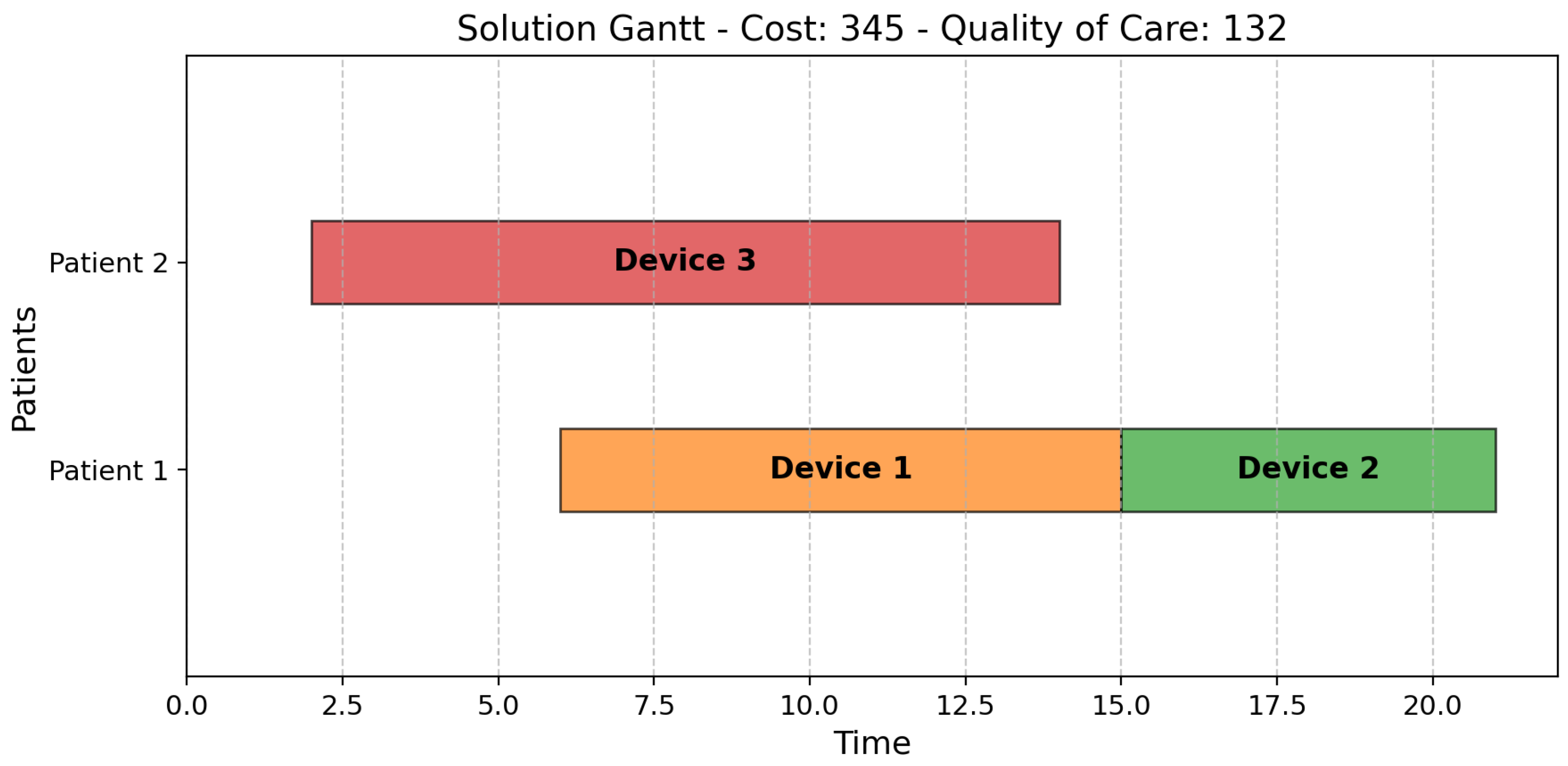
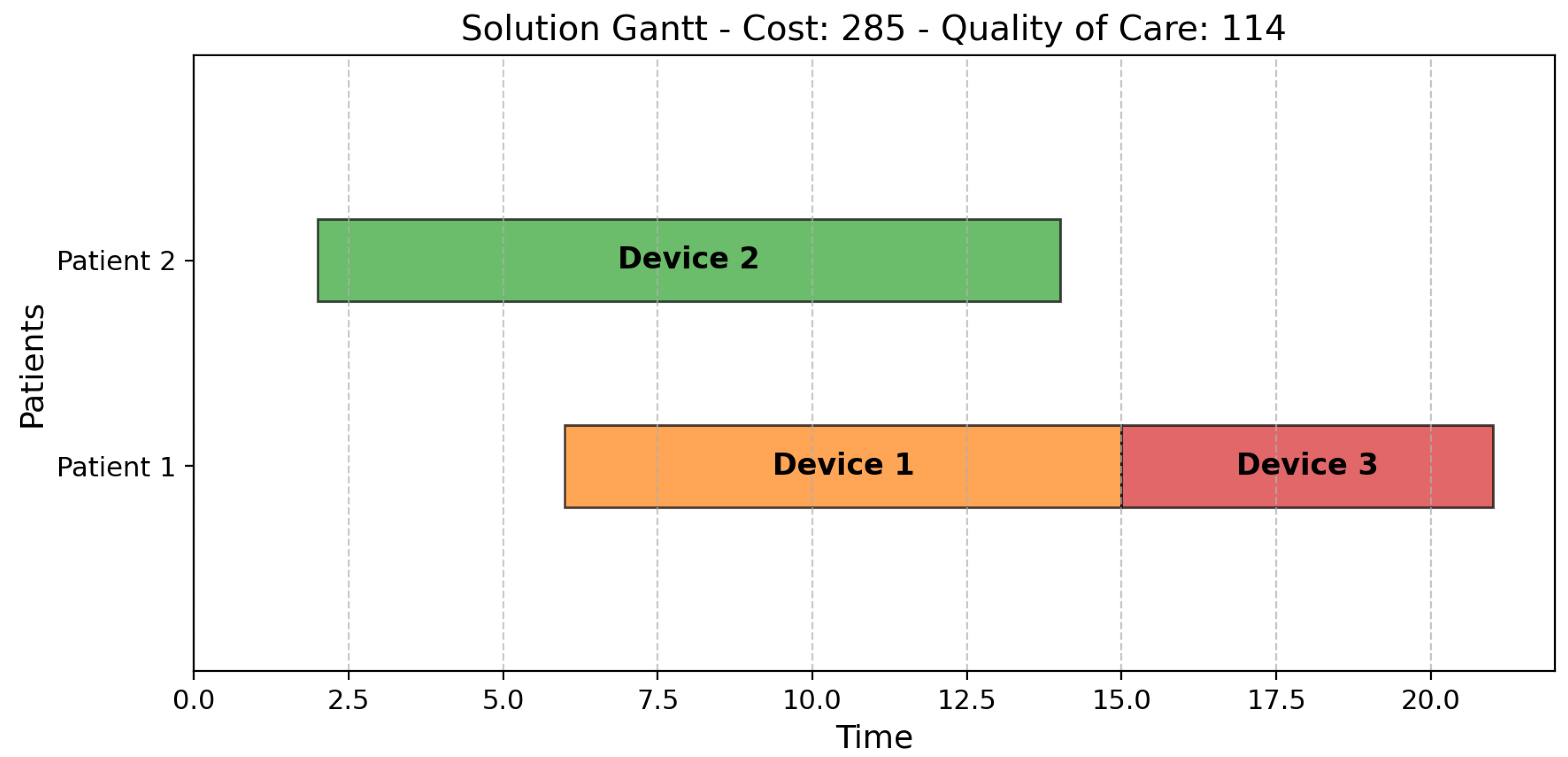
1. Introduction
The rapid advancement of Information Technology (IT) has fundamentally transformed healthcare, particularly in patient monitoring within hospital settings. As hospitals and medical facilities increasingly adopt digital solutions, the ability to continuously track patients’ vital signs, predict potential complications, and optimize healthcare operations has improved significantly. Advanced IT solutions, including cloud computing, IoT, AI, and edge computing, enable real-time data collection, remote monitoring, and predictive analytics, enhancing both patient outcomes and hospital efficiency [
1]. The incorporation of AI-driven decision support systems to enhance patient care is studied in [
2,
3].
