, 36, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Dosoky, N.S.; Setzer, W.N. Biological activities and safety of Citrus spp. essential oils. Int. J. Mol. Sci. 2018, 19, 1966. [Google Scholar] [CrossRef]
- Burnett, C.L.; Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; et al. Safety assessment of Citrus-derived peel oils as used in cosmetics. Int J Toxicol. 2019, 38, 33S–59S. [Google Scholar] [CrossRef]
- Brah, A.S.; Armah, F.A.; Obuah, C.; Akwetey, S.A.; Adokoh, C.K. Toxicity and therapeutic applications of Citrus essential oils (CEOs): A review. Int. J. Food Prop. 2023, 26, 301–326. [Google Scholar] [CrossRef]
- Park, H.; Hwang, Y.-H.; Choi, J.-G.; Ma, J.-Y. In vitro and in vivo evaluation of systemic and genetic toxicity of Citrus unshiu peel. J. Ethnopharmacol. 2018, 215, 120–123. [Google Scholar] [CrossRef]
- Kang, S.; Song, S.; Lee, J.; Chang, H.; Lee, S. Clinical investigations of the effect of Citrus unshiu peel pellet on obesity and lipid profile. Evid. Based Complement. Altern. Med. 2018, 2018, 4341961. [Google Scholar] [CrossRef]
- Ham, Y.-M.; Yoon, S.-A.; Hyeon, H.; Hyun, H.-B.; Kim, S.-C.; Go, B.; Jung, Y.-H.; Yoon, W.-J. Clinical evidence of effects of green mandarin (putgyul) extract on skin aging: A randomized, double blind, placebo-controlled study. Nutrients 2022, 14, 1352. [Google Scholar] [CrossRef]
- Klimek-Szczykutowicz, M.; Szopa, A.; Ekiert, H. Citrus limon (lemon) phenomenon-A review of the chemistry, pharmacological properties, applications in the modern pharmaceutical, food, and cosmetics industries, and biotechnological studies. Plants 2020, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Yarla, N.S.; Siddiqi, N.J.; Pereira, M.D.; Sharma, B. Features, pharmacological chemistry, molecular mechanism and health benefits of lemon. Med. Chem. 2021, 17, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Dongre, P.; Doifode, C.; Choudhary, S.; Sharma, N. Botanical description, chemical composition, traditional uses and pharmacology of Citrus sinensis: An updated review. Pharmacol. Res.-Mod. Chin. Med. 2023, 8, 100272. [Google Scholar] [CrossRef]
- Heo, J.-M.; Eun, C.-H.; Kim, I.-J. Identification of late ripening Citrus mutant, Ara-unshiu (Citrus unshiu), and its selectable marker. Plants 2023, 12, 3355. [Google Scholar] [CrossRef]
- Černi, S.; Hančević, K.; Škorić, D. Citruses in Croatia—Cultivation, major virus and viroid threats and challenges. Acta Bot. Croat. 2020, 79, 228–235. [Google Scholar] [CrossRef]
- Xiao, C.; He, L.; Qiu, W.; Wang, Z.; He, X.; Xiao, Y.; Sun, Z.; Tong, Z.; Jiang, Y. Guijing2501 (Citrus unshiu) has stronger cold tolerance due to higher photoprotective capacity as revealed by comparative transcriptomic and physiological analysis and overexpression of early light-induced protein. Int. J. Mol. Sci. 2023, 24, 15956. [Google Scholar] [CrossRef]
- Putnik, P.; Barba, F.J.; Lorenzo, J.M.; Gabrić, D.; Shpigelman, A.; Cravotto, G.; Kovačević, D.B. An integrated approach to mandarin processing: Food safety and nutritional quality, consumer preference, and nutrient bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1345–1358. [Google Scholar] [CrossRef]
- Ding, Y.; Chang, J.; Ma, Q.; Chen, L.; Liu, S.; Jin, S.; Han, J.; Xu, R.; Zhu, A.; Guo, J.; et al. Network analysis of postharvest senescence process in Citrus fruits revealed by transcriptomic and metabolomic profiling. Plant Physiol. 2015, 168, 357–376. [Google Scholar] [CrossRef]
- Inoue, T.; Yoshinaga, A.; Takabe, K.; Yoshioka, T.; Ogawa, K.; Sakamoto, M.; Azuma, J.-I.; Honda, Y. In situ detection and identification of hesperidin crystals in Satsuma mandarin (Citrus unshiu) peel cells. Phytochem. Anal. 2015, 26, 105–110. [Google Scholar] [CrossRef]
- Maslov Bandić, L.; Vlahoviček-Kahlina, K.; Sigurnjak Bureš, M.; Sopko Stracenski, K.; Jalšenjak, N.; Fruk, G.; Antolković, A.M.; Jurić, S. Fruit quality of Satsuma mandarins from Neretva valley and their flavonoid and carotenoid content. Horticulturae 2023, 9, 383. [Google Scholar] [CrossRef]
- Hwang, H.-J.; Kim, H.-J.; Ko, M.-J.; Chung, M.-S. Recovery of hesperidin and narirutin from waste Citrus unshiu peel using subcritical water extraction aided by pulsed electric field treatment. Food Sci. Biotechnol. 2021, 30, 217–226. [Google Scholar] [CrossRef]
- Šafranko, S.; Ćorković, I.; Jerković, I.; Jakovljević, M.; Aladić, K.; Šubarić, D.; Jokić, S. Green extraction techniques for obtaining bioactive compounds from mandarin peel (Citrus unshiu var. Kuno): Phytochemical analysis and process optimization. Foods 2021, 10, 1043. [Google Scholar]
- Inoue, T.; Tsubaki, S.; Ogawa, K.; Onishi, K.; Azuma, J.-I. Isolation of hesperidin from peels of thinned Citrus unshiu fruits by microwave-assisted extraction. Food Chem. 2010, 123, 542–547. [Google Scholar] [CrossRef]
- Kim, M.; Park, Y.; Yun, S.K.; Kim, S.S.; Joa, J.; Moon, Y.-E.; Do, G.-R. The anatomical differences and physiological responses of sunburned satsuma mandarin (Citrus unshiu Marc.) fruits. Plants 2022, 11, 1801. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Jiang, J.; Yin, C.; Li, G.; Jiang, Y.; Shan, Y. Effect of ozone treatment on flavonoid accumulation of satsuma mandarin (Citrus unshiu Marc.) during ambient storage. Biomolecules 2019, 9, 821. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kang, S.-B.; Yun, S.K.; Kim, S.K.; Joa, J.; Park, Y. Influence of excessively high temperatures on the fruit growth and physicochemical properties of shiranuhi mandarin in plastic-film greenhouse cultivation. Plants 2021, 10, 1525. [Google Scholar] [CrossRef]
- Kim, S.S.; Kim, H.-J.; Park, K.J.; Kang, S.B.; Park, Y.; Han, S.-G.; Kim, M.; Song, Y.H.; Kim, D.-S. Metabolomic profiling of Citrus unshiu during different stages of fruit development. Plants 2022, 11, 967. [Google Scholar] [CrossRef]
- Hunlun, C.; de Beer, D.; Sigge, G.O.; Van Wyk, J. Characterisation of the flavonoid composition and total antioxidant capacity of juice from different Citrus varieties from the Western Cape region. J. Food Compos. Anal. 2017, 62, 115–125. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.J. Antioxidant activities of premature and mature mandarin (Citrus unshiu) peel and juice extracts. Food Sci. Biotechnol. 2022, 31, 627–633. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, G.; Kato, M.; Yamawaki, K.; Takagi, T.; Kiriiwa, Y.; Ikoma, Y.; Matsumoto, H.; Yoshioka, T.; Nesumi, H. Regulation of carotenoid accumulation and the expression of carotenoid metabolic genes in citrus juice sacs in vitro. J. Exp. Bot. 2012, 63, 871–886. [Google Scholar] [CrossRef] [PubMed]
