1. Introduction
Biodiversity holds a crucial significance in the formation and maintenance of ecological functions. It not only reflects community composition, structure, successional stages, and habitat differences, but measures the interactions between biological communities and environmental factors. In addition, biodiversity optimizes resource allocation in the environment through mechanisms, such as complementarity and redundancy, ensuring ecosystem continuity. These mechanisms are particularly important in ecotones along some gradients. Therefore, studying the relationship between biodiversity and the environment is a focal point in ecological research [
1,
2]. Currently, in the field of community ecology, the diversity of plant communities holds significant importance, as it better reveals the structural and functional evolution of communities and ecosystems [
3,
4]. Plants, as vital components of an ecosystem, play an irreplaceable role in protecting the structure and carrying capacity of ecosystems, such as wetlands [
5,
6]. Plant diversity not only expresses the biodiversity at the species level, but reflects ecological diversity, including habitat heterogeneity, niche differentiation, and environmental complexity and variability. It is based on plant groups and encompasses the complex relationships formed by plants, plant species’ interactions with each other in competition and facilitation, population dynamics, with size and age relationships, and their interactions with the environment, as well as the ecological processes involved [
7]. This diversity reflects the functional roles of species within communities, the status of environmental resource utilization, and the mechanisms that sustain community stability, making it a crucial basis for biodiversity conservation [
8].
The relationship between plant diversity and the environment, including how environmental controls and external disturbances work together to shape spatial patterns of plant diversity along environmental gradients, represent an important issue in the field of plant ecology [
9,
10]. Most studies have suggested that environmental factors influence the diversity and distribution of plant communities [
11,
12]. Among these, soil factors are widely recognized as the dominant influences on vegetation pattern distribution at regional and local scales [
13]. Specifically, these factors, such as soil moisture content (SMC), soil pH (SPH), and soil electrical conductivity (SEC), strongly affect nutrient availability and plant physiological processes. Other important factors, like compositional makeup and pore space, also influence plant growth by regulating soil structure stability, water-holding capacity, and root-zone aeration dynamics. Soil can provide vital nutrients for plant survival, and significantly impacts plant physiology and vegetation distribution. Consequently, the effects of soil properties on communities and the spatial patterns of plants have been widely studied [
14]. In addition to the physicochemical properties of soil, factors influencing plant diversity also include topography [
15], climatic factors [
16], and human disturbances [
17,
18]. Plant diversity is closely linked to environmental factors. Exploring this relationship aids in uncovering the environmental factors that control plant community distribution. This understanding, in turn, can inform the development of appropriate management strategies to promote the conservation of plant diversity and the establishment of healthy, stable communities [
19].
The unique geographical environment of river deltas, as a dynamic interface of land–sea interaction, forms a complex ecosystem, which provides ideal conditions for the development of plant community transition zones [
20]. Among them, river ecosystems here have both water and land properties, with complex and diverse habitats and structures, and are breeding bases for many endangered plants [
21].The Yellow River Delta is located at the interface of land and sea, where the dynamics of terrestrial and marine interactions create a complex and diverse ecological environment [
20]. Various ecological processes in this region, such as soil development, water cycling, and vegetation succession, are affected by the combined effects of the Yellow River and marine dynamics. The delta not only features typical estuarine wetland ecosystems but is home to the most intact wetland ecosystems found in the warm temperate zone of China. The wetland vegetation types here are quite diverse, with extensive areas of naturally occurring vegetation in newly formed wetlands [
22,
23]. Due to coastal influences, the soil exhibits a high degree of salinization and relatively low nutrient levels in the Yellow River Delta. In littoral zones, plant diversity is particularly susceptible to significant impacts from environmental factors such as climate, soil, and water [
24]. Some studies have identified the salinity of soil as an important factor influencing the plant diversity in littoral zones [
25]. Additionally, the vegetation is characterized by its fragility, making it susceptible to backward and secondary succession caused by natural factors and human interference [
26]. Influenced by terrestrial and marine interactions, the Yellow River Delta displays strong environmental heterogeneity, hosting unique habitats and biological diversity [
27,
28]. It serves as a breeding ground for numerous endangered species, such as the
Grus japonensis,
Neophocaena sunameri, and
Glycine soja. The survival and reproduction of many species in this area are of significant importance for maintaining regional biodiversity. Currently, studies on the plant diversity of the Yellow River Delta, both domestically and internationally, primarily focus on the characteristics and succession of plant communities in estuarine wetlands or within the protected areas of the delta [
27,
29], as well as the relationship between meadow vegetation and soil factors [
30,
31,
32]. However, in-depth investigations into the spatial heterogeneity of plant diversity in the Yellow River Delta are relatively scarce, particularly along the longitudinal environmental gradient of the land–sea interface. Systematic studies addressing the composition and fundamental characteristics of plants are still lacking in the Yellow River Delta. Exploring the variations in plant community composition and species diversity along the land–sea gradient, as well as the driving mechanisms behind these changes, is crucial for understanding the structure and function of the Yellow River Delta ecosystem and for formulating effective ecological conservation and restoration strategies [
29].
Currently, issues such as water resource scarcity [
32], human activity interference [
22], and soil salinization [
33] are threatening the stability and development of ecosystems in the Yellow River Delta. This situation necessitates the implementation of effective measures to enhance conservation and management efforts, ensuring the healthy development of the wetland ecosystem in the Yellow River Delta. In response to these challenges, China introduced the “Ecological Protection and High-Quality Development Plan for the Yellow River Basin” in 2021, emphasizing the need to strengthen the protection and restoration of the wetland ecosystem in the Yellow River Delta, and to enhance the conservation of biological resources in tidal flats, salt marshes, and estuarine coastal wetlands. Therefore, exploring the spatial heterogeneity of plant diversity along the land–sea gradient and their responsive mechanisms to environmental factors is of significant importance for the integrated conservation and restoration of terrestrial and marine ecosystems in the Yellow River Delta.
