Experimental Investigation of Antibiotic Photodegradation Using a Nanocatalyst Synthesized via an Eco-Friendly Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Aqueous Pumpkin Extract and Synthesis of Green Nanoparticles
2.2. Photocatalytic Experiments with Green Nanoparticles
2.3. Characterization Analyses of Green Nanoparticles
3. Results
3.1. Investigation of Experimental Parameters for Catalytic Activity
3.2. Photodegradation Mechanisms
3.3. Comparative Analysis with Prior Studies
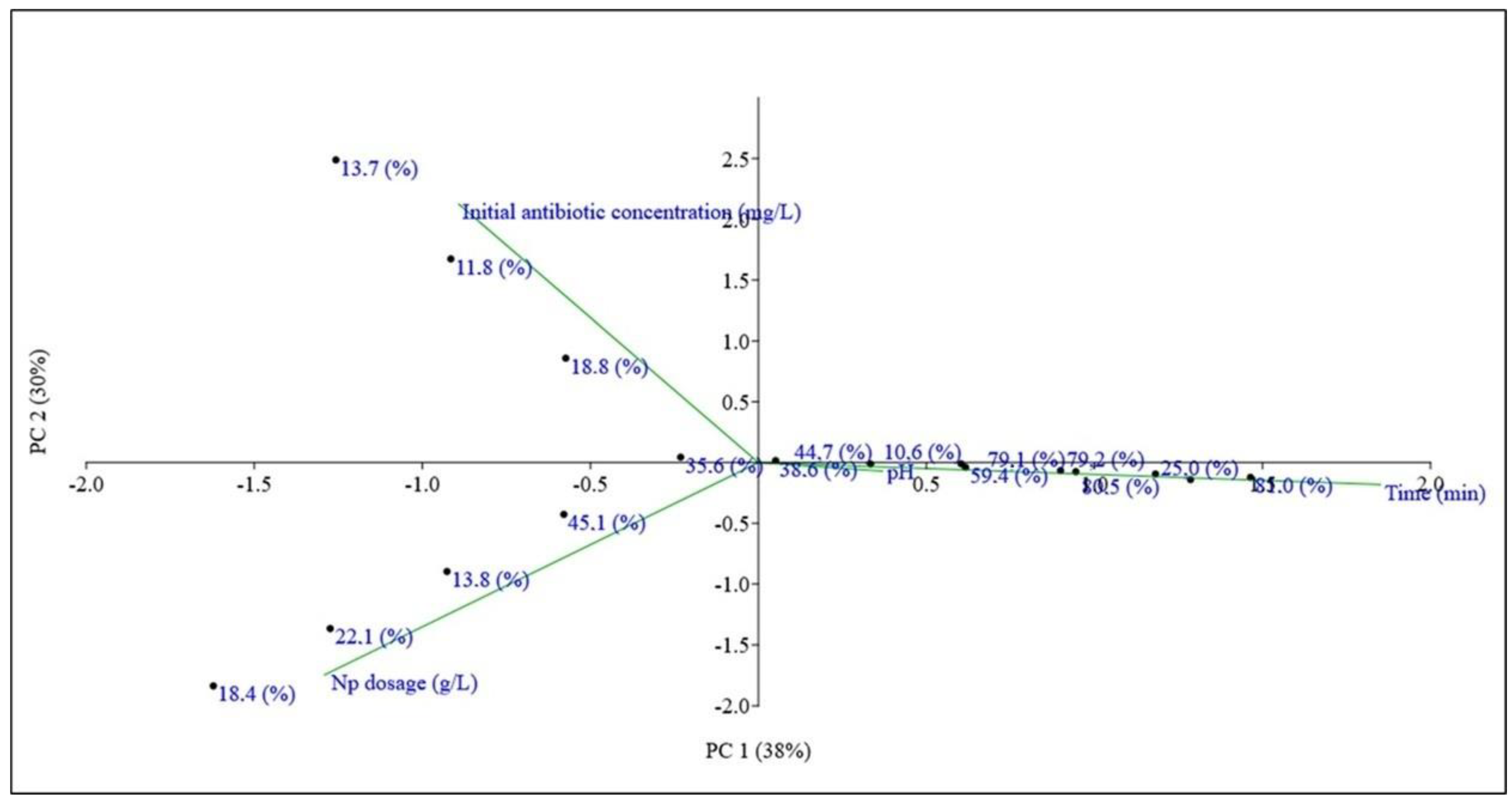
3.4. Principal Component Analysis (PCA)
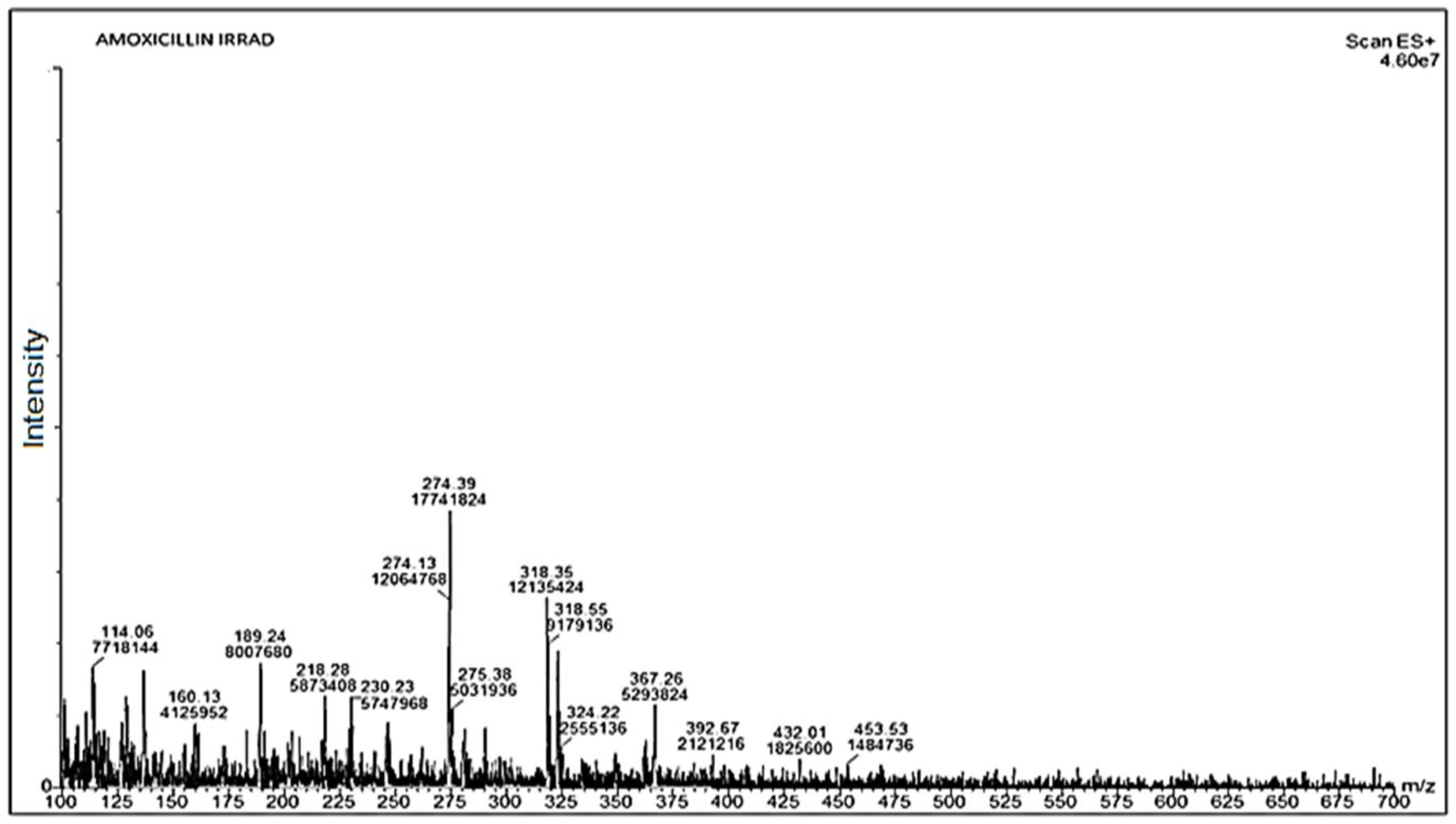
3.5. Mass Spectrometry (Q-TOF-MS/MS)
3.6. Electrical Energy Consumption per Order (EE/O)
3.7. Characterization of the Green Photocatalyst
3.7.1. Transmission Electron Microscopy (TEM)
3.7.2. Scanning Electron Microscopy (SEM)
3.7.3. FTIR Analysis
3.7.4. Thermogravimetric Analysis (TGA)
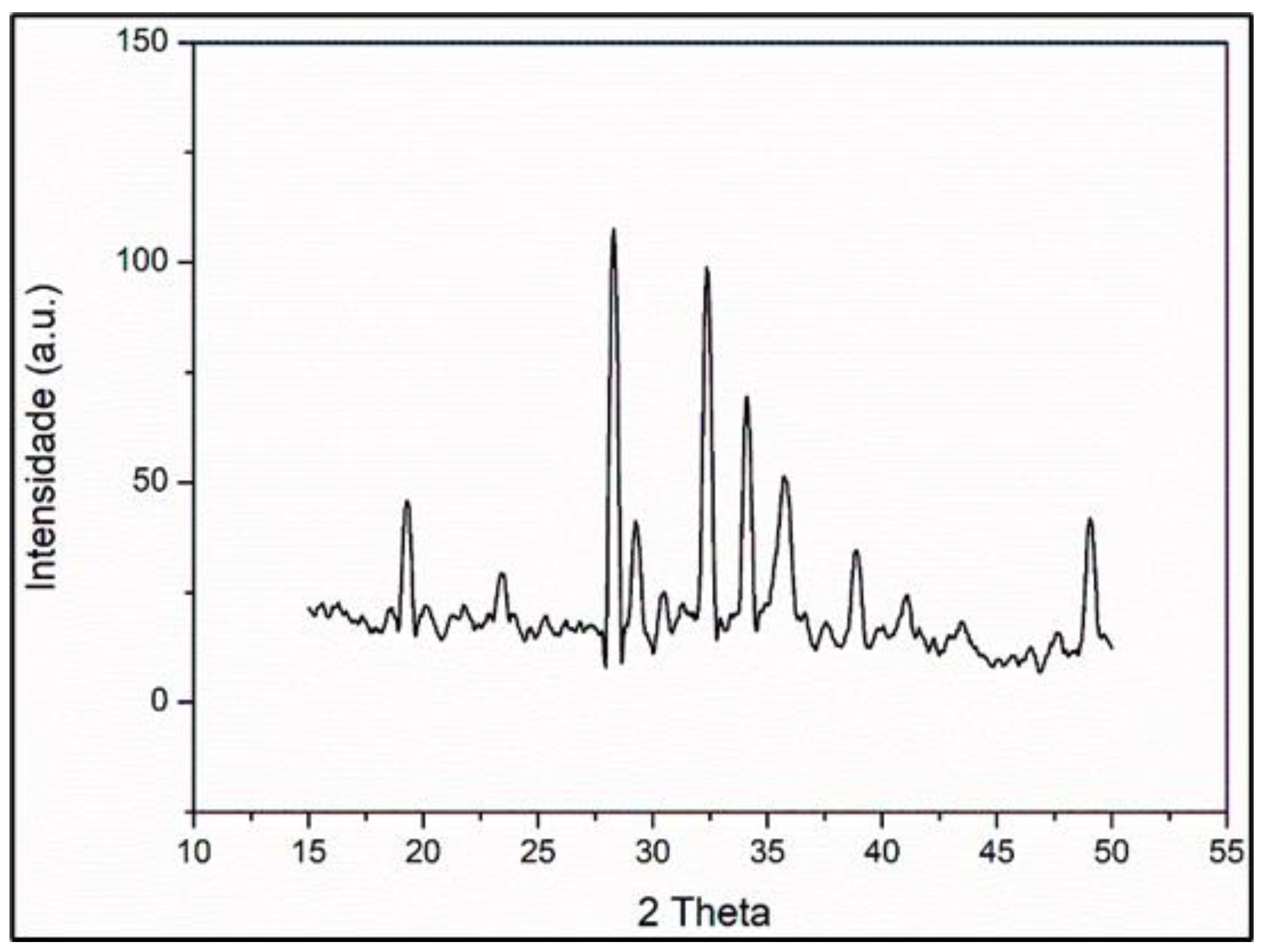
3.7.5. X-Ray Diffraction
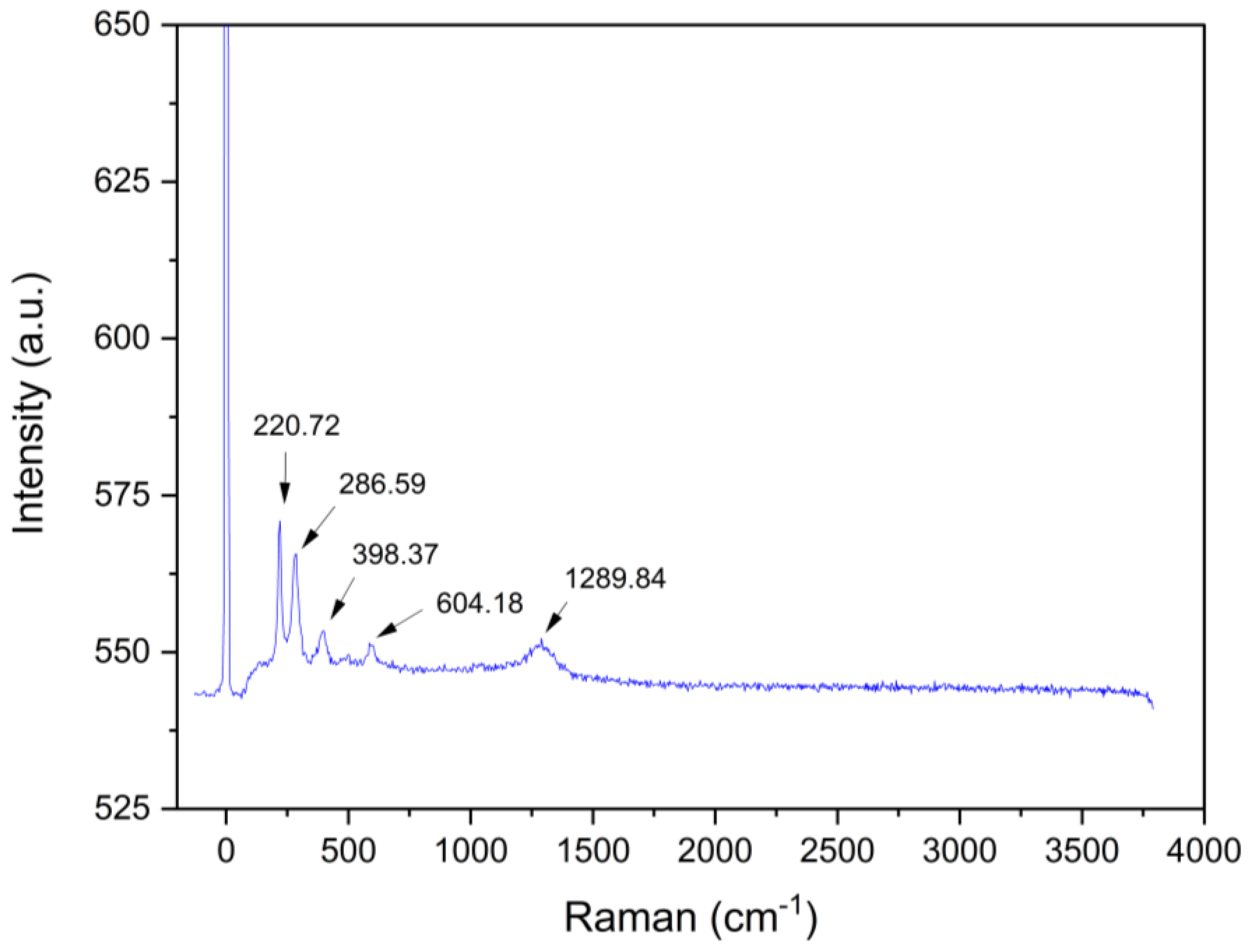
3.7.6. Raman Spectrum
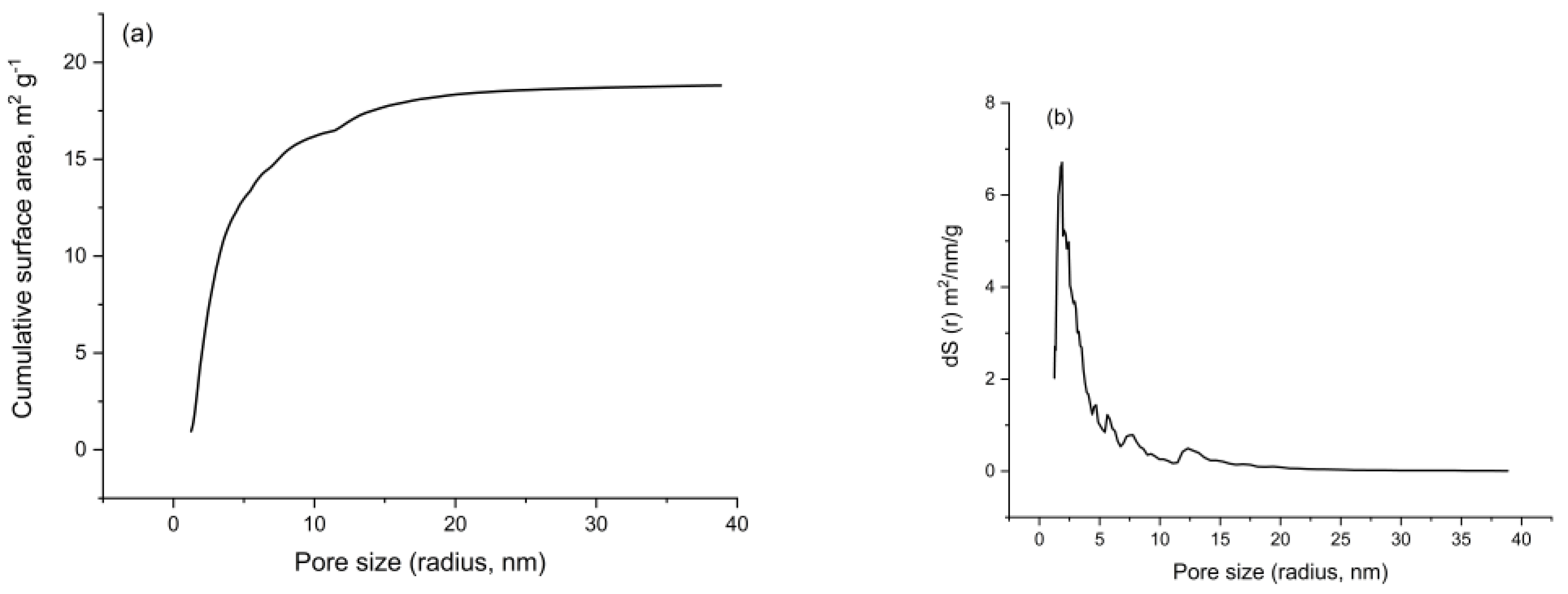
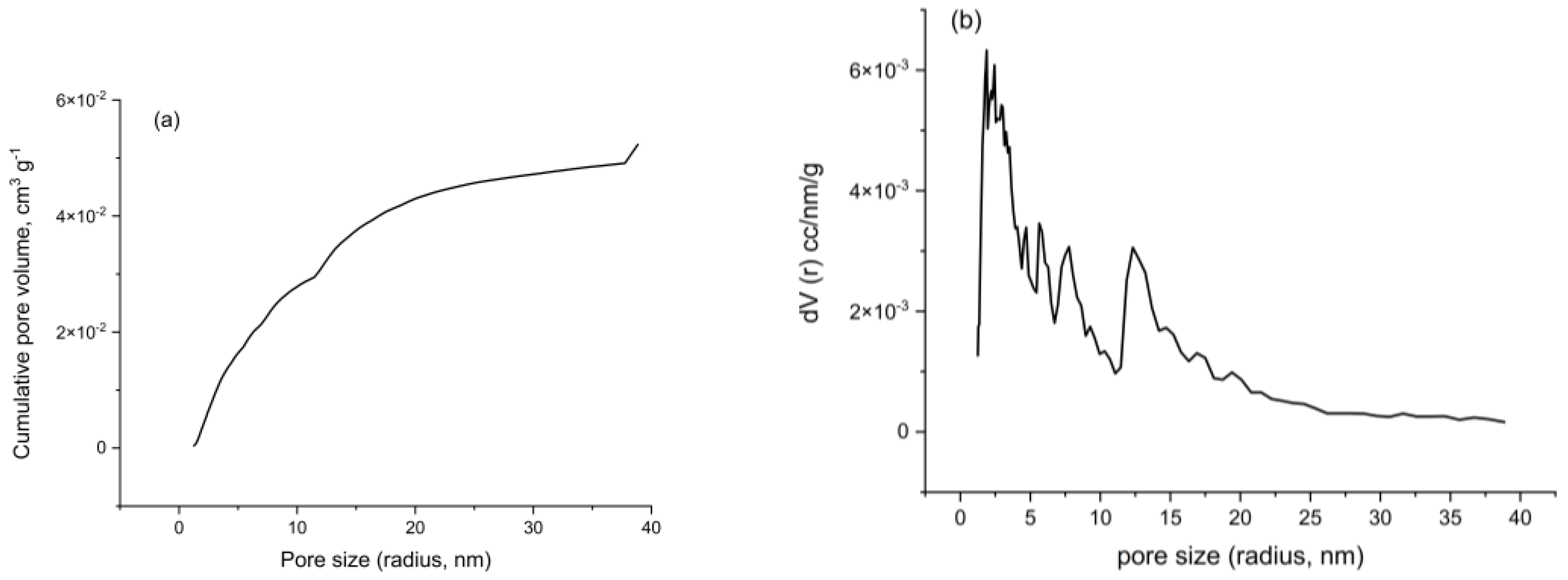
3.7.7. Surface Area
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sodhi, K.K.; Kumar, M.; Singh, D.K. Insight into the amoxicillin resistance, ecotoxicity, and remediation strategies. J. Water Process Eng. 2021, 39, 101858. [Google Scholar] [CrossRef]
- Elizalde-Velázquez, A.; Gómez-Oliván, L.M.; Galar-Martínez, M.; Islas-Flores, H.; Dublán-García, O.; SanJuan-Reyes, N. Amoxicillin in the Aquatic Environment, Its Fate and Environmental Risk, Environmental Health Risk; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Aryee, A.A.; Han, R.; Qu, L. Occurrence, detection and removal of amoxicillin in wastewater: A review. J. Clean. Prod. 2022, 368, 1221140. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Vaz-Moreira, I.; Della Giustina, S.V.; Llorca, M.; Barceló, D.; Schubert, S.; Berendonk, T.U.; Michael-Kordatou, I.; Fatta-Kassinos, D.; Martinez, J.L.; et al. Antibiotic residues in final effluents of European wastewater treatment plants and their impact on the aquatic environment. Environ. Int. 2020, 140, 105733. [Google Scholar] [CrossRef]
- Dias, I.M.; Mourão, L.C.; Andrade, L.A.; Souza, G.B.; Viana, J.C.; Oliveira, S.B.; Alonso, C.G. Degradation of antibiotic amoxicillin from pharmaceutical industry wastewater into a continuous flow reactor using supercritical water gasification. Water Res. 2023, 234, 119826. [Google Scholar] [CrossRef] [PubMed]
