Abstract
In Southwest China’s high-temperature, humid, and rainy climate, ancient sandstone structures face significant deterioration due to acid rain and water accumulation, which cause dynamic and static dissolution. This degradation weakens the sandstone’s physical and mechanical properties, threatening the preservation of cultural heritage sites. Dynamic dissolution is the process of matter and energy exchange during fluid–rock or fluid–mineral interactions under dynamic conditions. Under dynamic conditions, continuously renewed fluids supply chemicals for dissolution and remove dissolved products, sustaining reactions similar to acid rain dissolution. Static dissolution is the dissolution–erosion process between fluids and rocks or minerals in a relatively stationary fluid environment. Unlike dynamic dissolution, which involves moving fluids, static dissolution occurs in nearly stagnant fluids, where rising product concentrations from acid–rock reactions may hinder further dissolution, akin to static immersion dissolution. This study systematically examined how different dissolution conditions affect sandstone’s pore structure, mechanical properties, and hygroscopic behavior. Nuclear magnetic resonance (NMR) and scanning electron microscopy (SEM) were used to analyze pore structure changes, while ultrasonic testing and Leeb hardness measurements assessed mechanical strength. Hygroscopicity was evaluated through non-destructive moisture testing in controlled environments. The results show that dynamic dissolution has a greater impact on sandstone than static dissolution. Both conditions increased porosity in two stages, but dynamic dissolution enhanced pore connectivity while static dissolution caused gradual porosity growth and localized cracks. Dynamic dissolution significantly reduced surface hardness and P-wave velocity, increasing hardness heterogeneity, whereas static dissolution had a milder effect. Additionally, dynamic dissolution notably increased sandstone’s hygroscopicity, with moisture absorption rising over time. This study highlights the distinct effects of dynamic and static dissolution on sandstone deterioration, offering insights for the preventive conservation of ancient stone structures. Tailored preservation strategies are essential for addressing these varying degradation mechanisms.
1. Introduction
Rock-cut caves and stone carvings represent a significant portion of ancient stone architectural heritage, as their surface layers predominantly preserve critical cultural and historical information. Over time, the surface materials of these stone structures are continuously exposed to environmental factors, including water and moisture, resulting in a gradual decline in their physical and mechanical properties. This deterioration leads to various surface defects, significantly compromising the long-term durability and stability of these ancient structures. Therefore, a comprehensive investigation into the changes in the physical and mechanical properties of stone surfaces under natural environmental conditions is crucial for guiding preventive conservation and restoration efforts.
The climate in Southwest China (Figure 1) is characterized by year-round high temperatures, high humidity, and abundant rainfall, rendering it one of the regions most severely impacted by acid rain in the country. Sandstone, a natural material, is widely used in constructing rock-cut caves and stone carvings due to its fine-grained and soft texture, enabling intricate carving. However, prolonged exposure of sandstone to dynamic acid rain cycles and static immersion in residual rainwater has caused severe damage to the surface layers of sandstone-based rock-cut caves and stone carvings in this region. Understanding the mechanisms underlying this deterioration is crucial for developing effective preservation strategies to protect these invaluable cultural heritage sites.
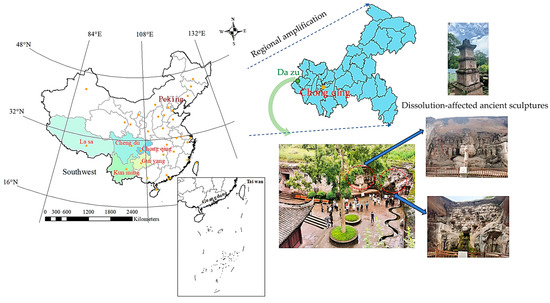
Figure 1.
Schematic diagram of the study area.
Sandstone, composed of calcium, argillaceous cement, and feldspar minerals, is highly susceptible to acidic dissolution, which gradually alters its pore structure [1]. Numerous studies have investigated this phenomenon. For example, Liu et al. [2] used CT imaging to show that acid immersion significantly increases sandstone porosity, accompanied by a notable rise in the number of interconnected pores, pore throats, and their equivalent radii. Similarly, Li et al. [3] conducted experiments on feldspathic sandstone immersed in acidic solutions (pH 2 and 5), showing that acid exposure increases porosity, reduces the proportion of small pores, and increases the proportion of medium and large pores. Other studies have also demonstrated that immersion in acidic solutions increases sandstone porosity, accompanied by a decline in mechanical parameters, including compressive strength, elastic modulus, peak strain, P-wave velocity, shear strength, internal friction angle, cohesion, and creep failure strength [4,5,6,7]. Furthermore, changes in the pore structure significantly affect the spontaneous moisture absorption process of sandstone surfaces. Moisture absorption acts as a critical medium for transporting erosive substances into the internal structure of building materials [8,9]. Zhang et al. [10] analyzed the correlation between porosity characteristics and adsorbed moisture content in conglomerate samples, finding that vapor adsorption positively correlates with porosity (a pore diameter > 0.2 μm significantly affects vapor adsorption). These findings highlight the complex interplay between acidic dissolution, pore structure evolution, and moisture absorption in sandstone, emphasizing the need for further research to guide conservation strategies for sandstone-based heritage structures.
Previous research indicates that dissolution processes primarily alter the pore structure of rocks, resulting in two significant outcomes: reduced surface strength and changes in the hygroscopic properties of the rock surface [11,12,13]. Based on methodologies used in prior studies, most researchers have focused on static immersion experiments with standard sandstone samples to investigate changes in physical and mechanical properties after acid-induced dissolution. These studies have offered valuable insights into the effects of static immersion on sandstone degradation. However, real-world exposure of sandstone structures involves both static and dynamic dissolution processes, including alternating cycles of rainfall and drying. This underscores the need for a more comprehensive approach incorporating dynamic dissolution conditions to better simulate natural environmental interactions and their impact on sandstone deterioration. Such an approach would enable a more accurate understanding of degradation mechanisms and guide effective conservation strategies for sandstone-based heritage structures.
For sandstone, static dissolution occurs under humid conditions with accumulated rainwater, while dynamic dissolution simulates the effects of rainfall. The dynamic testing device generates an unsaturated dynamic flow of the chemical solution, exposing sandstone to its effects and simulating acid rain precipitation on grotto stone carvings. Zhang et al. [14] comprehensively simulated carbonate rock dissolution under static and dynamic conditions to study the effects of condensed water. Their experimental results showed that dynamic dissolution, mimicking water vapor condensation, causes stronger microscopic water vapor intrusion, significantly increasing the dissolution rate and porosity of dolomite compared to static conditions. Similarly, Chen et al. [15] found that limestone porosity increased by 66% under dynamic acid rain erosion compared to only 28% under static immersion, indicating that dynamic dissolution significantly exacerbates rock degradation. These findings highlight the importance of considering both static and dynamic dissolution mechanisms in studying building material deterioration, as dynamic processes often significantly worsen rock property degradation. This dual approach is crucial for accurately assessing the long-term durability of sandstone and other rock-based structures in natural environments.
Previous studies have shown that the physical and mechanical properties of carbonate rocks change distinctly under dynamic and static dissolution conditions. Sandstone-based rock-cut caves and stone carvings frequently experience alternating cycles of rainfall (dynamic dissolution) and water accumulation (static dissolution). Therefore, comparative studies on sandstone’s physical and mechanical properties under these two dissolution modes are essential. This research is vital for understanding the different degradation mechanisms and their effects on sandstone structures. A systematic comparison of dynamic and static dissolution effects enables the development of more accurate models for predicting sandstone’s long-term behavior in natural environments. This knowledge lays a scientific foundation for developing targeted conservation strategies to mitigate the deterioration of sandstone-based heritage sites and ensure their long-term preservation.
To fully understand the impact of acid rain on the surface layers of rock-cut caves and stone carvings, this study simulates dynamic dissolution during acid rainfall and static immersion dissolution caused by residual water accumulation. Controlled laboratory experiments with dilute sulfuric acid solutions of identical concentration but different dissolution modes (dynamic and static) are conducted to elucidate key patterns of sandstone degradation under dynamic dissolution and compare its effects on pore structure, surface mechanical properties, and hygroscopic characteristics with those of static dissolution. This approach captures the distinct mechanisms of sandstone deterioration under realistic environmental conditions and provides critical insights into the differential impacts of dynamic and static dissolution. The findings enhance the understanding of sandstone degradation mechanisms and support the development of effective preservation strategies tailored to the challenges of acid rain and water accumulation.
2. Materials and Methods
In Southwest China, acid rain is primarily sulfuric-acid-based, with sulfate ions (SO42−) comprising 70% to 90% of the total anions [16]. Therefore, this study focuses on sulfuric acid rain as the primary research subject. Since dissolution damage in porous materials like sandstone typically progresses from the surface inward, a detailed investigation into surface-layer degradation mechanisms is essential. This research examines surface deterioration patterns to reveal the underlying processes driving sandstone degradation under sulfuric acid rain. This approach offers valuable insights into the early stages of sandstone deterioration, which are crucial for developing effective conservation strategies to mitigate the long-term effects of acid rain on sandstone-based heritage sites.