Based on the above background, this study explored plant species, communities, and habitat factors within the Yellow River Delta under the land–sea gradient using field techniques and multi-scale analysis. It aims to understand the situation of species composition, structural characteristics, and plant diversity in the ecological ecotone of the Yellow River Delta. Additionally, this study aims to elucidate how various factors, such as soil properties, other natural environmental factors, and human interference, influence plant diversity. The ultimate goal is to identify the key influencing factors that maintain plant diversity of the Yellow River Delta in riparian and regional areas, thereby providing theoretical references for restoration and sustainable development of river delta ecosystems.
5. Conclusions
Based on a field survey and statistical analysis of plant diversity, this study revealed the spatial differentiation of plant diversity in the Yellow River Delta under the land–sea gradients, and identified the key driving factors of plant diversity using regression analysis, redundancy analysis (RDA), and the Mantel test analysis. The results were as follows.
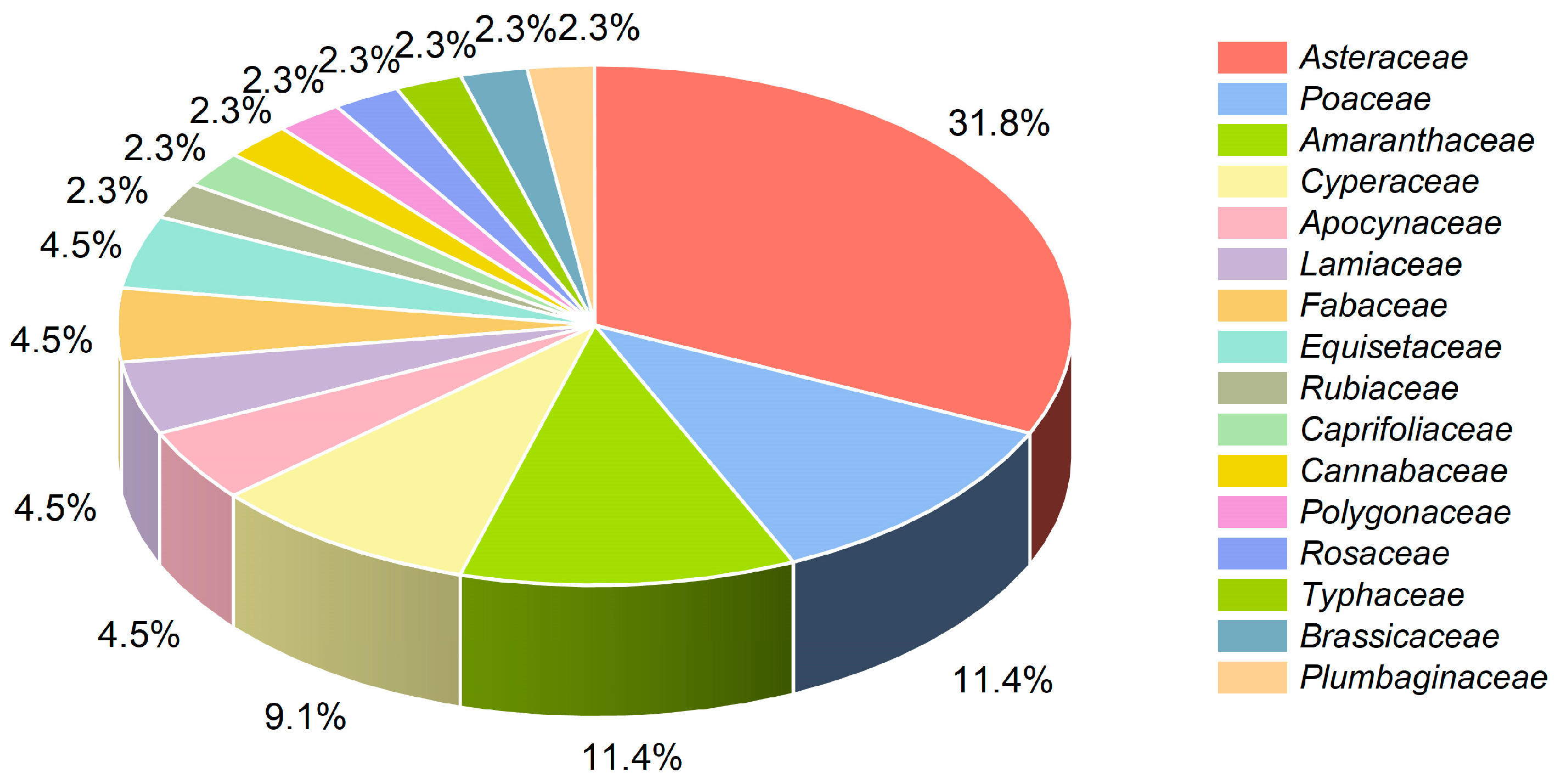
This study investigated 44 species of herbaceous plants of 36 genera and 16 families in the study area. Among them, plants of the Asteraceae, Poaceae, and Amaranthaceae families dominated. We recorded 30 species of plants of 26 genera and 13 families in the Yellow River Delta National Nature Reserve, indicating the richness of plant diversity in the reserve. The plants in the study area were mainly hygrophytes and halophytes, and the dominant communities were Suaeda salsa and Phragmites australis. With the increase of the distance from the sea, the community transitions from the monospecies dominance of Suaeda salsa to the herbaceous community, dominated by plants of the Poaceae family, such as Phragmites australis, Miscanthus sacchariflorus, and Calamagrostis pseudophragmites. Meanwhile, the plant species and distribution changed significantly in this process. The species similarity coefficient of the adjacent environment was higher than that of the non-adjacent environment. Additionally, the species similarity of the coastal area was higher, and the plant composition was mainly halophytes.