IT-based monitoring solutions bring clear benefits to healthcare. They boost workflow efficiency, improve early disease detection, and reduce human error. One key use is indoor positioning systems, which track patients and equipment in real time. These rely on wireless networks and cloud computing to support hospital navigation and emergency response [
4,
5]. Wearable IoT devices and sensor-based systems also allow continuous monitoring of vital signs. This supports personalized care and cuts down on manual checks [
6,
7]. A review in [
8] explores Industry 4.0 in healthcare, while [
9] focuses on IoT-based medical devices. As noted in [
10], the Healthcare IoT (H-IoT) enables real-time monitoring and quicker interventions, improving patient outcomes. It also simplifies data collection and analysis, making healthcare more efficient. According to [
11], IoT helps reduce hospital readmissions and supports remote care. Finally, AI-driven predictive analytics is transforming decision-making. It detects early warning signs and suggests timely actions [
12,
13]. These advancements have not only improved patient care but also reduced operational costs for healthcare providers.
Despite growing adoption of IoT and AI in healthcare, most existing scheduling solutions focus on general aspects like task timing, system responsiveness, or network performance. For instance, optimized scheduling models have been proposed to reduce delays and task failure rates [
14], while others introduced decision support systems to prioritize urgent care when resources are limited [
15]. Some approaches integrate deep learning or predictive analytics to improve sensing accuracy and tailor device usage based on patient needs [
16,
17]. However, these models often overlook the specific challenge of assigning individual IoT devices to patients in a way that balances cost with quality of care.
Studies from different regions highlight critical contextual considerations. For example, research in Japan shows that elderly users benefit from voice-based IoT interfaces due to ease of use [
18], while work in India emphasizes the need for scalable, cost-effective solutions in resource-limited healthcare environments [
19]. European reviews also stress the importance of adapting device allocation strategies to diverse care models and patient needs [
20].
Although these contributions have advanced the field, none offer a comprehensive framework that integrates operational cost, device characteristics, and patient well-being. This underscores the need for a new approach—one that formulates device–patient allocation as a multi-objective scheduling problem, explicitly balancing economic efficiency with high-quality, personalized care.
How can IoT medical devices be optimally allocated to patients in healthcare settings while simultaneously minimizing operational costs and maximizing quality of care? Specifically, we investigated whether a bi-objective scheduling approach can effectively balance these competing objectives while accounting for real-world constraints such as device compatibility, setup times, availability windows, and battery limitations.
This study aims to develop a comprehensive optimization framework for the allocation of IoT medical devices to patients that explicitly addresses the trade-off between cost efficiency and care quality. Our specific objectives are as follows:
Formulate the IoT device allocation problem as a bi-objective scheduling model that captures essential real-world constraints.
Design and implement an efficient heuristic solution approach based on NSGA-II that generates high-quality Pareto-optimal solutions within practical time constraints.
Evaluate the proposed approach against both existing methods and manual scheduling practices to demonstrate its practical value in healthcare settings.
This paper proposes a novel bi-objective optimization model that explicitly addresses the trade-off between operational costs and quality of care in IoT device scheduling. We develop a tailored heuristic solution based on the NSGA-II algorithm, enhanced with domain-specific constraints such as device compatibility, setup durations, availability windows, and battery lifespan.
Experimental results demonstrate the effectiveness of our approach. Compared to baseline heuristics and manual scheduling, our method achieves up to a 12% reduction in operational costs (with an average of 6.3%) and care quality improvements of up to 20% (with an average of 10%) based on weighted patient-priority metrics. The system rapidly produces diverse high-quality solutions within just a few seconds, and within less than a minute, it reliably approximates an accurate Pareto front—making it well suited for real-time decision support. These findings significantly advance the field by demonstrating that intelligent, constraint-aware scheduling can simultaneously optimize for clinical and economic outcomes in dynamic healthcare environments.
To address the challenge of allocating IoT medical devices in healthcare, we formulated the problem as a bi-objective scheduling model. This allowed us to represent key real-world elements—such as patients, devices, time windows, and care priorities—within a structured optimization framework, aligning them with classic scheduling constructs like jobs, machines, and processing times.
The model incorporates essential operational constraints, including device–patient compatibility, setup times, availability windows, continuous monitoring requirements, and battery limitations. These constraints ensure the formulation realistically captures the complexities of hospital environments. By modeling the trade-offs between cost and quality of care, the framework generates a Pareto front of optimal solutions. This empowers healthcare decision-makers with flexible, data-driven options that support efficient resource use and improved patient outcomes.
The proposed IoT device allocation model, which balances cost and quality of care, falls within the class of computationally intensive multi-objective scheduling problems. Hard constraints—such as time windows, device compatibility, battery limits, and setup times—further increase its complexity. As even single-objective variants are NP-hard, solving the bi-objective version requires navigating a large, non-convex solution space. Due to the limitations of exact methods for large instances, heuristic and metaheuristic approaches like evolutionary algorithms are preferred for efficiently approximating the Pareto front under realistic time constraints.
To address the complexity of allocating IoT medical devices in healthcare, this study presents a heuristic approach based on the NSGA-II algorithm, tailored for bi-objective scheduling. The method uses genetic representations to explore large solution spaces, optimizing for both operational cost and care quality. NSGA-II’s evolutionary operators—crossover, mutation, and elitism—refine solutions to generate a diverse Pareto front, giving decision-makers flexible, high-quality options.
Unlike single-objective or rigid heuristic methods, our approach dynamically adjusts NSGA-II parameters and integrates real-world constraints like device compatibility and battery limits. Its multi-run, time-bounded design ensures practicality in time-sensitive hospital environments. This enables scalable, interpretable scheduling with better resource use, improved patient–device matching, and transparent trade-off evaluation.