2.1. Experimental Rock Samples
Sandstone-based ancient stone structures are widespread in Southwest China, highlighting the importance of studying sandstone degradation patterns for cultural heritage preservation. In this study, sandstone samples were used as test specimens. Rock samples for this study were obtained from siltstone in Dazu District, Chongqing, Southwest China. During sampling, unweathered fresh sandstone was selected to prepare rock slabs for the experiment. The initial mineral composition and microscopic pore structure of the samples are detailed as follows:
As shown in Figure 2 and Figure 3 and summarized in Table 1, the sandstone samples in this study exhibit a clastic texture. The primary mineral is plagioclase, followed by quartz and calcite, with minor components, including clay minerals. This mineral composition is typical of sandstone and significantly influences its response to environmental stressors such as acid rain and moisture. The presence of plagioclase and calcite, which are highly susceptible to chemical weathering, underscores the sandstone’s vulnerability to dissolution. Understanding the sandstone’s mineral composition and texture is crucial for interpreting its degradation behavior under static and dynamic dissolution, as these factors directly affect pore structure evolution and mechanical properties over time.
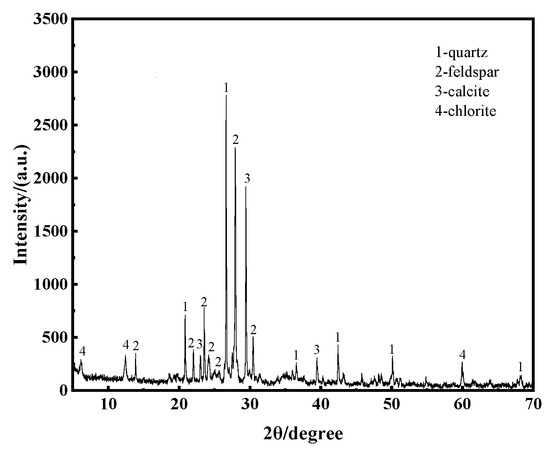
Figure 2.
XRD test results.

Figure 3.
Polarizing microscope.

Table 1.
Mineral composition of sandstone.
Since dissolution progresses from the surface inward, erosion is more pronounced along the surface width than depth, resulting in greater degradation of the sandstone surface layer. To accurately observe this phenomenon, 12 rock slabs measuring 12 × 12 × 3 cm were prepared, as shown in Figure 4.

Figure 4.
Schematic diagram of rock samples.
To ensure experimental accuracy and reliability, the initial rock slabs were tested for acoustic wave velocity, hardness, and porosity. Specimens with similar physical and mechanical properties were selected for further testing. The initial physical and mechanical properties of the selected sandstone samples are summarized in the Table 2 below.

Table 2.
Initial physical and mechanical properties.
To ensure a controlled comparison between static and dynamic dissolution processes, six specimens with closely matched initial physical and mechanical properties were selected for static immersion tests, and another six specimens were used for dynamic cyclic dissolution experiments. This balanced experimental design enables a direct comparison of the effects of static and dynamic dissolution on sandstone degradation while minimizing the influence of sample variability.
2.2. Experimental Procedure
To investigate the evolution of the pore structure, mechanical properties, and hygroscopic characteristics of sandstone under dynamic and static dissolution conditions, the following experimental procedure was designed:
(1) Sample Preparation: Sandstone slabs were dried in an oven at 105 °C and then cooled to room temperature in a desiccator to ensure a consistent initial state.
(2) Dissolution Experiments: Sandstone slabs were subjected to static immersion and dynamic cyclic dissolution using a 0.01 mol/L dilute sulfuric acid solution. To simulate natural conditions and accelerate deterioration, static immersion tests involved open immersion of slabs in a container for 0, 240, 480, and 720 h, while dynamic cyclic dissolution tests were conducted using a dynamic circulation device. To maintain consistent dynamic dissolution conditions, the liquid level in the reaction device was kept constant, and the flow rate was stabilized at 1.2 L/min. Each dynamic dissolution cycle lasted 24 h, with a cumulative duration of 192 h.
(3) Post-Dissolution Treatment: After dissolution experiments, the slabs were rinsed with deionized water. The sample requiring rinsing was placed into an appropriate container and distilled water was slowly added. The distilled water volume is typically 5–10 times that of the sandstone sample. Each sandstone sample was rinsed for 5–10 min, dried at 105 °C for 24 h, and cooled to room temperature in a desiccator. Twenty-five grid points were marked on each slab surface for ultrasonic wave velocity and hardness measurements to analyze mechanical strength distribution.
(4) Hygroscopicity Testing: Sandstone slabs were placed in a YB-60 constant temperature and humidity chamber set at 25 °C and 80% relative humidity for free moisture absorption. The moisture content of the slabs was monitored dynamically over time using a Concrete Moisture Encounter X5 moisture meter, Shanghai Laurier Instruments & Equipment Corporation, Shanghai, China.
(5) Microstructural Analysis: Finally, nuclear magnetic resonance (NMR) and Phenom desktop scanning electron microscopy (SEM) were used to analyze changes in the microscopic pore structure of sandstone after static and dynamic dissolution.
The pore structure of sandstone was analyzed using Niumag nuclear magnetic resonance (NMR) equipment, Suzhou Niumag Analytical Instrument Corporation, Suzhou, China. Niumag NMR systems, as advanced tools, are widely used in materials science, geology, petroleum exploration, and biomedical research. This method allows for non-destructive pore structure analysis, ideal for valuable or delicate samples.
Microstructural analysis was conducted with a Phenom Pro benchtop scanning electron microscope (SEM). The Phenom Pro, equipped with a high-brightness CeB6 filament, produces focused electron beams to scan sample surfaces, capturing backscattered (BSE) and secondary electron (SE) signals for high-resolution compositional and topographical imaging. This advanced, user-friendly benchtop SEM system supports a wide range of research applications.
This systematic experimental approach ensures a comprehensive understanding of sandstone degradation mechanisms under different dissolution conditions, offering valuable insights for preserving sandstone-based cultural heritage. The experimental procedure is illustrated in Figure 5.
Figure 5.
Experimental flowchart.
3. Results
3.1. Variation Laws of Surface Micro-Pore Structure Under Different Corrosion Mechanisms
Nuclear magnetic resonance (NMR) was used to analyze the pore structure of sandstone before and after exposure to different dissolution conditions, complemented by scanning electron microscopy (SEM) to observe microscopic morphological changes. In NMR analysis, the relaxation time (T2) and signal intensity of the T2 relaxation spectrum correspond to pore size and quantity, respectively. Specifically, relaxation times of [0.01, 3], [3, 50], and [50, 10,000] ms correspond to small, medium, and large pores in sandstone [17]. Using NMR technology, the evolution of the pore structure under static and dynamic dissolution conditions was systematically characterized. The T2 relaxation spectra, as shown in Figure 6, provide a detailed visualization of pore size distribution and connectivity changes, offering critical insights into sandstone degradation mechanisms. This combined NMR and SEM approach enables a comprehensive understanding of microstructural alterations induced by acidic dissolution, essential for developing effective preservation strategies for sandstone-based cultural heritage.
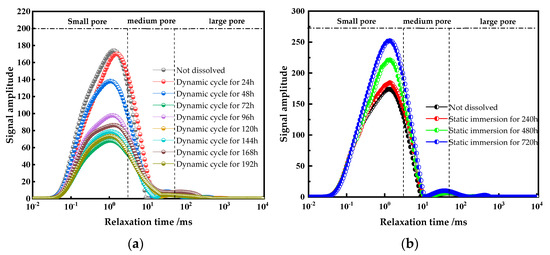
Figure 6.
NMR tests under different dissolution conditions. (a) dynamic cyclic dissolution, (b) static immersion dissolution.
As shown in Figure 6, the T2 relaxation spectra display a bimodal distribution. In untreated samples, the significant signal amplitude difference between the two peaks indicates a clear boundary between small pores and medium-to-large pores, suggesting poor pore connectivity [18]. With increasing dynamic cyclic dissolution time, the amplitude difference between the two peaks gradually decreases, and the boundary between small and medium-to-large pores becomes smoother, indicating improved pore connectivity. In contrast, under static immersion dissolution, the amplitude difference between the two peaks increases over time, and the boundary between small and medium-to-large pores remains distinct, suggesting that pore connectivity remains relatively poor. These observations highlight the distinct effects of dynamic and static dissolution on the sandstone pore structure: dynamic dissolution enhances pore connectivity by promoting the merging of small and large pores whereas static dissolution primarily enlarges existing pores without significantly improving connectivity. These findings offer valuable insights into the microstructural changes induced by different dissolution processes, critical for understanding sandstone degradation mechanisms and developing targeted conservation strategies.
Based on nuclear magnetic resonance (NMR) test results, Figure 7 illustrates the changes in porosity under different dissolution conditions.
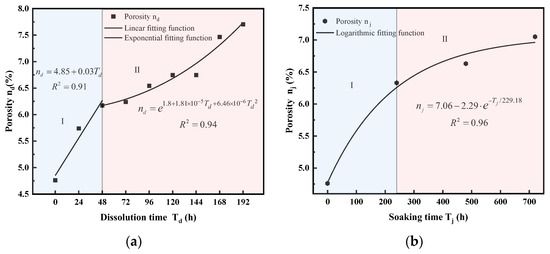
Figure 7.