- Dragull, K.; Breksa, A.P.; Cain, B. Synephrine content of juice from Satsuma mandarins (Citrus unshiu Marcovitch). J. Agric. Food Chem. 2008, 56, 8874–8878. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Zhu, Y.; Shu, C.; Gao, J.; Liu, F.; Pan, S. Extraction of pectin from Satsuma mandarin peel: A comparison of high hydrostatic pressure and conventional extractions in different acids. Molecules 2022, 27, 3747. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Lim, S.-B. Semi-continuous subcritical water extraction of flavonoids from Citrus unshiu peel: Their antioxidant and enzyme inhibitory activities. Antioxidants 2020, 9, 360. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Kim, H.H.; Preethi, V.; Moniruzzaman, M.; Lee, K.H.; Kalaiselvi, S.; Kim, G.S.; Min, T. Assessment of anti-inflammatory and antioxidant effects of Citrus unshiu peel (CUP) flavonoids on LPS-stimulated RAW 264.7 cells. Plants 2021, 10, 2209. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, G.-S.; Lee, J.H.; Park, S.; Jeong, W.Y.; Kim, Y.-H.; Kim, J.H.; Kim, S.T.; Cho, Y.A.; Lee, W.S.; et al. Determination of the change of flavonoid components as the defence materials of Citrus unshiu Marc. fruit peel against Penicillium digitatum by liquid chromatography coupled with tandem mass spectrometry. Food Chem. 2011, 128, 49–54. [Google Scholar]
- Adhikari-Devkota, A.; Elbashir, S.M.I.; Watanabe, T.; Devkota, H.P. Chemical constituents from the flowers of Satsuma mandarin and their free radical scavenging and α-glucosidase inhibitory activities. Nat. Prod. Res. 2019, 33, 1670–1673. [Google Scholar] [CrossRef]
- Méndez, L.J.L.; Martínez-Mota, L.; Cassani, J.; Mayagoitia-Novales, L.; Benítez-King, G.; Becerril-Villanueva, L.E.; Dorantes-Barrón, A.M.; Jurado-Hernández, N.; Estrada-Reyes, R. Antidepressant-like and beneficial effects of a neoponcirin-beta-cyclodextrin inclusion complex in mice exposed to prolonged stress. Int. J. Mol. Sci. 2024, 25, 8289. [Google Scholar] [CrossRef]
- Furukawa, Y.; Okuyama, S.; Amakura, Y.; Sawamoto, A.; Nakajima, M.; Yoshimura, M.; Igase, M.; Fukuda, N.; Tamai, T.; Yoshida, T. Isolation and characterization of neuroprotective components from Citrus peel and their application as functional food. Chem. Pharm. Bull. 2021, 69, 2–10. [Google Scholar] [CrossRef]
- Kang, G.-J.; Han, S.-C.; Ock, J.-W.; Kang, H.-K.; Yoo, E.-S. Anti-inflammatory effect of quercetagetin, an active component of immature Citrus unshiu, in HaCaT human keratinocytes. Biomol. Ther. 2013, 21, 138–145. [Google Scholar] [CrossRef]
- Takayanagi, K.; Morimoto, S.; Shirakura, Y.; Mukai, K.; Sugiyama, T.; Tokuji, Y.; Ohnishi, M. Mechanism of visceral fat reduction in Tsumura Suzuki obese, diabetes (TSOD) mice orally administered β-cryptoxanthin from Satsuma mandarin oranges (Citrus unshiu Marc). J. Agric. Food Chem. 2011, 59, 12342–12351. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Nishikawa, T.; Furukawa, A.; Itano, S.; Tamura, Y.; Hasei, T.; Watanabe, T. Antimutagenic effects of polymethoxy flavonoids of Citrus unshiu. Nat. Prod. Commun. 2017, 12, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, Y.; Tao, L.; Tao, X.; Su, X.; Wei, D. Tyrosinase inhibitory effects and inhibition mechanisms of nobiletin and hesperidin from Citrus peel crude extracts. J. Enzyme Inhib. Med. Chem. 2007, 22, 83–90. [Google Scholar] [CrossRef]
- Choi, E.O.; Lee, H.; HwangBo, H.; Kwon, D.H.; Kim, M.Y.; Ji, S.Y.; Hong, S.H.; Kim, G.-Y.; Park, C.; Hwang, H.-J.; et al. Citrus unshiu peel suppress the metastatic potential of murine melanoma B16F10 cells in vitro and in vivo. Phytother. Res. 2019, 33, 3228–3241. [Google Scholar] [CrossRef]
- Jin, H.; Lee, W.S.; Yun, J.W.; Jung, J.H.; Yi, S.M.; Kim, H.J.; Choi, Y.H.; Kim, G.; Jung, J.-M.; Ryu, C.H.; et al. Flavonoids from Citrus unshiu Marc. inhibit cancer cell adhesion to endothelial cells by selective inhibition of VCAM-1. Oncol. Rep. 2013, 30, 2336–2342. [Google Scholar] [CrossRef]
- Kim, M.Y.; Bo, H.H.; Choi, E.O.; Kwon, D.H.; Kim, H.J.; Ahn, K.I.; Ji, S.Y.; Jeong, J.-W.; Park, S.-H.; Hong, S.-H.; et al. Induction of apoptosis by Citrus unshiu peel in human breast cancer MCF-7 cells: Involvement of ROS-dependent activation of AMPK. Biol. Pharm. Bull. 2018, 41, 713–721. [Google Scholar] [CrossRef]
- Kim, M.Y.; Choi, E.O.; HwangBo, H.; Kwon, D.H.; Ahn, K.I.; Kim, H.J.; Ji, S.Y.; Hong, S.-H.; Jeong, J.-W.; Kim, G.Y.; et al. Reactive oxygen species-dependent apoptosis induction by water extract of Citrus unshiu peel in MDA-MB-231 human breast carcinoma cells. Nutr. Res. Pract. 2018, 12, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.; Kim, M.; Kim, J.H. Fermented extraction of Citrus unshiu peel inhibits viability and migration of human pancreatic cancers. J. Med. Food 2018, 21, 5–12. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.-H.; Kim, J.H. Combined administration of naringenin and hesperetin with optimal ratio maximizes the anti-cancer effect in human pancreatic cancer via down regulation of FAK and p38 signaling pathway. Phytomedicine 2019, 58, 152762. [Google Scholar] [CrossRef]
- Lee, S.; Ra, J.; Song, J.-Y.; Gwak, C.; Kwon, H.-J.; Yim, S.-V.; Hong, S.-P.; Kim, J.; Lee, K.-H.; Cho, J.-J.; et al. Extracts from Citrus unshiu promote immune-mediated inhibition of tumor growth in a murine renal cell carcinoma model. J. Ethnopharmacol. 2011, 133, 973–979. [Google Scholar] [CrossRef]
- Jannat, S.; Ali, M.Y.; Kim, H.-R.; Jung, H.A.; Choi, J.S. Protective effects of sweet orange, unshiu mikan, and mini tomato juice powders on t-BHP-induced oxidative stress in HepG2 cells. Prev. Nutr. Food Sci. 2016, 21, 208–220. [Google Scholar] [CrossRef]
- Tanaka, T.; Kohno, H.; Murakami, M.; Shimada, R.; Kagami, S.; Sumida, T.; Azuma, Y.; Ogawa, H. Suppression of azoxymethane-induced colon carcinogenesis in male F344 rats by mandarin juices rich in beta-cryptoxanthin and hesperidin. Int. J. Cancer 2000, 88, 146–150. [Google Scholar] [CrossRef]
