Artificial Intelligence for Image-Based Identification of Osteoclasts and Assessment of Their Maturation—Using the OC_Identifier
Abstract
Featured Application
Abstract
1. Introduction
AI-Based Image Analysis in (Biomaterials) Research and Medicine
2. Materials and Methods
2.1. Create an Osteoclast Specific Dataset
2.1.1. Culture of Human Monocytes and Osteoclasts
2.1.2. Monitoring of the Differentiation Process by Microscopic Analysis
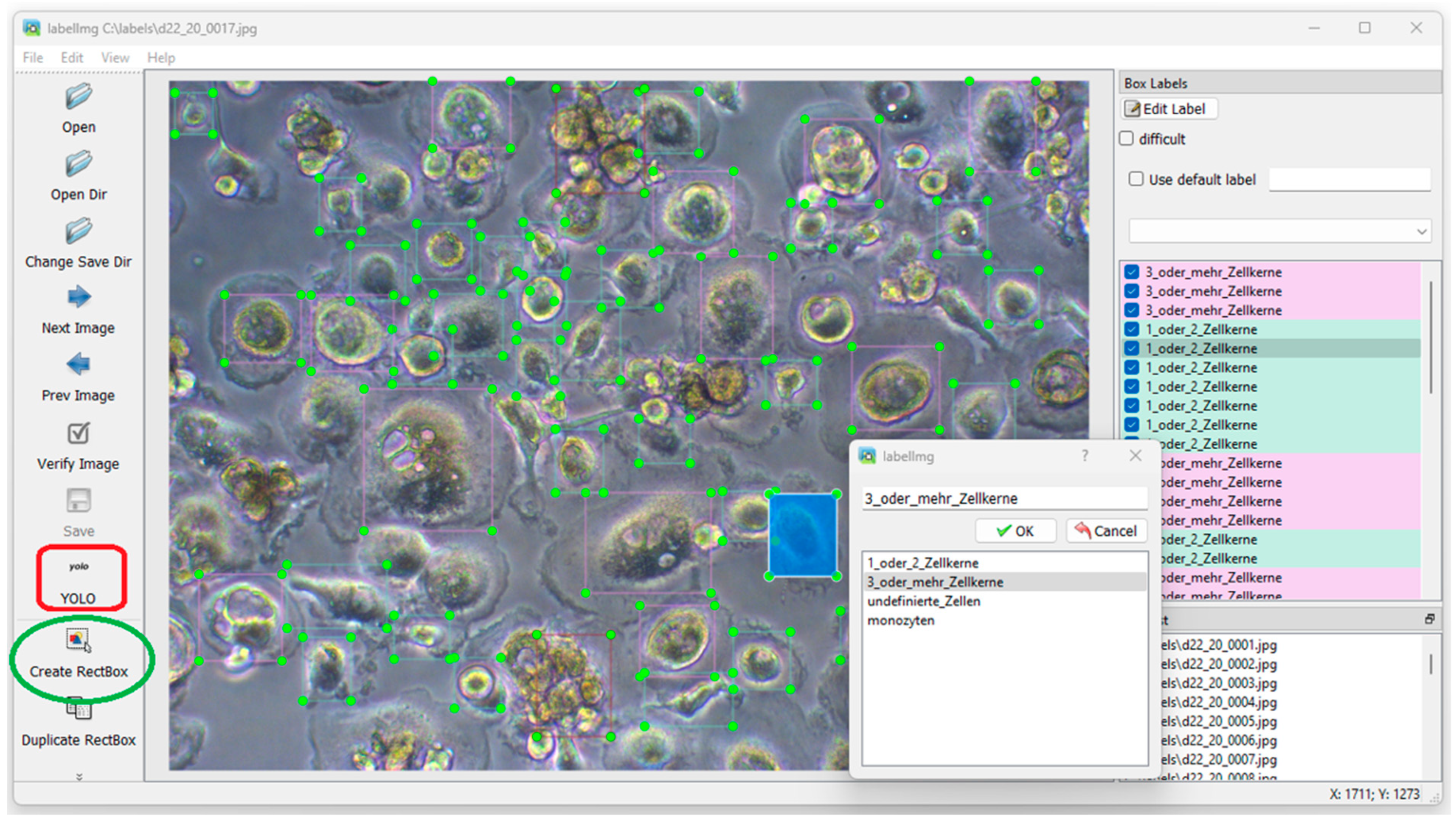
2.1.3. Performing Manual Labelling with LabelImg
2.2. Training of Osteoclast AI Based on YOLOv5
2.3. Osteoclast Cultivation with Varying Differentiation Additives
2.4. Biochemical Analysis
3. Results
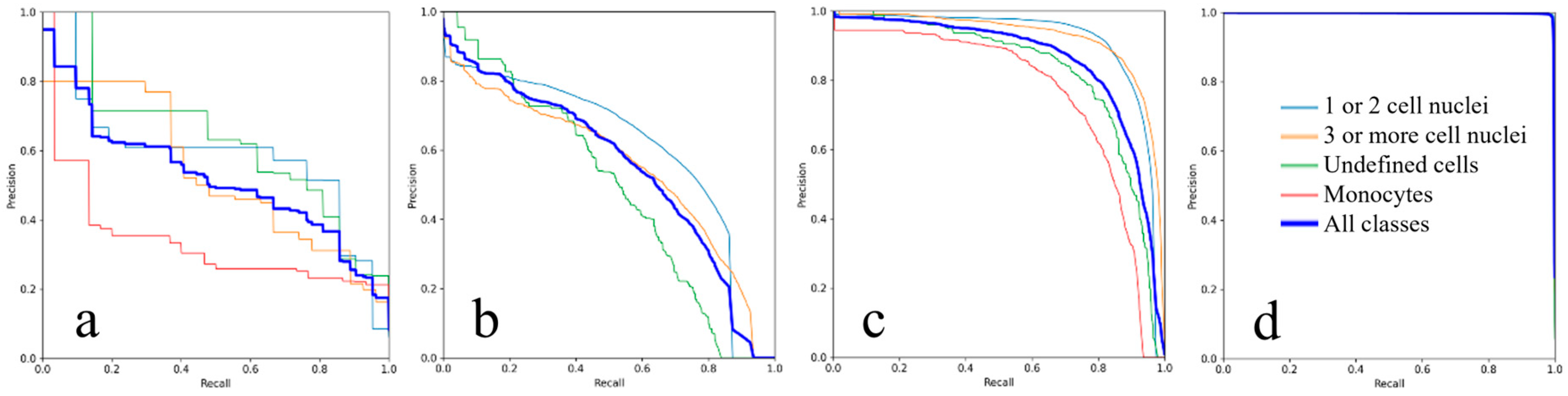
3.1. Evaluation of the AI Training
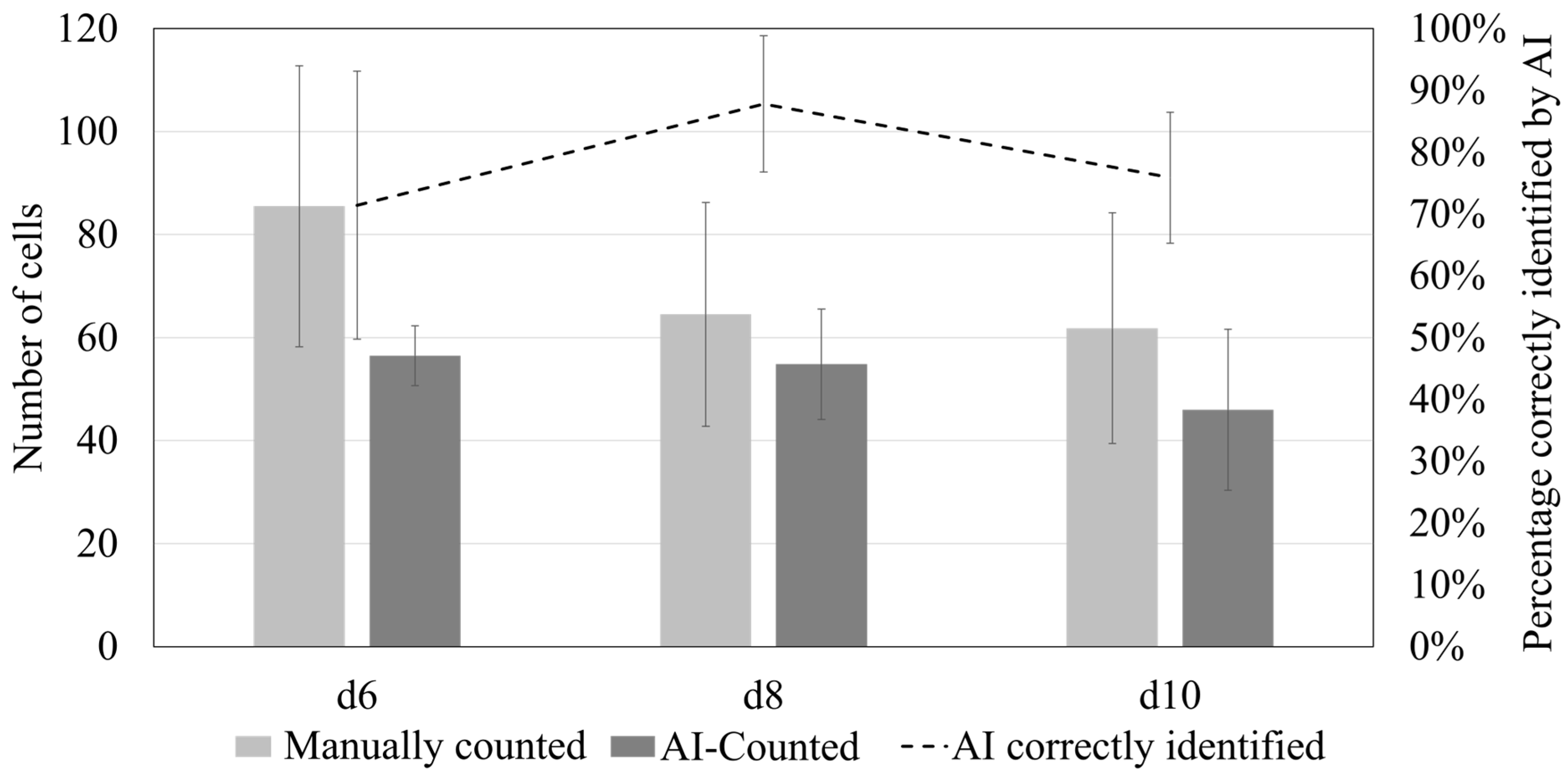
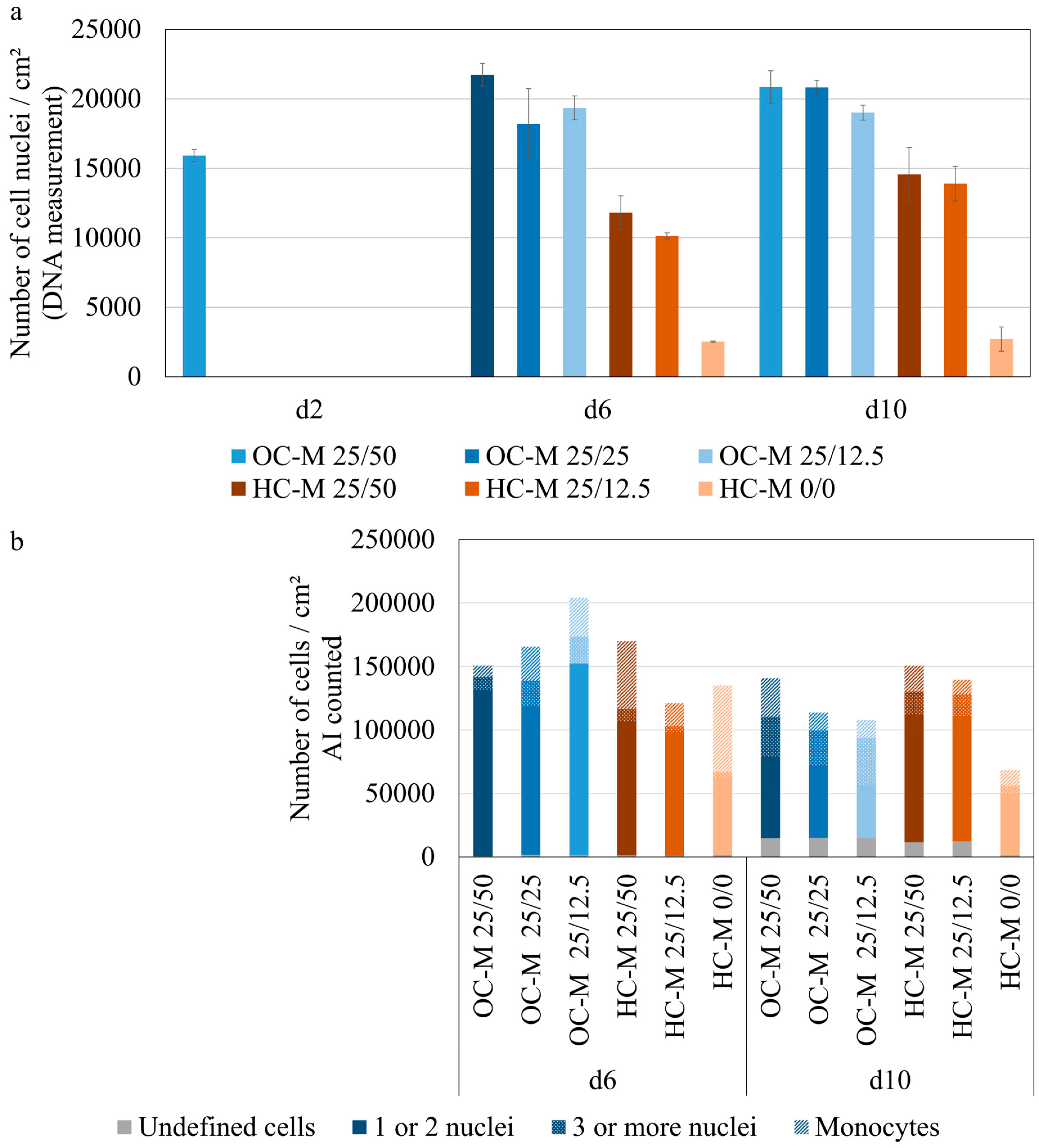
3.2. Comparison of Osteoclast Images: Manual Counting vs. AI Recognition
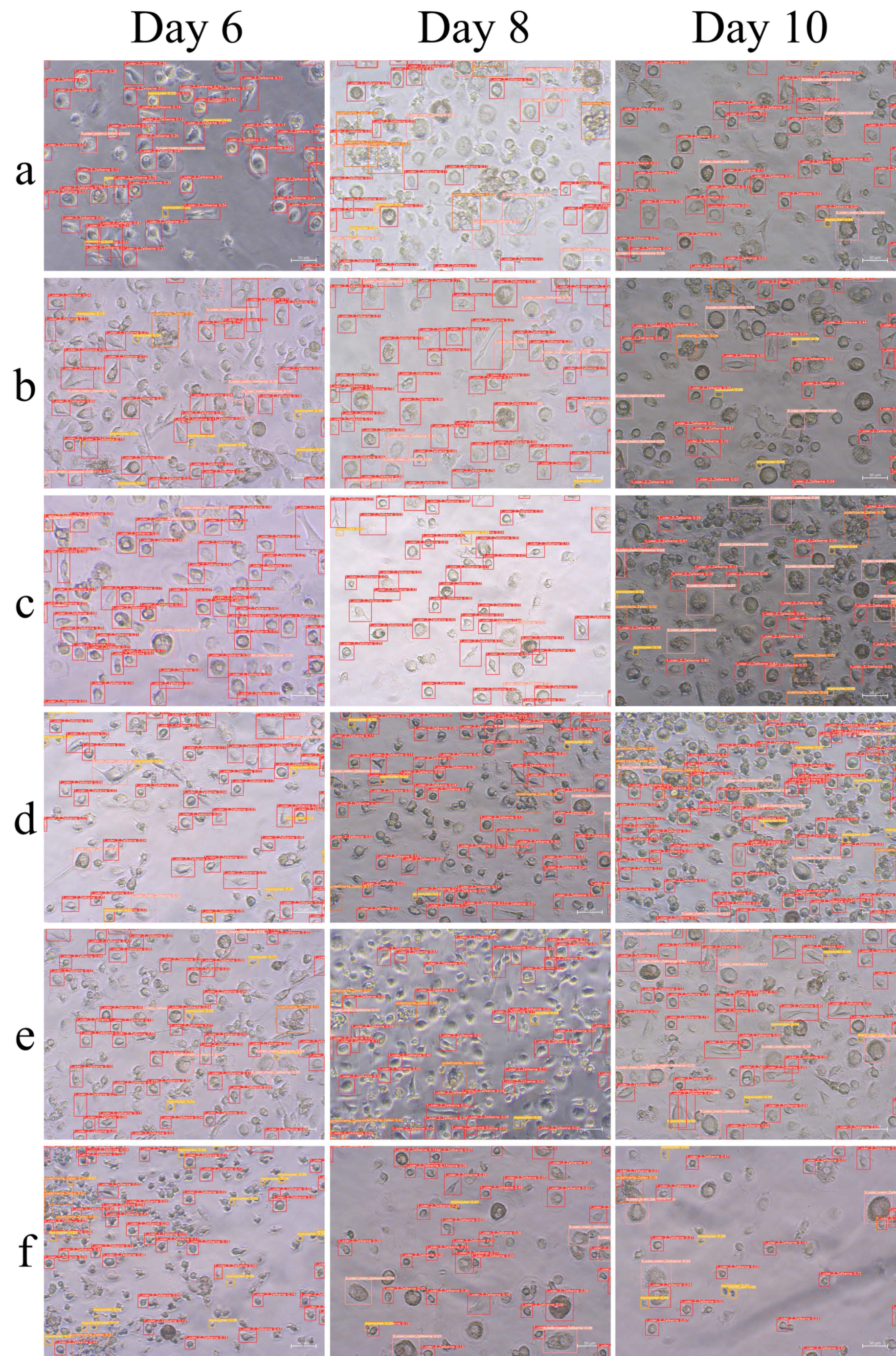
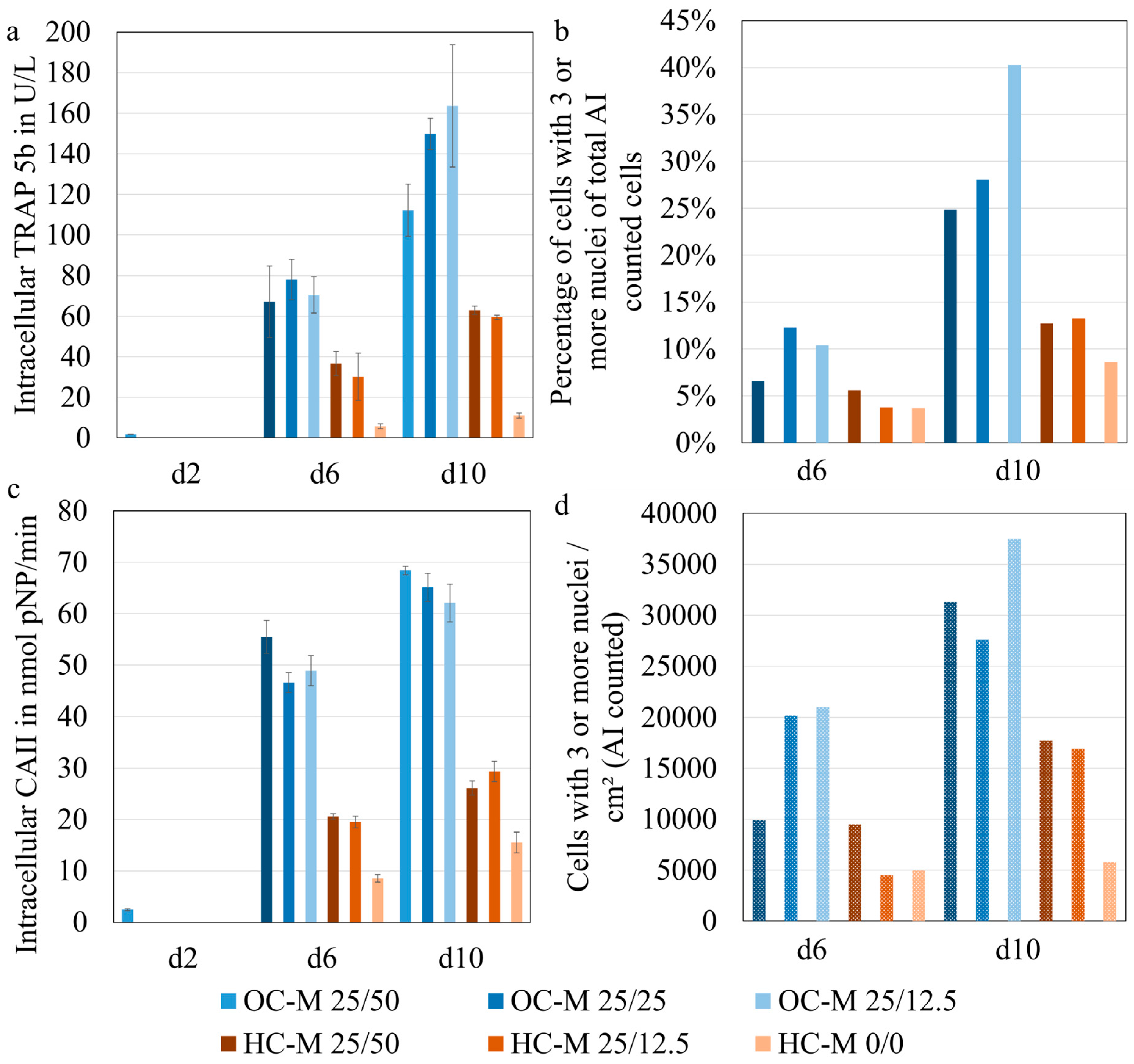