This paper makes several key contributions to the field of healthcare resource optimization through the design, formulation, and implementation of a bi-objective scheduling model for IoT device allocation. First, it introduces a novel optimization framework that explicitly balances operational costs with quality of care—two often conflicting objectives rarely addressed together in prior work. By modeling real-world constraints such as patient priority, device compatibility, setup times, and availability windows, the proposed formulation reflects the complexity of clinical environments more accurately than existing approaches. Second, the paper develops and tailors a heuristic algorithm based on NSGA-II, capable of generating a diverse set of high-quality solutions under realistic time constraints, which is essential given the NP-hard nature of the problem. Third, the method is designed to support real-time, adaptive re-optimization, allowing healthcare facilities to respond dynamically to changes such as new admissions or evolving patient conditions.
From a practical standpoint, the system has broad applicability in modernizing healthcare facility operations, offering a scalable and interpretable decision support tool that enhances efficiency and patient outcomes. It can be deployed across various healthcare settings—from small outpatient facilities to large multi-ward hospitals—where it automates complex planning tasks, reduces human error, and improves responsiveness to clinical needs.
By integrating intelligent scheduling into real-world medical center workflows, this work extends current scientific understanding of IoT-enabled healthcare optimization and bridges the gap between theoretical modeling and operational decision-making in digital healthcare environments.
The topic of this paper strongly aligns with the scope and objectives of ‘‘Applied Sciences” and its Special Issue ‘‘The Internet of Things (IoT) and Its Application in Monitoring”. By addressing a real-world challenge at the nexus of IoT deployment, healthcare optimization, and intelligent scheduling, our proposed framework directly contributes to the advancement of practical, data-driven solutions for patient monitoring. This work not only highlights the increasing importance of IoT in enhancing clinical operations but also introduces methodological innovations that support the journal’s mission of connecting applied research with tangible technological impact.
2. Background
Healthcare IoT software management is vital for hiding complexity and enabling easy deployment [
21]. Smart resource allocation is equally crucial for cost control and performance amid fluctuating device demand. Poor allocation can harm care during peaks and waste resources during lulls. Effective resource management ensures efficient and cost-effective device use. Proper system design is key for successful IoT adoption and better healthcare service [
22]. Strong resource allocation is also essential for reliable remote monitoring [
23]. Management must balance scalability and cost within financial and performance limits. IoT’s healthcare benefits come with rising operating costs [
24], making efficient resource use critical. Device variety in cost and comfort impacts patient acceptance, requiring scheduling to balance cost, comfort, and clinical needs.
Recent studies address IoT scheduling in healthcare, especially for home visits and monitoring. The authors of [
14] presented a smart PHMS with optimized scheduling, reducing task starvation (14%) and failure (17%). A review in [
25] analyzed real-time IoT healthcare scheduling, comparing approaches and outlining future directions. The authors of [
26] proposed Prioritized Scheduling (PS) for delay-sensitive tasks, outperforming EDF variations. The study in [
15] presented a decision support system to allocate limited devices, prioritizing critical cases. The architecture described in [
16] combines IoT with deep learning to improve sensing accuracy and personalize resource allocation based on patient needs. Finally, the authors of [
17] integrated machine learning with IoT to predict demand, enhancing care and operations.
To present a well-rounded view of the global research landscape, it is essential to highlight key contributions from international research centers, especially those in Europe and Asia. For instance, a study by [
18] in Japan evaluated various user interfaces for IoT devices among senior citizens, finding that voice interfaces were more suitable for elderly users due to shorter setting times and higher usability scores. In India, [
19] discussed the impact of IoT in healthcare, emphasizing the challenges and opportunities in adopting digital healthcare solutions in resource-constrained environments. Additionally, the authors of [
20] reviewed IoT-based ambient assisted living solutions for the growing elderly population and their care needs, categorizing them by health focus, IoT technologies, aims, and experimental evaluation. The work in [
27] performed heart disease prediction using an IoT-based framework and an improved deep learning approach, applied to the Hungarian dataset.
While this body of work demonstrates significant advancements in IoT-based healthcare scheduling, most studies focus on general resource use, network performance, or task timing. Crucially, none directly address the problem of assigning specific IoT devices to patients in a way that balances operational cost with quality of care. This gap in the literature highlights the need for novel approaches that integrate clinical priorities, device characteristics, and patient comfort. Even recent international studies, while innovative in their respective domains, do not provide comprehensive frameworks for patient-specific device allocation that optimizes both economic and clinical outcomes.
The systematic analysis of this expanded literature reveals several methodological patterns crucial to our research design. First, successful implementations consistently incorporate multi-objective optimization techniques that balance technical efficiency with patient-centered outcomes. Second, regional variations in healthcare delivery models significantly impact device allocation strategies, necessitating adaptable frameworks. This methodological synthesis directly informs our research approach by establishing the assessment metrics, constraint parameters, and evaluation frameworks employed in our study. Our proposed multi-objective optimization framework for IoT device allocation therefore builds upon a comprehensive understanding of global research trends. Unlike prior work, it explicitly considers both financial constraints and the delivery of high-quality care through appropriate device assignment, addressing the identified gaps in current approaches.
3. Materials and Methods
3.1. Research Problem
In the context of our study, a healthcare facility accommodates patients for defined periods, and IoT devices must be allocated to patients throughout their stay to optimize both operational costs and quality of care.
Each patient requires a specific class of IoT device tailored to monitor particular parameters. The allocation of these devices incurs costs related to their operational expenses, which vary depending on the type of device used. In addition, the devices’ quality and characteristics—such as weight, size, and battery life—directly influence patient quality of care. These factors affect the ease with which patients can wear and use the devices, thereby impacting their overall experience. Consequently, it is essential—particularly when considering the patient’s priority level—to utilize devices that maximize the patient experience. However, it is important to recognize that cost minimization and quality of care are often competing objectives.
The overarching goal is to effectively schedule the allocation of available IoT devices to patients over time in a manner that minimizes operational costs while simultaneously maximizing patient well-being.
The mathematical formulation of the scheduling problem is described as follows.
A list of patients is provided, with each patient assigned a priority level by healthcare professionals based on medical assessments, urgency, and institutional protocols. Additionally, each patient specifies their stay duration in the facility, including start and end times, as well as the required device class.
A set of IoT devices is available, each characterized by a specific quality level that impacts the comfort and well-being of the wearer. Each device is also associated with an hourly operational cost, a limited operational lifespan (battery duration), and a predefined availability window (start and end times) during which it can be utilized. Additionally, the device’s class parameter specifies the subset of patients for whom it is suitable, while the setup time parameter represents the time required to transfer the device from one patient to another.
The constraints are of various types. First, the device–patient allocation is valid only if it falls within the availability window of both the device and the patient. Second, the allocation is valid if the device class matches the patient class. If the device is reassigned to another patient, a setup activity must occur between the two allocations. Within the time window that identifies the patient’s stay in the facility, the patient must always be assigned to a device. Therefore, if a device needs to be replaced because it has reached its operational lifespan, a new device must be immediately allocated to the patient. Finally, the total periods during which a device is allocated to multiple patients must not exceed the device’s operational lifespan.