- Suzuki, R.; Kohno, H.; Yasui, Y.; Hata, K.; Sugie, S.; Miyamoto, S.; Sugawara, K.; Sumida, T.; Hirose, Y.; Tanaka, T. Diet supplemented with Citrus unshiu segment membrane suppresses chemically induced colonic preneoplastic lesions and fatty liver in male db/db mice. Int. J. Cancer 2007, 120, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Im, M.; Gu, M.J.; Ma, J.Y. Citrus unshiu peel extract alleviates cancer-induced weight loss in mice bearing CT-26 adenocarcinoma. Sci. Rep. 2016, 6, 24214. [Google Scholar] [CrossRef]
- Tahaghoghi-Hajghorbani, S.; Ebrahimzadeh, M.A.; Rafiei, A.; Golpour, M.; Hosseini-Khah, Z.; Akhtari, J. Improvement of chemotherapy through reducing of cachexia by using Citrus unshiu peel extract. J. Ethnopharmacol. 2019, 242, 111929. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.I.; Choi, E.O.; Kwon, D.H.; HwangBo, H.; Kim, M.Y.; Kim, H.J.; Ji, S.Y.; Hong, S.-H.; Jeong, J.-W.; Park, C.; et al. Induction of apoptosis by ethanol extract of Citrus unshiu Markovich peel in human bladder cancer T24 cells through ROS-mediated inactivation of the PI3K/Akt pathway. Biosci. Trends 2017, 11, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Han, J.M.; Kang, Y.J.; Jung, H.J. Chloroform extract of Citrus unshiu Markovich peel induces apoptosis and inhibits stemness in HeLa human cervical cancer cells. Mol. Med. Rep. 2021, 23, 86. [Google Scholar] [CrossRef]
- Lim, H.; Yeo, E.; Song, E.; Chang, Y.-H.; Han, B.-K.; Choi, H.-J.; Hwang, J. Bioconversion of Citrus unshiu peel extracts with cytolase suppresses adipogenic activity in 3T3-L1 cells. Nutr. Res. Pract. 2015, 9, 599–605. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, Y.-S.; Song, M.; Lee, M.; Park, J.; Kim, H. A herbal formula HT048, Citrus unshiu and Crataegus pinnatifida, prevents obesity by inhibiting adipogenesis and lipogenesis in 3T3-L1 preadipocytes and HFD-induced obese rats. Molecules 2015, 20, 9656–9670. [Google Scholar] [CrossRef]
- Kim, J.K.; Jeong, H.W.; Kim, A.Y.; Hong, Y.D.; Lee, J.H.; Choi, J.K.; Hwang, J.S. Green satsuma mandarin orange (Citrus unshiu) extract reduces adiposity and induces uncoupling protein expression in skeletal muscle of obese mice. Food Sci. Biotechnol. 2018, 28, 873–879. [Google Scholar] [CrossRef]
- Im, S.T.; Kang, H.; Kim, J.; Kim, S.-R.; Kim, K.-N.; Lee, S.-H. Narirutin-rich celluclast extract from mandarin (Citrus unshiu) peel alleviates high-fat diet-induced obesity and promotes energy metabolism in C57BL/6 mice. Int. J. Mol. Sci. 2024, 25, 4475. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kang, S.-I.; Ko, H.C.; Park, J.; Jun, W. Anti-obesity effect of jeju roasted Citrus peel extract in high-fat diet-induced obese mice and 3T3-L1 adipocytes via lipid metabolism regulation. J. Med. Food 2024, 27, 369–378. [Google Scholar] [CrossRef]
- Sugiura, M.; Ohshima, M.; Ogawa, K.; Yano, M. Chronic administration of Satsuma mandarin fruit (Citrus unshiu Marc.) improves oxidative stress in streptozotocin-induced diabetic rat liver. Biol. Pharm. Bull. 2006, 29, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Ogawa, K.; Yano, M. Effect of chronic administration of fruit extract (Citrus unshiu Marc.) on glucose tolerance in GK rats, a model of type 2 diabetes. Biosci. Biotechnol. Biochem. 2006, 70, 293–295. [Google Scholar] [CrossRef]
- Park, H.-J.; Jung, U.J.; Cho, S.-J.; Jung, H.-K.; Shim, S.; Choi, M.-S. Citrus unshiu peel extract ameliorates hyperglycemia and hepatic steatosis by altering inflammation and hepatic glucose-and lipid-regulating enzymes in db/db mice. J. Nutr. Biochem. 2013, 24, 419–427. [Google Scholar] [CrossRef]
- Lee, G.-H.; Peng, C.; Park, S.-A.; Hoang, T.-H.; Lee, H.-Y.; Kim, J.; Kang, S.-I.; Lee, C.-H.; Lee, J.-S.; Chae, H.-J. Citrus peel extract ameliorates high-fat diet-induced NAFLD via activation of AMPK signaling. Nutrients 2020, 12, 673. [Google Scholar] [CrossRef]
- Shin, M.-R.; Shin, S.H.; Roh, S.-S. Diospyros kaki and Citrus unshiu mixture improves disorders of lipid metabolism in nonalcoholic fatty liver disease. Can. J. Gastroenterol. Hepatol. 2020, 2020, 8812634. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.W.; Lee, Y.; Kim, Y.T. Preventive effects of Citrus unshiu peel extracts on bone and lipid metabolism in OVX rats. Molecules 2014, 19, 783–794. [Google Scholar] [CrossRef]
- Iwata, E.; Hotta, H.; Goto, M. Hypolipidemic and bifidogenic potentials in the dietary fiber prepared from Mikan (Japanese mandarin orange: Citrus unshiu) albedo. J. Nutr. Sci. Vitaminol. 2012, 58, 175–180. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.A.; Shin, M.-R.; Park, H.-J.; Roh, S.-S. Citrus unshiu peel attenuates dextran sulfate sodium-induced ulcerative colitis in mice due to modulation of the PI3K/Akt signaling pathway and MAPK and NF-κ B. Evid. Based Complement. Altern. Med. 2022, 2022, 4041402. [Google Scholar] [CrossRef]
- Lee, S.-H.; Seo, D.; Lee, K.-H.; Park, S.-J.; Park, S.; Kim, H.; Kim, T.; Joo, I.H.; Park, J.-M.; Kang, Y.-H.; et al. Biometabolites of Citrus unshiu peel enhance intestinal permeability and alter gut commensal bacteria. Nutrients 2023, 15, 319. [Google Scholar] [CrossRef]
- Ren, Y.; Mao, S.; Zeng, Y.; Chen, S.; Tian, J.; Ye, X. Pectin from Citrus unshiu Marc. alleviates glucose and lipid metabolism by regulating the gut microbiota and metabolites. Foods 2023, 12, 4094. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Gao, X.; Xie, Z.; Xia, C.; Gu, Q.; Li, P. Soluble dietary fiber from Citrus unshiu peel promotes antioxidant activity in oxidative stress mice and regulates intestinal microecology. Foods 2024, 13, 1539. [Google Scholar] [CrossRef]
- Gu, Q.; Gao, X.; Zhou, Q.; Li, Y.; Li, G.; Li, P. Characterization of soluble dietary fiber from Citrus peels (Citrus unshiu), and its antioxidant capacity and beneficial regulating effect on gut microbiota. Int. J. Biol. Macromol. 2023, 246, 125715. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.H.; Lee, H.-T. Effects of dried Citrus unshiu peels on gastrointestinal motility in rodents. Arch Pharm Res. 2013, 36, 641–648. [Google Scholar] [CrossRef]
