Development of Ultra-Fast Surface Acoustic Wave-Based NO2 Sensor Incorporating a Monolayered Graphene: MoS2 Sensing Material and a Microheater for Spacecraft Applications
Abstract
1. Introduction
2. Optimal Design Consideration and Analytical Modeling
2.1. Operating Mechanism
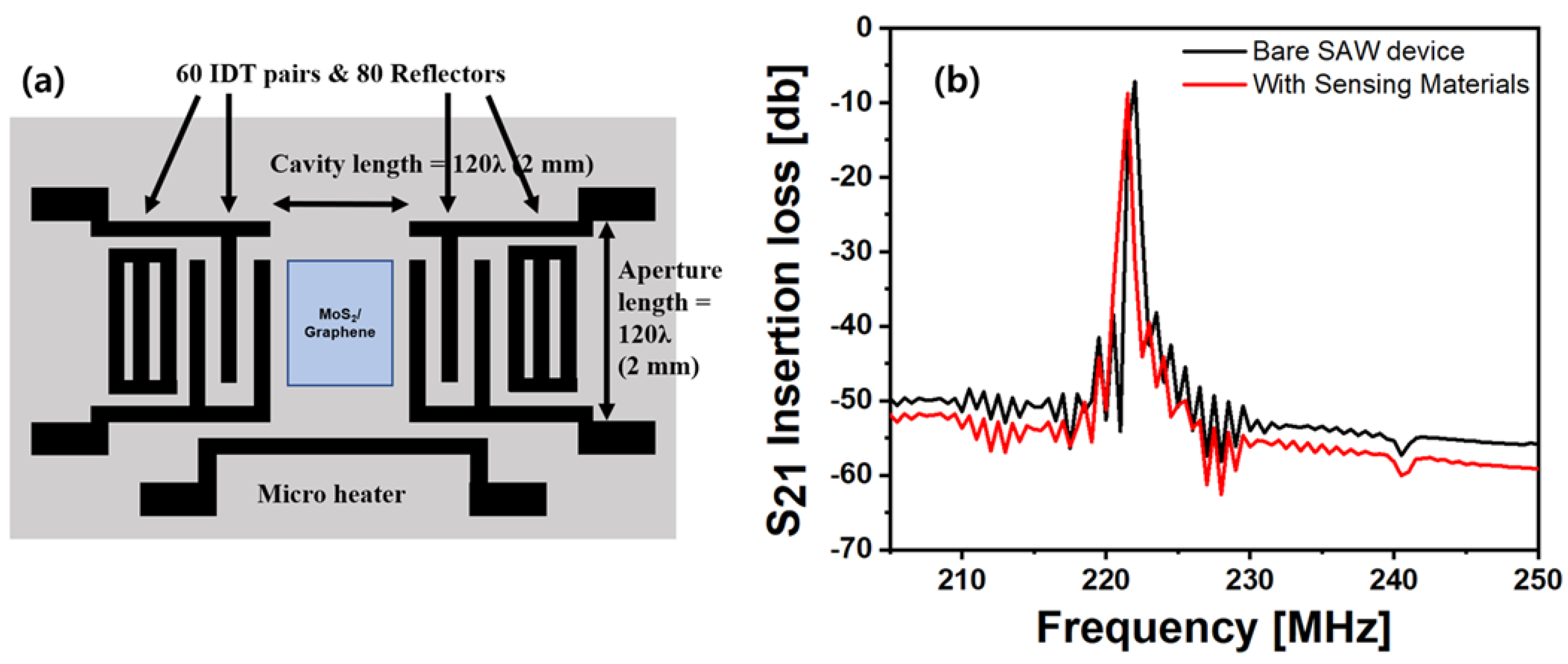
2.2. COM Modeling of the SAW Sensor Platform
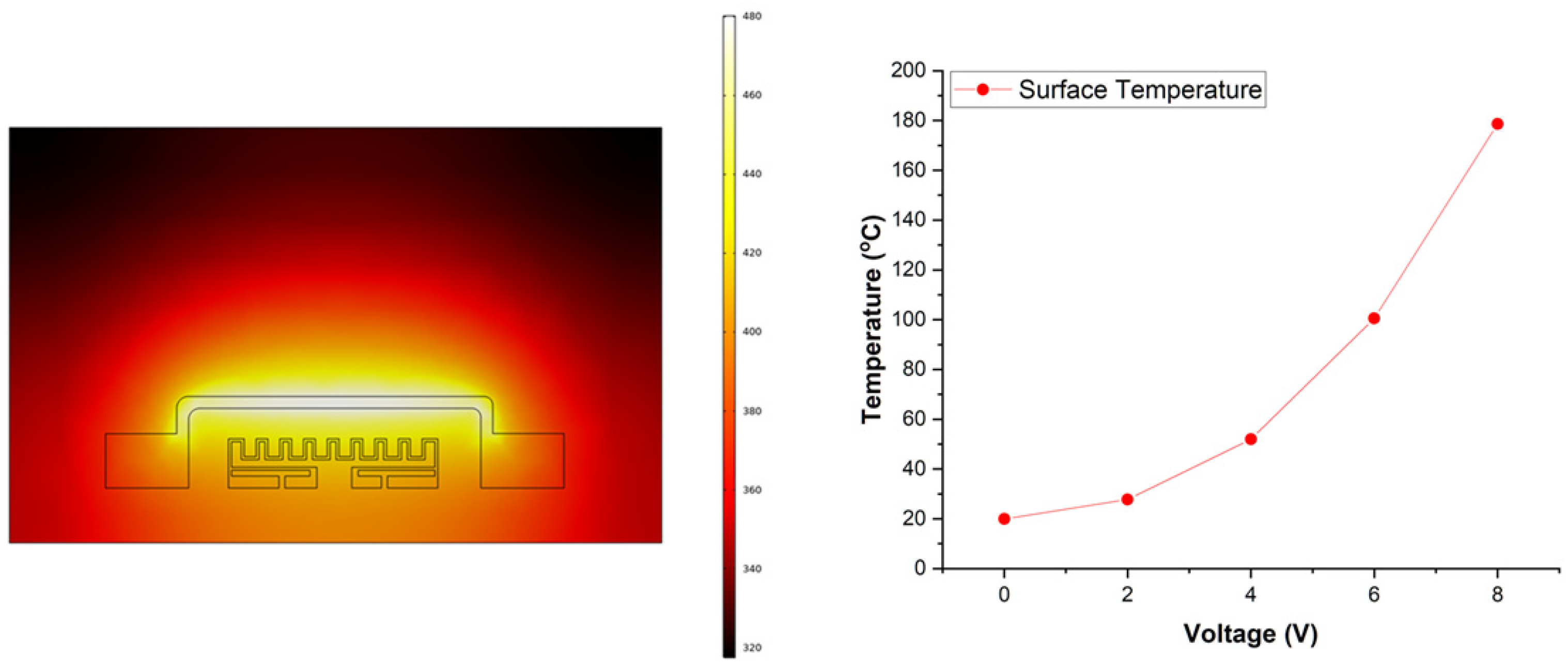
2.3. COMSOL Simulation of the Microheater
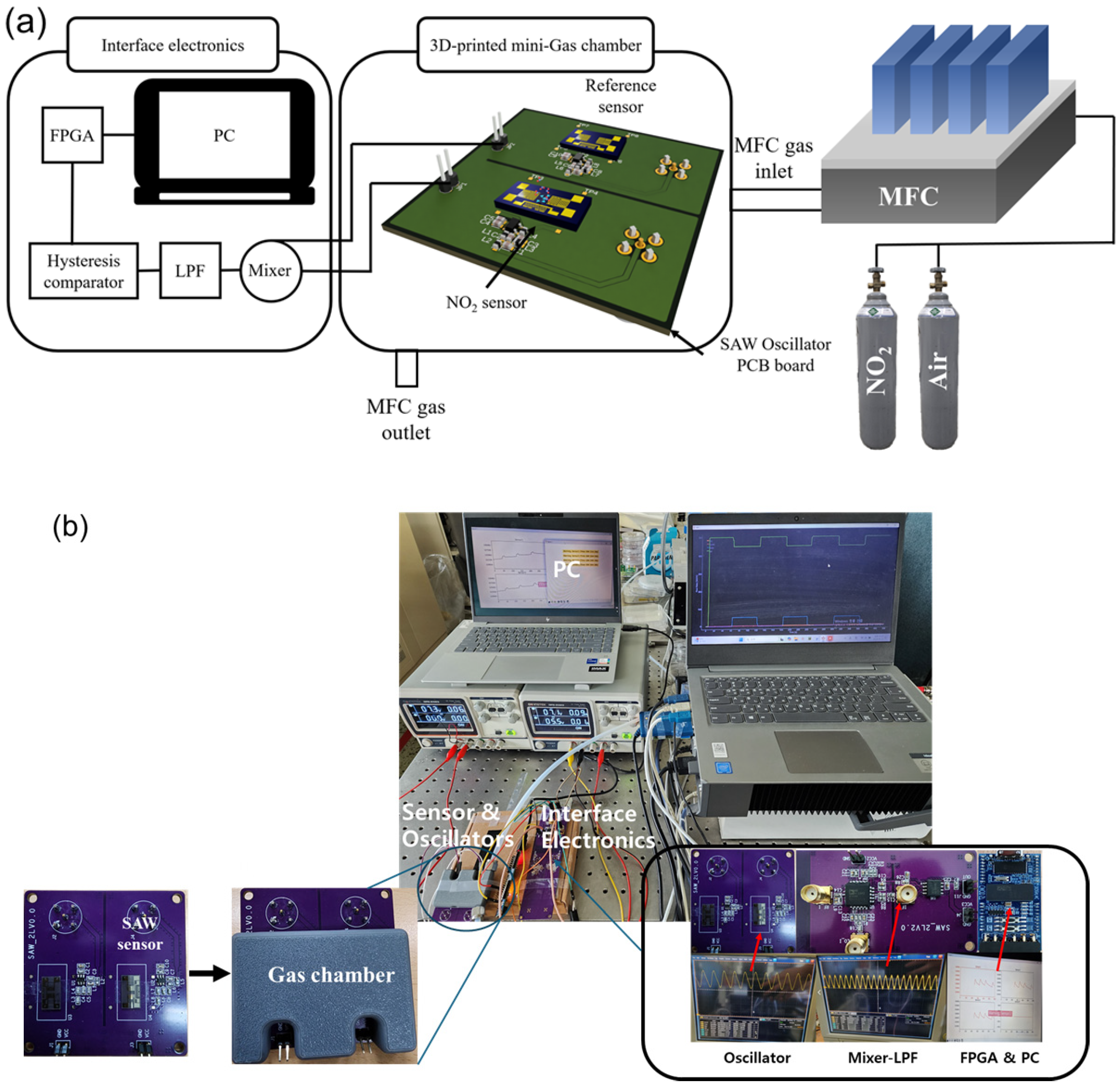
3. Experimental Methods