- Nisha, A.R. Antibiotic residues—A global health hazard. Vet. World 2008, 2, 375–377. [Google Scholar] [CrossRef]
- Chowdhury, J.; Mandal, T.K.; Mondal, S. Genotoxic impact of emerging contaminant amoxicillin residue on zebra fish (Danio rerio) embryos. Heliyon 2020, 6, e05379. [Google Scholar] [CrossRef]
- Ayanda, O.S.; Aremu, O.H.; Akintayo, C.O.; Sodeinde, K.O.; Igboama, W.N.; Oseghe, E.O.; Nelana, S.M. Sonocatalytic degradation of amoxicillin from aquaculture effluent by zinc oxide nanoparticles. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100513. [Google Scholar] [CrossRef]
- Tung, M.H.T.; Phuong, T.T.T.; Le Chi, N.T.P.; The, D.M.; Quoc, N.T.; Khan, D.T.; Pham, T.-D.; Khoa, N.V.; Hien, T.T.T.; Cam, N.T.D. Novel amoxicillin degradation via photocatalysis of WO3/AgI heterojunction decorated on rGO. Ceram. Int. 2022, 49, 10881–10888. [Google Scholar] [CrossRef]
- Alwared, A.I.; Sulaiman, F.A.; Raad, H.; Al-Musawi, T.J.; Mohammed, N.A. Ability of FeNi3/SiO2/TiO2 nanocomposite to degrade amoxicillin in wastewater samples in solar light-driven processes. S. Afr. J. Bot. 2022, 153, 195–202. [Google Scholar] [CrossRef]
- Mohapatra, S.; Snow, D.; Shea, P.; Gálvez-Rodríguez, A.; Kumar, M.; Padhye, L.P.; Mukherji, S. Photodegradation of a mixture of five pharmaceuticals commonly found in wastewater: Experimental and computational analysis. Environ. Res. 2022, 216, 114659. [Google Scholar] [CrossRef]
- Ma, X.; Liu, G.; Wang, L.; Xin, S.; Zhang, B.; Chen, Q.; Yan, Q.; Zhang, G.; Zhang, Y.; Xin, Y. High efficiency 2D/0D/3D Z-scheme rGO@g-C3N4/TiO2 nanobelt-tubes heterojunction for tetracycline degradation under visible light: Electrochemical synthesis, performance, and mechanisms. J. Environ. Chem. Eng. 2023, 11, 110483. [Google Scholar] [CrossRef]
- Hirami, Y.; Hunge, Y.M.; Suzuki, N.; Rodríguez-González, V.; Kondo, T.; Yuasa, M.; Fujishima, A.; Teshima, K.; Terashima, C. Enhanced degradation of ibuprofen using a combined treatment of plasma and Fenton reactions. J. Colloid Interface Sci. 2023, 642, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Anjali, R.; Shanthakumar, S. Simultaneous degradation of amoxicillin, ciprofloxacin and acetaminophen in a mixture by ozonation: Kinetics and mechanisms pathway. J. Clean. Prod. 2022, 378, 134509. [Google Scholar] [CrossRef]