- Shimamura, Y.; Sei, S.; Nomura, S.; Masuda, S. Protective effects of dried mature Citrus unshiu peel (Chenpi) and hesperidin on aspirin-induced oxidative damage. J. Clin. Biochem. Nutr. 2021, 68, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Lee, S.J.; Gim, H.; Kim, H.J.; Han, T.; Kim, J.G.; Lim, E.Y.; Kim, Y.T.; Kim, B.J. Regulation of the pacemaker activities in cultured interstitial cells of Cajal by Citrus unshiu peel extracts. Mol. Med. Rep. 2016, 14, 3908–3916. [Google Scholar] [CrossRef]
- Ihara, H.; Yamamoto, H.; Ida, T.; Tsutsuki, H.; Sakamoto, T.; Fujita, T.; Okada, T.; Kozaki, S. Inhibition of nitric oxide production and inducible nitric oxide synthase expression by a polymethoxyflavone from young fruits of Citrus unshiu in rat primary astrocytes. Biosci. Biotechnol. Biochem. 2012, 76, 1843–1848. [Google Scholar] [CrossRef]
- Lim, D.W.; Um, M.Y.; Han, T.; Lee, J.; Kim, Y.T.; Cho, S.; Kim, I.-H.; Han, D.; Lee, C. Standardized Citrus unshiu peel extract ameliorates dexamethasone-induced neurotoxicity and depressive-like behaviors in mice. Metab. Brain Dis. 2018, 33, 877–1886. [Google Scholar] [CrossRef]
- Jang, A.; Choi, G.-E.; Kim, Y.-J.; Lee, G.-H.; Hyun, K.-Y. Neuroprotective properties of ethanolic extract of Citrus unshiu Markovich peel through NADPH oxidase 2 inhibition in chemotherapy-induced neuropathic pain animal model. Phytother. Res. 2021, 35, 6918–6931. [Google Scholar] [CrossRef]
- Bae, J.T.; Ko, H.J.; Kim, G.B.; Pyo, H.B.; Lee, G.S. Protective effects of fermented Citrus unshiu peel extract against ultraviolet-A-induced photoageing in human dermal fibrobolasts. Phytother. Res. 2012, 26, 1851–1856. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-I.; Jeong, Y.-U.; Kim, J.-H.; Park, Y.-J. 3,5,6,7,8,3′,4′-Heptamethoxyflavone, a Citrus flavonoid, inhibits collagenase activity and induces type I procollagen synthesis in HDFn Cells. Int. J. Mol. Sci. 2018, 19, 620. [Google Scholar] [CrossRef]
- Kang, G.-J.; Han, S.-C.; Yi, E.-J.; Kang, H.-K.; Yoo, E.-S. The inhibitory effect of premature Citrus unshiu extract on atopic dermatitis in vitro and in vivo. Toxicol. Res. 2011, 27, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Park, K.J.; An, H.J.; Choi, Y.H. Phytochemical, antioxidant, and antibacterial activities of fermented Citrus unshiu byproduct. Food Sci. Biotechnol. 2017, 26, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Ji, J.; Baek, S.H.; Lee, J.H.; Ha, I.J.; Lim, S.S.; Yoon, H.J.; Nam, Y.J.; Ahn, K.S. Fermented dried Citrus unshiu peel extracts exert anti-inflammatory activities in LPS-induced RAW264.7 macrophages and improve skin moisturizing efficacy in immortalized human HaCaT keratinocytes. Pharm. Biol. 2019, 57, 392–402. [Google Scholar] [CrossRef]
- Fujita, T.; Shiura, T.; Masuda, M.; Tokunaga, M.; Kawase, A.; Iwaki, M.; Gato, T.; Fumuro, M.; Sasaki, K.; Utsunomiya, N.; et al. Anti-allergic effect of a combination of Citrus unshiu unripe fruits extract and prednisolone on picryl chloride-induced contact dermatitis in mice. J. Nat. Med. 2008, 62, 202–206. [Google Scholar] [CrossRef]
- Kim, J.-K.; Park, N.-H.; Hwang, J.-S. Skin lightening effect of the dietary intake of Citrus peel extract against UV-induced pigmentation. Nat. Product. Commun. 2019, 14, 1934578X19859979. [Google Scholar] [CrossRef]
- Tamaru, E.; Watanabe, M.; Nomura, Y. Dietary immature Citrus unshiu alleviates UVB-induced photoaging by suppressing degradation of basement membrane in hairless mice. Heliyon 2020, 6, e04218. [Google Scholar] [CrossRef]
- Lee, J.; Yang, D.-S.; Han, S.-I.; Yun, J.H.; Kim, I.-W.; Kim, S.J.; Kim, J.H. Aqueous extraction of Citrus unshiu peel induces proangiogenic effects through the FAK and ERK1/2 signaling pathway in human umbilical vein endothelial cells. J. Med. Food 2016, 19, 569–577. [Google Scholar] [CrossRef]
- Kim, J.-J.; Kim, K.; Jung, Y.-R.; Bian, Y.; Ngo, T.; Bae, O.-N.; Lim, K.-M.; Chung, J.-H. Co-existence of hypertensive and anti-hypertensive constituents, synephrine, and nobiletin in Citrus unshiu peel. Molecules 2019, 24, 1197. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tanabe, S. Evaluation of the anti-allergic activity of Citrus unshiu using rat basophilic leukemia RBL-2H3 cells as well as basophils of patients with seasonal allergic rhinitis to pollen. Int. J. Mol. Med. 2006, 17, 511–515. [Google Scholar] [CrossRef]
- Oku, H.; Kitagawa, F.; Kato, Y.; Miyashita, T.; Hara, M.; Minetoki, T.; Yamada, S. Anti-allergic effects of the subcritical water extract powder of Citrus unshiu in mouse and guinea pig models. J. Med. Food 2021, 24, 533–540. [Google Scholar] [CrossRef]
- Yang, X.; Kang, S.-M.; Jeon, B.-T.; Kim, Y.-D.; Ha, J.-H.; Kim, Y.-T.; Jeon, Y.-J. Isolation and identification of an antioxidant flavonoid compound from citrus-processing by-product. J. Sci. Food Agric. 2011, 91, 1925–1927. [Google Scholar] [CrossRef]
- Ma, Y.-Q.; Ye, X.-Q.; Fang, Z.-X.; Chen, J.-C.; Xu, G.-H.; Liu, D.-H. Phenolic compounds and antioxidant activity of extracts from ultrasonic treatment of Satsuma Mandarin (Citrus unshiu Marc.) peels. J. Agric. Food Chem. 2008, 56, 5682–5690. [Google Scholar] [CrossRef]
- Oh, Y.-C.; Cho, W.-K.; Jeong, Y.H.; Im, G.Y.; Yang, M.C.; Hwang, Y.-H.; Ma, J.Y. Anti-inflammatory effect of Citrus unshiu peel in LPS-stimulated RAW 264.7 macrophage cells. Am. J. Chin. Med. 2012, 40, 611–629. [Google Scholar] [CrossRef]
- Kim, M.G.; Kim, S.; Boo, K.-H.; Kim, J.-H.; Kim, C.-S. Anti-inflammatory effects of immature Citrus unshiu fruit extracts via suppression of NF-κB and MAPK signal pathways in LPS-induced RAW264.7 macrophage cells. Food Sci. Biotechnol. 2024, 33, 903–911. [Google Scholar] [CrossRef]
- Nishi, K.; Ito, T.; Kadota, A.; Ishida, M.; Nishiwaki, H.; Fukuda, N.; Kanamato, N.; Yagata, Y.; Sugahara, T. Aqueous extract from leaves of Citrus unshiu attenuates lipopolysaccharide-induced inflammatory responses in a mouse model of systemic inflammation. Plants 2021, 10, 1708. [Google Scholar] [CrossRef]
| Bioactive Compounds | Part Used | Solvents | Extraction Methods | Bioactive Values | Ref. |
|---|---|---|---|---|---|
| Hesperidin | Peel | Methanol | Raman microscopy | Hesperidin: 64.3 mg/g | [30] |