Porosity variation under different dissolution conditions. (a) Dynamic cyclic dissolution, (b) static immersion dissolution.
As shown in the figure, sandstone porosity progressively increases with dissolution time under both dynamic cyclic dissolution and static immersion conditions. For dynamic cyclic dissolution, porosity shows a strong linear relationship with dissolution time during Stage I (for example, 0–48 h as shown in Figure 7a), followed by an exponential relationship in Stage II (for example, 48~192 h as shown in Figure 7a). In contrast, static immersion dissolution leads to an exponential increase in porosity over time. During Stage I (for example, 0~240 h as shown in Figure 7b) of static immersion, porosity rapidly increases to 1.33 times the initial value, while, in Stage II (for example, 240~720 h as shown in Figure 7b), the rate of porosity increase slows, reaching 1.48 times the initial value after 720 h of dissolution. These trends highlight the distinct mechanisms of porosity evolution under dynamic and static dissolution: dynamic dissolution accelerates porosity growth through enhanced pore connectivity whereas static immersion results in a more gradual increase, primarily driven by the enlargement of existing pores. These findings offer critical insights into the time-dependent degradation of sandstone under different environmental conditions, providing a scientific basis for preserving sandstone-based cultural heritage.
Furthermore, the proportions of small, medium, and large pores under dynamic and static dissolution conditions were analyzed as a function of dissolution time.
As shown in Figure 8, the proportions of small, medium, and large pores exhibit distinct trends under dynamic and static dissolution conditions.
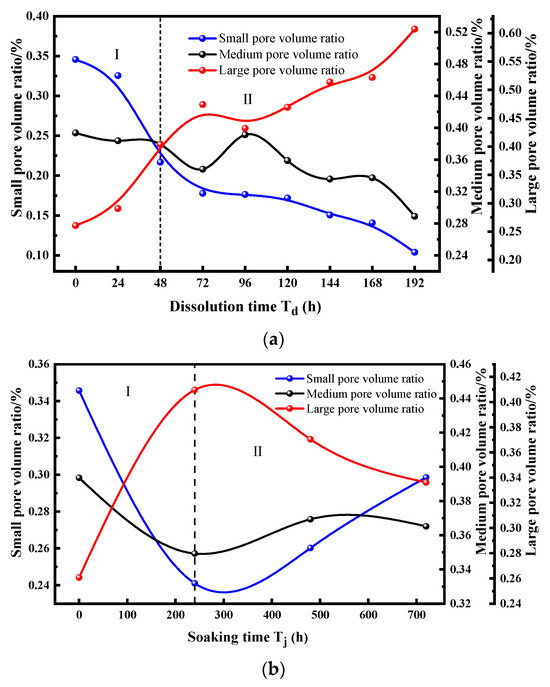
Figure 8.
Variation in small, medium, and large pore proportions under different dissolution conditions. (a) Dynamic cyclic dissolution, (b) dynamic cyclic dissolution.
During Stage I of dynamic cyclic dissolution, the proportion of small pores decreases, while large pores increase rapidly, and medium pores show a slight decline. This stage is primarily characterized by large pore development. In Stage II, the proportion of large pores continues to increase exponentially, albeit at a slower rate than in Stage I, while small pores decrease linearly, and medium pores decline due to the continued expansion of large pores. In contrast, under static immersion dissolution, Stage I is marked by a rapid and significant decrease in small pores, accompanied by a sharp increase in large pores and a slight decline in medium pores.
In Stage II, large pores gradually decrease while small pores begin to rise, and medium pores fluctuate. These trends highlight the distinct mechanisms of pore structure evolution under dynamic and static dissolution: dynamic dissolution promotes the merging of small pores into larger ones, enhancing pore connectivity, whereas static dissolution primarily enlarges existing pores without significantly improving connectivity. These findings offer critical insights into the time-dependent degradation of sandstone under different environmental conditions, providing a scientific basis for preserving sandstone-based cultural heritage.
To investigate microscopic changes in the pore structure, scanning electron microscopy (SEM) was used to analyze the surface morphology of sandstone samples subjected to dynamic and static dissolution, e.g., the SEM images in Figure 9.
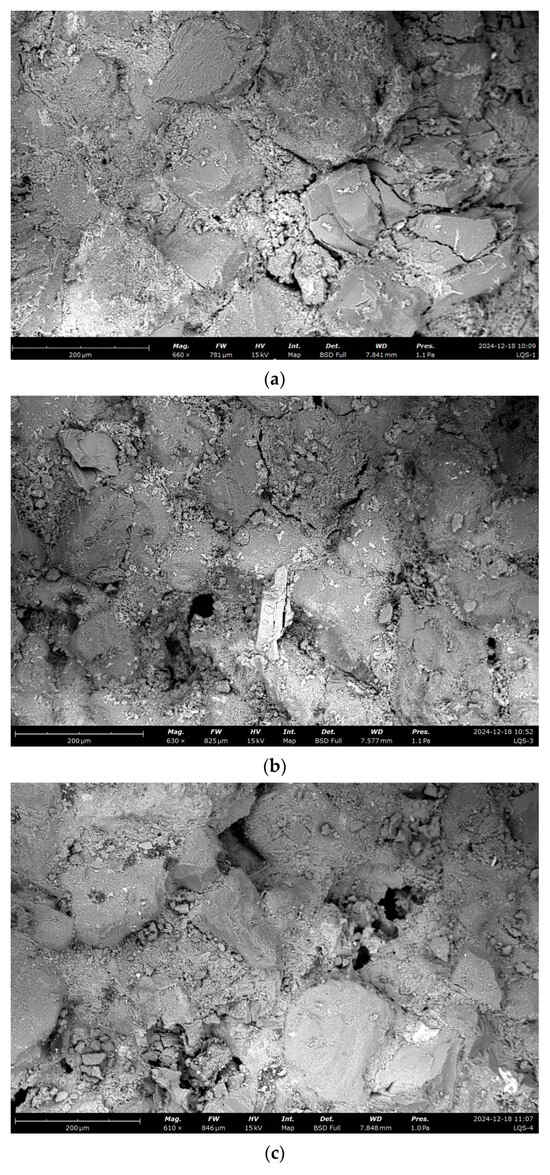
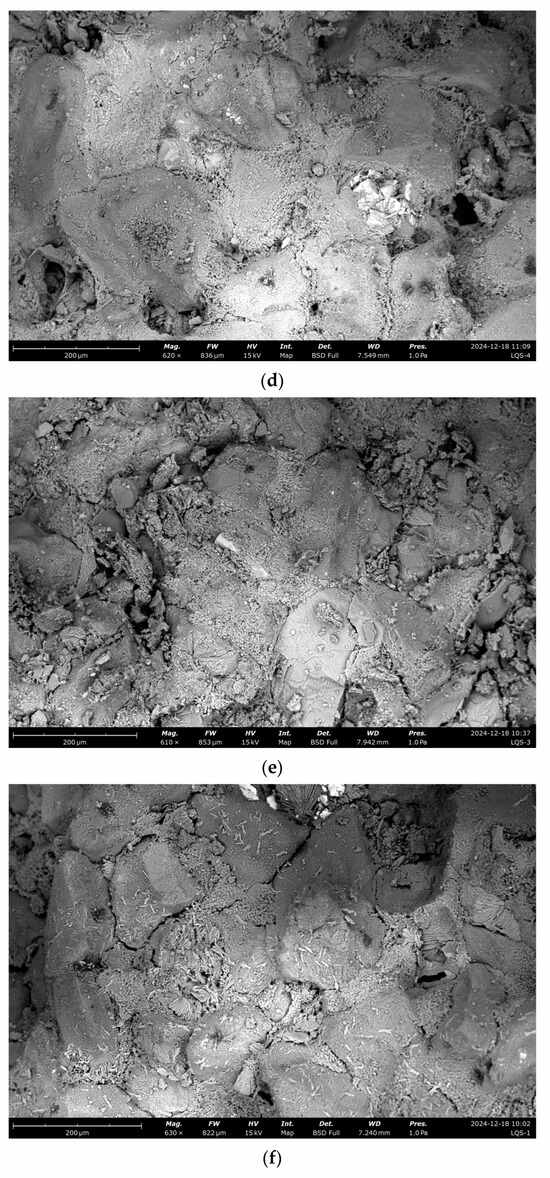
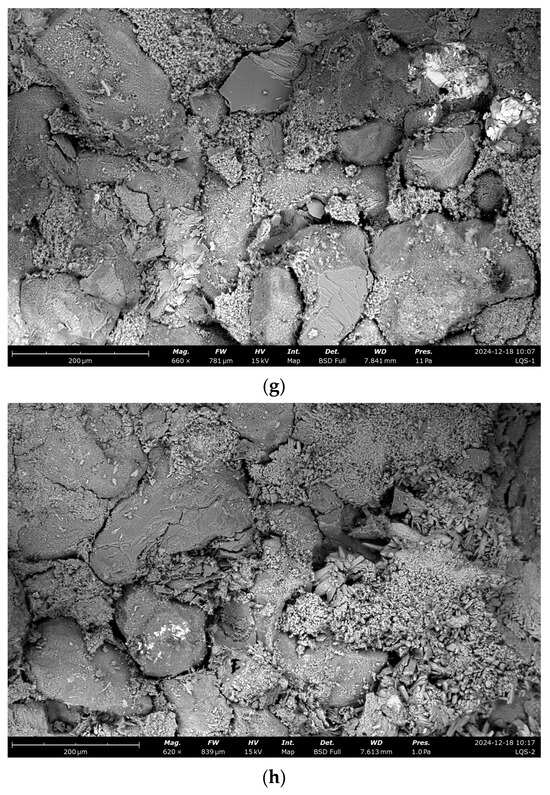
Figure 9.