3.2. Research Model
Our approach transforms the scheduling challenge into a bi-objective optimization problem, a methodology that simultaneously addresses two potentially competing goals. In bi-objective optimization, improving one performance metric can necessitate a trade-off in another, which is particularly characteristic of complex systems in manufacturing, logistics, and project management.
In our context, efficiency is quantified by minimizing assignment costs, while quality of care is defined by the level of benefit patients receive from the assigned devices. Specifically, higher quality of care is achieved by allocating patients to more advanced devices that offer enhanced features directly impacting their experience and well-being. For instance, lighter and more compact models improve patient comfort and ease of use, thus contributing to a higher quality of care. The system prioritizes allocating the most suitable devices, especially those with superior features, to high-priority patients whenever feasible. This dual-objective optimization framework mirrors the complex realities of medical device scheduling, where financial limitations must be carefully balanced with the imperative to maximize positive clinical outcomes and patient experience.
Mathematically formulating the two-objective scheduling problem requires a precise definition of variables, constraints, and objective functions, which are comprehensively detailed in the accompanying parameters in
Table 1. Variables are listed in
Table 2.
Constraints are a core component of our optimization model, as they determine which scheduling solutions are feasible. They ensure compliance with device availability windows (Equations (
1) and (2)) and patient availability windows (Equations (3) and (4)), enforce device–patient class compatibility (Equation (5)), incorporate setup times between assignments (Equation (6)), maintain continuous device coverage throughout a patient’s stay (Equation (7)), and respect device lifespan constraints (Equation (8)).
It is important to highlight that constraints enforce distinct availability requirements for devices and patients. Specifically, device–patient allocations must fall within the device’s availability window (Equations (
1) and (2)), while each patient must be continuously assigned to a device throughout their entire availability window (Equations (3), (4) and (7)). This distinction is critical: devices may remain idle during their availability, but patients require uninterrupted device coverage.
Regarding the bi-objective function, the two key performance indicators (KPIs) are the total cost and the overall quality of care .
Equation (
9) calculates
, which represents the total operational cost of device usage across all patients. For each device
i, the formula sums the durations of all assignments
across all patients
j to which the device was assigned (set
). Each device’s total usage time is then multiplied by its hourly cost
. The final value
is obtained by summing these cost calculations across all devices in the system. In summary,
provides a comprehensive measure of the economic cost incurred from device utilization throughout the scheduling period, accounting for both assignment durations and device-specific operational costs.
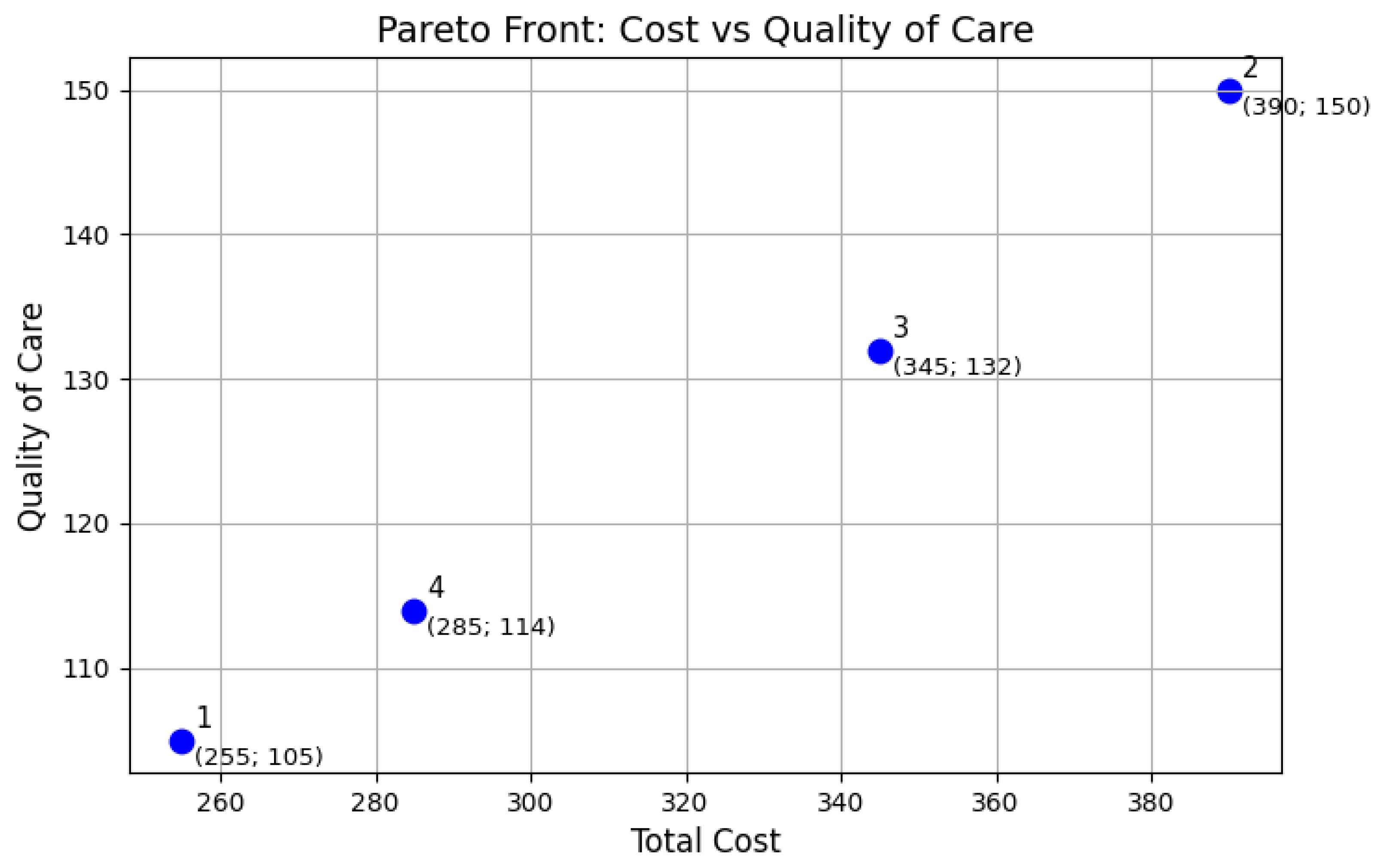
Equation (10) calculates , which represents a weighted measure of the quality of service provided to patients. For each patient j, the algorithm computes the sum of quality-weighted assignment durations across all devices i assigned to that patient. This sum is then normalized by the patient’s total stay duration and weighted by the patient’s priority . The final value is obtained by summing these weighted quality measures across all patients. Notably, if a patient j is assigned only a single device i during their stay, the summation naturally reduces to a single term, and the normalized, weighted quality for that patient’s assignment simplifies to . In essence, serves as a comprehensive service quality metric, integrating factors such as assignment durations, the inherent quality of the assigned devices, and the clinical priority of the patients. A higher value of signifies an improved overall service quality, reflecting a greater emphasis on accommodating the needs of higher-priority patients and the allocation of devices with superior features and functionalities.
This metric is designed to capture the multifaceted nature of service quality in our context, ensuring that both the efficiency of the assignment and the tangible benefits experienced by patients are appropriately valued.