| Hesperidin, narirutin, naringin, nobiletin, tangeretin | Peel, pulp | Methanol | High-performance liquid chromatography | Hesperidin: 51.9–73.8 mg/g; Narirutin: 8.0–19.8 mg/g; Naringin: 0.35–0.40 mg/g; Nobiletin: 0.2–0.6 mg/g; Tangeretin: 0.13–0.25 mg/g | [31] |
| Hesperidin, narirutin | Peel | Methanol, water | Subcritical water extraction | Hesperidin: 46.96 mg/g; Narirutin: 8.76 mg/g | [32] |
| Hesperidin, narirutin, rutin, heptamethoxyflavone | Peel | Water | High-performance liquid chromatography, supercritical CO2 | Hesperidin: 0.16–15.07 mg/g; Narirutin: 0.11–4.87 mg/g; Rutin: 0.18–4.27 mg/g; HMF: 0.01–14.33 mg/g | [33] |
| Hesperidin, narirutin | Peel | Methanol | Microwave-assisted extraction | Hesperidin: 5860 mg/100 g; Narirutin: 1310 mg/100 g | [34] |
| Hesperidin, narirutin, naringin, nobiletin, rutin, tangeretin | Peel | Ethanol | High-performance liquid chromatography | Hesperidin: 4297 mg/100 g; Narirutin: 3469 mg/100 g; Naringin: 24.6 mg/100 g; Nobiletin: 107.5 mg/100 g; Rutin: 1098.6 mg/100 g; Tangeretin: 41.7 mg/100 g | [35] |
| Hesperidin, narirutin, nobiletin, rutin, tangeretin | Peel | Methanol | Ultra-high-performance liquid chromatography | Hesperidin: 16,522.2 mg × kg−1; Narirutin: 8686.6 mg × kg−1; Nobiletin: 759.9 mg × kg−1; Rutin: 3068.7 mg × kg−1; Tangeretin: 311.4 mg × kg−1 | [36] |
| Hesperidin, narirutin, nobiletin, rutin, tangeretin | Peel, pulp | Ethanol | High-performance liquid chromatography | Hesperidin: 1685.6–2023.6 mg/100 g; Narirutin: 504.4–883.4 mg/100 g; Nobiletin: 131.2–185.2 mg/100 g; Rutin: 44.2–66.9 mg/100 g; Tangeretin: 21.3–26.3 mg/100 g | [37] |
| Hesperetin, nobiletin, tangeretin, quercetin, β-cryptoxanthin | Peel | Methanol | High-performance liquid chromatography, gas chromatography–mass spectrometry, ultra-performance liquid chromatography–quadrupole time-of-flight mass spectrometer | Hesperetin, nobiletin, tangeretin, quercetin: N/A; βCX: 680.93–1989.13 μg/100 g | [38] |
| Quercetin, rutin, naringin | Peel | Water | High-performance liquid chromatography | Quercetin, rutin: N/A; Naringin: 748 mg/L | [39] |
| Quercetin, hesperidin, hesperetin, narirutin | Peel | Methanol | High-performance liquid chromatography | Quercetin: 0.09–43.99 mg/g; Hesperidin: 76.81 mg/g; Hesperetin: 0.1 mg/g; Narirutin: 51.35 mg/g | [40] |
| β-cryptoxanthin | Pulp, juice | Ethanol | High-performance liquid chromatography | βCX: 0.8–5.5 μg/g−1 | [41] |
| Synephrine | Juice | Water | High-performance liquid chromatography | Synephrine: 73.3–158 mg/L−1 | [42] |
| Pectin | Peel | Ethanol | High hydrostatic pressure-assisted citric acid, hydrochloric acid | Pectin: 15.34–18.99% | [43] |
| Classification | Chemical Structure | Ref. |
|---|---|---|
| Flavanones | 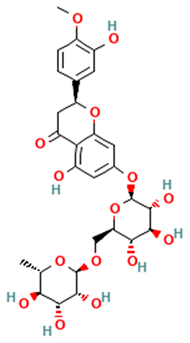 Hesperidin | [44,45,46,47] |
| Flavanones | 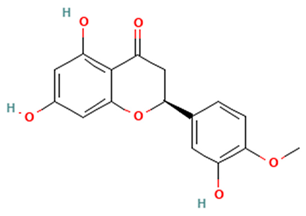 Hesperetin | [44,45,46,47] |
| Flavanones | 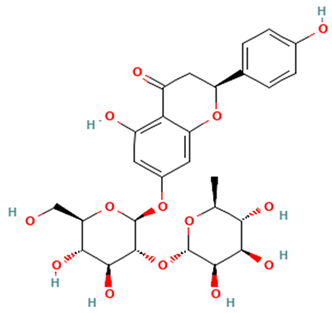 Naringin | [45,46] |
| Flavanones | 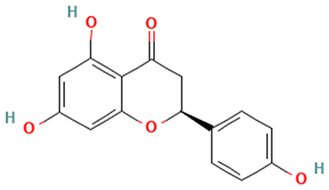 Naringenin | [44] |
| Flavanones | 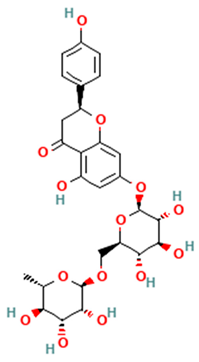 Narirutin | [44,46,47] |
| Flavanones | 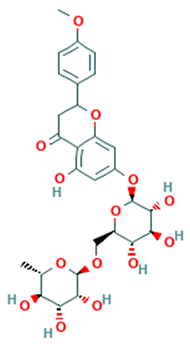 Neoponcirin | [48] |
| Polymethoxylated flavones | 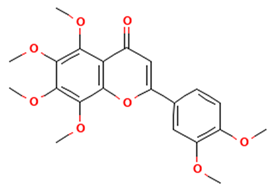 Nobiletin | [44,46] |
| Polymethoxylated flavones |  Tangeretin | [44,46] |
| Polymethoxylated flavones |  Heptamethoxyflavone | [49] |
| Alkaloids | 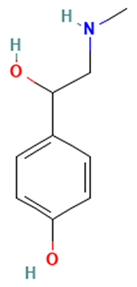 Synephrine | [42] |
| Flavonols | 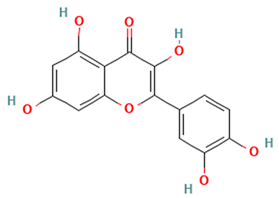 Quercetin | [45,47] |
| Flavonols | 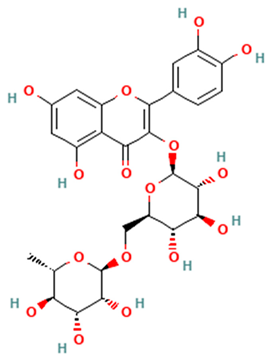 Rutin | [45,47] |
| Flavonols | 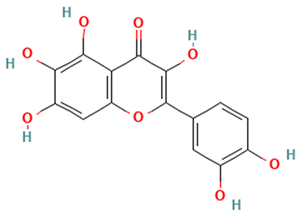 Quercetagetin | [50] |
| Carotenoids | 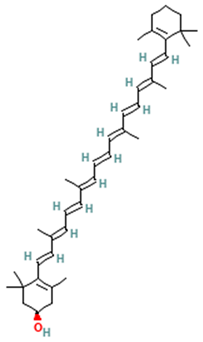 β-cryptoxanthin | [51] |
| Dietary fibers | 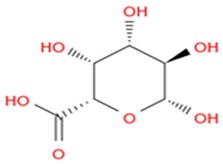 Pectin | [13] |
| Cancers | Model Type | Bioactive Compounds | Extraction Techniques and Application in Treatment | Anticancer Activity/Mechanisms | Target Genes/Signaling Pathways | Ref. |
|---|---|---|---|---|---|---|
| Melanoma | B16 mouse MSC cells | Hesperidin, nobiletin | B16 cells were treated with crude extracts from C. unshiu by petroleum ether and 95% ethanol at 15.63–250 µg/mL concentration for 72 h incubation at 70 °C. High-performance liquid chromatography was used to analyze the extracts. | Anti-proliferative | N/A | [53] |
| Melanoma | B16F10 mouse MSC cells, B16F10 cells-inoculated C57BL/6 mice | Hesperidin, naringin | B16F10 cells were treated with extracts from C. unshiu peel by 70% ethanol at different concentrations (0, 20, 40, 60, 80, and 100 µg/mL) for 24 h and incubated with 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetra-zolium bromide solution for 2 h. The extracts were stored at −80 °C. High-performance liquid chromatography was conducted to identify the extracts. Mice were injected with phosphate-buffered saline and then randomized into 3 groups: B16 + control group (100 μL of distilled water), B16 + C. unshiu peel extracts 100 group (100 μL of C. unshiu peel extracts 100 mg/kg/day), and B16 + C. unshiu peel extracts 200 group (100 μL of C. unshiu peel extracts 200 mg/kg/day). Mice were orally administered for 21 days. | Apoptosis, anti-metastatic, anti-migration, anti-invasion, anti-inflammatory, inhibition of cell growth and colony formation | Bax, TIMP-1/2 ↑; Bcl-2, MMP-2/9, TNF-α, LDH ↓ | [54] |
| Breast | MDA-MB-231 human BRC cells | Hesperidin, naringin | MDA-MB-231 cells were treated with C. unshiu extracts by 70% aqueous methanol and ethyl acetate elution at different concentrations (0, 10, 50, 100, and 200 µg/mL) for 24 h. The extracts were identified by high-performance liquid chromatography using a C18 column. | Anti-metastatic, anti-adhesion, anti-invasion | VCAM-1, PKC ↓ | [55] |
| Breast | MCF-7 human BRC cells | Hesperidin, naringin | MCF-7 cells were treated with C. unshiu ethanolic extracts at different concentrations (0, 0.25, 0.5, 1, and 1.5 mg/mL) for 72 h. The extracts were pulverized into powder, boiled with water for 3 h, and then freeze-dried at −80 °C. The extracts were analyzed using high-performance liquid chromatography. | Anti-proliferative, apoptosis | caspase-8/9, Bax, acetyl-CoA carboxylase, PARP, ROS, AMPK, cytochrome c ↑; Bcl-2 ↓ | [56] |