SEM analysis under different dissolution conditions. (a) Undissolved, (b) dynamic dissolution 48 h, (c) dynamic dissolution 120 h, (d) dynamic dissolution 192 h, (e) undissolved, (f) static dissolution 240 h, (g) static dissolution 480 h, (h) static dissolution 720 h.
Scanning electron microscopy (SEM) observations reveal distinct changes in the sandstone microstructure induced by dissolution processes. In untreated samples, the sandstone structure appears intact, with cementitious fragments filling spaces between mineral grains. Under dynamic cyclic dissolution, dissolution cavities and pits form within minerals and at mineral boundaries, indicating significant material loss and enhanced pore connectivity. In contrast, static immersion dissolution causes the gradual disappearance of cementitious materials at mineral edges, accompanied by widening and deepening boundary cracks. Additionally, as immersion time increases, gaps between minerals become progressively filled with secondary minerals. These microstructural changes highlight the distinct degradation mechanisms under dynamic and static dissolution: dynamic dissolution promotes interconnected pore network development through dissolution feature formation, whereas static dissolution results in localized damage and secondary mineral deposition, partially mitigating further degradation. These findings offer critical insights into the microscopic processes driving sandstone deterioration, providing a scientific basis for preserving sandstone-based cultural heritage.
In summary, dissolution processes cause a gradual increase in porosity, with distinct two-stage evolution patterns observed under dynamic and static dissolution conditions.
(1) Dynamic Dissolution
Stage I: The formation of dissolution cavities and pits in the sandstone microstructure reduces the proportion of small pores and increases the proportion of large pores, leading to a linear increase in porosity.
Stage II: The development of larger and more extensive dissolution features drives an exponential increase in large pores, accompanied by a continued decrease in small pores and a slight reduction in medium pores. This stage is characterized by an exponential rise in porosity.
(2) Static Dissolution
Stage I: The rapid dissolution of intergranular cement due to solution diffusion causes a sharp increase in large pores and a significant decrease in small pores. This stage is marked by a rapid rise in porosity, with microscopic observations revealing dissolved debris and localized dissolution features.
Stage II: Mineral separation becomes more pronounced, with widening and deepening cracks at grain boundaries. Simultaneously, secondary minerals fill existing fractures, causing a gradual decline in large pores and an increase in small pores. Medium pores fluctuate due to the combined effects of dissolution and secondary mineral deposition, resulting in a decelerated increase in porosity.
These findings highlight the distinct mechanisms of pore structure evolution under dynamic and static dissolution, offering critical insights into sandstone degradation processes. Understanding these differences is crucial for developing targeted conservation strategies to mitigate the deterioration of sandstone-based cultural heritage under varying environmental conditions.
3.2. Variation Laws of Surface Mechanical Properties Under Different Corrosion Mechanisms
Dissolution-induced microstructural changes significantly weaken the mechanical properties of sandstone. Previous studies have shown a strong correlation between ultrasonic wave velocity, hardness, and sandstone mechanical strength [19,20]. Therefore, ultrasonic wave velocity and hardness measurements can serve as reliable indicators for indirectly assessing sandstone strength degradation following dissolution. Furthermore, rock failure is typically not a sudden, catastrophic event but a gradual process initiated at localized weak zones [21]. Consequently, when evaluating the durability and stability of stone-based cultural heritage, it is crucial to monitor not only the overall decline in mechanical strength but also the distribution and evolution of localized mechanical properties. A comprehensive understanding of both global and localized strength degradation is essential for developing effective conservation strategies to mitigate the progressive deterioration of sandstone structures.
3.2.1. Variation Laws of Surface Hardness Under Different Corrosion Mechanisms
Dissolution processes induce chemical reactions and material loss on sandstone surfaces, resulting in gradual softening and a decline in mechanical properties. To visualize the spatial distribution of surface hardness under static and dynamic dissolution conditions, hardness contour maps were generated, as shown in Figure 10 and Figure 11.
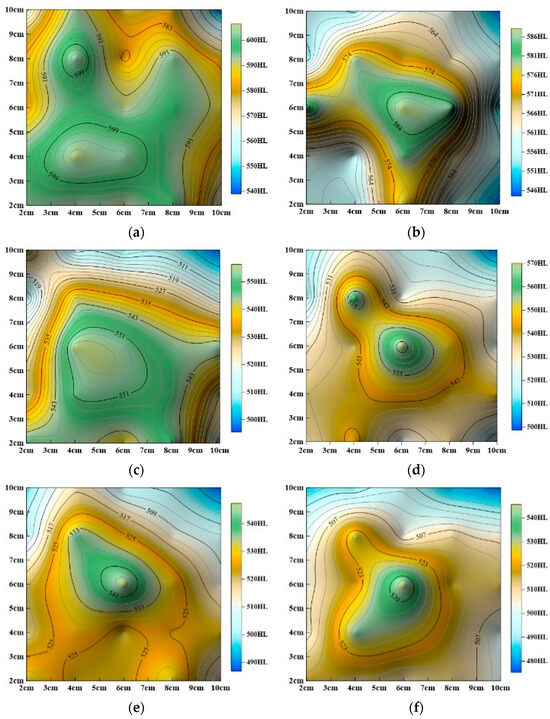
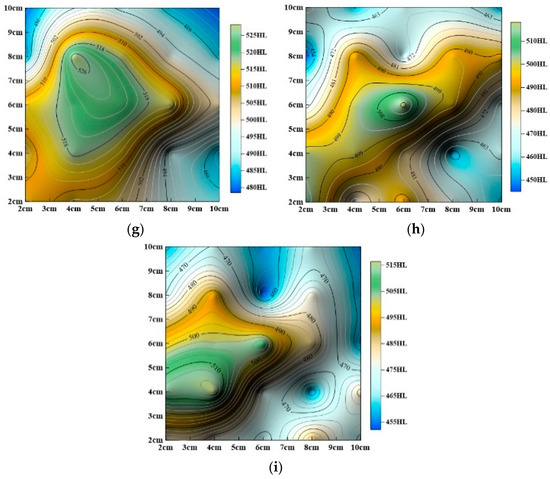
Figure 10.
Evolution of surface hardness contour maps with dynamic cyclic time (a) Undissolved, (b) dynamic dissolution for 24 h, (c) dynamic dissolution for 48 h, (d) dynamic dissolution for 72 h, (e) dynamic dissolution for 96 h, (f) dynamic dissolution for 120 h, (g) dynamic dissolution for 144 h, (h) dynamic dissolution for 168 h, (i) dynamic dissolution for 192 h.
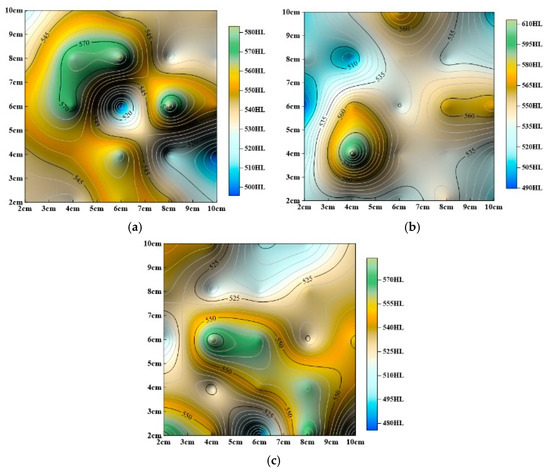
Figure 11.
Evolution of surface hardness contour maps with static immersion time. (a) Static dissolution for 240 h, (b) static dissolution for 480 h, (c) static dissolution for 720 h.
The studies above (Figure 10 and Figure 11) show that surface hardness gradually decreases under both dynamic and static dissolution conditions, but the specific patterns of change differ significantly:
(1) Dynamic Cyclic Dissolution:
As shown in Figure 10, overall surface hardness progressively decreases, with hardness values significantly lower than those of untreated samples. The lowest hardness values consistently appear in the upper-right corner while the highest values are located in the central region. Hardness decreases radially from the center toward the edges. Initially, hardness values in the inner and outer regions are similar, but, as cyclic dissolution continues, contour lines become more localized, the central high-hardness area shrinks, and overall surface hardness heterogeneity increases.