3.3. Evaluation of Osteoclast Image Analysis Using Osteoclast AI
3.4. Determining the Degree of Maturation of Osteoclasts
4. Discussion
4.1. AI for Osteoclast Identification and Determination of Maturation
4.2. The YOLO Framework, Limitations and Future Solutions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| DNA | Deoxyribonucleic acid |
| CA II | Carbonic anhydrase II |
| NLP | Natural language processing |
| CV | Computer vision |
| PCR | Polymerase chain reaction |
| GPU | Graphics processing unit |
| BSA | Bovine serum albumin |
| EDTA | Ethylenediaminetetraacetic acid |
| PBS | Phosphate-buffered saline |
| FCS | Fetal calf serum |
| hMSC | Human mesenchymal stem cells |
| OC-M | Osteoclast medium |
| HC-M | hMSC-conditioned medium |
| MON | Monocytes |
| M-CSF | Macrophage colony-stimulating factor |
| RANKL | Receptor activator of nuclear factor kappa-Β ligand |
| TRAP | Tartrate-resistant acid phosphatase |
| OC | Osteoclast |
References
- Nijweide, P.J.; van der Plas, A.; Scherft, J.P. Biochemical and histological studies on various bone cell preparations. Calcif. Tissue Int. 1981, 33, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Auf’mkolk, B.; Hauschka, P.V.; Schwartz, E.R. Characterization of human bone cells in culture. Calcif. Tissue Int. 1985, 37, 228–235. [Google Scholar] [CrossRef]
- Heinemann, C.; Heinemann, S.; Worch, H.; Hanke, T. Development of an osteoblast/osteoclast co-culture derived by human bone marrow stromal cells and human monocytes for biomaterials testing. Eur. Cell Mater. 2011, 21, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Schedle, A.; Matejka, M.; Rausch-Fan, X.; Andrukhov, O. The proliferation and differentiation of osteoblasts in co-culture with human umbilical vein endothelial cells: An improved analysis using fluorescence-activated cell sorting. Cell Mol. Biol. Lett. 2010, 15, 517–529. [Google Scholar] [CrossRef]