- Hu, J.; Bian, X.; Xia, Y.; Weng, M.; Zhou, W.; Dai, Q. Application of response surface methodology in electrochemical degradation of amoxicillin with Cu-PbO2 electrode: Optimization and mechanism. Sep. Purif. Technol. 2020, 250, 117109. [Google Scholar] [CrossRef]
- Xie, W.; Huang, Z.; Zhou, F.; Li, Y.; Bi, X.; Bian, Q.; Sun, S. Heterogeneous fenton-like degradation of amoxicillin using MOF-derived Fe0 embedded in mesoporous carbon as an effective catalyst. J. Clean. Prod. 2021, 313, 127754. [Google Scholar] [CrossRef]
- Brito, N.N.; Silva, V.B.M. Advanced oxidative process and environmental application. Rev. Eletrônica Eng. Civ. 2012, 1, 36–47. [Google Scholar]
- Fioreze, M.; dos Santos, E.P.; Schmachtenberg, N. Processos oxidativos avançados: Fundamentos e aplicação ambiental. Rev. Cent. Cienc. Nat. Exatas 2014, 18, 19–91. [Google Scholar] [CrossRef]
- Choudhary, S. Role of nano catalysts in green chemistry. J. Sci. Innov. Nat. Earth 2024, 4, 8–11. [Google Scholar] [CrossRef]
- Xiao, Z.; Yuan, M.; Yang, B.; Liu, Z.; Huang, J.; Sun, D. Plant-mediated synthesis of highly active iron nanoparticles for Cr (VI) removal: Investigation of the leading biomolecules. Chemosphere 2016, 150, 357–364. [Google Scholar] [CrossRef]
- Bolade, O.P.; Williams, A.B.; Benson, N.U. Green synthesis of iron-based nanomaterials for environmental remediation: A review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100279. [Google Scholar] [CrossRef]
- AlSalka, Y.; Granone, L.I.; Ramadan, W.; Hakki, A.; Dillert, R.; Bahnemann, D.W. Iron-based photocatalytic and photoelectrocatalytic nano-estructures: Facts, perspectives, and expectations. Appl. Catal. B Environ. 2019, 244, 1065–1095. [Google Scholar] [CrossRef]
- Lalnunthari, C.; Devi, L.M.; Amami, E.; Badwaik, L.S. Valorisation of pumpkin seeds and peels into biodegradable packaging films. Food Bioprod. Process. 2019, 118, 58–66. [Google Scholar] [CrossRef]