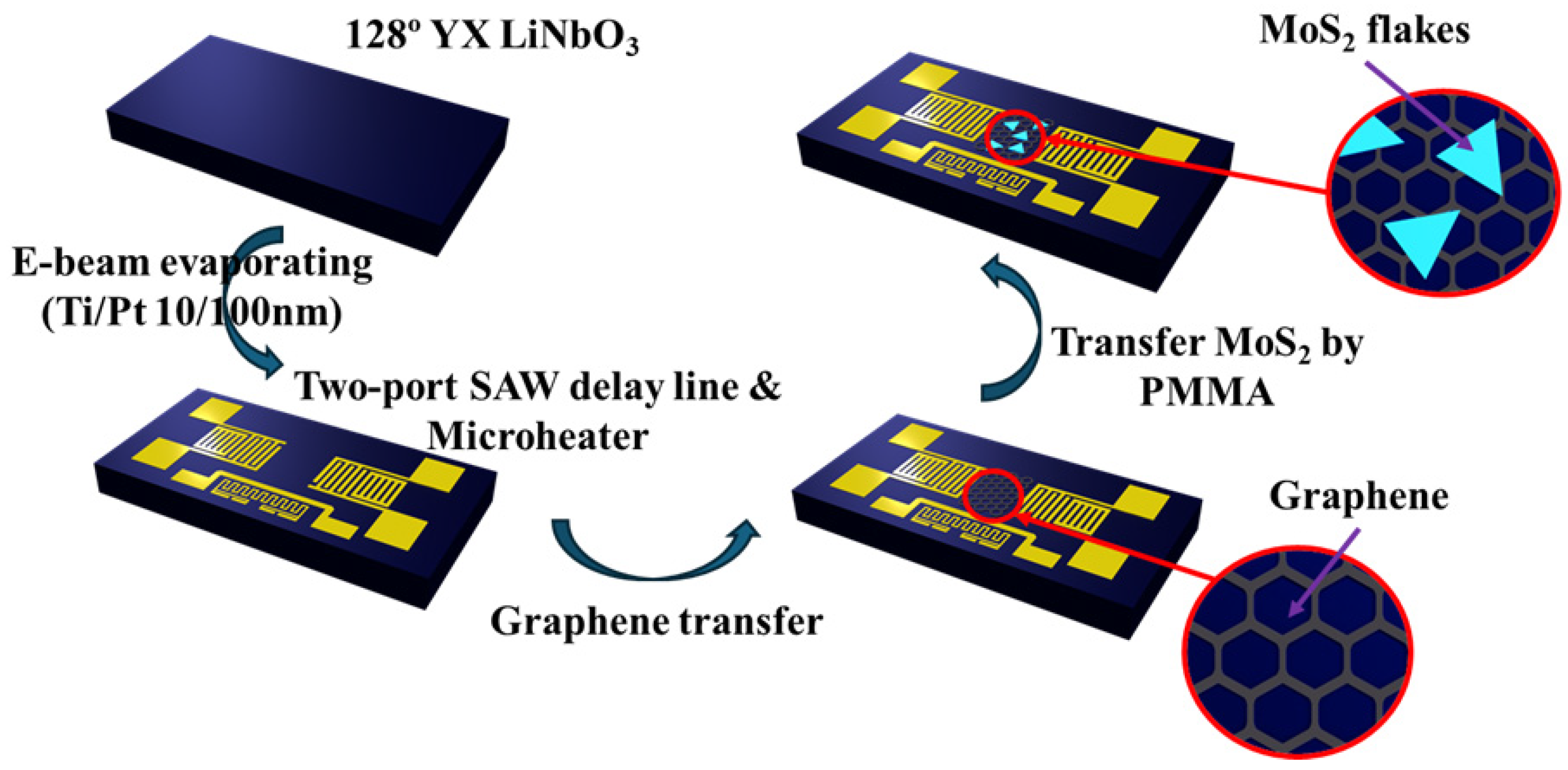
3.1. SAW Sensor Platform Fabrication
3.2. Sensing Material Fabrication
3.3. Interface Electronics Fabrication
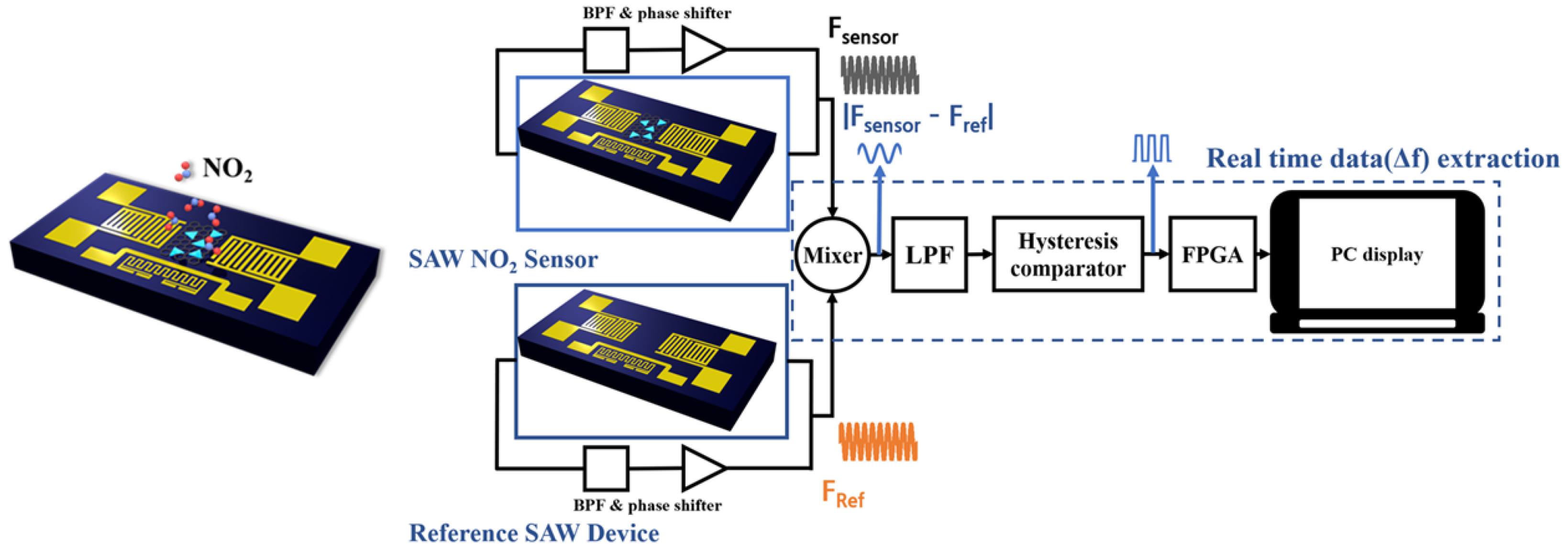
3.4. Testing Setup
4. Results
4.1. Fabricated SAW Sensor
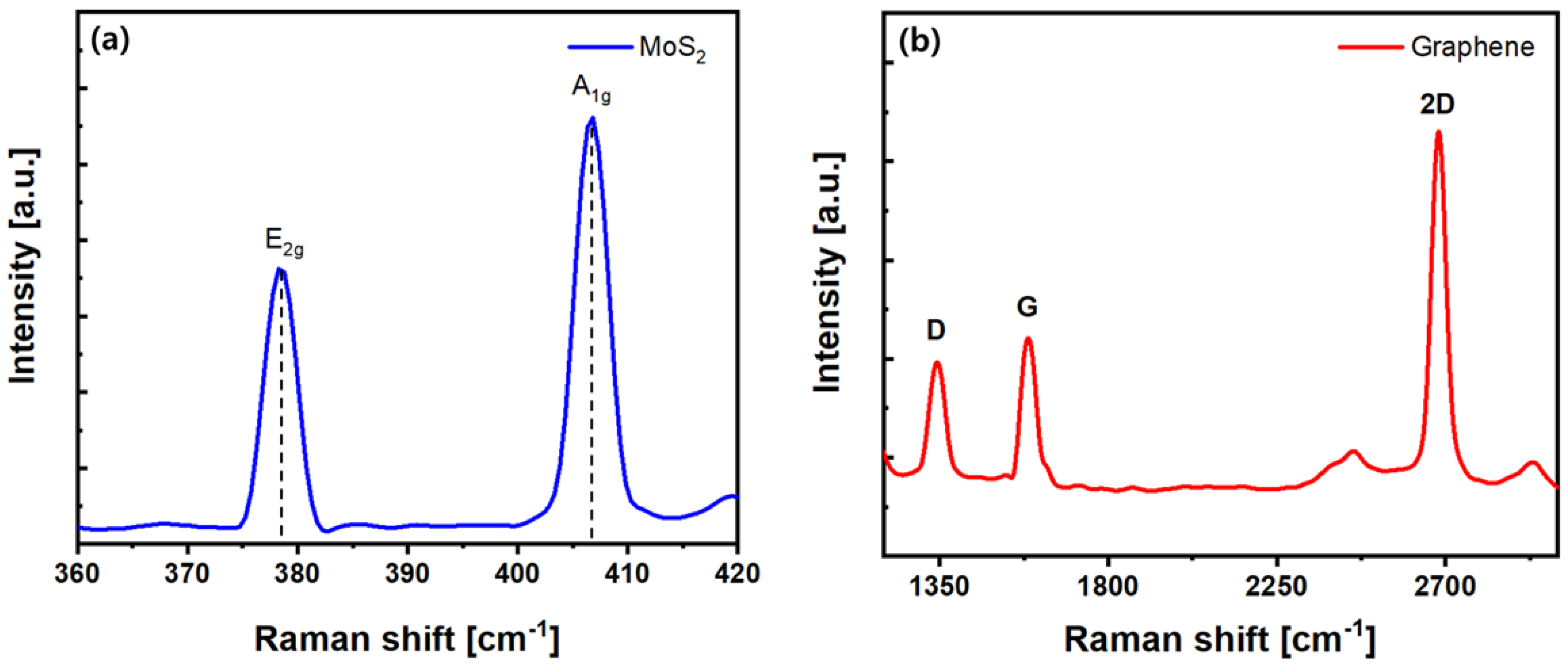
4.2. Analysis of the NO2 Sensing Material
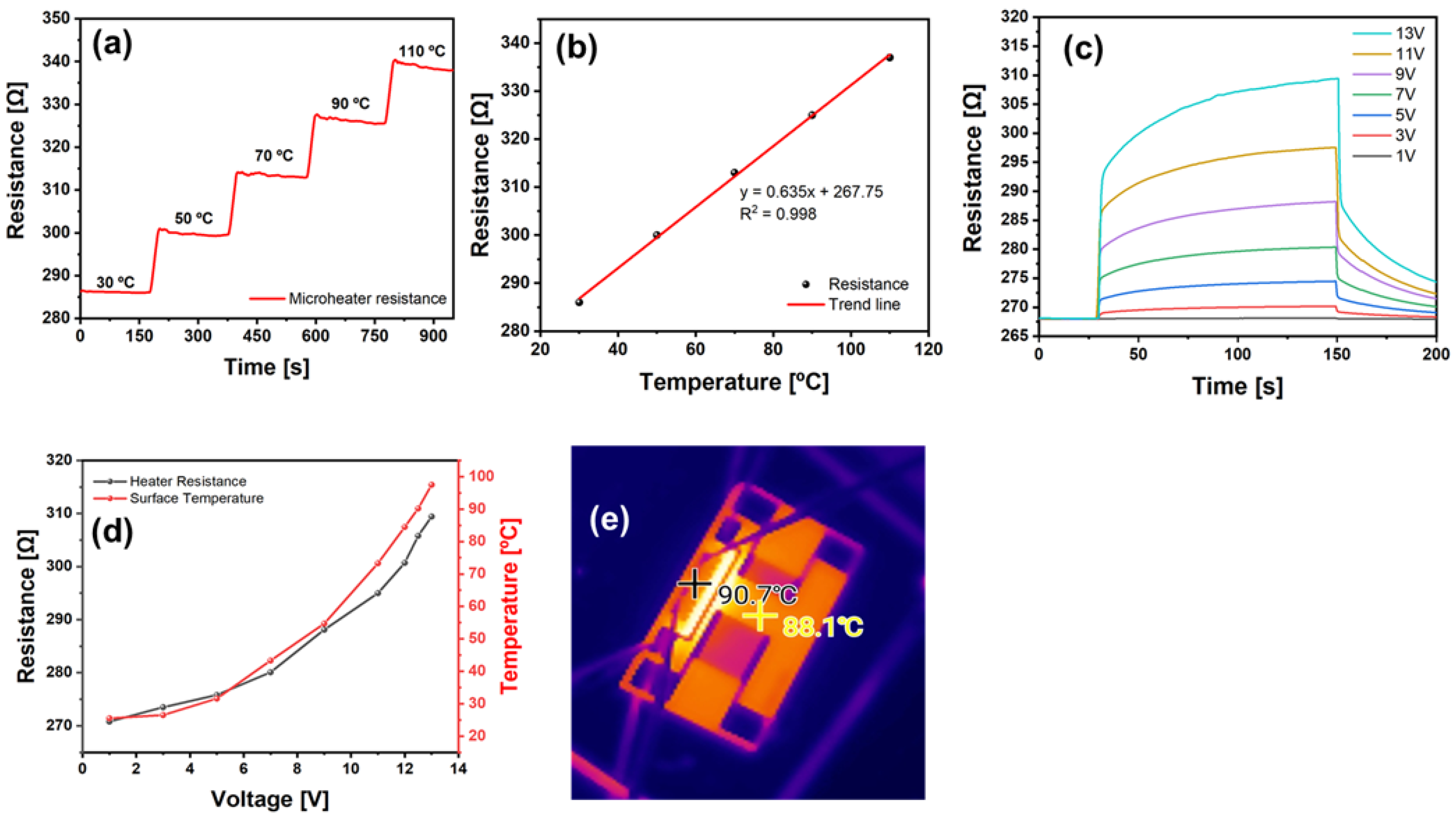
4.3. Microheater Characterization
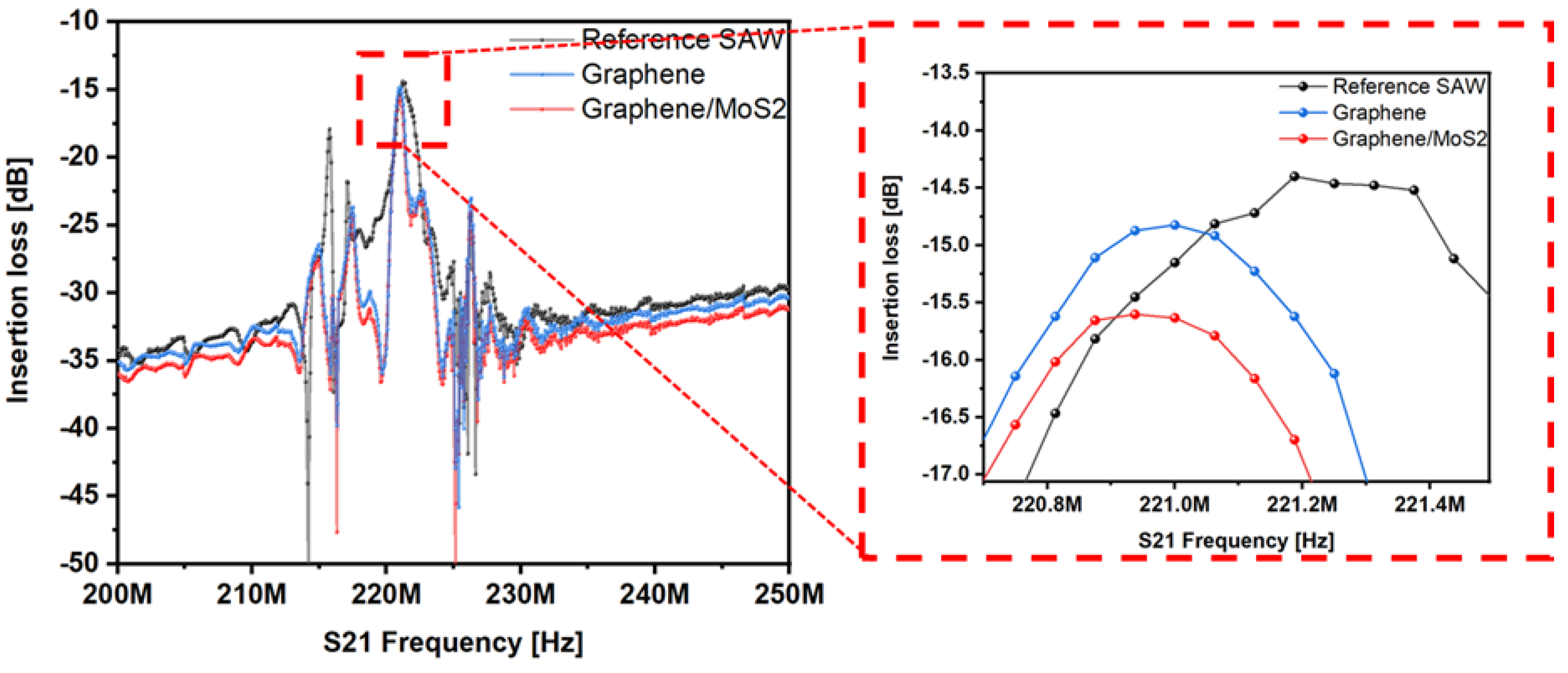
4.4. Sensor Characterizations with Interface Electronics
4.5. Humidity/Temperature Compensations via Interface Electronics
4.6. Field Test Simulating Space Environment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perry, J.L.; George, J.L. Spacecraft Cabin Air Quality Control and Its Application to Tight Buildings. AIAA J. 1995, 95, 1050. [Google Scholar]
- James, J.T.; Limero, T.F. Air Quality in NASA’s Manned Spacecraft: The Artemis Challenge. Aerosp. Med. Hum. Perform. 2021, 92, 537–550. [Google Scholar]
- Jones, B.W.; Mason, E.A. Spacecraft Cabin Air Contaminants and Their Control. J. Spacecr. Rocket. 1985, 22, 25–33. [Google Scholar]
- Limero, T.F.; Wilson, T. Off-Gassing and Trace Contaminants in Manned Spacecraft Environments. Aerospace Med. Hum. Perform. 2018, 89, 567–575. [Google Scholar]
- James, J.T.; Gardner, D.P. Spacecraft Maximum Allowable Concentrations for Airborne Contaminants: 2020 Update; NASA Technical Reports; NASA/TP-2020-50001913; National Aeronautics and Space Administration: Washington, DC, USA, 2020.