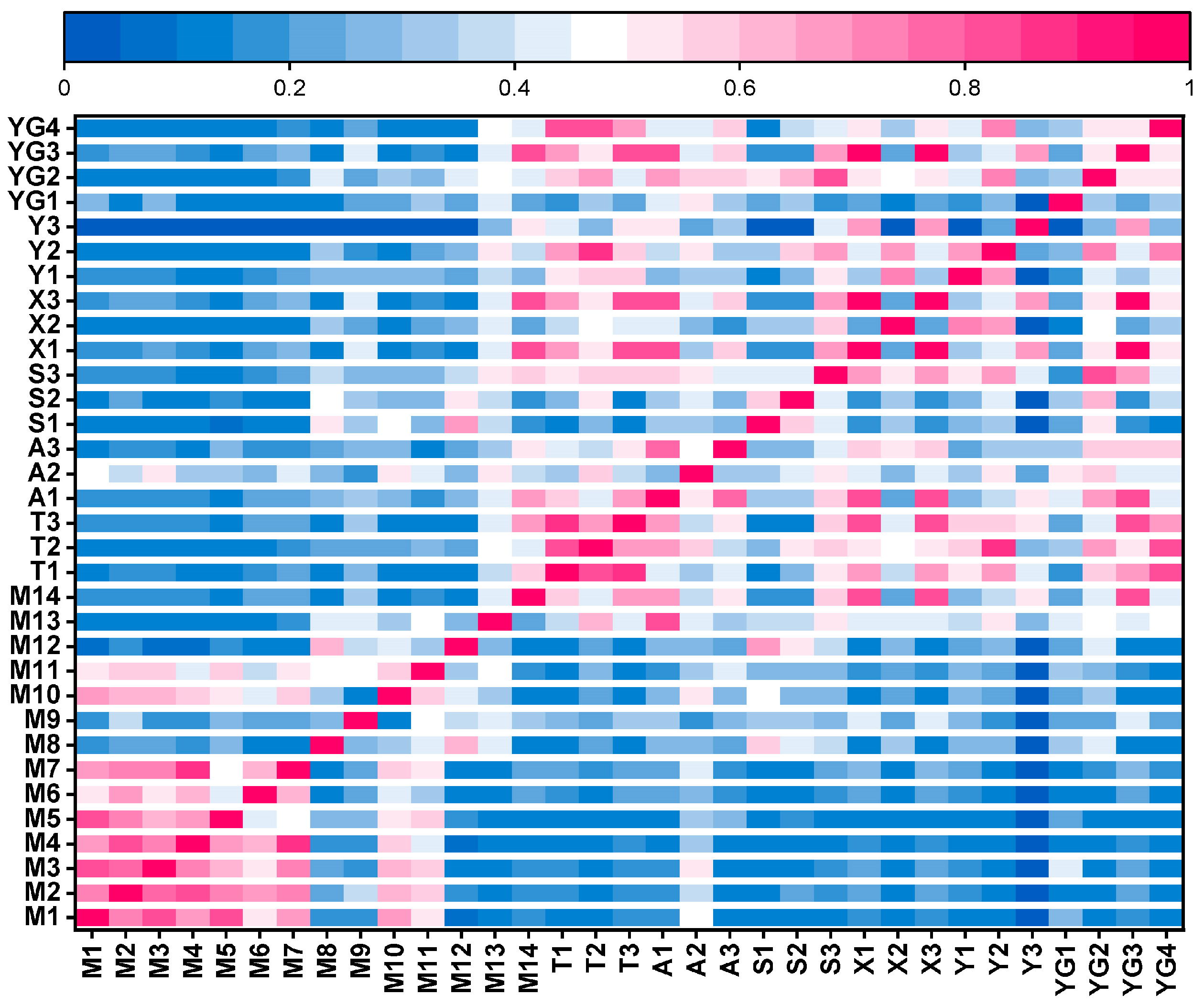
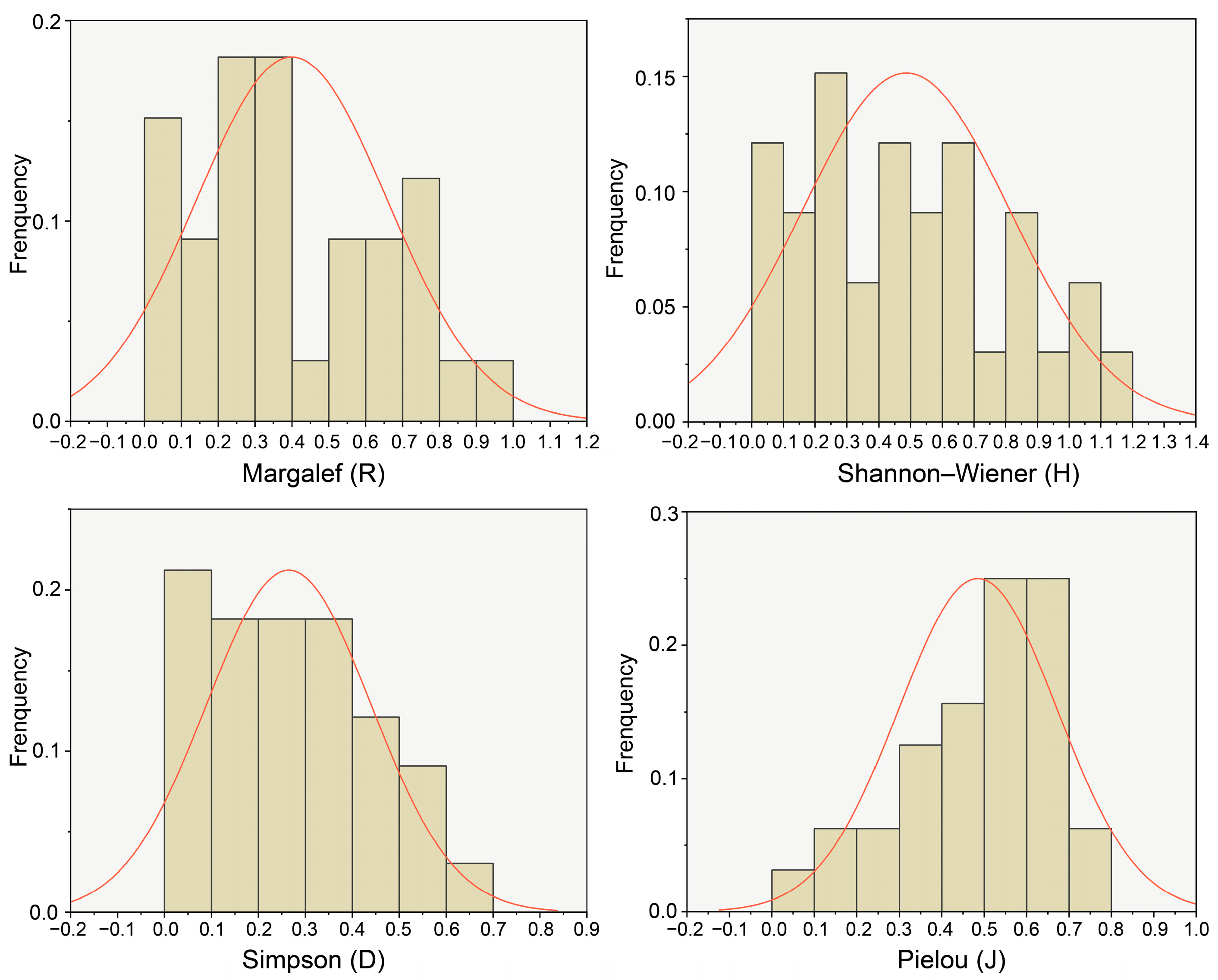
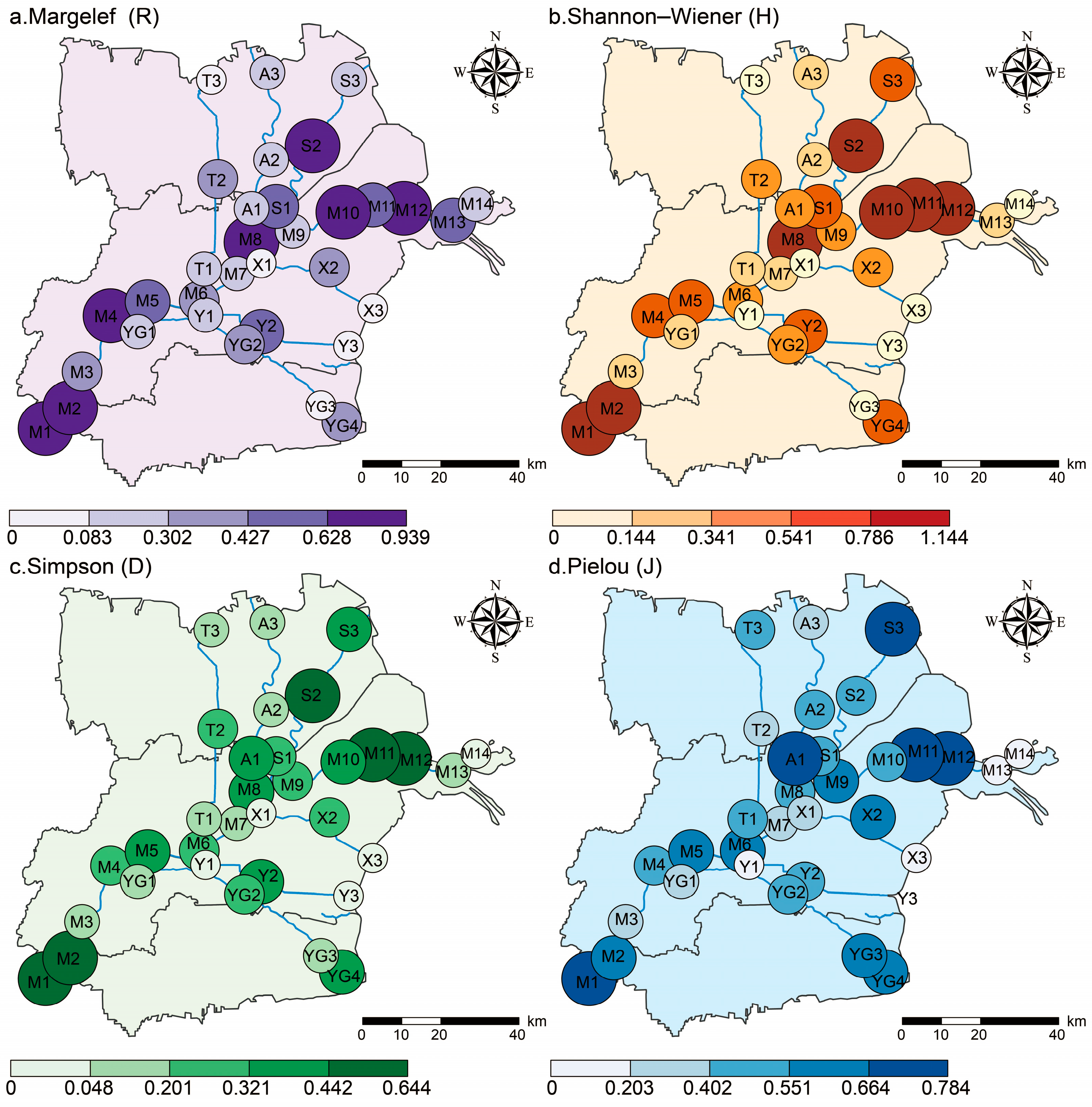
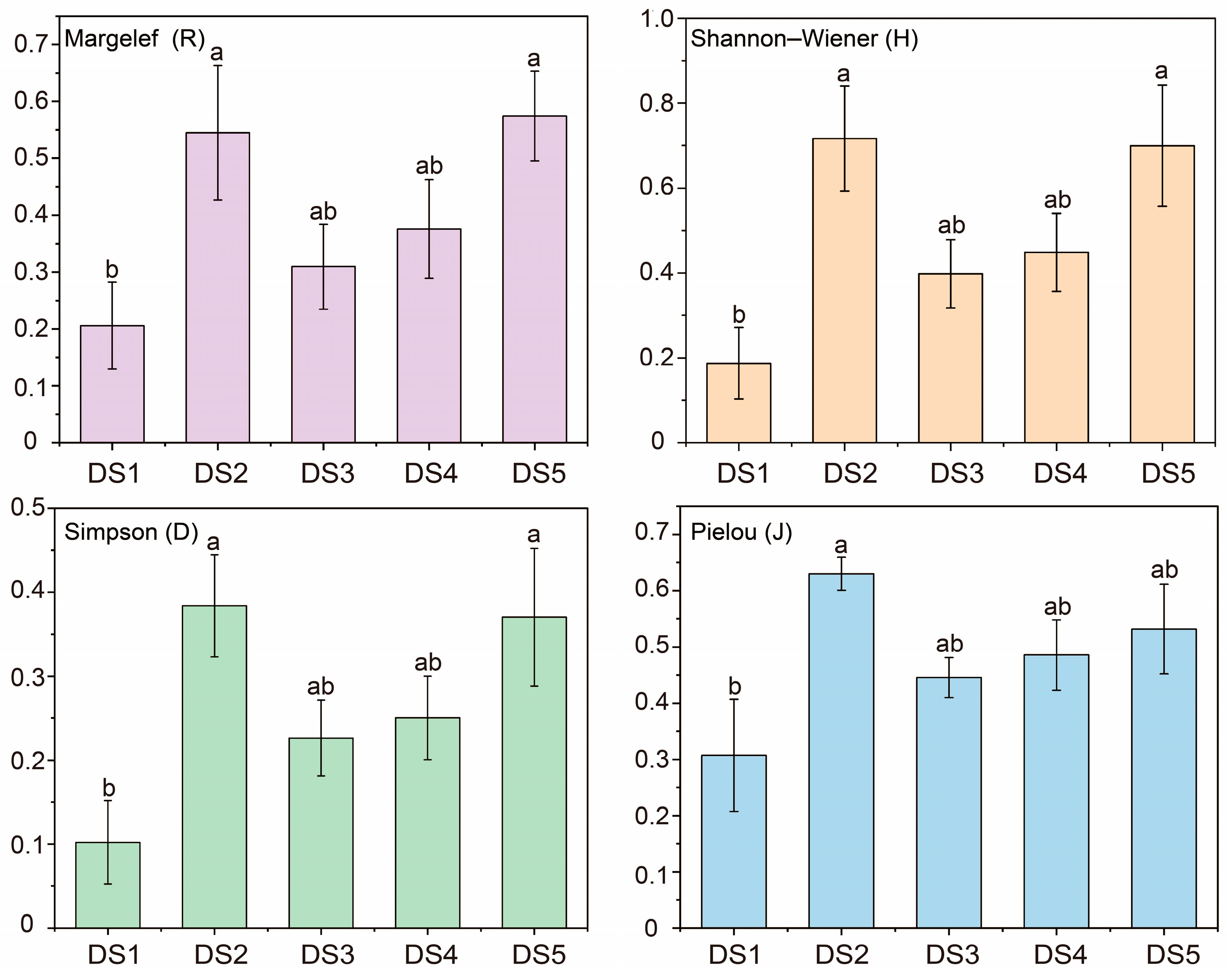
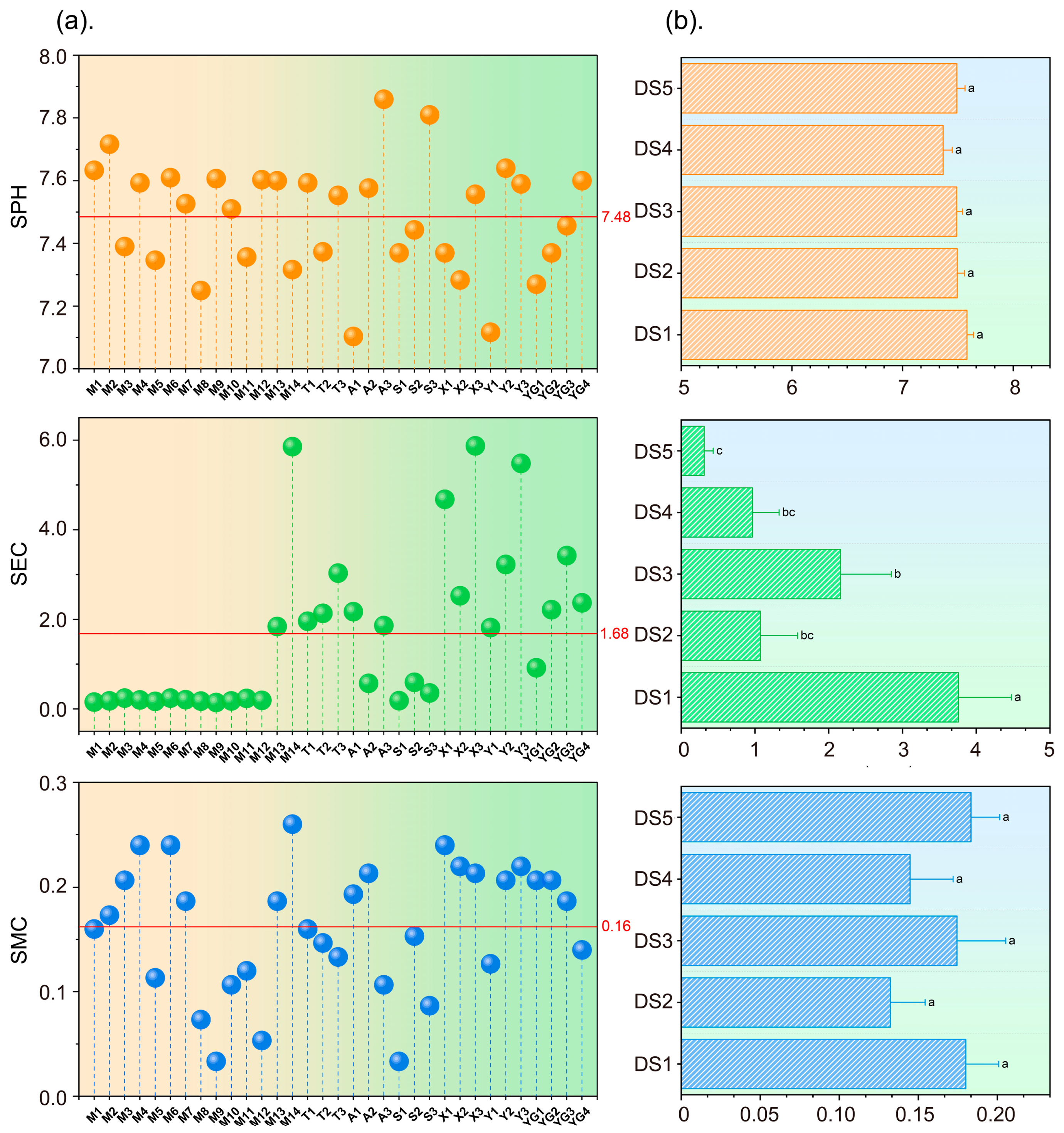
Overall, the level of plant diversity in the Yellow River Delta was relatively low. From the perspective of spatial distribution, the longitudinal change trends of the Margalef index (R), the Shannon–Wiener index (H), the Simpson index (D), and the Pielou index (J) were basically the same, and the level of plant diversity showed a fluctuating downward trend from land to sea. The plant diversity in the reserve also showed a decreasing trend from land to sea. Additionally, it indicated a peak value in the region of 3–17 km from the sea (DS2) and the region of > 42 km from the sea (DS5), which may be influenced by such comprehensive factors as habitat level, soil conditions, and policy protection. From the riverbank perspective, the overall plant diversity in the main stream of the Yellow River was significantly higher than that in the tributaries. Human interference was the key factor affecting the level of plant diversity in the main stream, and soil electrical conductivity (SEC) was the key factor affecting the level of plant diversity in the tributaries.