| Breast | MDA-MB-231 human BRC cells | NA | MDA-MB-231 cells were treated with dried C. unshiu peel water extracts at different concentrations (0, 0.25, 0.5, 1, and 1.5 mg/mL) for 72 h. The dried peel was pulverized into powder, boiled with water for 3 h, and then freeze-dried at −80 °C. | Anti-viability, apoptosis | caspase-8/9, Bax, PARP, cytochrome c, ROS ↑; Bcl-2, cIAP-1/2, XIAP, mitochondrial membrane potential ↓ | [57] |
| Pancreatic | Panc-1 and SNU-213 PaC cells, BALB/c nude mice | Hesperidin, hesperetin, narirutin, naringenin | PaC cells were treated with C. unshiu peel extracts at different concentrations (0, 2.5, and 5 mg/mL) for 48 h. C. unshiu peel extracts were dissolved in sodium acetate buffer; incubated at 45 °C with β-glucosidase (90 U/g), cellulase (54 U/g), pectinase (120 U/g), and at 30 °C with fermivin. The quantification of C. unshiu peel extracts was conducted using limit of quantification and limit of detection values based on high-performance liquid chromatography. Mice were randomized into two groups to receive phosphate buffered saline or C. unshiu peel extracts (50 mg/kg). | Anti-viability, anti-migration, apoptosis | caspase-3, p38, MKK 3/6 ↑; PCNA ↓ | [58] |
| Pancreatic | Panc-1, Detroit551, Miapaca-2, and SNU-213 PaC cells; BALB/c nude mice | Hesperetin, naringenin | PaC cells were treated with hesperetin and naringenin isolated from C. unshiu peel at different concentrations (0, 1, 5, 10, and 20 µM) for 48 and 72 h. The extraction was performed in sodium acetate buffer. The extracts were added to buffer and incubated with β-glucosidase (90 U/g), cellulase (54 U/g), pectinase (120 U/g) at 45 °C, and with fermivin at 30 °C. The extracts were analyzed using high-performance liquid chromatography. Mice were randomized to receive phosphate-buffered saline, 30 mg/kg hesperetin, 30 mg/kg naringenin, and 10/30 mg/kg naringenin/hesperetin mixture. | Anti-viability, anti-migration, apoptosis | caspase-3 ↑; FAK, p38 ↓ | [59] |
| Renal | Balb/C mice renal cancer cell line Renca | Hesperidin, narirutin | Mice were supplemented with a standard diet and then randomized to receive phosphate-buffered saline plus water, or C. unshiu peel extracts (3 and 30 mg/kg) for 32 days, plus phosphate-buffered saline injection of Renca cells. The extracts were concentrated using a rotary evaporator and dried in a freeze-dryer, then dissolved in 400 mL of distilled water for 24 h at 4 °C and filtered. High-performance liquid chromatography–ultraviolet method was used for the detection of extracts. | Anti-proliferative, inhibition of tumor growth | IFN-γ ↑; TNF-α ↓ | [60] |
| Liver | HepG2 human hepatic cells | Hesperidin, narirutin | HepG2 cells were treated with C. unshiu pulp juice extracts at different concentrations up to 200 µg/mL for 24 h. The pulp juice was filtered, concentrated using an evaporator at 60 °C, and then freeze-dried at −52 °C. High-performance liquid chromatography was used to detect the extracts. | Antioxidants | HO-1 ↑; ROS, GSH ↓ | [61] |
| Colorectal | F344 rats | Hesperidin, β-cryptoxanthin | Rat were first injected with azoxymethane (20 mg/kg body weight) for 2 weeks to induce colonic neoplasms and then treated with a commercial C. unshiu juice rich in hesperidin and βCX (100 mg/kg) for 36 weeks. The centrifugation was applied to C. unshiu juice twice. The juice was pressed from the pulp and then frozen and thawed. This procedure was conducted again with adding 0.01% pectin to increase βCX levels. | Anti-proliferative, apoptosis | PCNA, cyclin D1 ↓ | [62] |
| Colorectal | C57BL/KsJ-db/db mice | Hesperidin | Mice were injected with azoxymethane (15 mg/kg body weight) for 5 weeks and then supplemented with a diet containing C. unshiu segment membrane rich in hesperidin at concentration levels of 0.02%, 0.1%, and 0.5% for 7 weeks. Powdered C. unshiu consisted of 2.2 g hesperidin, 2.3 g ash, 5.5 g glucose, 6.1 g D-fructose, 15 g D-sucrose, 2.4 g moisture, 0.3 g fat, 5.5 g protein, 51 g fiber, and 9.7 g other flavonoids and unknown compounds. | Anti-proliferative | PCNA ↓ | [63] |
| Colorectal | CT-26 mouse CRC cells, BALB/c mice | Hesperidin, naringin, narirutin, nobiletin | CRC cells were injected into the abdominal region of BALB/c mice. After tumor injection, mice were randomized to receive saline or C. unshiu aqueous extracts daily at concentrations of 250 and 500 mg/kg. The dried peel was dissolved in 1000 mL distilled water, extracted at 115 °C for 3 h, and then freeze-dried at 4 °C. | Anti-cachectic, anti-inflammatory | IL-6, TNF-α, IL-1β, MAFbx, MuRF-1 ↓ | [64] |
| Colorectal | CT-26 mouse CRC cells, BALB/c mice | Hesperidin, naringin | CRC cells were injected with fetal bovine serum (10%), streptomycin (100 μg/mL), and penicillin (100 μg/mL) at 5% CO2. Mice were randomized to receive saline or C. unshiu peel aqueous extracts at a concentration of 350 mg/kg/day for 10 days. The peel was first dried and powdered. The extracts were steamed into the balloon, filtered, and stored at −20 °C. | Anti-cachectic, anti-inflammatory, anti-tumor growth | IL-6, TNF-α, IL-1β, MDA ↓ | [65] |
| Bladder | T24 human BC cells | NA | BC cells were treated with C. unshiu peel ethanolic extracts at different concentrations (0, 100, 200, 400, 600, 800, and 1000 µg/mL) for 48 h. The dried peel was first powdered and extracted in 1 L of 70% ethanol for 24 h. The extracts were then concentrated using a vacuum rotary evaporator and freeze-dried at −80 °C. | Anti-proliferative, anti-viability, inhibition of colony formation, apoptosis | caspase-3/8/9, cytochrome c, Bax, PARP, ROS, TRAIL, FasL, tBid ↑; Bcl-2, Bid, XIAP, cIAP, PI3K, Akt ↓ | [66] |
| Cervical | HeLa human CC cells | Hesperidin, heperetin | CC cells were treated with chloroform extracts of C. unshiu at different concentrations (0, 2, 4, 8, 16, 31, 63, 125, 250, and 500 µg/mL) for 72 h. The extracts were prepared using dimethyl sulfoxide at a concentration of 100 mg/mL. High-performance liquid chromatography analysis was conducted to detect hesperidin and heperetin contents. | Anti-proliferative, anti-migration, inhibition of colony formation, apoptosis, induction of cell cycle arrest | caspase-8/9, Bax, Bad, Fas ↑; Akt, PI3K, p21, p53, ERK1/2, cyclin B1/D1, Bcl-2, Bcl-XL ↓ | [67] |