3.3. Solution Method
The bi-objective nature of the proposed IoT device allocation model—balancing operational cost and quality of care—places it within the class of multi-objective scheduling problems, which are well known for their computational complexity. The inclusion of hard constraints such as time windows, device compatibility, battery limitations, and setup times further increases the problem’s difficulty. Even in single-objective forms, many variants of scheduling problems are classified as NP-hard. Extending these to a bi-objective framework significantly compounds the computational burden, as it requires exploring a large, non-convex solution space to identify a diverse set of Pareto-optimal solutions.
Given this complexity, exact methods (e.g., integer programming or branch-and-bound) often become infeasible for realistic problem sizes due to exponential runtime. As a result, heuristic and metaheuristic approaches, such as evolutionary algorithms, are commonly adopted to provide high-quality solutions within acceptable time frames. These methods are especially suited for multi-objective problems, as they can efficiently approximate the Pareto front while navigating complex constraint landscapes.
In [
28], a Mixed Integer Linear Programming (MILP) model was proposed to address critical scheduling challenges, including staff assignments, patient distribution, resource allocation, and overtime management. The primary objective was to control healthcare costs while enhancing the quality of patient care.
In [
29], the Genetic Algorithm (GA), a metaheuristic inspired by natural selection, is applied to various healthcare scheduling problems, including patient admission scheduling, nurse scheduling, operating room scheduling, surgery scheduling, and other related tasks.
In [
30], the authors investigate the application of biomimicry concepts in cancer treatment to improve appointment scheduling and therapeutic outcomes. Their approach leverages automation through three nature-inspired algorithms: Wolf Optimization, Firefly Optimization, and Genetic Algorithm.
Simulated Annealing (SA), a probabilistic technique for approximating the global optimum of a function, is introduced in [
29]. This method is particularly effective for evaluating a broad set of potential schedules, iteratively refining them to achieve an optimal or near-optimal solution.
Multi-Objective Particle Swarm Optimization (MOPSO) enhances the Particle Swarm Optimization (PSO) algorithm to handle multiple objectives [
31]. It maintains a repository of non-dominated solutions and employs a leader selection mechanism to guide the swarm toward optimal outcomes. MOPSO is particularly effective in exploring the solution space and identifying a set of Pareto-optimal schedules.
Pareto Simulated Annealing (PSA), an adaptation of SA for multi-objective optimization, applies a Pareto dominance criterion to accept or reject solutions, as discussed in [
32]. PSA is valuable for balancing competing objectives by evaluating different scheduling configurations.
Non-dominated Sorting Genetic Algorithm II (NSGA-II) is a widely adopted approach for multi-objective optimization, designed to identify a diverse set of optimal solutions. It is particularly useful for balancing conflicting objectives, such as minimizing travel time and reducing patient visit delays, while generating Pareto-optimal schedules. In [
31], NSGA-II is applied to scheduling problems through a simulation-based approach.
To address the inherent complexity of allocating IoT medical devices in healthcare environments, this study introduces a heuristic optimization approach based on the NSGA-II algorithm, tailored to solve bi-objective scheduling problems. At its core, the method leverages genetic representations of patient–device assignments, enabling the intelligent exploration of vast solution spaces that traditional methods struggle to handle. Each candidate solution encodes device assignments across time windows, with objectives that aim to minimize operational costs while maximizing the quality of care based on patient priorities and device capabilities. Through evolutionary mechanisms—such as crossover, mutation, and elitism—NSGA-II continuously refines the population of solutions, ultimately producing a Pareto front that captures a range of trade-offs. This allows decision-makers to choose from multiple high-quality solutions rather than being forced to accept a single, potentially suboptimal result.
Unlike existing methods that often optimize a single objective or rely on rigid heuristics, this approach offers several key innovations. By dynamically adjusting NSGA-II parameters across multiple runs and incorporating domain-specific constraints—such as device compatibility, setup time, and battery limits—the algorithm adapts to the real-world variability of clinical environments. Its multi-run design and time-bound execution also make it highly practical for deployment in resource-constrained healthcare settings, where timely decisions are crucial. In doing so, the approach overcomes the limitations of static or overly simplified models, offering a flexible, scalable, and interpretable solution framework. The anticipated benefits include more efficient use of healthcare facility resources, improved patient outcomes through better device matching, and greater transparency in the decision-making process through access to a spectrum of optimal solutions.
Algorithm 1 outlines the optimization process for assigning IoT devices to patients using the NSGA-II algorithm. The procedure begins by receiving input lists of patients and devices, along with a time constraint (line 1). The goal is to produce an optimized set of device–patient assignments and a final Pareto-optimal front (line 2). The algorithm first defines the attributes of both patients and devices (line 3) and introduces an assignment class to manage their pairings (line 4). A multi-objective fitness function is then defined (lines 5–8), which aims to minimize the overall cost (
), maximize quality of care (
)—based on patient priority and device quality—and respect time window constraints. The genetic representation of a solution is set up next (lines 9–11): each chromosome is a list of assignment objects, where each gene includes the device ID, patient ID, and corresponding assignment time window. Storage for Pareto-optimal solutions is initialized (line 12), and a timer is started (line 13). The main loop runs until the specified time limit is reached (line 14). Within this loop, NSGA-II parameters—
,
,
,
,
,
, and
—are randomly generated as defined in Equations (
11)–(17) (line 15), and the algorithm (as detailed in Algorithm 2) is executed using these parameters (line 16). The resulting Pareto front and logbook from each execution are stored (line 17). Once the time limit expires, the algorithm returns the accumulated set of Pareto-optimal solutions and the complete logbook of all evolutionary runs (line 19).
| Algorithm 1 Optimization of IoT device assignment using NSGA-II. |
- 1:
Input: List of patients, List of devices, Time limit - 2:
Output: List of optimized assignments of devices to patients and Pareto optimal set - 3:
Define the patient and device classes with attributes - 4:
Define an Assignment class to store device-patient assignments - 5:
Define the fitness function: - 6:
Minimize total cost of device usage - 7:
Maximize quality of care , based on patient priority and device quality - 8:
Respect time window constraints - 9:
Define the genetic representation of a solution: - 10:
A chromosome is a list of device-patient assignments - 11:
Each gene represents an assignment (device ID, patient ID, start time, end time) - 12:
Initialize storage for Pareto optimal solutions - 13:
Initialize the timer - 14:
while
do - 15:
Randomly generate the NSGA-II parameters , , , , , , and according to Equations ( 11)–(17). - 16:
Run Execution of NSGA-II with the generated parameters as detailed in Algorithm 2 - 17:
Store the Pareto front and logbook obtained from the execution - 18:
end while - 19:
Return the final Pareto optimal set and logbook containing all evolutionary results
|
Algorithm 2 details the practical implementation of the NSGA-II optimization, including specific parameters such as population size and number of generations, and more. It methodically walks through the evolutionary process, highlighting how NSGA-II applies tournament selection with Pareto dominance ranking to effectively navigate the multi-objective solution space. The genetic operators—crossover, mutation, and elitism—work in concert to explore diverse assignment possibilities while preserving high-quality solutions.