- Ramos, R.M.B.; Paludo, L.C.; Monteiro, P.I.; da Rocha, L.V.M.; de Moraes, C.V.; Santos, O.O.; Alves, E.R.; Dantas, T.L.P. Amoxicillin degradation by iron photonanocatalyst synthetized by green route using pumpkin (Tetsukabuto) peel extract. Talanta 2023, 260, 124658. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.M.B.; Monteiro, P.I.; Biz, A.P.; Paludo, L.C.; Tasso, I.d.S.; da Rocha, L.V.M.; Alves, E.R.; Dantas, T.L.P. Green synthesis and characterization of magnesium nanoparticles using pumpkin peel extract (Tetsukabuto). Obs. Econ. Latinoam. 2024, 22, e573. [Google Scholar] [CrossRef]
- Liu, J.; Liang, R.; Hu, Z.; Zhang, X.; Zhou, M. Photoelectrocatalysis/photoelectro-Fenton system based on cone-like TiO2/nickel foam photoanode for efficient degradation of carbamazepine: Comparison with DSA. Chem. Eng. J. 2024, 491, 152088. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, X.; Hao, J.; Wang, Z.; Huo, B.; Qi, J.; Wang, Y.; Meng, F. Sustainable self-powered degradation of antibiotics using Fe3O4@MoS2/PVDF modified pipe with superior piezoelectric activity: Mechanism insight, toxicity assessment and energy consumption. Appl. Catal. B Environ. 2023, 331, 122655. [Google Scholar] [CrossRef]
- Wahyuni, E.T.; Cahyono, R.N.; Nora, M.; Alharissa, E.Z.; Kunarti, E.S. Degradation of amoxicillin residue under visible light over TiO2 doped with Cr prepared from tannery wastewater. Results Chem. 2024, 7, 101302. [Google Scholar] [CrossRef]
- Mohammadi, A.; Kazemeini, M.; Sadjadi, S. A developed novel Hal′@MIL-PMA triple composite utilized as a robust photocatalyst for activated removal of antibiotic contaminants: A new chemical mechanism for Tetracycline degradation. J. Environ. Chem. Eng. 2024, 12, 112941. [Google Scholar] [CrossRef]
- Harikumar, B.; Khan, S.S. Hierarchical construction of ZrO2/CaCr2O4/BiOIO3 ternary photocatalyst: Photodegradation of antibiotics, degradation pathway, toxicity assessment, and genotoxicity studies. Chem. Eng. J. 2022, 442, 136107. [Google Scholar] [CrossRef]
- Khalaji, A.D. NiFe2O4 prepared by the chitosan-assisted route for the photodegradation of methylene blue under simulated sun light irradiation. Chem. Phys. Impact 2024, 8, 00652. [Google Scholar] [CrossRef]
- Wahyuni, E.T.; Yulikayani, P.Y.; Aprilita, N.H. Enhancement of visible-light photocatalytic activity of Cu-doped TiO2 for photodegradation of amoxicillin in water. J. Mater. Environ. Sci. 2020, 11, 670–683. [Google Scholar]
- Safari, M.; Rezaee, R.; Soltani, R.D.C.; Ghorbani, M.; Faridan, M.; Wantala, K.; Shivaraju, H.; Shahmoradi, B. Solar photocatalytic degradation of amoxicillin using Ni:TiO2 nanocatalyst stabilized on ceramic plates. Desalination Water Treat. 2022, 270, 163–171. [Google Scholar] [CrossRef]