- Frampton, M.W.; Boscia, J.; Roberts, N.J. Nitrogen Dioxide Exposure: Effects on Airway and Immune Cells. Am. J. Respir. Crit. Care Med. 2002, 166, 1298–1305. [Google Scholar]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 3rd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2016; pp. 872–880. [Google Scholar]
- Devlin, R.B.; Horstman, D.P.; Gerrity, T.R. NO2 Effects on Lung Function and Airway Inflammation. Am. J. Respir. Crit. Care Med. 1999, 159, 315–325. [Google Scholar]
- Tunnicliffe, W.S.; Harrison, R.M.; Kelly, F.J. NO2 Exposure and Effects on Lung Inflammation and Cardiovascular Function. Thorax 2001, 56, 567–574. [Google Scholar]
- Yang, S.; Li, Y.; Wang, C.; Xie, G. Recent Advances in NO2 Gas Sensors: Challenges and Future Directions. Sensors 2020, 20, 3147. [Google Scholar]
- Verma, A.; Prasad, M. 2D Materials for Gas Sensing Applications: A Review on Graphene Oxide, MoS2, WS2 and Phosphorene. Nanomaterials 2018, 8, 736. [Google Scholar]
- Liu, C.; Zhou, Y. Highly Sensitive NO2 Gas Sensors Based on MoS2@MoO3 Ferromagnetic Heterostructures. Nanomaterials 2022, 12, 1441. [Google Scholar]
- Zhang, H.; Wang, J. Robust Room-Temperature NO2 Sensors from Exfoliated 2D Few-Layered WSe2 Field-Effect Transistors. ACS Appl. Mater. Interfaces 2021, 13, 12334–12344. [Google Scholar]
- Sharma, S.; Kumar, N. A Systematic Review on 2D MoS2 for Nitrogen Dioxide (NO2) Sensing at Room Temperature. Mater. Today Commun. 2020, 25, 101586. [Google Scholar]
- Lee, H.; Park, Y. Strategies and Challenges for Improving the Performance of Two-Dimensional Material-Based Gas Sensors. Emerg. Mater. Res. 2023, 12, 2288353. [Google Scholar]
- Smith, A.; Brown, K. Unencapsulated and Washable Two-Dimensional Material Electronic Textiles for Health and Environmental Monitoring. Sci. Rep. 2022, 12, 13002. [Google Scholar]
- Kim, D.; Lee, J. Mechanisms of NO2 Detection in Hybrid Structures Containing Reduced Graphene Oxide. Sensors 2022, 22, 5316. [Google Scholar] [CrossRef]
- Park, T.; Choi, H. Chemical Sensing of 2D Graphene/MoS2 Heterostructure Device. ACS Appl. Mater. Interfaces 2015, 7, 19295–19304. [Google Scholar]
- Zhao, Y.; Wang, S. Graphene-Based Sensors for Detecting NO2 at Room Temperature. Sensors 2020, 20, 3175. [Google Scholar]