- Okamoto, M.; Inagaki, Y.; Okamura, K.; Uchihara, Y.; Saito, K.; Kawai, A.; Ogawa, M.; Kido, A.; Mori, E.; Tanaka, Y. Identifying the best reference gene for RT-qPCR analyses of the three-dimensional osteogenic differentiation of human induced pluripotent stem cells. Bone Rep. 2024, 23, 101816. [Google Scholar] [CrossRef]
- Yang, X.; Hatfield, J.T.; Hinze, S.J.; Mu, X.; Anderson, P.J.; Powell, B.C. Bone to pick: The importance of evaluating reference genes for RT-qPCR quantification of gene expression in craniosynostosis and bone-related tissues and cells. BMC Res. Notes 2012, 5, 222. [Google Scholar] [CrossRef]
- Henss, A.; Hild, A.; Rohnke, M.; Wenisch, S.; Janek, J. Time of flight secondary ion mass spectrometry of bone—Impact of sample preparation and measurement conditions. Biointerphases 2016, 11, 02A302. [Google Scholar] [CrossRef] [PubMed]
- Kern, C.; Ray, S.; Gelinsky, M.; Bellew, A.T.; Pirkl, A.; Rohnke, M. New insights into ToF-SIMS imaging in osteoporotic bone research. Biointerphases 2020, 15, 031005. [Google Scholar] [CrossRef]