(2) Static Immersion Dissolution:
As shown in Figure 11, overall surface hardness also decreases, but, unlike dynamic dissolution, the rate of hardness reduction slows over time. After 240 h of immersion, the decrease in hardness becomes less pronounced. Static immersion significantly alters surface hardness distribution, even after 240 h. The locations of the highest and lowest hardness values shift continuously during immersion, with the highest values following a pattern of center (Figure 10a) → upper-left (Figure 11a) → lower-left (Figure 11b) → center (Figure 11c) and the lowest values following a pattern of upper-right (Figure 10a) → lower-right (Figure 11a) → upper-left (Figure 11b) → upper-right (Figure 11c). Despite these changes, the final hardness distribution tends to revert to a pattern similar to the initial untreated state.
To further elucidate differences in surface hardness evolution under static and dynamic dissolution, area ratios of hardness distribution (relative to the untreated state) were calculated from hardness contour maps. These ratios are summarized in Table 3 and visualized in Figure 12, illustrating changes in hardness distribution over time. This analysis highlights the increasing heterogeneity of surface hardness under dynamic dissolution and the more uniform but slower degradation under static dissolution, offering critical insights into the distinct mechanisms of sandstone deterioration under different environmental conditions.

Table 3.
Area proportion of hardness segments under different dissolution methods.
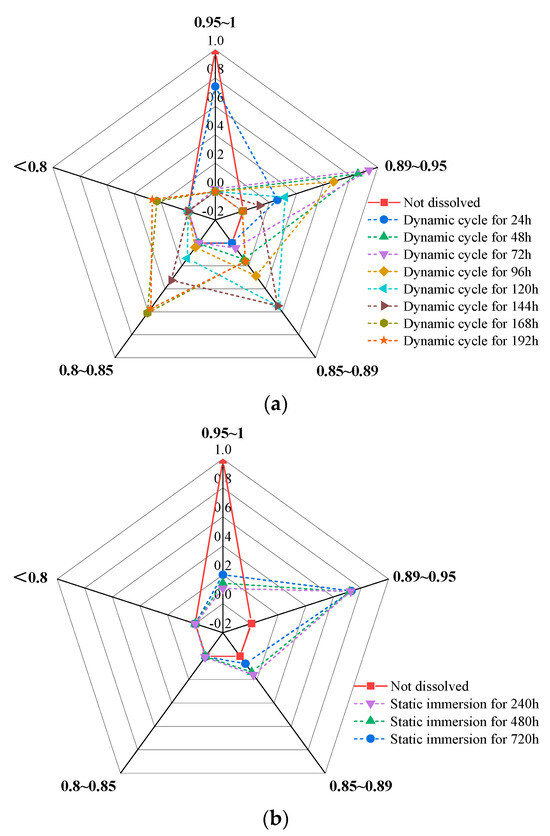
Figure 12.
Variation in hardness ratio under different dissolution conditions. (a) Dynamic cyclic dissolution, (b) static immersion dissolution.
Based on the data described above, area ratios of hardness distribution (expressed as the ratio of hardness to the initial untreated state) were calculated for static and dynamic dissolution conditions. These ratios are presented in the histogram in Figure 12, illustrating the evolution of hardness distribution over dissolution time. The histogram reveals distinct trends in surface hardness degradation under static and dynamic dissolution.
The above analysis shows that the distribution of hardness ratios progressively shifts toward lower values in a staged manner.
(1) Dynamic Cyclic Dissolution:
In the radar chart (Figure 12a), a finer distribution indicates a more concentrated pattern. As cyclic dissolution time increases, the hardness ratio decreases, and the distribution range gradually expands, reflecting an enlargement of the affected area. Critical turning points occur at 72 h, 120 h, and 168 h of cyclic dissolution, where hardness ratios exhibit relatively concentrated distributions. At 72 h, hardness ratios primarily range from 0.89 to 0.95. By 120 h, the main distribution shifts to 0.85–0.9. At 168 h, hardness ratios are no longer concentrated in a single range but are nearly evenly distributed between 0.8–0.85 and below 0.8. Combined with contour map analysis, the upper-right corner of the rock slab is identified as the region with the lowest dynamic dissolution intensity and the most vulnerable to potential damage.
(2) Static Immersion Dissolution:
As shown in Figure 12b, a significant decrease in hardness is observed at 240 h of immersion, with hardness ratios predominantly distributed between 0.89 and 0.95. Subsequently, although hardness ratios continue to decrease, the rate of decline slows, and no significant changes in hardness distribution are observed.
In conclusion, dynamic cyclic dissolution has a more pronounced impact on rock slab surface hardness compared to static immersion dissolution, making surface damage more likely under dynamic conditions. These findings highlight the importance of considering dissolution mechanisms when assessing the durability and stability of sandstone structures in acidic environments.
3.2.2. Variation Laws of Surface Longitudinal Wave Velocity Under Different Corrosion Mechanisms
P-wave velocity is a reliable indicator of pore and micro-fissure development within the rock, as well as the degree of specimen damage [22]. To compare changes in surface acoustic wave distribution under different dissolution conditions, acoustic wave contour maps for dynamic and static dissolution are presented in Figure 13 and Figure 14.
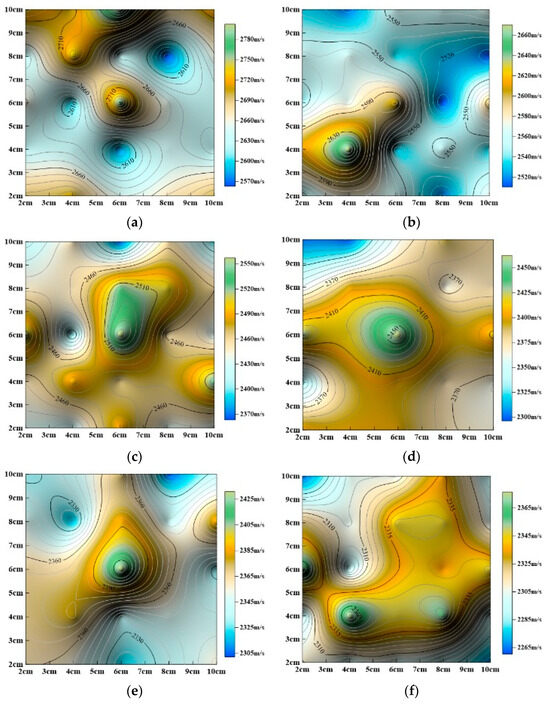
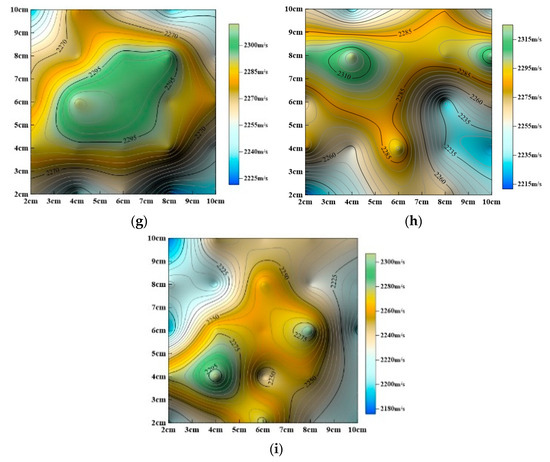
Figure 13.
Evolution of P–wave velocity contour maps with dynamic cyclic time. (a) Undissolved, (b) dynamic dissolution for 24 h, (c) dynamic dissolution for 48 h, (d) dynamic dissolution for 72 h, (e) dynamic dissolution for 96 h, (f) dynamic dissolution for 120 h, (g) dynamic dissolution for 144 h, (h) dynamic dissolution for 168 h, (i) dynamic dissolution for 192 h.
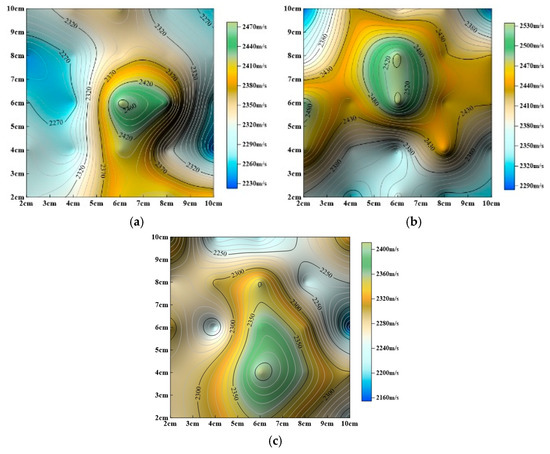
Figure 14.
Evolution of P–wave velocity contour maps with static immersion time (a) Static dissolution for 240 h, (b) static dissolution for 480 h, (c) static dissolution for 720 h.
The aforementioned studies (Figure 13 and Figure 14) show that surface P–wave velocity under different dissolution methods gradually decreases as corrosion progresses. However, the specific patterns of change differ significantly, as described below:
(1) Dynamic Cyclic Dissolution:
As shown in Figure 13, overall P-wave velocity gradually decreases with increasing dissolution time. Analysis shows that minimum P–wave velocity values become more concentrated and their distribution range becomes more defined. Maximum P–wave velocity values remain largely unchanged in position, consistently concentrated in the central region, but their distribution range gradually expands. The areas where minimum P-wave velocity occurs follow a sequence: periphery (Figure 13a) → upper right (Figure 13b and c) → upper left (Figure 13d) → upper right (Figure 13e) → upper left (Figure 13f and g) → lower right (Figure 13h) → upper left (Figure 13i), indicating significant fluctuations in the locations of minimum values. This suggests that dynamic cyclic dissolution induces fluctuating changes in the rock’s internal pore structure, attributed to the staged evolution of porosity.