- Rocha, H.F.; Silva, V.; Lima, D.L.; Calisto, V. Evaluation of the impact of photodegradation processes on the environmental persistence of amoxicillin. Case Stud. Chem. Environ. Eng. 2024, 9, 100724. [Google Scholar] [CrossRef]
- Mahlaule-Glory, L.M.; Mapetla, S.; Makofane, A.; Mathipa, M.M.; Hintsho-Mbita, N.C. Biosynthesis of iron oxide nanoparticles for the degradation of methylene blue dye, sulfisoxazole antibiotic and removal of bacteria from real water. Heliyon 2022, 8, e10536. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Haritha, E.; Roopan, S.M.; Madhavi, G.; Elango, G.; Al-Dhabi, N.A.; Arasu, M.V. Green chemical approach towards the synthesis of SnO2 NPs in argument with photocatalytic degradation of diazo dye and its kinetic studies. J. Photochem. Photobiol. B Biol. 2016, 162, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, S.H.; Miah, M.Y.; Paul, S.C.; Das Aka, T.; Saha, O.; Rahaman, M.; Sharif, J.I.; Habiba, O.; Ashaduzzaman, M. Green synthesis of iron oxide nanoparticle using Carica papaya leaf extract: Application for photocatalytic degradation of remazol yellow RR dye and antibacterial activity. Heliyon 2020, 6, e04603. [Google Scholar] [CrossRef]
- Verbruggen, S.W. TiO2 photocatalysis for the degradation of pollutants in gas phase: From morphological design to plasmonic enhancement. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 64–82. [Google Scholar] [CrossRef]
- Rosales, M.; Orive, J.; Espinoza-González, R.; de Luis, R.F.; Gauvin, R.; Brodusch, N.; Rodríguez, B.; Gracia, F.; García, A. Evaluating the bi-functional capacity for arsenic photo-oxidation and adsorption on anatase TiO2 nanostructures with tunable morphology. Chem. Eng. J. 2021, 415, 128906. [Google Scholar] [CrossRef]
- Zheng, F.; Queirós, J.M.; Martins, P.M.; de Luis, R.F.; Fidalgo-Marijuan, A.; Vilas-Vilela, J.L.; Lanceros-Méndez, S.; Reguera, J. Au-sensitised TiO2 and ZnO nanoparticles for broadband pharmaceuticals photocatalytic degradation in water remediation. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 671, 131594. [Google Scholar] [CrossRef]