| Model Type | Bioactive Compounds | Extraction Techniques and Application in Treatment | Therapeutic Activities/Mechanisms | Target Genes/Signaling Pathways | Ref. |
|---|---|---|---|---|---|
| Obese mouse model | β-cryptoxanthin | Mice were assigned to two groups: tsumura suzuki obese diabetes group (experiment) and tsumura suzuki non-obese diabetes group (control). The experimental groups were fed with enzyme-processed Satsuma mandarin suspended in olive oil (400 mg/kg/day; 0.8 mg of βCX/kg/day). The control groups were supplemented with olive oil only. | Reduced adipocyte hypertrophy, body weight, cell proliferation, inflammatory chemokines, and excess immune responses in adipose tissue | Steroid metabolism genes: Hmgcs1, Cyp51, Idi1 ↑; Hdlbp, Abca1 ↓ DNA replication initiation genes: Mcm 2/4/5/6 ↓ Chemotaxis, cell cycle, and immune system development genes: Cdk1, Ccna2, Ccnb1, Ccnb2, Cxcl2/10, Ccl2/3/4/7/12 ↓ Inflammatory/anti-inflammatory genes: adiponectin ↑; TNF-α, MCP-1 ↓ Lipid transport, wound response, fatty acid biosynthesis, and muscle contraction genes: Myh2/7, Tpm3, Tnnc1 ↑; Elovl6, Scd1, Fasn, ApoA 1/2, ApoC 1/3, Fga, Kng, Serpinc1 ↓ | [51] |
| 3T3-L1 adipocyte cells | Hesperetin, naringenin, | 3T3-L1 cells were treated with C. unshiu, C. unshiu with cytolase, and Sinetrol at a concentration of 0.5 mg/mL for 24 h during 10-day adipocyte differentiation. Extraction of 100 g dried C. unshiu peel was conducted with 190 units/g cytolase PCL5 in 2 L of d-H2O by incubation for 14 h at 60 °C. The peel was enzymatically treated and filtered using a filter paper, which was then extracted in 8 L of 80% ethanol for 8 h at 60 °C. High-performance liquid chromatography was used to analyze C. unshiu peel extracts. | Anti-adipogenic, lipolytic | PPARγ, C/EBPα, SREBP1c ↓ | [68] |
| 3T3-L1 adipocyte cells, Sprague–Dawley rats | Hesperidin | 3T3-L1 cells were treated with HT048 (C. unshiu peel extracts and Crataegus pinnatifida leaf) at different concentrations (0, 50, 100, 200, 400, and 800 μg/mL). HT048 was extracted in 30% ethanol at 90 °C for 4 h, filtered, and freeze-dried to produce a dark-yellow powder. HT048 extracts were analyzed using high-performance liquid chromatography. Rats were randomized to receive a chow diet, high-fat diet, high-fat diet with orlistat, and high-fat diet supplemented with HT048 (0.2%, 0.4%) for 12 weeks. | Reduced adipocyte differentiation, body and fat weight, and serum lipid levels; promoted glycerol release | β-oxidation genes: PPARα, CPT-1 ↑ Adipogenic genes: PPARγ, C/EBPα mRNA ↓ Lipogenic genes: SREBP1c, FAS mRNA ↓ | [69] |
| C2C12 mouse myocytes, C57BL/6 obese mice | Hesperidin | C2C12 cells were treated with C. unshiu at different concentrations (10, 50, and 100 μg/mL) and hesperidin (35 μg/mL) for 24 h. The dried C. unshiu peel was extracted in 4 L of 70% aqueous ethanol at 80 °C for 1 h. Mice were randomized to receive a normal diet (AIN-76A) for 10 weeks, high-fat diet, and high-fat diet (60% calories from fat) supplemented with C. unshiu extracts (75 mg/kg/day). | Reduced fat mass/body weight and average fat cell size | UCP3 ↑ | [70] |
| C57BL/6 obese mice | Narirutin | Mice were randomized to receive a high-fat diet (containing 60% kcal from fat), low-dose high-fat diet supplemented with C. unshiu extracts (125 mg/kg/bw), and high-dose high-fat diet supplemented with C. unshiu extracts (200 mg/kg/bw) once daily for 11 weeks. A total of 10 g of dried C. unshiu powder was first mixed with 100 μL of Celluclast enzyme and 1 L of distilled water, and then incubated for 24 h at 50 °C. | Inhibited adipogenesis, lipogenesis, and hepatic lipid accumulation; reduced serum lipid levels | Lipogenic genes/enzymes: SREBP1c, FAS ↓ Adipogenic genes/enzymes: PPARγ, C/EBPα ↓ β-oxidation genes/enzymes: AMPK, ACC ↑ | [71] |
| 3T3-L1 adipocyte cells, C57BL/6 obese mice | NA | 3T3-L1 cells were treated with Jeju roasted peel extract at concentrations range from 50 to 200 μg/mL. Mice were randomized to receive a normal diet, high-fat diet (containing 60% kcal from fat), and high-fat diet supplemented with Jeju roasted peel extract (25/50 mg/kg/bw). | Reduced lipid accumulation in adipocytes | Enzymes: ALT, ASAT, γ-GTP ↑ Lipogenic genes: SREBP1c, FAS ↓ Adipogenic genes: PPARγ, C/EBPα ↓ | [72] |
| Wistar rats | NA | Rats were first fed with a standard diet (Oriental yeast) for 10 weeks and injected with Streptozotocin (75 mg/kg) dissolved in a citrate buffer. Rats were then assigned to three groups: 0%, 1%, or 3% (wt/wt) C. unshiu-treated diabetic groups. | Inhibited hyperglycemia-induced liver dysfunction | Antioxidant enzymes: SOD, GSSG, GSH ↑ Liver enzymes: ALT, γ-GTP ↓ | [73] |
| Goto-Kakizaki rat model of type 2 diabetes | NA | Rats were fed with 1% and 3% (wt/wt) C. unshiu for 10 weeks. | Reduced glucose/non-fasting blood glucose levels; improved glucose tolerance | NA | [74] |
| C57BL/KsJ-db/db type 2 diabetic mice | Hesperidin, narirutin | Mice were supplemented with rosiglitazone (0.001 g/100 g diet) or C. unshiu peel ethanol extract (2 g/100 g diet) for 6 weeks. The extraction of 50 g dried C. unshiu peel was performed with 1 kg of 60% ethanol by incubation at 70 °C for 3 h. The extracts were then cooled, filtered, and freeze-dried at −40 °C. | Reduced blood glucose levels, body fat mass, body weight gain, plasma lipid levels, hypertriglyceridemia, and hepatic steatosis | Hepatic lipid regulating enzymes: FAS, ME, PAP, HMGR ↓ Hepatic inflammatory genes: adiponectin and IL-10 ↑; IL-6, TNF-α, IFN-γ, MCP-1↓ | [75] |
| Model Type | Bioactive Compounds | Extraction Techniques and Application in Treatment | Therapeutic Activities/Mechanisms | Target Genes/Signaling Pathways | Ref. |
|---|---|---|---|---|---|
| Human dermal fibroblasts | Hesperetin, naringenin | Dermal fibroblasts were treated with the aqueous extracts of C. unshiu peel at different concentrations (0.025–0.1%, w/v) for 24h in the submerged culture of Schizophyllum commune QG143 strain. C. unshiu peel (100 g) was extracted with distilled water (1 L) at 85 °C and then filtered and evaporated to produce a raw residue. High-performance liquid chromatography analysis was carried out to identify the extracts. | Increased the biosynthesis of collagen from fibroblast; decreased photoaging skin | MMP-1, SA-β-gal ↓ | [91] |