(2) Static Immersion Dissolution:
As shown in Figure 14, overall P–wave velocity also decreases gradually with increasing immersion time. Maximum P–wave velocity values remain relatively concentrated in the central region, with no significant positional changes, but their distribution range gradually expands. Minimum P–wave velocity values consistently appear around the edges of the rock slab.
To further elucidate the differential impacts of static and dynamic dissolution on surface acoustic waves, area proportions of P–wave velocity distribution (relative to the non-corroded state) were calculated from P–wave velocity contour maps and are summarized in Table 4. Figure 15 illustrates changes in the area distribution proportions of different wave–velocity ratios over dissolution time. These analyses reveal distinct variation patterns of surface P–wave velocity under static and dynamic dissolution, highlighting the non-uniformity of acoustic wave distribution during weathering. These insights are crucial for understanding sandstone degradation mechanisms and developing targeted conservation strategies for sandstone-based cultural heritage.

Table 4.
Area proportion of surface wave velocity segments under different dissolution methods.
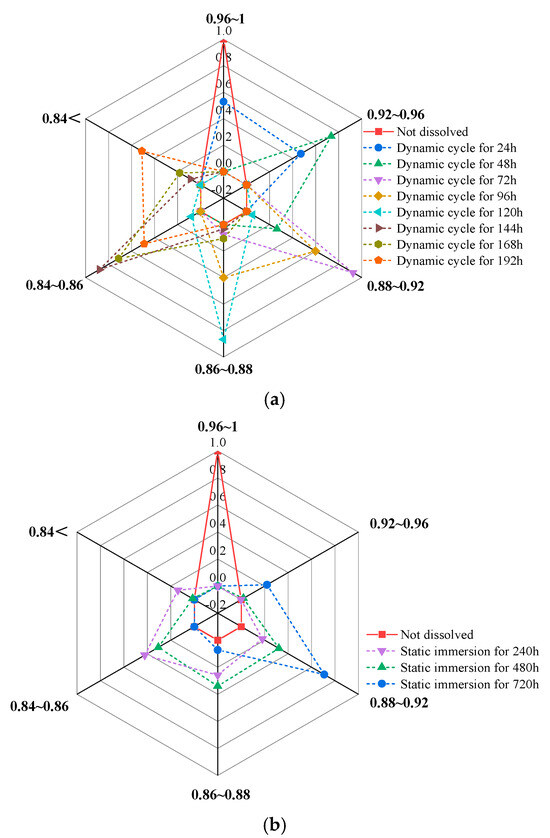
Figure 15.
Variation in P–wave velocity ratio under different dissolution conditions. (a) Dynamic cyclic dissolution, (b) static immersion dissolution.
The above analysis shows that the distribution of P-wave velocity ratios progressively shifts toward lower values, with distinct differences between static and dynamic dissolution.
(1) Dynamic Cyclic Dissolution:
The shift toward lower values occurs in a staged and continuous manner. As cyclic dissolution time increases, the P–wave velocity ratio decreases, and the distribution range gradually expands, indicating an enlargement of the affected area. Critical turning points occur at 48 h, 72 h, 120 h, and 144 h of cyclic dissolution (equivalent to the duration of acid rain in Southwest China), where P–wave velocity ratios exhibit relatively concentrated distributions. At 48 h, ratios primarily range from 0.92 to 0.96; at 72 h, they shift to 0.88–0.92; at 120 h, the main distribution moves to 0.85–0.9; and, at 144 h, ratios are predominantly in the range of 0.84–0.86. Combined with contour map analysis, the regions above the two sides of the rock slab are identified as areas with the lowest dynamic dissolution intensity.
(2) Static Immersion Dissolution:
A significant decrease in P-wave velocity is observed at 240 h of immersion, with ratios mainly distributed between 0.88 and 0.92. Subsequently, although P–wave velocity ratios continue to decrease, the distribution range broadens, and no concentrated areas of P–wave velocity ratios emerge.
In summary, dynamic cyclic dissolution results in a more complex P–wave velocity distribution, reflecting significant changes in the internal pore structure. Consequently, dynamic dissolution has a greater impact on pore structure evolution compared to static immersion dissolution. These findings highlight the importance of considering dissolution mechanisms when assessing sandstone degradation and developing preservation strategies for sandstone-based cultural heritage.
3.2.3. Research on Differences in Distribution Laws of Mechanical Parameters Under Different Corrosion Mechanisms
To comprehensively analyze the variation in corrosion influence range with static and dynamic corrosion time and the differences between static and dynamic corrosion, the distribution of mechanical strength affected by static and dynamic corrosion is analyzed by combining surface hardness and surface P–wave velocity analyses.
From the above analysis, the following can be concluded:
(1) During dynamic cyclic corrosion, the distributions of surface hardness and surface P–wave velocity show relatively consistent changes. Specifically, the maximum values of surface hardness and surface P–wave velocity are concentrated in the central area, and the positions of minimum values correspond closely, with the strength distribution showing good integrity. However, the distribution range of low P–wave velocity values gradually decreases, while that of low surface hardness values gradually increases.
(2) During static immersion corrosion, the consistency of surface hardness and surface P–wave velocity shows fluctuating changes. Initially, the distributions of surface hardness and P–wave velocity are inconsistent, but they gradually become consistent with increasing immersion time.
From the above analysis, it is evident that both static and dynamic corrosion lead to decreased surface mechanical strength, deteriorated surface stability, and reduced material durability. However, differences exist in the non-uniformity of mechanical strength distribution. The more concentrated the surface hardness ratio and P–wave velocity ratio are in one area, the more uniform they become.
Therefore, the following can be concluded:
(1) During dynamic cyclic corrosion, the distribution range of the surface hardness ratio gradually increases, and uniformity gradually decreases. The distribution range of the surface P–wave velocity ratio fluctuates, leading to fluctuating uniformity.
(2) During static immersion corrosion, the distribution range of surface hardness increases slightly, but the overall distribution remains relatively concentrated with good uniformity; the distribution range of surface P–wave velocity gradually expands, and uniformity gradually decreases.
For testing purposes, more non-uniform indicators are more suitable as danger signals. Therefore, for dynamic cyclic corrosion, it is advisable to focus on testing hardness distribution and hardness ratio changes; for static immersion corrosion, it is advisable to focus on testing P–wave velocity distribution and the P–wave velocity ratio.
3.3. Variation Regularities of Surface Moisture –Absorption Water –Content Curves Under Different Corrosion Mechanisms
When exposed to atmospheric conditions, sandstone materials are perpetually subjected to dynamic fluctuations in moisture content. Therefore, an indepth investigation into changes in the hygroscopic properties of sandstone following dissolution damage is of significant importance. The variations in moisture absorption curves under different dissolution conditions are shown in Figure 16.
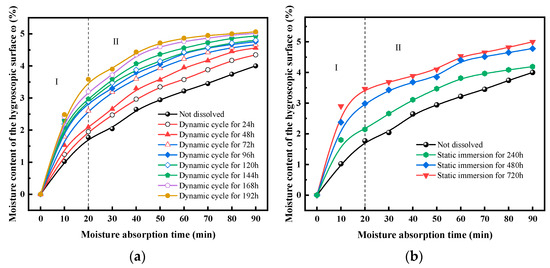
Figure 16.
Variation in surface moisture content under different dissolution conditions. (a) Dynamic cyclic dissolution, (b) static immersion dissolution.
Moisture absorption curves under different dissolution conditions exhibit a similar growth pattern, characterized by an initial rapid increase followed by gradual deceleration, eventually approaching equilibrium. The effects of static and dynamic dissolution on moisture absorption are as follows:
(1) Dynamic cyclic dissolution progressively enhances sandstone hygroscopicity. This is evidenced by the gradual increase in sandstone moisture content during absorption as the dynamic cyclic dissolution duration extends; longer cyclic dissolution times result in higher moisture absorption rates at any given time. A clear positive correlation exists between cyclic dissolution duration and hygroscopicity.
(2) Static immersion dissolution leads to a gradual increase in moisture absorption. This is reflected in higher moisture absorption rates of sandstone post-immersion compared to pre-dissolution rates at all stages, with longer immersion times resulting in greater surface moisture absorption rates.
The moisture content in porous materials, when in equilibrium with air humidity, is referred to as equilibrium moisture content. It is evident that equilibrium moisture content under dynamic cyclic dissolution increases significantly during the initial phase, with the rate of increase slowing down subsequently. In contrast, static immersion dissolution shows a smaller initial increase, followed by an accelerated rate of increase.
As shown in the figure, both static and dynamic dissolution moisture contents exhibit a rapid increase within the first 20 min, after which the rate of increase significantly slows down. Therefore, the growth stages of static and dynamic moisture content are divided into two phases. Additionally, to compare differences between them, the growth rates of different dissolution methods are contrasted.
As shown in Figure 17, the two phases of moisture content growth rate under static and dynamic dissolution exhibit a strong linear relationship with dissolution duration.