- Thomas, J.; Worch, H.; Kruppke, B.; Gemming, T. Contribution to understand the biomineralization of bones. J. Bone Miner. Metab. 2020, 38, 456–468. [Google Scholar] [CrossRef]
- Brent, M.B.; Emmanuel, T. Contemporary Advances in Computer-Assisted Bone Histomorphometry and Identification of Bone Cells in Culture. Calcif. Tissue Int. 2022, 112, 1–12. [Google Scholar] [CrossRef]
- Lu, L.; Ono, N.; Welch, J.D. Linking transcriptome and morphology in bone cells at cellular resolution with generative AI. J. Bone Miner. Res. 2024, 40, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Xu, J.; Wen, X.; Sun, L.; Xiu, Y.; Zhang, Z.; Liu, T.; Zhang, D.; Wang, P.; Xing, D. The future of bone regeneration: Artificial intelligence in biomaterials discovery. Mater. Today Commun. 2024, 40, 109982. [Google Scholar] [CrossRef]
- Kolomenskaya, E.; Butova, V.; Poltavskiy, A.; Soldatov, A.; Butakova, M. Application of Artificial Intelligence at All Stages of Bone Tissue Engineering. Biomedicines 2024, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Paek, K.; Kim, S.; Tak, S.; Kim, M.K.; Park, J.; Chung, S.; Park, T.H.; Kim, J.A. A high-throughput biomimetic bone-on-a-chip platform with artificial intelligence-assisted image analysis for osteoporosis drug testing. Bioeng. Transl. Med. 2023, 8, e10313. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Mak, K.L.; Chen, L.Y.; Chou, M.P.; Ho, C.K. Heterogeneity of human blood monocyte: Two subpopulations with different sizes, phenotypes and functions. Immunology 1992, 77, 298. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC1421624/ (accessed on 22 November 2024). [PubMed]
- Bar-Shavit, Z. The osteoclast: A multinucleated; hematopoietic-origin; bone-resorbing osteoimmune cell. J. Cell Biochem. 2007, 102, 1130–1139. [Google Scholar] [CrossRef]
- Rubin, J.; Greenfield, E.M. Osteoclast: Origin and Differentiation. Bone Resorpt. 2005, 1–23. Available online: https://api.semanticscholar.org/CorpusID:4681387 (accessed on 27 January 2025).