- Aba-Guevara, C.G.; Medina-Ramírez, I.E.; Hernández-Ramírez, A.; Jáuregui-Rincón, J.; Lozano-Álvarez, J.A.; Rodríguez-López, J.L. Comparison of two synthesis methods on the preparation of Fe, N-Co-doped TiO2 materials for degradation of pharmaceutical compounds under visible light. Ceram. Int. 2017, 43, 5068–5079. [Google Scholar] [CrossRef]
- Liang, J.; Chen, K.; Duan, X.; Zhao, L.; Qiu, H.; Xu, X.; Cao, X. pH-dependent generation of radical and nonradical species for sulfamethoxazole degradation in different carbon/persulfate systems. Water Res. 2022, 224, 119113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, M.; Liang, J.; Xiong, Z.; Liu, Y.; Peng, J.; Yuan, Y.; Zhang, H.; Zhou, P.; Lai, B. pH-modulated oxidation of organic pollutants for water decontamination: A deep insight into reactivity and oxidation pathway. J. Hazard. Mater. 2024, 471, 134393. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhu, Q.; Han, C.; Yang, Y.; Jiang, W.; Zhang, Z. Photocatalytic degradation of recalcitrant organic pollutants in water using a novel cylindrical multi-column photoreactor packed with TiO2-coated silica gel beads. J. Hazard. Mater. 2015, 285, 398–408. [Google Scholar] [CrossRef]
- MubarakAli, D. Microwave irradiation mediated synthesis of needle-shaped hydroxyapatite nanoparticles as a flocculant for Chlorella vulgaris. Biocatal. Agric. Biotechnol. 2019, 17, 203–206. [Google Scholar] [CrossRef]
- Panneerselvam, C.; Alshehri, M.A.; Saif, A.; Faridi, U.; Khasim, S.; Mohammedsaleh, Z.M.; Parveen, H.; Omer, N.; Alasmari, A.; Mukhtar, S.; et al. Green synthesis of Abutilon indicum (L) derived iron oxide (FeO) nanoparticles with excellent biological, anticancer and photocatalytic activities. Polyhedron 2024, 257, 117022. [Google Scholar] [CrossRef]
- Sayed, F.N.; Polshettiwar, V. Facile and sustainable synthesis of shaped iron oxide nanoparticles: Effect of iron precursor salts on the shapes of iron oxides. Sci. Rep. 2015, 5, 9733. [Google Scholar] [CrossRef]
- Bensy, A.D.; Christobel, G.J.; Muthusamy, K.; Alfarhan, A.; Anantharaman, P. Green synthesis of iron nanoparticles from Ulva lactuca and bactericidal activity against enteropathogens. J. King Saud Univ.-Sci. 2022, 34, 101888. [Google Scholar] [CrossRef]
- Dash, A.; Ragavendran, C.; Rajendran, R. Biogenic Nickel Oxide Nanoparticles: Synthesis, Characterization and Biomedical Potential. Mol. Biotechnol. 2025. [Google Scholar] [CrossRef]