- Lin, J.; Zhu, L. Enhanced NO2 Gas Sensing Performance of MoS2 Nanosheets by Surface Decoration with Au Nanoparticles. Sensors 2019, 19, 1296. [Google Scholar]
- Zhang, R.; Zhou, Z.; Shi, L. NO2 Sensing Mechanisms of 2D Transition Metal Dichalcogenide Materials: A First-Principles Study. J. Phys. Chem. C 2022, 126, 5460–5471. [Google Scholar]
- Zhang, C.; Wang, Y.; Li, H. High-Performance MoS2-Based Gas Sensors for NO2 Detection at Room Temperature. ACS Sens. 2019, 4, 2171–2180. [Google Scholar]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R. The Electronic Properties of Graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar]
- Liu, B.; Wang, X.; Sun, H. Two-Dimensional MoS2-Based Composite Materials for High-Performance Gas Sensors. J. Mater. Chem. C 2021, 9, 5475–5487. [Google Scholar]
- Cheng, X.; Tang, W.; Ma, X. The Influence of Defects on NO2 Sensing Performance of 2D Materials: A Density Functional Theory Study. J. Phys. Chem. C 2023, 127, 2340–2350. [Google Scholar]
- Ma, X.; Tang, W.; Cheng, X. Recent Advances in 2D NO2 Sensing Materials: Performance, Mechanisms, and Challenges. ACS Appl. Mater. Interfaces 2023, 15, 20345–20360. [Google Scholar]
- Nawaz, F.; Kim, S.; Lee, H.; Park, J.; Song, J.; Choi, C.; Lee, K. Toward Ultra-Fast Response and Recovery Characteristics through ZnO Multilayer Thin Films in Surface Acoustic Wave UV Sensor. IEEE Sens. J. 2025, 25, 343–353. [Google Scholar]
- Kim, S.; Nawaz, F.; Choi, C.; Jeon, I.; Lee, K. Synthesis and Characterization of High-Quality Ta2O5 Nanoparticles for Highly Selective SAW-Based DUV Sensor and Corona Detection Application. Adv. Mater. Technol. 2024, 9, 2301486. [Google Scholar]
- Kondalkar, V.; Li, X.; Park, I.; Yang, S.; Lee, K. Development of Chipless Wireless Current Sensor System based on Giant Magnetoimpedance Magnetic Sensor and Surface Acoustic Wave Transponder. Sci. Rep. 2018, 8, 2401. [Google Scholar]
- Yalon, E.; McClellan, C.J.; Smithe, K.K.H.; Muñoz Rojo, M.; Xu, R.L.; Suryavanshi, S.V.; Gabourie, A.J.; Neumann, C.M.; Xiong, F.; Farimani, A.B.; et al. Energy Dissipation in Monolayer MoS2 Electronics. Nano Lett. 2017, 17, 3429–3433. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal Properties of Graphene and Nanostructured Carbon Materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef]