- Xu, F.; Teitelbaum, S.L. Osteoclasts: New insights. Bone Res. 2013, 1, 11–26. [Google Scholar] [CrossRef]
- Marie, A.; Ockernahl, Ø. Development of a Proof of Concept for Automatically Counnting Osteoclasts in a Web Application. Master’s Thesis, NTNU Norwegian University of Science and Technology, Trondheim, Norway, 2022. [Google Scholar]
- Davies, B.K.; Hibbert, A.P.; Roberts, S.J.; Roberts, H.C.; Tickner, J.C.; Holdsworth, G.; Arnett, T.R.; Orriss, I.R. A Machine Learning-Based Image Segmentation Method to Quantify In Vitro Osteoclast Culture Endpoints. Calcif. Tissue Int. 2023, 113, 437–448. [Google Scholar] [CrossRef]
- Wang, X.; Kittaka, M.; He, Y.; Zhang, Y.; Ueki, Y.; Kihara, D. OC_Finder: A deep learning-based software for osteoclast segmentation, counting, and classification. bioRxiv 2021, bioRxiv2021.10.25.465786. [Google Scholar] [CrossRef]
- Wang, X.; Kittaka, M.; He, Y.; Zhang, Y.; Ueki, Y.; Kihara, D.; Segmentation, O. Counting, and Classification Using Watershed and Deep Learning. Front. Bioinform. 2022, 2, 819570. [Google Scholar] [CrossRef]
- Heinemann, C.; Adam, J.; Kruppke, B.; Hintze, V.; Wiesmann, H.P.; Hanke, T. How to get them off?—Assessment of innovative techniques for generation and detachment of mature osteoclasts for biomaterial resorption studies. Int. J. Mol. Sci. 2021, 22, 1329. [Google Scholar] [CrossRef]
- Cavaillon, J.-M.; Adib-Conquy, M. Monocytes/macrophages and sepsis. Crit. Care Med. 2005, 33, S506–S509. [Google Scholar] [CrossRef] [PubMed]
- Szulc, P. Bone turnover: Biology and assessment tools. Best. Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Osdoby, P.; Martini, M.C.; Caplan, A.I. Isolated osteoclasts and their presumed progenitor cells, the monocyte, in culture. J. Exp. Zool. 1982, 224, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Muschter, D. Modulation der zellulären Eigenschaften und Osteoklastogenese von Knochenmark-Makrophagen durch Kollagen Typ II-induzierte Arthritis und sympathische Neurotransmitterstimulation. Doctoral Dissertation, Universität Regensburg, Regensburg, Germany, 2015. Available online: https://epub.uni-regensburg.de/33660/1/Dissertation_Muschter%20Dominique.pdf (accessed on 23 January 2025).
- Bernhardt, A.; Schumacher, M.; Gelinsky, M. Formation of Osteoclasts on Calcium Phosphate Bone Cements and Polystyrene Depends on Monocyte Isolation Conditions. Tissue Eng. Part. C Methods 2015, 21, 160–170. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Bone Resorption by Osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef]
- Lv, G. OC_Identifier (A YOLOv5 GUI for Object Detection), Version 1.0.0; TUD Dresden University of Technology: Dresden, Germany, 2024. [CrossRef]
- Lv, G. Utilisation of Artificial Intelligence for Image-Based Identification of Osteoclasts and Assessment of Their State of Maturation. Diploma Thesis, TU Dresden, Dresden, Germany, 2024. [Google Scholar]
- Piper, K.; Boyde, A.; Jones, S. The relationship between the number of nuclei of an osteoclast and its resorptive capability in vitro. Anat. Embryol. 1992, 186, 291–299. [Google Scholar] [CrossRef]
- Heinemann, C.; Brünler, R.; Kreschel, C.; Kruppke, B.; Bernhardt, R.; Aibibu, D.; Cherif, C.; Wiesmann, H.-P.; Hanke, T. Bioinspired calcium phosphate mineralization on net-shape-nonwoven chitosan scaffolds stimulates human bone marrow stromal cell differentiation. Biomed. Mater. 2019, 14, 045017. [Google Scholar] [CrossRef]
- Rother, S.; Samsonov, S.A.; Moeller, S.; Schnabelrauch, M.; Rademann, J.; Blaszkiewicz, J.; Köhling, S.; Waltenberger, J.; Pisabarro, M.T.; Scharnweber, D.; et al. Sulfated Hyaluronan Alters Endothelial Cell Activation in Vitro by Controlling the Biological Activity of the Angiogenic Factors Vascular Endothelial Growth Factor-A and Tissue Inhibitor of Metalloproteinase-3. ACS Appl. Mater Interfaces 2017, 9, 9539–9550. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.S.; Eckert, H.; Hess, R.; Gelinsky, M.; Rancourt, D.; Krawetz, R.; Cuniberti, G.; Scharnweber, D. Developing a Customized Perfusion Bioreactor Prototype with Controlled Positional Variability in Oxygen Partial Pressure for Bone and Cartilage Tissue Engineering. Tissue Eng. Part. C Methods 2017, 23, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Kruppke, B.; Heinemann, C.; Gebert, A.; Rohnke, M.; Weiß, M.; Henß, A.; Wiesmann, H.-P.; Hanke, T. Strontium substitution of gelatin modified calcium hydrogen phosphates as porous hard tissue substitutes. J. Biomed. Mater. Res. A 2021, 109, 722–732. [Google Scholar] [CrossRef]