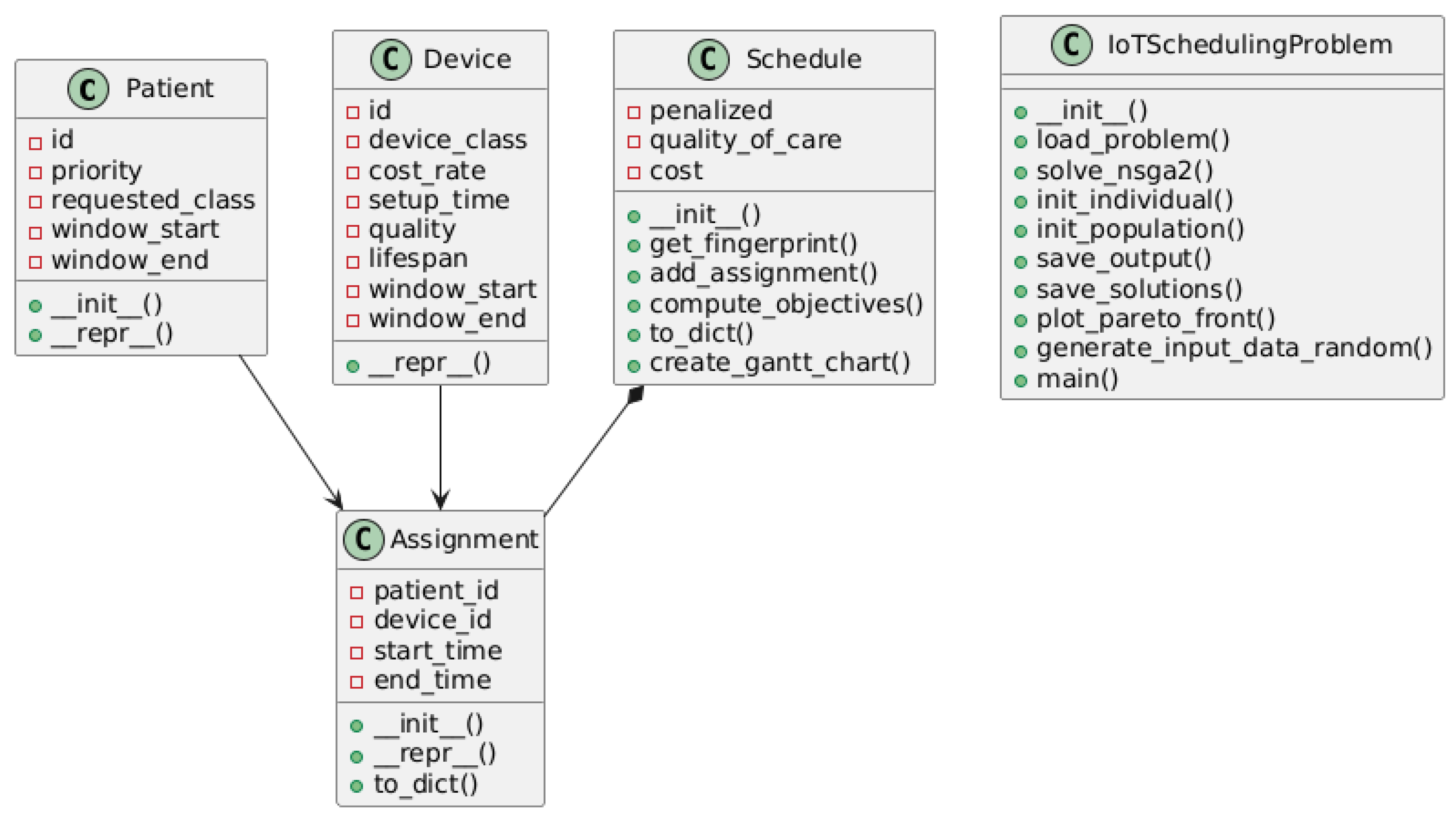
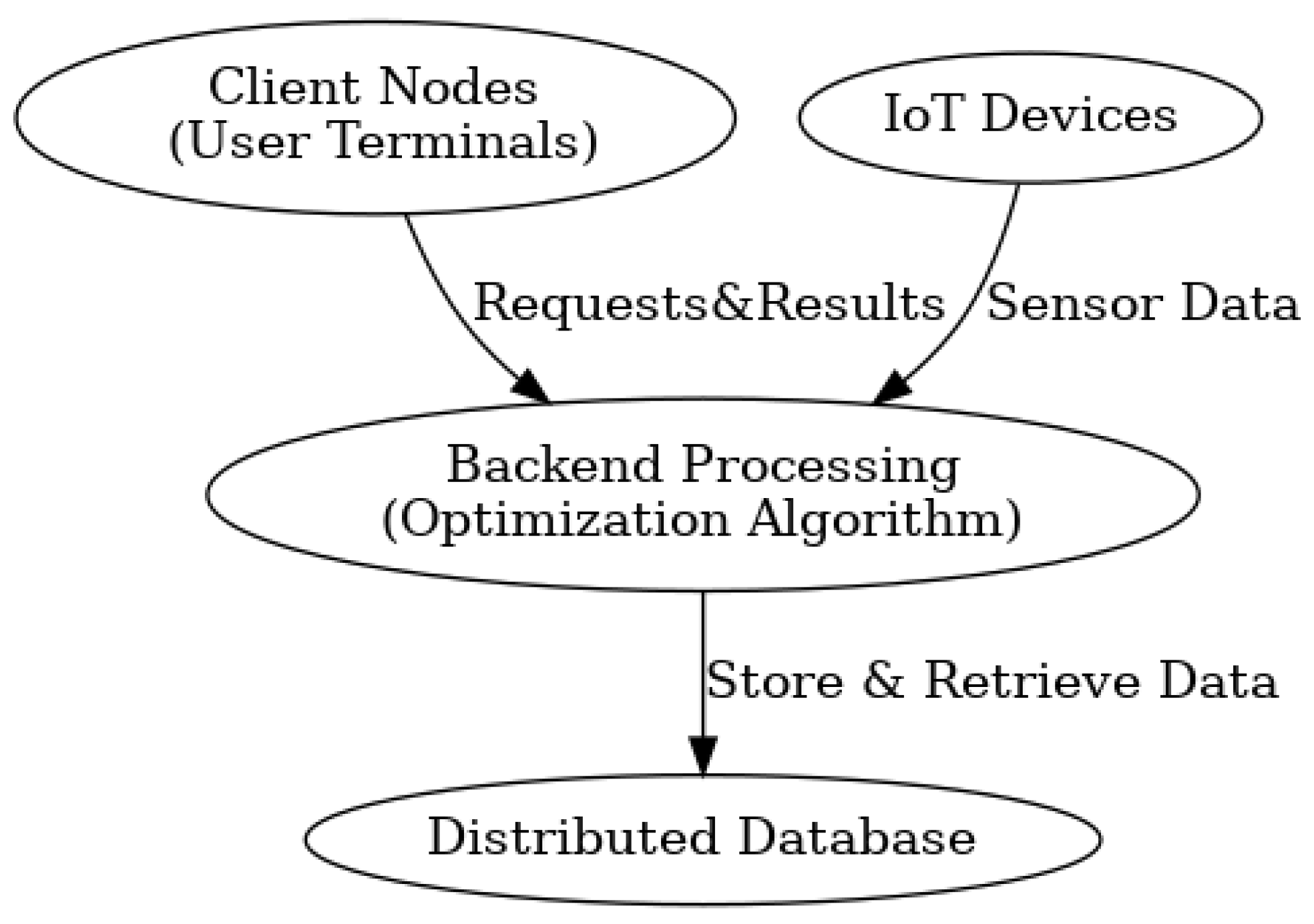
This implementation demonstrates how evolutionary algorithms can tackle complex healthcare resource allocation problems by mimicking natural selection processes to iteratively improve assignments across multiple competing objectives. For further details regarding the developed code, refer to
Appendix A. For a comprehensive description of the system architecture and workflow, consult
Appendix B.