- Shiue, R.-J.; Gao, Y.; Tan, C.; Peng, C.; Zheng, J.; Efetov, D.K.; Kim, Y.D.; Hone, J.; Englund, D. Thermal Radiation Control from Hot Graphene Electrons Coupled to a Photonic Crystal Nanocavity. Nat. Commun. 2019, 10, 109. [Google Scholar] [CrossRef]
- Chen, X.-K.; Zeng, Y.-J.; Chen, K.-Q. Thermal Transport in Two-Dimensional Heterostructures. Front. Mater. 2020, 7, 578791. [Google Scholar] [CrossRef]
- Pasupuleti, K.S.; Reddeppa, M.; Chougule, S.S.; Bak, N.; Nam, D.-J.; Jung, N.; Cho, H.D.; Kim, S.-G.; Kim, M.-D. High Performance Langasite Based SAW NO2 Gas Sensor Using 2D G-C3N4@TiO2 Hybrid Nanocomposite. J. Hazard. Mater. 2022, 427, 128174. [Google Scholar] [PubMed]
- Xiong, S.; Zhou, J.; Wu, J.; Li, H.; Zhao, W.; He, C.; Liu, Y.; Chen, Y.; Fu, Y.; Duan, H. High Performance Acoustic Wave Nitrogen Dioxide Sensor with Ultraviolet Activated 3D Porous Architecture of Ag-Decorated Reduced Graphene Oxide and Polypyrrole Aerogel. ACS Appl. Mater. Interfaces 2021, 13, 42094–42103. [Google Scholar] [PubMed]
- Pasupuleti, K.S.; Reddeppa, M.; Nam, D.-J.; Bak, N.-H.; Peta, K.R.; Cho, H.D.; Kim, S.-G.; Kim, M.-D. Boosting of NO2 Gas Sensing Performances Using GO-PEDOT:PSS Nanocomposite Chemical Interface Coated on Langasite-Based Surface Acoustic Wave Sensor. Sens. Actuators B Chem. 2021, 344, 130267. [Google Scholar]
- Sadek, A.Z.; Wlodarski, W.; Kalantar-zadeh, K.; Powell, D.A.; Hughes, W.L.; Buchine, B.A.; Wang, Z.L. H2 and NO2 Gas Sensors with ZnO Nanobelt Layer on 36° LiTaO3 and 64° LiNbO3 SAW Transducers. IEEE Sens. J. 2005, 5, 1343–1346. [Google Scholar]

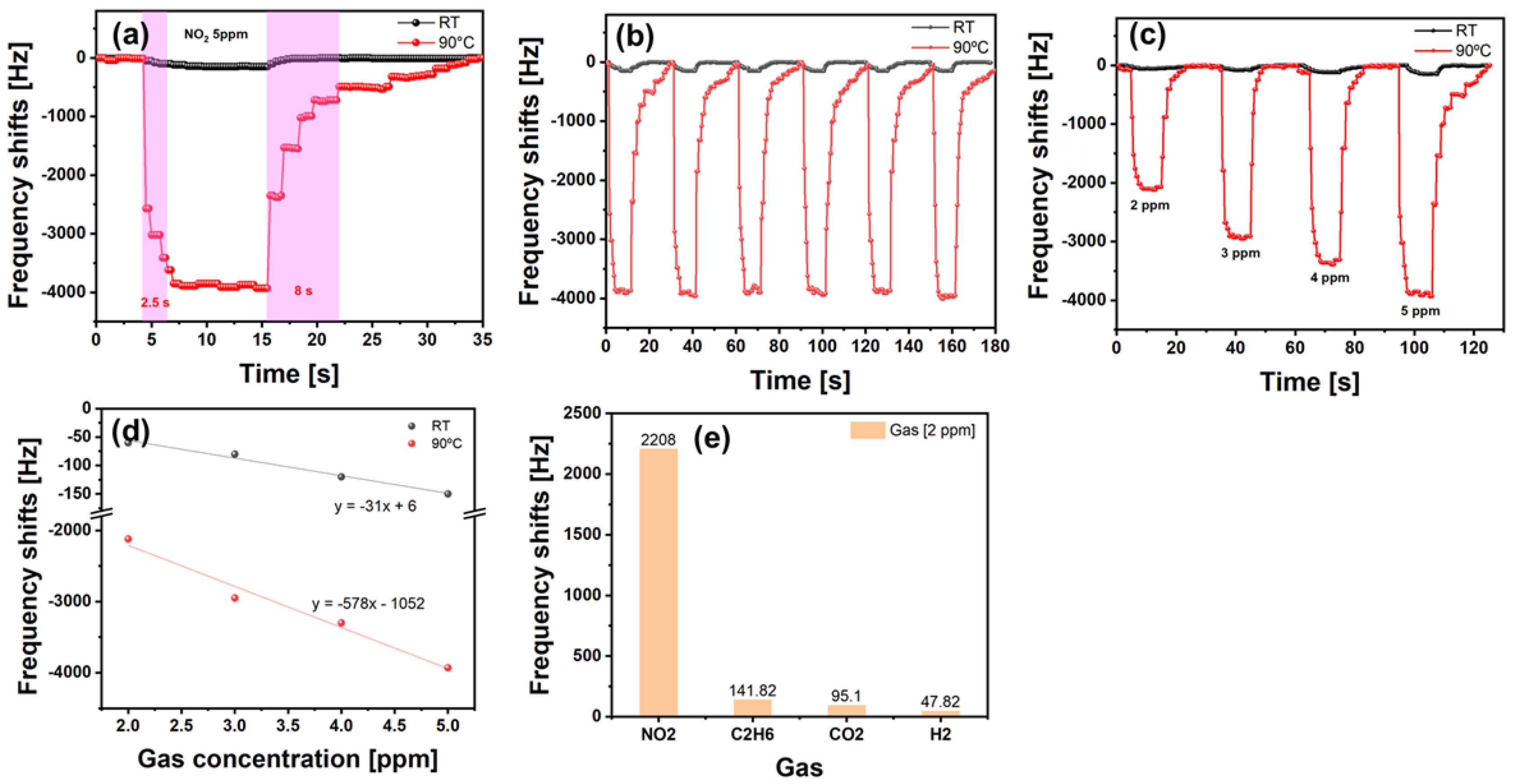
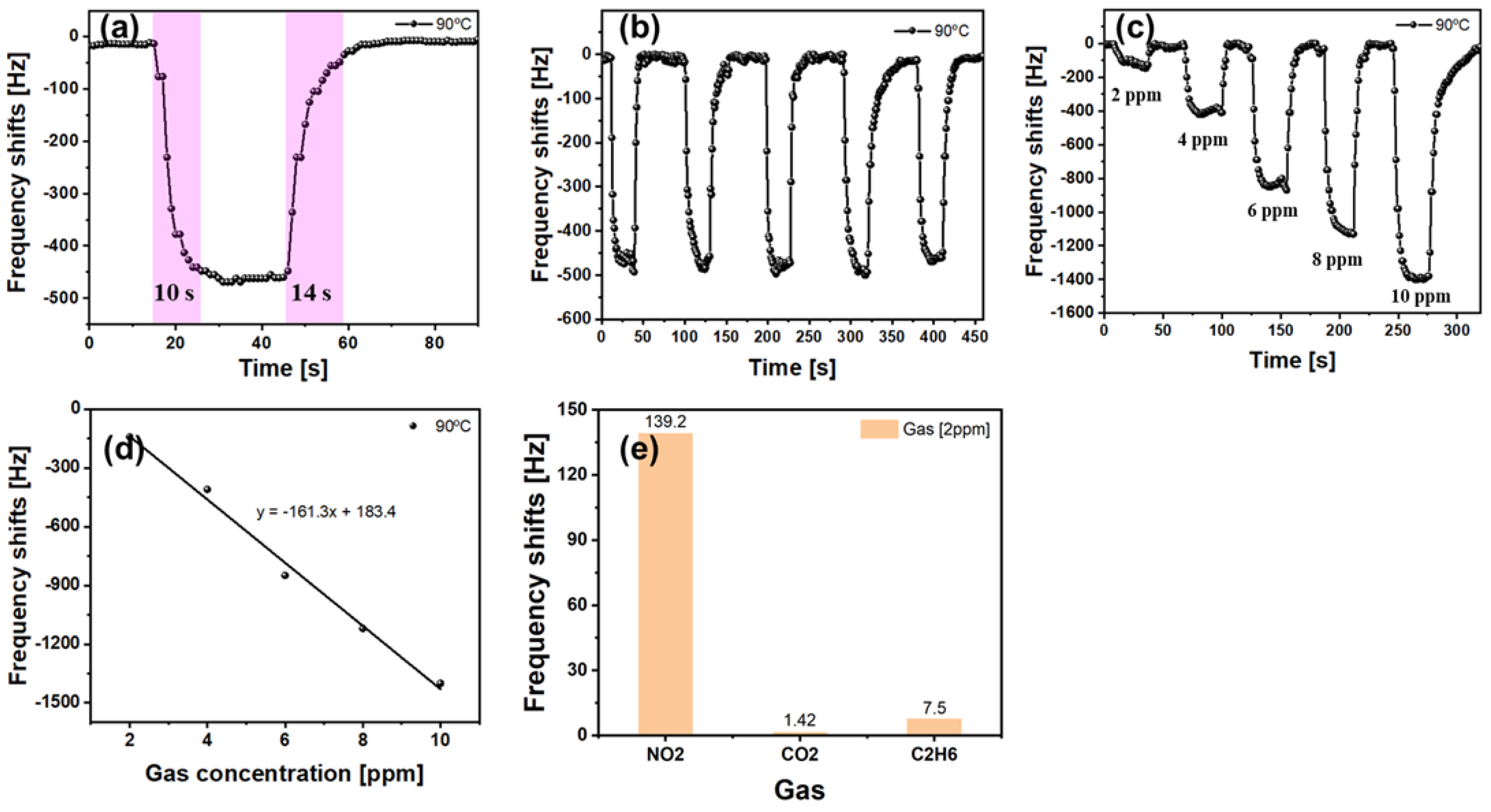
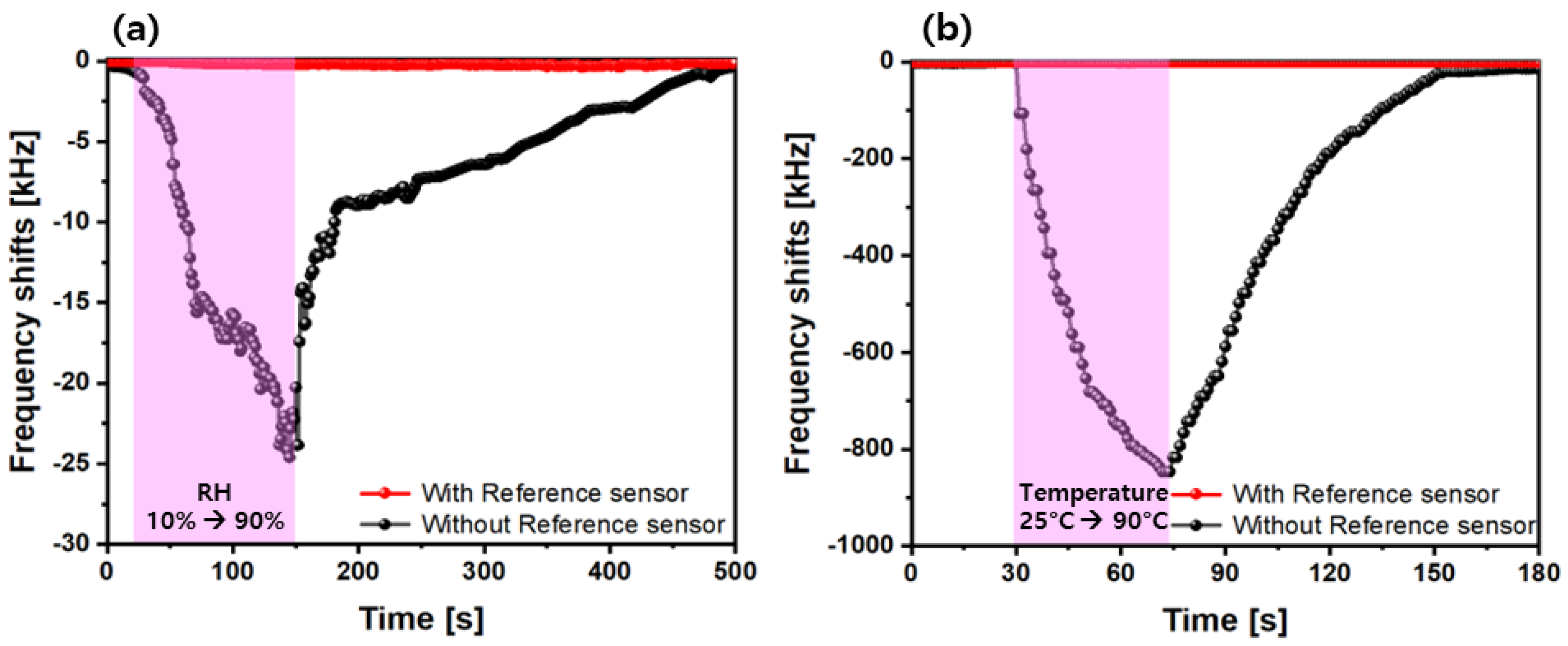
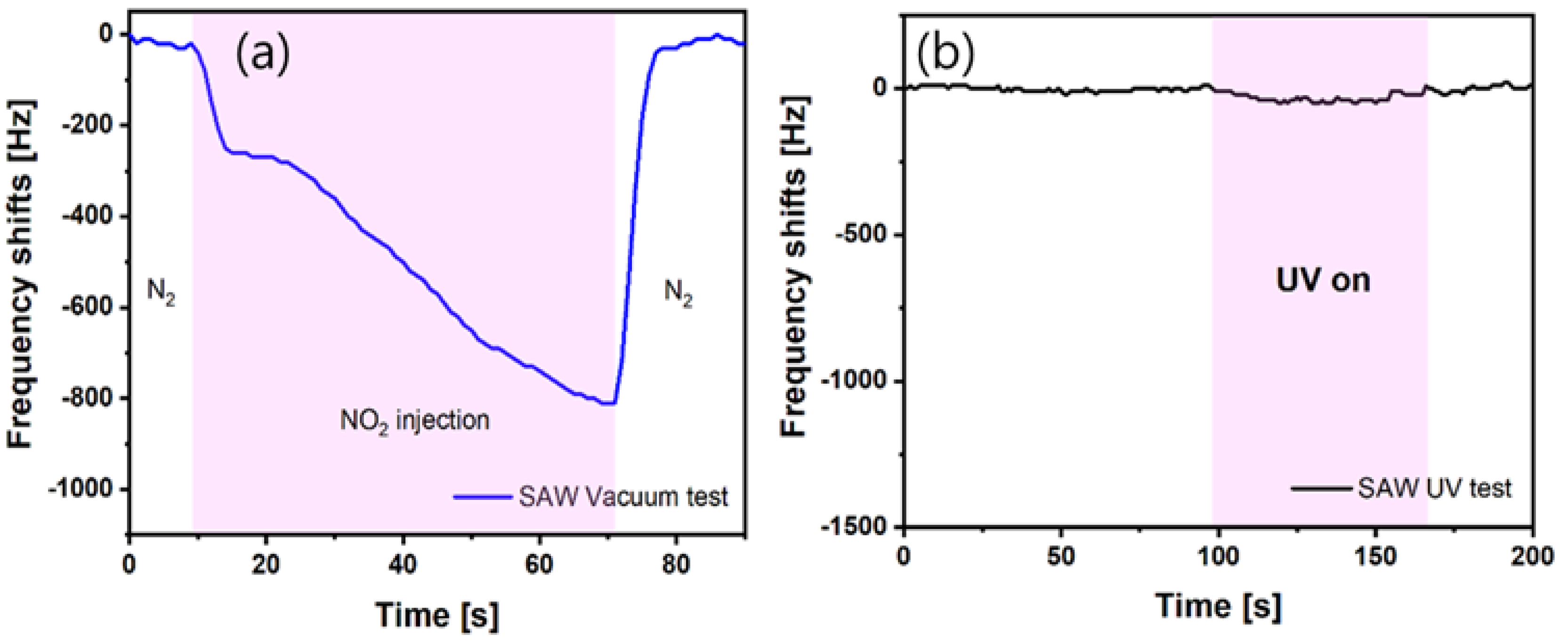
| Gas | Formula | 10-Day SMAC ppm | 6-h SMAC ppm |
|---|---|---|---|
| Hydrogen | H2 | 3000 | 12,000 |
| Carbon dioxide | CO2 | 7000 | 13,000 |
| Ethanol | CH3CH2OH | 240 | 1000 |
| Methane | CH4 | 5000 | 5000 |
| 2-propanol | CH3CH[OH]CH3 | 50 | 500 |
| Dichloromethane | CH2Cl | 25 | 100 |
| Toluene | C6H5CH3 | 20 | 100 |
| Carbon monoxide | CO | 20 | 50 |
| Trichloroethylene | CHCl CCl2 | 12 | 50 |
| Ammonia | NH3 | 25 | 25 |