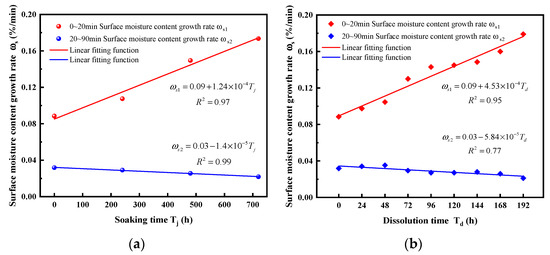
Figure 17.
Growth rate of surface moisture content under different dissolution conditions. (a) Dynamic cyclic dissolution, (b) static immersion dissolution.
Specifically,
(1) During the first phase of moisture content growth (0~20 min), the growth rates of static and dynamic dissolution increase linearly with dissolution damage. The growth rate of dynamic cyclic dissolution shows some fluctuations during this phase, ultimately increasing to 0.18%/min, 2.02 times the initial growth rate. In contrast, the growth rate of static immersion dissolution increases almost linearly, reaching 1.96 times the initial growth rate. This indicates that dynamic cyclic dissolution results in a greater moisture content increase during this phase compared to static immersion dissolution.
(2) In the second phase of moisture content growth (20~90 min), the growth rates of static and dynamic dissolution decrease linearly with dissolution damage. During this phase, the growth rates of static and dynamic dissolution decrease steadily without significant fluctuations, with dynamic cyclic dissolution decreasing more substantially. This suggests that dynamic cyclic dissolution allows moisture content to reach equilibrium more quickly during this phase.
3.4. Mass Loss Rate Under Different Dissolution Conditions
Mass loss indicates rock deterioration under dissolution conditions. The dissolution-induced mass loss rate is a key metric for evaluating rock resistance to dissolution and structural stability. In heritage stone conservation, surface mass loss accelerates the progressive degradation of grottoes and carved features. This necessitates a systematic analysis of sandstone mass loss dynamics under dissolution conditions. The mass loss rate is determined as follows:
Here, represents the initial mass and denotes the mass that decreases progressively during the corrosion process.
Figure 18 reveals that mass loss rates rise progressively over time in both dynamic and static dissolution processes. Dynamic cyclic dissolution shows phased increases in mass loss, with growth rates decreasing and a notable inflection point at 72 h. After 192 h of dynamic cycling, the mass loss rate reaches 0.74%. Static immersion dissolution exhibits a nearly linear mass loss trend, reaching 0.7% after 480 h. After 480 h of immersion, the rate of mass loss increase exhibits a slight deceleration.
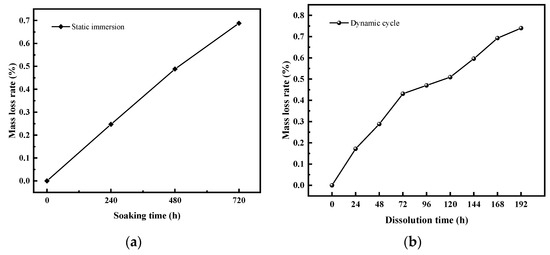
Figure 18.
Mass loss rate under different dissolution conditions. (a) Static immersion dissolution, (b) dynamic cyclic dissolution.
Comparative analysis shows that the average mass loss rate in the dynamic process (0.00385% per hour) is four times that of static dissolution (0.00097% per hour). This necessitates enhanced protective measures for exposed heritage stone surfaces to reduce morphological degradation due to mass loss.
4. Discussion
4.1. Analysis of Sandstone Corrosion Mechanism Under Acid Rain Exposure
The primary mineral constituents of the rock samples are quartz, plagioclase, and calcite. Among these, the main chemical compositions of plagioclase and calcite are CaAl2Si2O8, NaAl2Si3O8, and CaCO3, respectively. During the experimental process, the following chemical reactions predominantly occur [23,24]:
Due to the rapid flow of water after dynamic dissolution, the dissolution solution does not reach saturation, enabling the dissolution process to continue. Consequently, soluble minerals in the rock are continuously lost, increasing the number and size of pores within the rock. This is manifested as increased porosity with dynamic dissolution duration. Simultaneously, under prolonged rainwater immersion, sandstone minerals are gradually dissolved, and small pores and micro-fractures progressively develop, resulting in an exponential increase in porosity, with the rate of increase gradually slowing down.
The mechanism underlying the staged changes in porosity with increasing dissolution time is analyzed as follows:
(1) Under dynamic cyclic dissolution, erosion by the dynamic solution carries reaction products away with the water, driving the sandstone’s chemical reactions forward. This results in increased secondary minerals, some of which are carried away while others remain on the mineral surface. Overall, this leads to pore enlargement and an improved sandstone microstructure.
(2) In the initial stages of static immersion dissolution, the sandstone microstructure is severely disrupted, leading to a significant increase in porosity. As the immersion time progresses, secondary minerals generated by the reaction remain near the reaction sites, accumulating and precipitating, gradually blocking the pores. The deposition of secondary minerals closes the pores, reducing the contact area for subsequent reactions and slowing the rate of porosity increase.
(3) Therefore, dynamic dissolution significantly alters the sandstone microstructure, causing a substantial increase in porosity. In contrast, static dissolution impacts the sandstone microstructure more gradually, with the rate of porosity increase slowing over time.
4.2. Mechanistic Analysis of Sandstone Deterioration in Physical and Mechanical Properties Under Acid Rain Exposure
Physicochemical reactions lead to a continuous decline in particle bonding strength, gradual disappearance of soluble particles, increasing mass loss, and cumulative porosity increase, resulting in the progressive deterioration of overall physical and mechanical parameters. Due to significant changes in the pore structure, the number of pores increases and their size gradually enlarges. Macroscopically, this is reflected in the gradual rise in the moisture absorption curve with increasing porosity and the decline in mechanical properties as porosity increases. Previous studies indicate that the rapid water absorption phase is primarily controlled by capillary action (rapid water penetration along capillary pores) while the stable phase is mainly governed by water diffusion through interlayer pores in sandstone [25,26].
The following mechanistic analysis explains the deterioration of surface physical and mechanical properties during static and dynamic dissolution processes:
(1) Under dynamic cyclic dissolution, capillary pore numbers gradually increase and both surface hardness and surface longitudinal wave velocity decrease as porosity increases. In a dynamic environment, rock slab surface hardness is significantly affected, exhibiting uneven distribution. During the first phase, surface moisture content increases with dissolution time due to capillary action under dynamic cyclic dissolution. As internal capillary pores expand and the pore structure becomes more uniform and interconnected after dynamic cyclic dissolution, the second phase allows for faster water saturation. Fluctuations in porosity proportion result in variations in surface longitudinal wave velocity uniformity.
(2) Under static immersion dissolution, porosity also gradually increases and both surface hardness and surface longitudinal wave velocity decrease with increasing porosity. Since static immersion primarily relies on solution diffusion, surface hardness decreases relatively uniformly and stably. Additionally, as porosity increases, surface moisture content gradually rises with immersion time. With prolonged immersion, porosity increases logarithmically and the rate of increase slows, reflected in the moisture absorption curve as gradual deceleration in moisture content growth. The improvement in pore connectivity under static immersion is limited, making surface longitudinal wave velocity distribution less uniform than surface hardness distribution.
In summary, dynamic dissolution causes more severe and uneven damage to sandstone whereas static dissolution has a slower and more uniform impact. Acidic dissolution significantly threatens the long-term preservation of sandstone materials, necessitating a detailed investigation into changes in the microscopic pore structure, mechanical properties, and hygroscopic behavior under different dissolution conditions. By systematically studying pore structure evolution, mechanical strength decline, and hygroscopicity enhancement under static and dynamic dissolution processes, this research aims to establish a comprehensive framework for assessing the durability of sandstone–based cultural heritage. These insights are crucial for designing targeted preservation strategies to address the specific challenges posed by acidic environments, ensuring the sustainable protection of these invaluable historical structures.
5. Conclusions
Static dissolution refers to the chemical weathering process in which sandstone is submerged in stagnant acidic solutions for extended durations. Dynamic dissolution involves the combined chemical–mechanical erosion process that takes place when sandstone is exposed to flowing acidic aqueous solutions. This study compares the effects of static immersion and dynamic cyclic dissolution on the sandstone surface pore structure, mechanical strength distribution, and moisture absorption properties, revealing the mechanisms and differences between the two dissolution methods.
The main conclusions are as follows:
(1) Under different dissolution methods, sandstone porosity shows a gradual increasing trend, but the effects of dynamic and static dissolution on pore structure modification differ significantly. Under dynamic cyclic dissolution, porosity increase can be divided into two phases: Phase I is characterized by linear growth, with the proportion of large pores gradually increasing; Phase II shows exponential growth in the proportion of large pores, leading to an exponential increase in porosity. In contrast, under static immersion dissolution, porosity increases logarithmically, with the growth rate gradually slowing, and the proportion of large pores initially rises rapidly before gradually declining. Overall, dynamic cyclic dissolution has a significantly stronger effect on pore structure modification than static dissolution.