A systematic benchmark analysis was conducted, comparing our proposed NSGA-II-based heuristic algorithm against the exact solver SCIP in terms of solution quality, computational efficiency (runtime), and scalability (performance on increasing problem sizes).
SCIP was chosen as a benchmark for comparison against the metaheuristic algorithm due to its capability to find high-quality, and ideally provably optimal, solutions for mixed integer programming problems [
33]. Unlike metaheuristics, which provide approximate solutions, SCIP strives to find the absolute best possible outcome within the given time constraints. However, it also allows a maximum time limit to be set, enabling the retrieval of a high-quality solution even if its optimality is not fully proven.
| Algorithm 2 Execution of NSGA-II. |
- 1:
Input: Population size , Number of generations , Crossover probability , Mutation probability , Crossover operator , Mutation operator , Selection method - 2:
Output: Optimized solutions and logbook of evolutionary process - 3:
Step 1: Define Genetic Algorithm Components - 4:
Register the individual structure: List of assignments - 5:
Register the fitness function: Multi-objective (min cost , max quality of care ) - 6:
Register genetic operators: , , - 7:
Set NSGA-II parameters: , , , - 8:
Step 2: Initialize the Population - 9:
Create an initial population of individuals - 10:
Each individual is randomly generated with assignments - 11:
Step 3: Evolutionary Process - 12:
for generation = 1 to do - 13:
Evaluate the fitness of each individual in the population - 14:
Apply NSGA-II selection using : - 15:
Apply Crossover with probability using : - 16:
Apply Mutation with probability using : - 17:
Randomly modify some assignments (change device or time slot) - 18:
Apply Elitism: - 19:
end for - 20:
Return the final population and logbook containing evolutionary data
|
Solving a multi-objective problem with a primarily single-objective MILP solver like SCIP presents challenges due to the need to handle multiple conflicting objectives simultaneously. While SCIP is designed for single-objective optimization, various techniques can be employed to adapt it for multi-objective problems. One effective approach is the
-constraint method, a well-established technique in multi-objective optimization, as described in [
34]. This method transforms a multi-objective problem into a series of single-objective optimizations by iteratively reformulating the problem: one objective is selected as the primary function to minimize, while the remaining objectives are converted into constraints with dynamically adjusted bounds. This allows the solver to systematically explore the trade-offs between competing objectives and generate a set of Pareto-optimal solutions. We refer to the method of employing the SCIP solver to solve a bi-objective optimization problem using the
-constraint method as ‘SCIP 2-OBJ’.
5. Discussion
The experimental results offer significant insights into the comparative performance of the NSGA-II heuristic and the SCIP 2-OBJ exact approach for multi-objective healthcare resource allocation. These findings contribute to our understanding of algorithmic performance in complex decision-making and provide practical guidance for real-world applications.
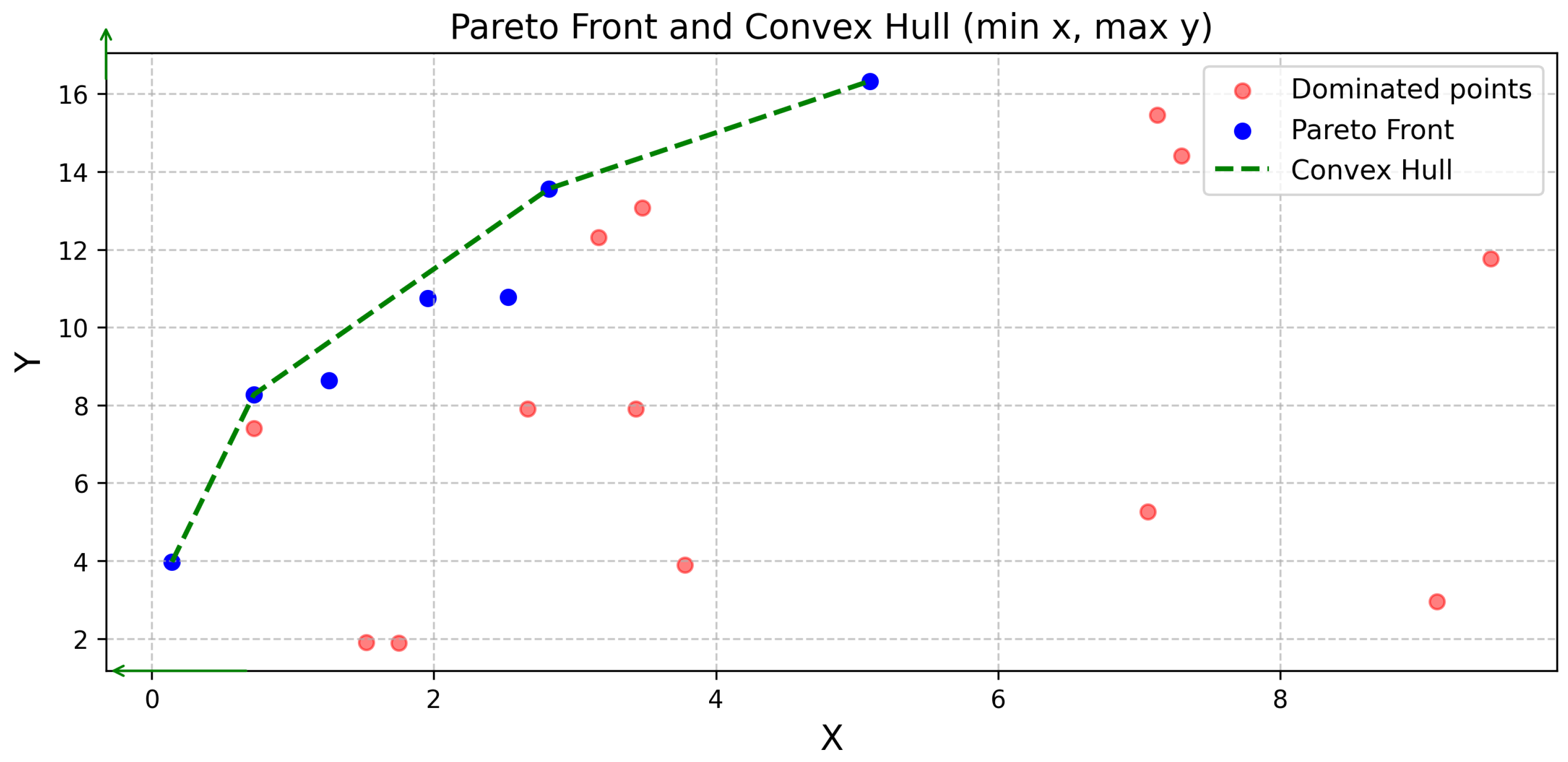
Consistently, NSGA-II outperformed SCIP 2-OBJ regarding solution quality, as indicated by superior hypervolume and convex hull representation, particularly in moderate complexity scenarios (experiments 1–3). This aligns with previous research highlighting NSGA-II’s efficacy in navigating complex Pareto fronts [
31].