- Duan, Y.-T.; Soni, K.; Patel, D.; Choksi, H.; Sangani, C.B.; Saeed, W.S.; Ameta, K.L.; Ameta, R.K. Green synthesis of iron oxide nanoparticles using Nicotiana plumbaginifolia and their biological evaluation. J. Mol. Liq. 2024, 396, 123985. [Google Scholar] [CrossRef]
- Yassin, M.T.; Al-Otibi, F.O.; Al–Askar, A.A. Green synthesis, characterization and antimicrobial activity of iron oxide nanoparticles with tigecycline against multidrug resistant bacterial strains. J. King Saud Univ.-Sci. 2024, 36, 103131. [Google Scholar] [CrossRef]
- Kumari, T.; Phogat, D.; Shukla, V. Exploring the multipotentiality of plant extracts for the green synthesis of iron nanoparticles: A study of adsorption capacity and dye degradation efficiency. Environ. Res. 2023, 229, 116025. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Habib, F.; Darwish, N.; Shanableh, A. Iron sulfide nanoparticles prepared using date seed extract: Green synthesis, characterization and potential application for removal of ciprofloxacin and chromium. Powder Technol. 2021, 380, 219–228. [Google Scholar] [CrossRef]
- Joshi, D.P.; Pant, G.; Arora, N.; Nainwal, S. Effect of solvents on morphology, magnetic and dielectric properties of (α-Fe2O3SiO2) core-shell nanoparticles. Heliyon 2017, 3, e00253. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Jadaun, A.; Arora, V.; Sinha, R.K.; Biyani, N.; Jain, V. In vitro toxicity assessment of chitosan oligosaccharide coated iron oxide nanoparticles. Toxicol. Rep. 2015, 2, 27–39. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Li, F.-A. Kinetic study on removal of copper(II) using goethite and hematite nano-photocatalysts. J. Colloid Interface Sci. 2010, 347, 277–281. [Google Scholar] [CrossRef]
- Faria, D.L.A.; Silva, S.V.; Oliveira, M.T. Raman Microspectroscopy of Some Iron Oxides and Oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Chamritski, I.; Burns, G. Infrared- and Raman-active phonons of magnetite, maghemite, and hematite: A computer simulation and spectroscopic study. J. Phys. Chem. B 2005, 109, 4965–4968. [Google Scholar] [CrossRef]
- Kamath, V.; Chandra, P.; Jeppu, G.P. Comparative study of using five different leaf extracts in the green synthesis of iron oxide nanoparticles for removal of arsenic from water. Int. J. Phytoremediat. 2020, 22, 1278–1294. [Google Scholar] [CrossRef]