- Kotlarz, M.; Jordan, R.; Wegener, E.; Dobrzyński, P.; Neunzehn, J.; Lederer, A.; Wolf-Brandstetter, C.; Pamula, E.; Scharnweber, D. One step 3D printing of surface functionalized composite scaffolds for tissue engineering applications. Acta Bioeng. Biomech. 2018, 20, 35–45. [Google Scholar] [CrossRef]
- Lutter, A.-H.; Hempel, U.; Wolf-Brandstetter, C.; Garbe, A.I.; Goettsch, C.; Hofbauer, L.C.; Jessberger, R.; Dieter, P. A novel resorption assay for osteoclast functionality based on an osteoblast-derived native extracellular matrix. J. Cell Biochem. 2010, 109, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Wenisch, S.; Stahl, J.-P.J.-P.; Horas, U.; Heiss, C.; Kilian, O.; Trinkaus, K.; Hild, A.; Schnettler, R. In vivo mechanisms of hydroxyapatite ceramic degradation by osteoclasts: Fine structural microscopy. J. Biomed. Mater. Res. 2003, 67A, 713–718. [Google Scholar] [CrossRef]
- Trinkaus, J.P.; Groves, P.W. Differentiation in culture of mixed aggregates of dissociated tissue cells. Proc. Natl. Acad. Sci. USA 1955, 41, 787–795. [Google Scholar] [CrossRef]
- Kruppke, B.; Wagner, A.-S.; Rohnke, M.; Heinemann, C.; Kreschel, C.; Gebert, A.; Wiesmann, H.-P.; Mazurek, S.; Wenisch, S.; Hanke, T. Biomaterial based treatment of osteoclastic/osteoblastic cell imbalance—Gelatin-modified calcium/strontium phosphates. Mater. Sci. Eng. C 2019, 104, 109933. [Google Scholar] [CrossRef]
- Kruppke, B.; Farack, J.; Wagner, A.-S.; Beckmann, S.; Heinemann, C.; Glenske, K.; Rößler, S.; Wiesmann, H.-P.; Wenisch, S.; Hanke, T. Gelatine modified monetite as a bone substitute material: An in vitro assessment of bone biocompatibility. Acta Biomater. 2016, 32, 275–285. [Google Scholar] [CrossRef]


| Round | Epochs | Training Time | Images for Training Part | Images for Validation Part | ||||
|---|---|---|---|---|---|---|---|---|
| OC | MON | Background | OC | MON | Background | |||
| 1. | 1–300 | 114.70 Std. | 3800 | 100 | 500 | 800 | 50 | 500 |
| 2. | 301–350 | 26.34 Std. | 4600 | 100 | 500 | 4600 | 50 | 100 |
| 3. | 351–500 | 78.38 Std. | 4900 | 100 | 500 | 4700 | 50 | 100 |
| 4. | 501–600 | 41.31 Std | 4100 | 100 | 500 | 900 | 50 | 100 |
| Times for | Osteoclast Medium (OC-M) | hMSC-Conditioned Medium (HC-M) | |||
|---|---|---|---|---|---|
| Biochemical Analyses | AI Image Analysis | M-CSF/ng/mL | RANKL/ng/mL | M-CSF/ng/mL | RANKL/ng/mL |
| (2), 6, 10 | 6, 8, 10 | 25 | 50 | 25 | 50 |
| 25 | 25 | 25 | 12.5 | ||
| 25 | 12.5 | 0 | 0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, G.; Heinemann, C.; Wiesmann, H.-P.; Kruppke, B. Artificial Intelligence for Image-Based Identification of Osteoclasts and Assessment of Their Maturation—Using the OC_Identifier. Appl. Sci. 2025, 15, 4159. https://doi.org/10.3390/app15084159
Lv G, Heinemann C, Wiesmann H-P, Kruppke B. Artificial Intelligence for Image-Based Identification of Osteoclasts and Assessment of Their Maturation—Using the OC_Identifier. Applied Sciences. 2025; 15(8):4159. https://doi.org/10.3390/app15084159
Chicago/Turabian StyleLv, Guofan, Christiane Heinemann, Hans-Peter Wiesmann, and Benjamin Kruppke. 2025. "Artificial Intelligence for Image-Based Identification of Osteoclasts and Assessment of Their Maturation—Using the OC_Identifier" Applied Sciences 15, no. 8: 4159. https://doi.org/10.3390/app15084159
APA StyleLv, G., Heinemann, C., Wiesmann, H.-P., & Kruppke, B. (2025). Artificial Intelligence for Image-Based Identification of Osteoclasts and Assessment of Their Maturation—Using the OC_Identifier. Applied Sciences, 15(8), 4159. https://doi.org/10.3390/app15084159