However, in highly complex scenarios (experiments 4–6), the exact method showed improved relative performance, especially in solution quality, supporting the theoretical notion that exact methods may excel in certain complex domains [
35].
A key observation was the distinct convergence patterns. NSGA-II demonstrated rapid initial convergence, ideal for time-sensitive healthcare applications, as emphasized in previous studies [
36]. Conversely, the exact method exhibited gradual improvement with increased computational resources, suggesting its suitability for offline analysis, consistent with observed time–performance trade-offs [
37].
Scalability experiments (experiments 4–6) revealed that NSGA-II maintained its ability to find high-quality solutions with increasing complexity, though with some relative performance degradation, extending previous scalability studies [
38]. Interestingly, the exact method’s sampling improved in larger solution spaces, suggesting that the
-constraint approach benefits from increased solution diversity, mitigating time constraints.
The dominance of NSGA-II solutions in the convex hull underscored the importance of comprehensive evaluation metrics. Combining hypervolume analysis with convex hull representation, building on previous hypervolume indicator work [
39], provided a more robust evaluation framework.
Practically, NSGA-II’s rapid high-quality solution generation makes it ideal for time-critical healthcare resource allocation. Its ability to balance competing objectives suits dynamic healthcare environments.
The proposed system has several practical implications for real-world healthcare operations. First, it streamlines the patient monitoring workflow by automating the assignment of IoT devices, which reduces reliance on manual planning and minimizes the risk of human error. This leads to more accurate and efficient resource use, especially in high-demand or time-sensitive scenarios. Second, the dynamic re-optimization feature allows the system to adapt in real time to changes such as new patient admissions or shifts in clinical conditions. This ensures that device allocation remains optimal even in unpredictable or rapidly evolving environments, supporting better responsiveness in patient care. Finally, the scalable architecture makes the solution suitable for a wide range of healthcare settings—from small medical centers to large hospitals—offering long-term benefits in terms of operational efficiency, cost savings, and improved patient outcomes. This positions the system as a valuable tool for modernizing healthcare resource management and delivering higher-quality care with reduced administrative burden.
6. Conclusions
This study addressed the complex challenge of optimally allocating IoT medical devices to patients in healthcare environments by formulating the task as a bi-objective scheduling problem that explicitly balances operational cost with quality of care. We incorporated a rich set of real-world constraints—such as device compatibility, setup durations, time availability windows, and battery limitations—into a novel optimization framework. To solve this NP-hard problem efficiently, we developed a tailored heuristic based on the NSGA-II algorithm.
The results of our study confirm the successful achievement of all research objectives. First, the proposed model offers a realistic and flexible structure that captures the trade-offs inherent in clinical scheduling, enabling decision-makers to make data-driven choices. Second, the NSGA-II-based approach generates diverse high-quality solutions rapidly: meaningful options appear within seconds, and a refined Pareto front is available in under a minute, making it suitable for real-time use. Third, our evaluation shows that the method outperforms both baseline heuristics and manual scheduling approaches, with an average reduction of 6.3% in operational costs (reaching up to 12%) and improvements in care quality of up to 20% (with an average of 10%) based on patient-priority metrics. These findings provide a clear and positive answer to our research question, demonstrating that a bi-objective scheduling strategy can effectively balance economic and clinical considerations, even under realistic constraints.
The practical implications of this research are considerable. Healthcare facilities can deploy the system as part of real-time scheduling dashboards to assist staff in planning and revising device assignments efficiently. In high-pressure environments like emergency rooms or intensive care units, the system’s rapid re-optimization capability can support immediate responses to shifting clinical needs. Moreover, by embedding the algorithm into medical center information systems, healthcare providers can automate the scheduling of connected devices in smart healthcare settings. In mobile or remote care environments, where resource constraints are more severe, the model supports strategic, data-driven allocation to maximize both coverage and impact.
Despite promising results in optimizing IoT medical device allocation via bi-objective scheduling, limitations exist. The simulation might not fully reflect real-world unpredictability like sudden patient changes or device failures. The model’s accuracy depends on reliable input data, potentially limiting it in data-scarce settings. While NSGA-II performed well, its scalability for much larger systems needs further study. Lastly, user adoption and integration into current hospital workflows were not evaluated, which could affect real-world implementation.
Looking ahead, the proposed framework opens up multiple promising avenues for both theoretical advancement and practical deployment in the broader context of smart and connected healthcare. Beyond immediate healthcare facility scheduling needs, this approach can serve as a foundation for developing fully autonomous healthcare logistics systems, where device assignment, personnel scheduling, and patient flow are coordinated in real time. Its adaptability positions it as a core component in next-generation hospital management platforms, potentially integrating with electronic health records (EHRs), remote monitoring systems, and AI-driven clinical decision support. Furthermore, the methodology is applicable to other resource-constrained domains beyond healthcare, such as eldercare facilities, disaster response units, or home-based telehealth services, where balancing efficiency with service quality is equally critical.
Future research will focus on investigating how priority configurations influence the trade-offs between cost and quality of care, with the goal of identifying optimal weight settings and dynamic adjustment strategies. Understanding these relationships is essential for tailoring the system to different medical contexts, where clinical priorities, patient demographics, and resource availability may vary. Building on these insights, future versions of the system could incorporate adaptive priority calibration mechanisms, enabling scheduling policies to dynamically respond to real-time clinical needs and patient conditions.
Beyond this, further research should explore richer and more dynamic scheduling environments. Integrating patient mobility data, urgency updates, and predictive analytics could transform the framework into a proactive system capable of anticipating needs and reallocating resources before bottlenecks arise. The use of reinforcement learning, hybrid metaheuristics, or adaptive online optimization techniques may further enhance system performance under high-uncertainty conditions. Additionally, extending the scope to multi-hospital networks or regional healthcare systems could support collaborative device sharing and cross-institution scheduling, bringing added value to public health infrastructure. Incorporating human-centered factors—such as caregiver fatigue, ethical prioritization, and patient comfort—would also broaden the framework’s applicability and acceptance.
To realize this potential, strong collaboration with healthcare professionals, IT developers, and policymakers will be essential. Pilot studies in diverse healthcare settings—from technologically advanced hospitals to under-resourced clinics—will help validate the system’s effectiveness, uncover real-world challenges, and guide further refinement. Ultimately, this work not only offers a solution to a complex and timely healthcare problem, but also establishes a flexible, extensible platform for future innovations in intelligent, patient-centered resource optimization.