| Benzene | C6H6 | 2 | 10 |
| Nitric dioxide | NO2 | 0.5 | - |
| Parameter | Value |
|---|---|
| Number of input and output IDT pairs | 60 |
| Number of reflector pairs | 80 |
| Aperture length (λ) | 120 |
| Cavity length (μm) | 2000 |
| Wavelength λ (μm) | 17.6 |
| IDT Ti/Pt thickness (nm) | 10/100 |
| Acoustic velocity (m/s) of 128° YX LiNbO3 | 3890 |
| Electromechanical coupling factor, K2 (%) | 5.5 |
| Effective dielectric coefficient εp/ε0 | 55 |
| Sheet resistance (Ω-m) | 0.939 × 108 |
| Sensing Material | Center Freq. (MHz) | Res. (kHz/ppm) | Res./Rec. Time (s) | Temp. | Ref. |
|---|---|---|---|---|---|
| TiO2 NP | 135.8 | 8.5/100 | 310/182 | RT | [34] |
| g-C3N4@TiO2 NP | 135.7 | 19.7/100 | 143/114 | RT | [34] |
| Ag/rGO-ppy | 246.99 | 13.2/100 | 68/190 | RT | [35] |
| GO-PEDOT:PSS | 135.78 | 5.7/100 | 35/10 | RT | [36] |
| ZnO nano belt | 111 | 3.5/10 | 90/140 | 160 °C | [37] |
| Graphene/MoS2 film | 222 | 0.14/2 | 10/14 | 90 °C | This work |
| Graphene/MoS2 flakes | 222 | 2.1/2 | 2.5/8 | 90 °C | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawaz, F.; Lee, H.; Wang, W.; Lee, K. Development of Ultra-Fast Surface Acoustic Wave-Based NO2 Sensor Incorporating a Monolayered Graphene: MoS2 Sensing Material and a Microheater for Spacecraft Applications. Appl. Sci. 2025, 15, 4050. https://doi.org/10.3390/app15074050
Nawaz F, Lee H, Wang W, Lee K. Development of Ultra-Fast Surface Acoustic Wave-Based NO2 Sensor Incorporating a Monolayered Graphene: MoS2 Sensing Material and a Microheater for Spacecraft Applications. Applied Sciences. 2025; 15(7):4050. https://doi.org/10.3390/app15074050
Chicago/Turabian StyleNawaz, Faisal, Hyunho Lee, Wen Wang, and Keekeun Lee. 2025. "Development of Ultra-Fast Surface Acoustic Wave-Based NO2 Sensor Incorporating a Monolayered Graphene: MoS2 Sensing Material and a Microheater for Spacecraft Applications" Applied Sciences 15, no. 7: 4050. https://doi.org/10.3390/app15074050
APA StyleNawaz, F., Lee, H., Wang, W., & Lee, K. (2025). Development of Ultra-Fast Surface Acoustic Wave-Based NO2 Sensor Incorporating a Monolayered Graphene: MoS2 Sensing Material and a Microheater for Spacecraft Applications. Applied Sciences, 15(7), 4050. https://doi.org/10.3390/app15074050