(2) Under both dissolution methods, surface hardness and longitudinal wave velocity decrease gradually with increasing dissolution time, but their distributions are affected differently. For dynamic cyclic dissolution, surface hardness decreases and shifts toward lower values, with a decline in distribution uniformity; longitudinal wave velocity also shifts toward lower values, with significant fluctuations in uniformity. For static immersion dissolution, surface hardness decreases and shifts toward lower values, with little change in distribution uniformity; longitudinal wave velocity decreases and shifts toward lower values, with an expanding distribution range and gradually declining uniformity (Figure 12 and Figure 15). Dynamic cyclic dissolution has a more intense and uneven impact on sandstone mechanical properties, while static immersion dissolution has a slower and more uniform effect.
(3) Moisture absorption curves under different dissolution methods rise gradually with increasing porosity. They can be divided into two phases (Figure 16 and Figure 17): Phase I (0–20 min) shows an increasing rate of moisture absorption with a longer dissolution time and higher porosity; Phase II (20–90 min) exhibits a decreasing rate of moisture absorption with a longer dissolution time and higher porosity, indicating that dissolution accelerates the attainment of equilibrium in surface moisture content. Additionally, experiments demonstrate that dynamic cyclic dissolution results in greater moisture absorption and a faster attainment of equilibrium.
(4) This study provides critical insights into the degradation mechanisms of sandstone under dynamic and static dissolution conditions, which are prevalent in high-temperature, humid, and acidic environments like Southwest China. The findings offer a scientific basis for developing targeted preservation strategies for ancient sandstone structures, such as rock-cut caves and stone carvings, which are integral to cultural heritage. By understanding the differential effects of dynamic (acid rain) and static (water immersion) dissolution on pore structure, mechanical properties, and hygroscopic behavior, conservation efforts can be tailored to mitigate specific degradation patterns. This research is particularly relevant for heritage sites exposed to acid rain and prolonged moisture, enabling the design of preventive measures to enhance the long-term durability and stability of these invaluable structures.
Author Contributions
Conceptualization, Q.L. and C.L.; methodology, C.L. and D.H.; software, D.H. and C.W.; validation, Q.L. and C.W.; formal analysis, C.L. and D.H.; investigation, Q.L. and D.H.; writing—original draft preparation, Q.L. and C.L.; writing—review and editing, C.L., D.H. and C.W.; funding acquisition, C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 41272300).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hong, J.; Zhu, Y.; Zhang, Y.; Huang, J.Z.; Peng, N.B. Differentiation Study of the Damage Characteristics of Rock Cultural Heritage Sites Due to the Sulfate Weathering Process. Appl. Sci. 2023, 13, 12831. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, L.; Su, X.B.; Zhao, L.X.; Wang, Y. Micro-CT characterization on pore structure evolution of low-permeability sandstone under acid treatment. Appl. Geochem. 2023, 152, 105633. [Google Scholar] [CrossRef]
- Liang, Y.L.; Huo, R.K.; Song, S.S.; Song, Z.P.; Li, G.Y.; Mu, Y.H. Effect of the surface-area-to-volume ratio on dissolution and deterioration of acid-corroded sandstone. J. Build. Eng. 2024, 86, 108789. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, D.; Xu, J.; Cheng, L.; Li, J.X. Corrosion and degradation of feldspar sandstone in acidic environment. Coal Geol. Explor. 2020, 48, 165–173. [Google Scholar]
- Li, P.; Liu, J.; Li, G.H.; Zhu, J.B.; Liu, S.G. Experimental study for shear strength characteristics of sandstone under water-rock interaction effects. Rock Soil Mech. 2011, 32, 380–386. [Google Scholar]
- Han, T.L.; Chen, Y.S.; Shi, J.P.; Yu, J.Q. Experimental study of mechanical characteristics of sandstone subjected to hydrochemical erosion. Chin. J. Rock Mech. Eng. 2013, 32, 3065–3072. [Google Scholar]
- Xue, Y.C.; Xu, T.; Heap, M.J.; Zhu, W.C.; Ranjith, P.G.; Li, Z.G. Effect of pH on primary and secondary crack propagation in sandstone under constant stress (creep) loading. Constr. Build. Mater. 2024, 421, 135727. [Google Scholar] [CrossRef]
- Tamimi, A.K.; Abdalla, J.A.; Sakka, Z.I. Prediction of long-term chloride diffusion of concrete in harsh environment. Constr. Build. Mater. 2008, 22, 829–836. [Google Scholar] [CrossRef]
- TorrentiI, J.M.; Granger, L.; Diruy, M.; Genin, P. Modeling concrete shrinkage under variable ambient conditions. ACI Mater. J. 1999, 96, 35–39. [Google Scholar]
- Zhang, N.; He, M.; Liu, P. Water vapor sorption and its mechanical effect on clay-bearing conglomerate selected from China. Eng. Geol. 2012, 141, 1–8. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Qin, W.J.; Jin, A.B.; Wu, H.Y.; Chen, Z. Research and analysis of the impact of the pore structure on the mechanical properties and fracture mechanism of sandstone. Mater. Today Commun. 2024, 38, 107753. [Google Scholar] [CrossRef]
- Wang, H.S.; Bi, J.; Zhao, Y.; Wang, C.L.; Ma, J.B. NMR-based analysis of the effect of moisture migration on sandstone pore structure under alternating wetting and drying conditions. Int. J. Min. Sci. Technol. 2024, 34, 1135–1150. [Google Scholar] [CrossRef]
- Rabat, Á.; Tomás, R.; Cano, M. Evaluation of mechanical weakening of calcarenite building stones due to environmental relative humidity using the vapour equilibrium technique. Eng. Geol. 2020, 278, 105849. [Google Scholar] [CrossRef]
- Zhang, A.; Fang, Y.; Chen, J.; Fan, Z. Dissolution experiment and numerical simulation analysis for condensation water on carbonate rocks. Chin. J. Rock Mech. Eng. 2014, 33, 3648–3656. [Google Scholar]
- Chen, W.C.; Li, L.; Shao, M.S.; Liang, X.Z.; Olatayo, A.L. Experimental study on carbonate dissolution and erosion effect under attack of simulated sulphuric acid rain. Chin. J. Geotech. Eng. 2017, 39, 2058–2067. [Google Scholar]
- Wang, T.J.; Jin, L.S.; Li, Z.K.; Lam, K.S. A Modeling study on acid rain and recommended emission control strategies in China. Atmos. Environ. 2000, 34, 4467–4477. [Google Scholar] [CrossRef]
- Qin, Q.C.; Li, K.G.; Li, M.L.; Li, W.; Liu, B. Study on the deterioration mechanism of dolomite microscopic damage based on NMR technique. Chin. J. Rock Mech. Eng. 2022, 41, 2944–2954. [Google Scholar]
- Ghomeshi, S.; Kryuchkov, S.; Kantzas, A. An investigation into the effects of pore connectivity on T2 NMR relaxation. J. Magn. Reson. 2018, 289, 79–91. [Google Scholar] [CrossRef]
- El-Gohary, M.A. Evaluation of treated and un-treated Nubia Sandstone using ultrasonic as a non-destructive technique. J. Archaeol. Sci. 2013, 40, 2190–2195. [Google Scholar] [CrossRef]
- Corkum, A.G.; Jeans, B.; Ivars, D.M. Leeb hardness test as a tool for joint wall compressive strength (JCS) evaluation. Eng. Geol. 2025, 344, 107851. [Google Scholar] [CrossRef]
- Liu, X.H.; Zheng, Y.; Guo, J.Y.; Hao, Q.J.; Xue, Y. Deformation and failure characteristics of loading and unloading rock based on volume crack strain. Earth Sci. 2022, 10, 911823. [Google Scholar] [CrossRef]
- Aghaei, H.; Penkov, G.M.; ASolomoichenko, D.; Toorajipour, A.; Petrakov, D.G.; Jafarpour, H.; Ghosh, S. Density-dependent relationship between changes in ultrasonic wave velocities, effective stress, and petrophysical-elastic properties of sandstone. Ultrasonics 2023, 132, 106985. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yan, Z.Y.; Chen, D.Y.; He, Y.; Liang, Z.Z.; Li, Y.H.; Han, Y.Y. The mechanism of mineral dissolution and its impact on pore evolution of CO2 flooding in tight sandstone: A case study from the Chang 7 member of the Triassic Yanchang formation in the Ordos Basin, China. Geoenergy Sci. Eng. 2024, 235, 212715. [Google Scholar] [CrossRef]
- Yu, Z.C.; Yang, S.Y.; Liu, K.Y.; Zhuo, Q.G.; Yang, L.L. An Experimental and Numerical Study of CO2–Brine-Synthetic Sandstone Interactions under High-Pressure (P)–Temperature (T) Reservoir Conditions. Appl. Sci. 2019, 9, 3354. [Google Scholar] [CrossRef]
- Akkay, Y.; Shah, S.P.; Ghandehari, M. Influence of fiber dispersion on the performance of microfiber reinforced cement composites. ACI Spec. Publ. 2003, 216, 1–18. [Google Scholar]
- Tyson, B.M.; Al-Rub, R.K.A.; Yazdanbakhsh, A.; Grasley, Z. Carbon nanotubes and carbon nanofibers for enhancing the mechanical properties of nanocomposite cementitious materials. J. Mater. Civ. Eng. 2011, 23, 1028–1035. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).