| Human dermal fibroblast neonatal cells | Heptamethoxyflavone | Cells were treated with dried C. unshiu peel ethanolic extracts at different concentrations (50, 100, 200, and 400 µg/mL) for 24h. The peel (20 g) was extracted with 50% ethanol (5 L) at room temperature for 7 days. The extracts were evaporated and mixed with hexane/water (1 L) to yield the hexane-soluble fraction. | Decreased photoaging skin; increased type I procollagen; inhibited collagenase activity | Type I procollagen protein, Smad3 ↑; MMP-1, Smad7, MAPK, ERK, JNK, c-Fos ↓ | [92] |
| HaCaT human keratinocyte cells, atopic dermatitis mouse model | NA | Cells were first induced with TNF-α and IFN-γ (10 ng/mL) in the presence or absence of premature C. unshiu ethanolic extracts at different concentrations (25, 50, 100, and 200 μg/mL) and then treated with 20 μL 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide for 4 h. The powder (37.95 kg) was extracted with 80% ethanol (5 L) at 60 °C for 4 h. The extracts were evaporated for 3 h, filtered, and freeze-dried for 60 h Mice first received a standard diet and ad libitum water and then assigned to four groups (induction, positive control, normal, and C. unshiu). Mice in the dinitrochlorobenzene (DNCB) group (induction) were sensitized by applying 100 μL of 1% DNCB in acetone and 100 μL of 0.5% DNCB to the dorsal skin for 36 days. Premature C. unshiu extracts were administrated every 2 days from day 16. Mice in the positive control group received HYDCORT cream (2 mg/g hydrocortisone valerate). | Anti-atopic, anti-inflammatory | TNF-α, IFN-γ, IL-4, TARC/CCL17, MDC/CCL22, STAT1 ↓ | [93] |
| HaCaT human keratinocyte cells | Quercetagetin | Cells were first stimulated with TNF-α and IFN-γ (10 ng/mL) for 24 h in the presence or absence of immature C. unshiu ethanolic extracts and other flavonoids at different concentrations (12.5, 25, and 50 μM) and then treated with 10 μL 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide for 4 h. High-performance liquid chromatography analysis was used to identify quercetagetin. | Anti-atopic; reduced inflammatory response | TNF-α, IFN-γ, IL-4, TARC/CCL17, MDC/CCL22, JAK, STAT1 ↓ | [50] |
| HaCaT human keratinocyte cells | Hesperetin, naringenin, quercetin | Cells were treated with fermented C. unshiu byproduct by 70% ethanol (25 °C for 24 h) at a concentration of 100 μg/mL, followed by induction with H2O2 at a dose of 500 μM. | Protected against oxidative stress | H2O2 ↓ | [94] |
| HaCaT human keratinocyte cells, RAW264.7 murine macrophages | Hesperidin, hesperetin, nobiletin, tangeretin, narirutin, narigenin, rutin | Cells were pretreated with the isolated Bacillus subtilis strains WE(1-4) and AL(2-1), which were added to dried C. unshiu peel ethanolic extracts at a concentration of 10% (v/v) for 96 h RAW264.7 macrophages were pretreated with WE(1-4) and AL(2-1) for 2 h and then stimulated with 1 μg/mL LPS for 22 h. | Reduced inflammatory response; moisturizing effect | Hyaluronic acid, SPT, filaggrin ↑; NO, iNOS, COX-2, TNF-α, IL-6, PGE2 ↓ | [95] |
| Swiss albino mice | Hesperidin | Mice were first sensitized by a topical administration of 0.1 mL picryl chloride solution (7%) in ethanol to the shaved abdomen. C. unshiu extracts or hesperidin were suspended in 0.2% carboxymethylcellulose sodium and administered orally at a dose of 0.2 mL/10 g from day 1 to 5 over 7 days. C. unshiu extracts or hesperidin in combination with prednisolone (0.1 mL/10 g) were then administrated for 7 days. | Inhibited ear swelling of contact dermatitis induced by picryl chloride | NA | [96] |
| Melana melanocytes, brown guinea pigs | Hesperidin, heptamethoxyflavone, β-cryptoxanthin | Cells were treated with C. unshiu ethanolic extracts (70%) at different concentrations (1, 5, 10, and 50 μg/mL) or vitamin C (50 μg/mL) for 72 h. Pigs were exposed to ultraviolet B radiation (380 mJ/cm2) three times per week over two weeks, and then randomized into groups to receive water (control group) and C. unshiu extracts (50 and 250 mg/kg). | Inhibited melanin synthesis; reduced oxidative stress and skin pigmentation | Tyrosinase enzyme, ROS ↓ | [97] |
| Hos:HR-1 hairless mice | Hesperidin, narirutin | Mice were given access to a laboratory diet and water, and then assigned to ultraviolet non-irradiated/irradiated control mice and C. unshiu groups (200 mg/kg for seven weeks). | Improved photoaging skin | NA | [98] |
| Pharmacological Activities | Bioactive Compounds |
|---|---|
 | Hesperidin, hesperetin, nobiletin, naringin, naringenin, narirutin, β-cryptoxanthin |
 | Hesperidin, hesperetin, naringenin, narirutin, β-cryptoxanthin |
 | Hesperidin, narirutin |
 | Hesperidin, dietary fiber |
 | Hesperidin, dietary fiber |
 | Hesperidin, hesperetin, naringenin, narirutin, dietary fiber, pectin |
 | Hesperidin, narirutin, nobiletin, heptamethoxyflavone, tangeretin |
 | Hesperidin, hesperetin, nobiletin, naringin, naringenin, narirutin, quercetin, quercetagetin, rutin, heptamethoxyflavone, tangeretin, β-cryptoxanthin |
 | Hesperidin, narirutin |
 | Hesperetin, nobiletin |
 | Hesperidin, nobiletin, naringin, narirutin, quercetin, quercetagetin, rutin, tangeretin, β-cryptoxanthin |
 | Hesperetin, naringin |
 | Hesperidin, nobiletin, tangeretin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsharairi, N.A. A Review on Bioactive Compounds and Pharmacological Activities of Citrus unshiu. Appl. Sci. 2025, 15, 4475. https://doi.org/10.3390/app15084475
Alsharairi NA. A Review on Bioactive Compounds and Pharmacological Activities of Citrus unshiu. Applied Sciences. 2025; 15(8):4475. https://doi.org/10.3390/app15084475
Chicago/Turabian StyleAlsharairi, Naser A. 2025. "A Review on Bioactive Compounds and Pharmacological Activities of Citrus unshiu" Applied Sciences 15, no. 8: 4475. https://doi.org/10.3390/app15084475
APA StyleAlsharairi, N. A. (2025). A Review on Bioactive Compounds and Pharmacological Activities of Citrus unshiu. Applied Sciences, 15(8), 4475. https://doi.org/10.3390/app15084475