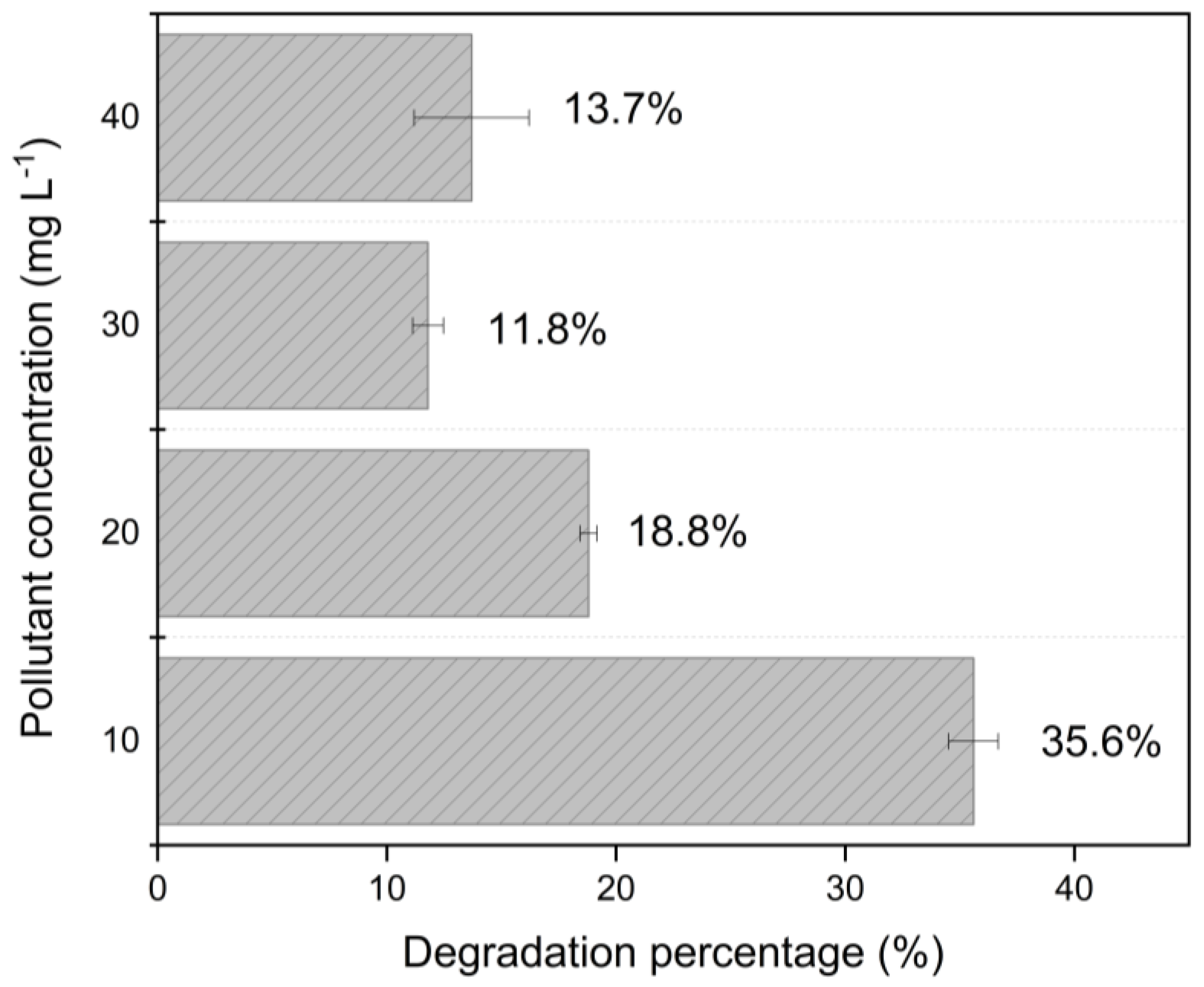
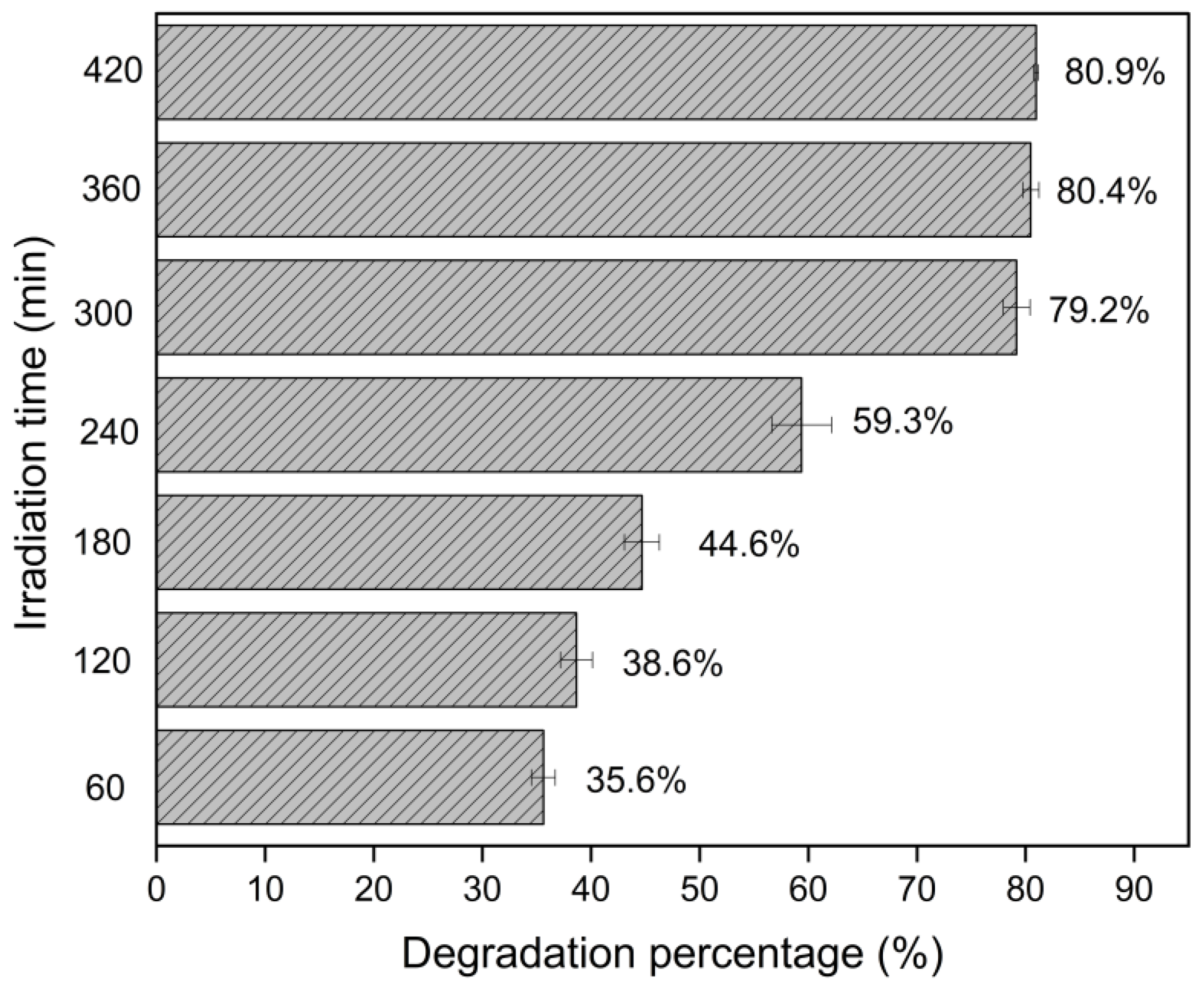
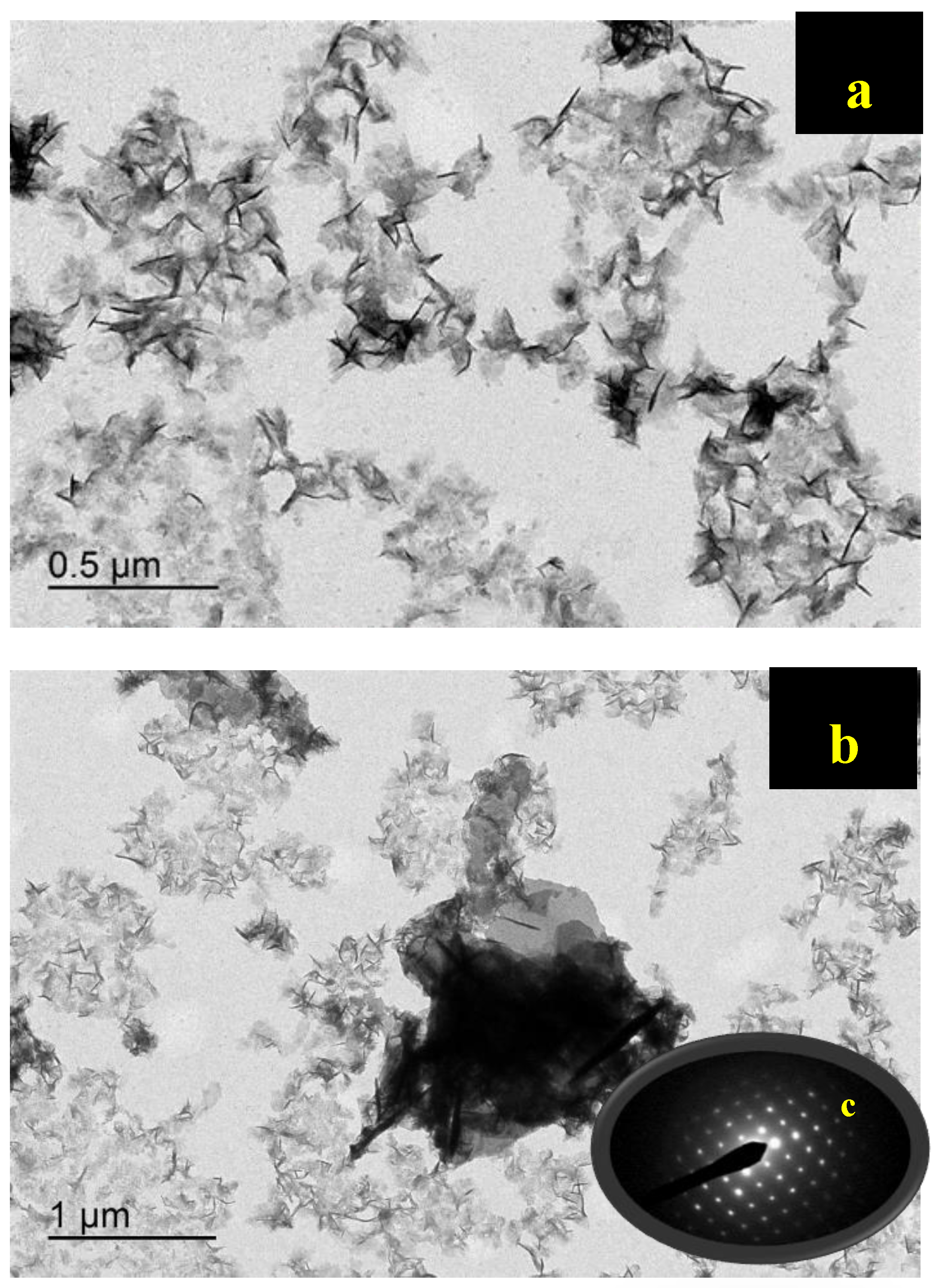
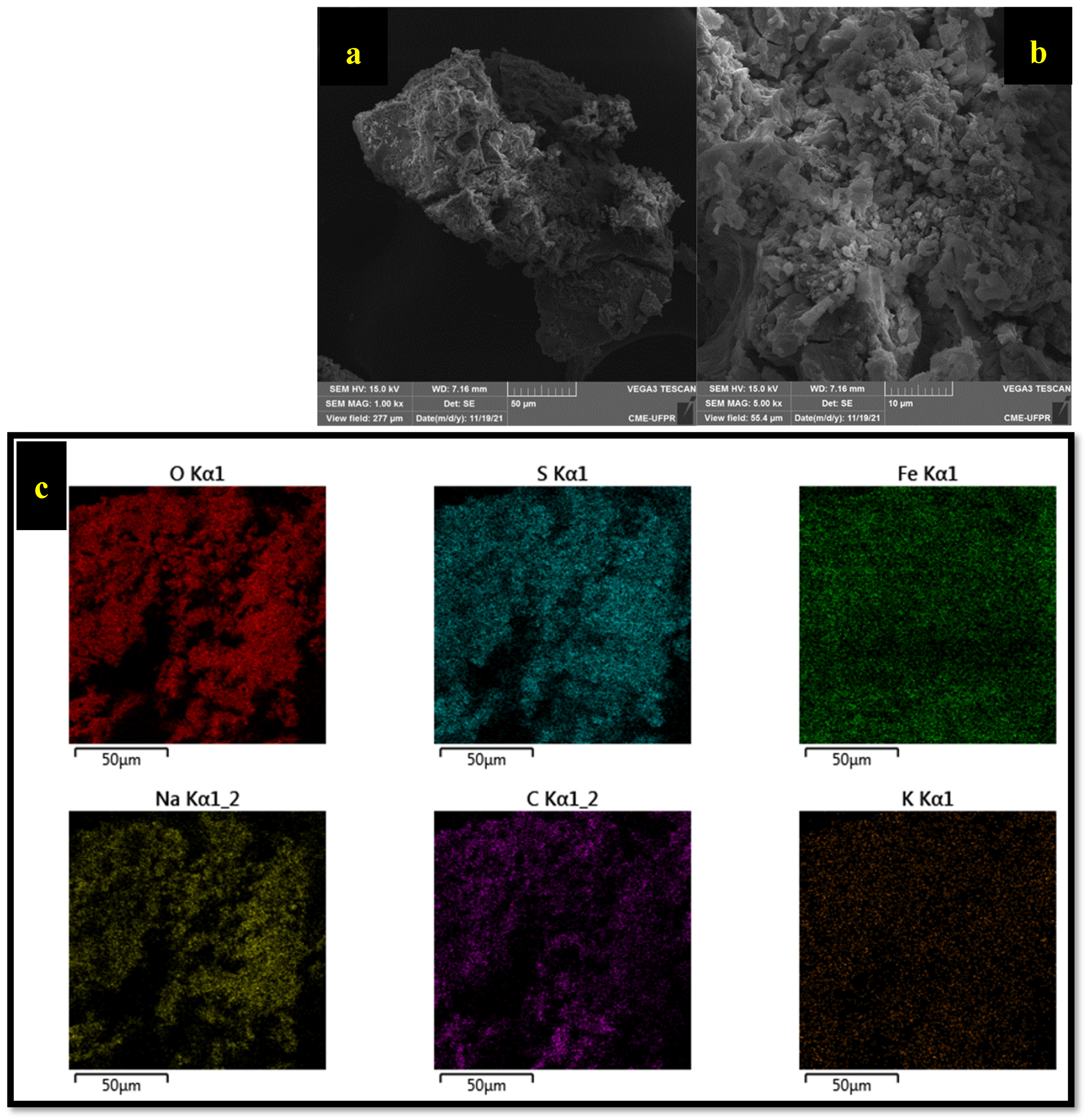
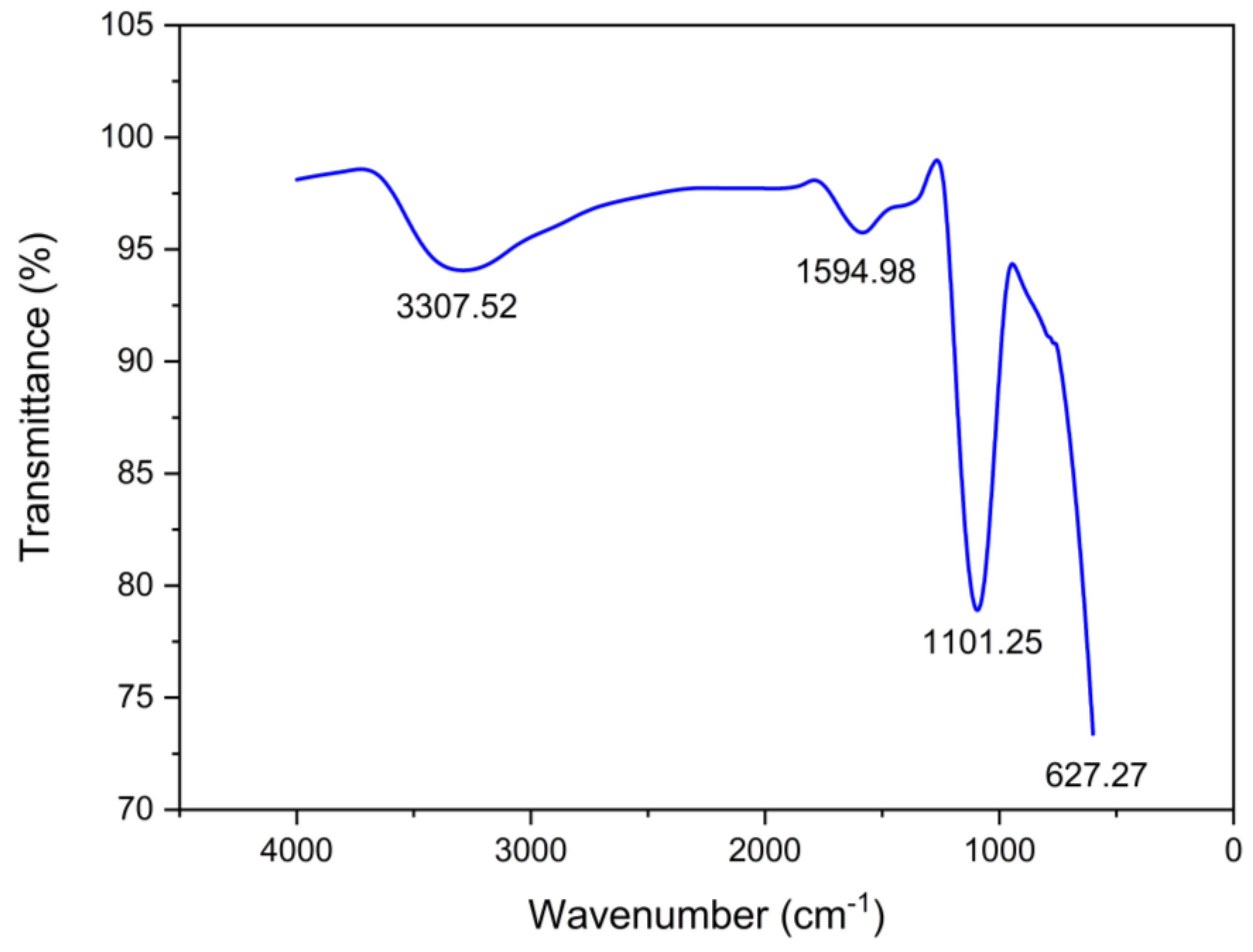
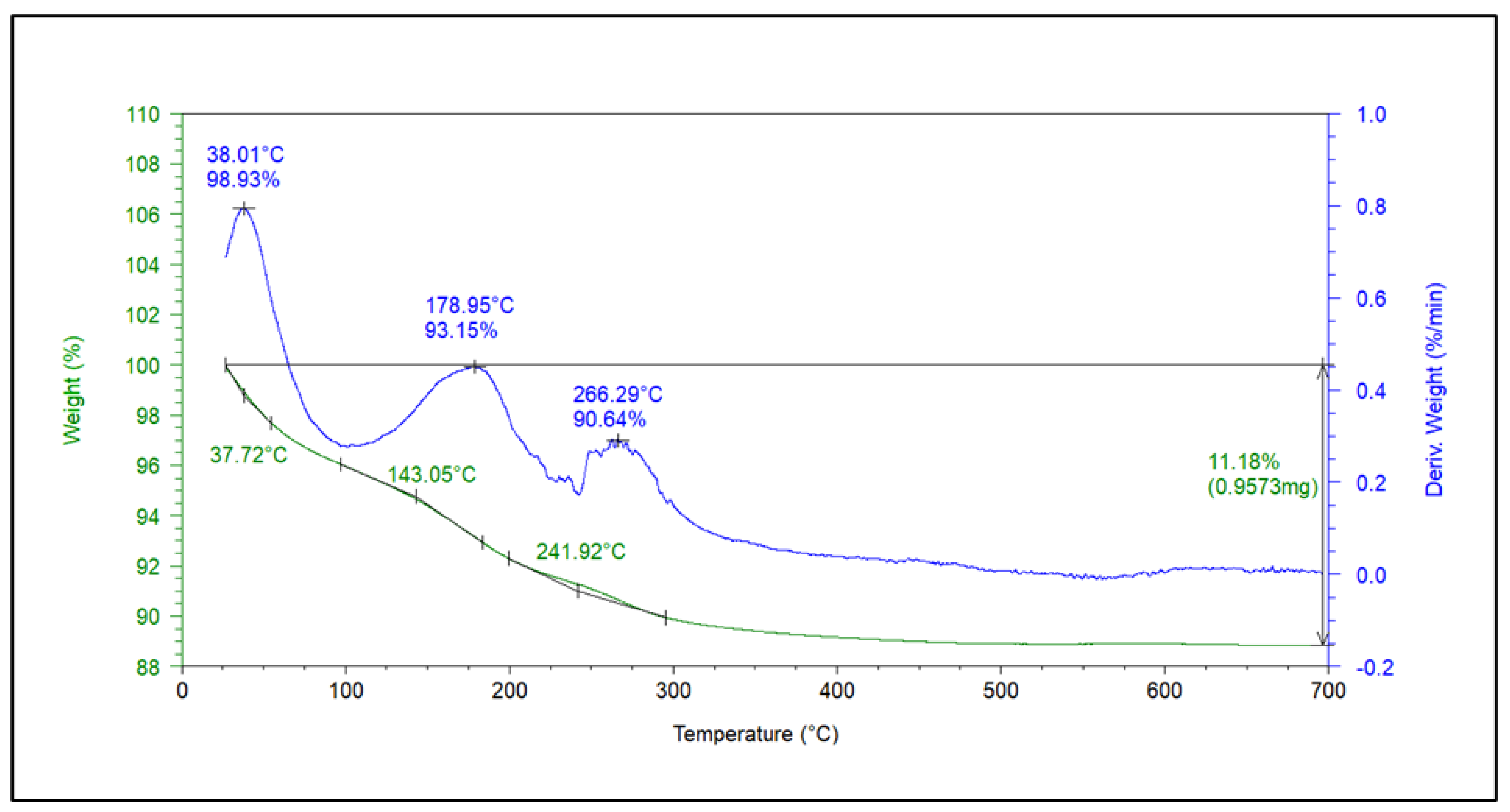

| pH | Amoxicillin Charge | Green Photocatalyst (Surface Charge) | Degradation |
|---|---|---|---|
| 3.0 | + | + | 10.6 ± 0.8871 |
| 6.0 | − | + | 79.2 ± 1.2173 |
| 9.0 | − | − | 25.0 ± 2.3648 |
| Synthesis Method | Degradation (%.) | Time (min) | Light | pH | Reference |
|---|---|---|---|---|---|
| Sol–gel | 58.61 | 240 | Visible | 3.50 | [42] |
| Microwave | 46.12 | 240 | Visible | 3.50 | [42] |
| Microwave | 69.15 | 300 | Visible | 3.50 | [42] |
| Green synthesis | 80.00 | 300 | UV | 6.00 | This study |
| 2 Theta Values (Degrees) | Crystallographic Planes (h k l) | FWHM (Degrees) |
|---|---|---|
| 19.3 | 111 | 0.4733 |
| 23.4 | 012 | 0.1950 |
| 28.3 | 311 | 1.0593 |
| 29.3 | 222 | 0.0122 |
| 32.4 | 104 | 0.5943 |
| 34.1 | 110 | 0.2372 |
| 35.8 | 113 | 1.0142 |
| 38.9 | 400 | 0.5357 |
| 49.1 | 422 | 0.8975 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, R.M.B.; Monteiro, P.I.; Rocha, L.V.M.d.; Santos, O.O.; Alves, E.R.; Dantas, T.L.P. Experimental Investigation of Antibiotic Photodegradation Using a Nanocatalyst Synthesized via an Eco-Friendly Process. Appl. Sci. 2025, 15, 4308. https://doi.org/10.3390/app15084308
Ramos RMB, Monteiro PI, Rocha LVMd, Santos OO, Alves ER, Dantas TLP. Experimental Investigation of Antibiotic Photodegradation Using a Nanocatalyst Synthesized via an Eco-Friendly Process. Applied Sciences. 2025; 15(8):4308. https://doi.org/10.3390/app15084308
Chicago/Turabian StyleRamos, Rúbia Martins Bernardes, Pablo Inocêncio Monteiro, Lizandra Viana Maurat da Rocha, Oscar Oliveira Santos, Evandro Roberto Alves, and Tirzhá Lins Porto Dantas. 2025. "Experimental Investigation of Antibiotic Photodegradation Using a Nanocatalyst Synthesized via an Eco-Friendly Process" Applied Sciences 15, no. 8: 4308. https://doi.org/10.3390/app15084308
APA StyleRamos, R. M. B., Monteiro, P. I., Rocha, L. V. M. d., Santos, O. O., Alves, E. R., & Dantas, T. L. P. (2025). Experimental Investigation of Antibiotic Photodegradation Using a Nanocatalyst Synthesized via an Eco-Friendly Process. Applied Sciences, 15(8), 4308. https://doi.org/10.3390/app15084308