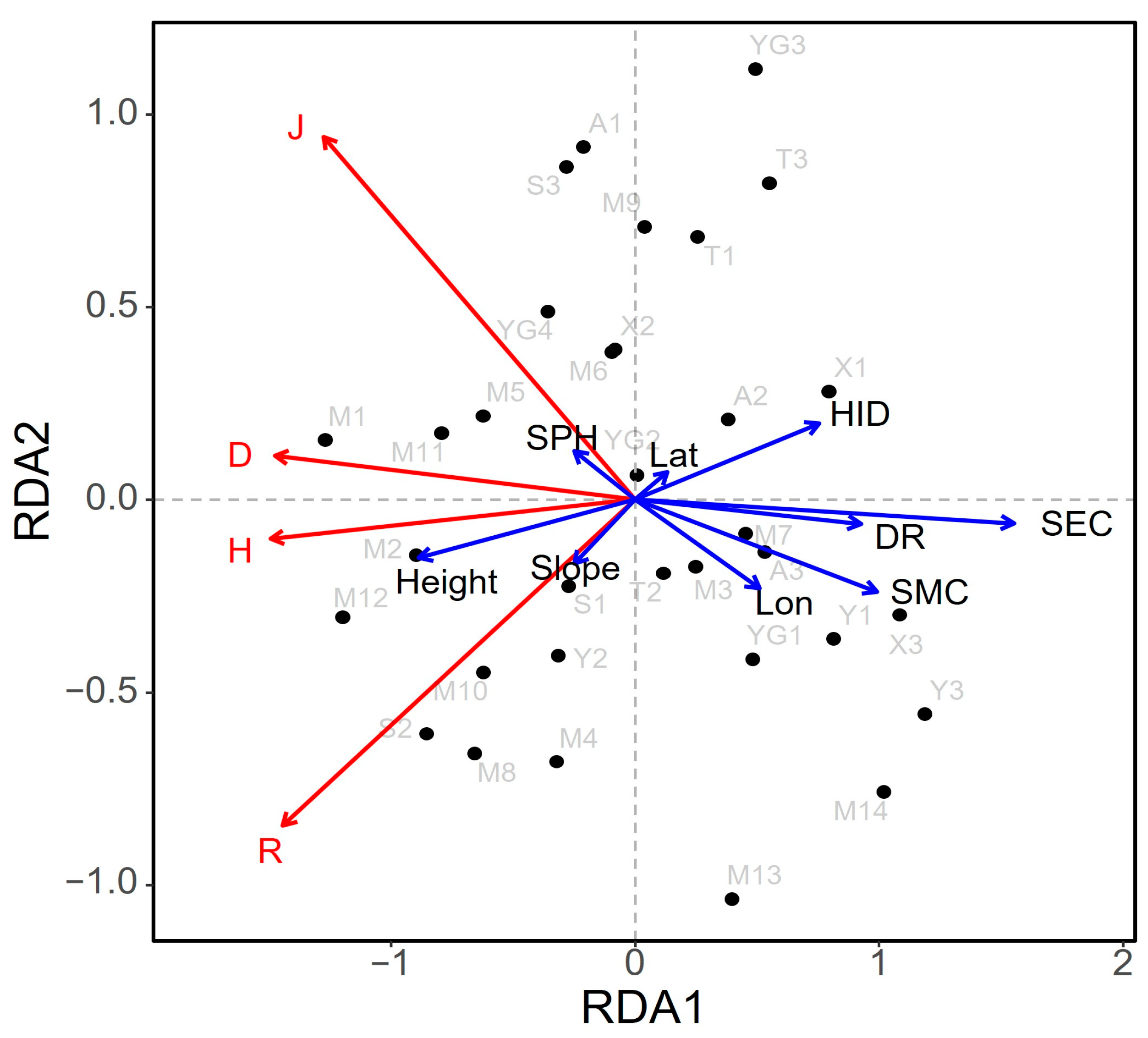
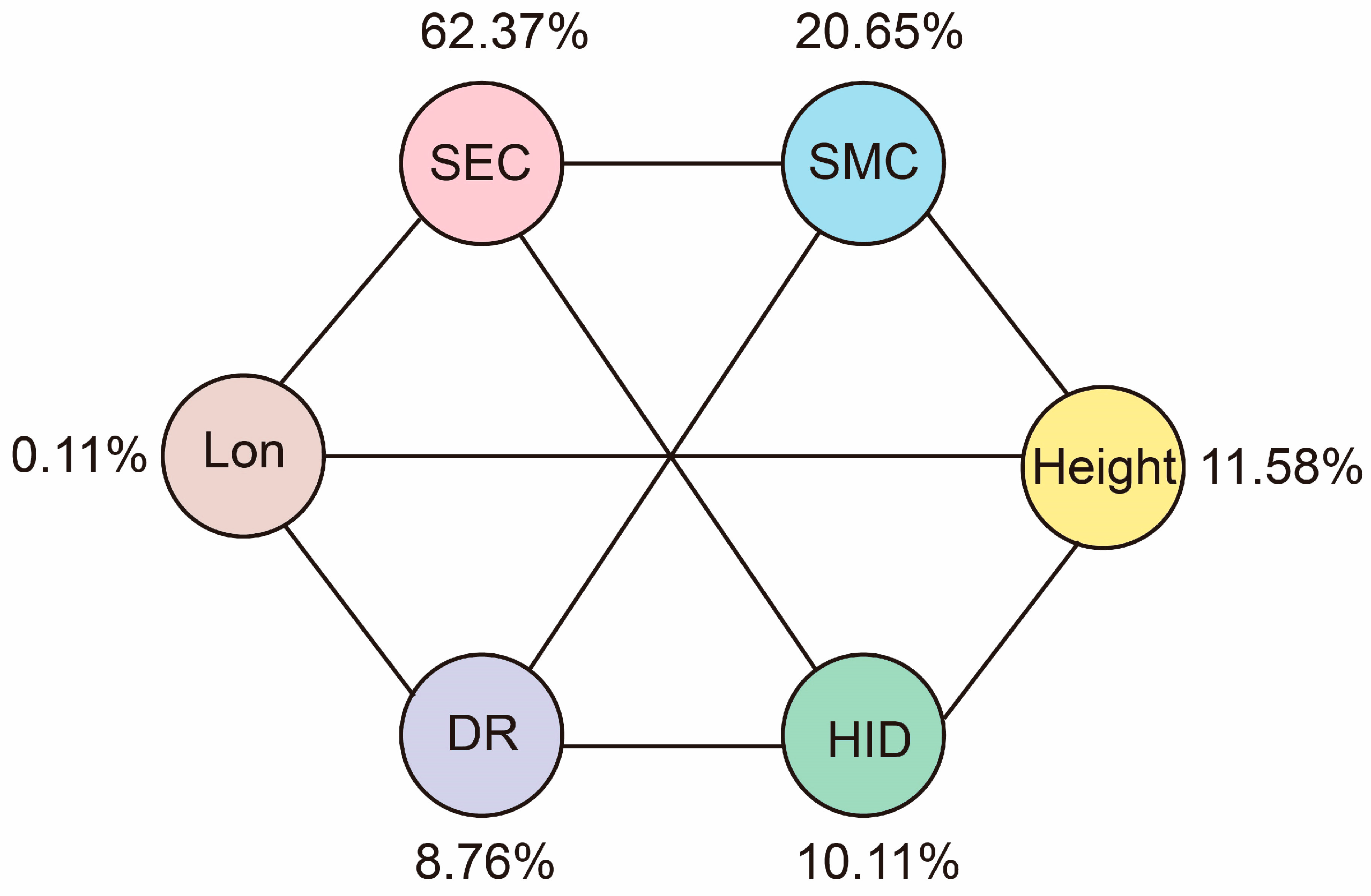
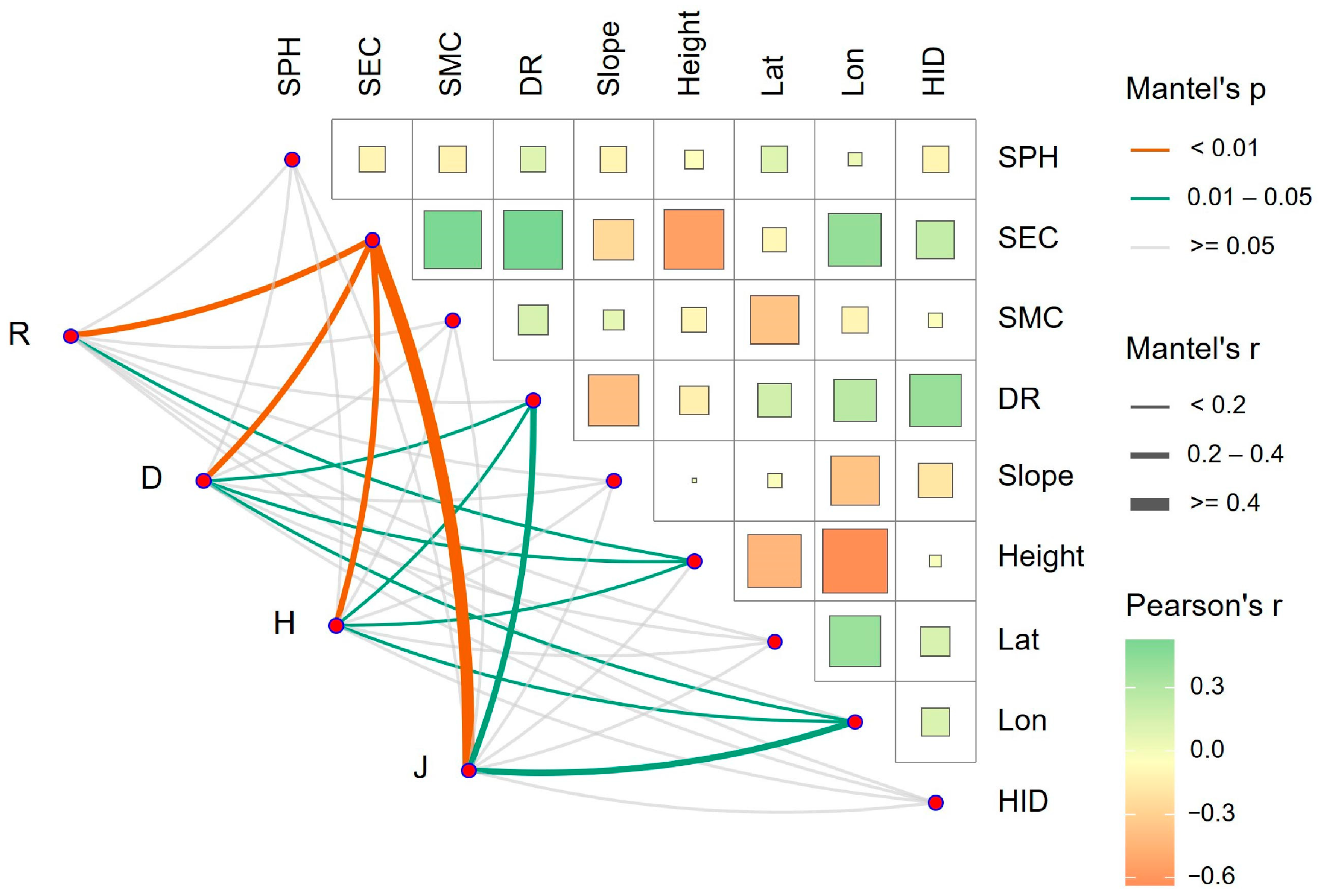
The results of the RDA and Mantel test analysis found that different plant diversity indexes had different responses to different environmental factors. In general, the plant diversity index had a strong negative correlation with SEC, soil moisture content (SMC), the nearest distance from the Yellow River (DR), human interference degree (HID), and longitude (Lon), and a strong positive correlation with height. Among them, SEC had the greatest impact on species diversity in the Yellow River Delta, and its comprehensive contribution rate to plant diversity was 62.37%. Therefore, SEC was a key limiting factor for plant diversity in the Yellow River Delta. Therefore, the implementation of reasonable soil improvement measures to reduce the risk of soil salinization is crucial for the conservation and restoration of ecosystems in the Yellow River Delta.