Comparable Analysis of Natural and Modified Starches from Kazakhstan: Physicochemical Properties, Applications, and Insights on Biodegradable Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Physicochemical Properties
2.2.1. Starch Composition Analysis
2.2.2. Moisture and Ash Content Determination
2.2.3. Mineral Content Analysis
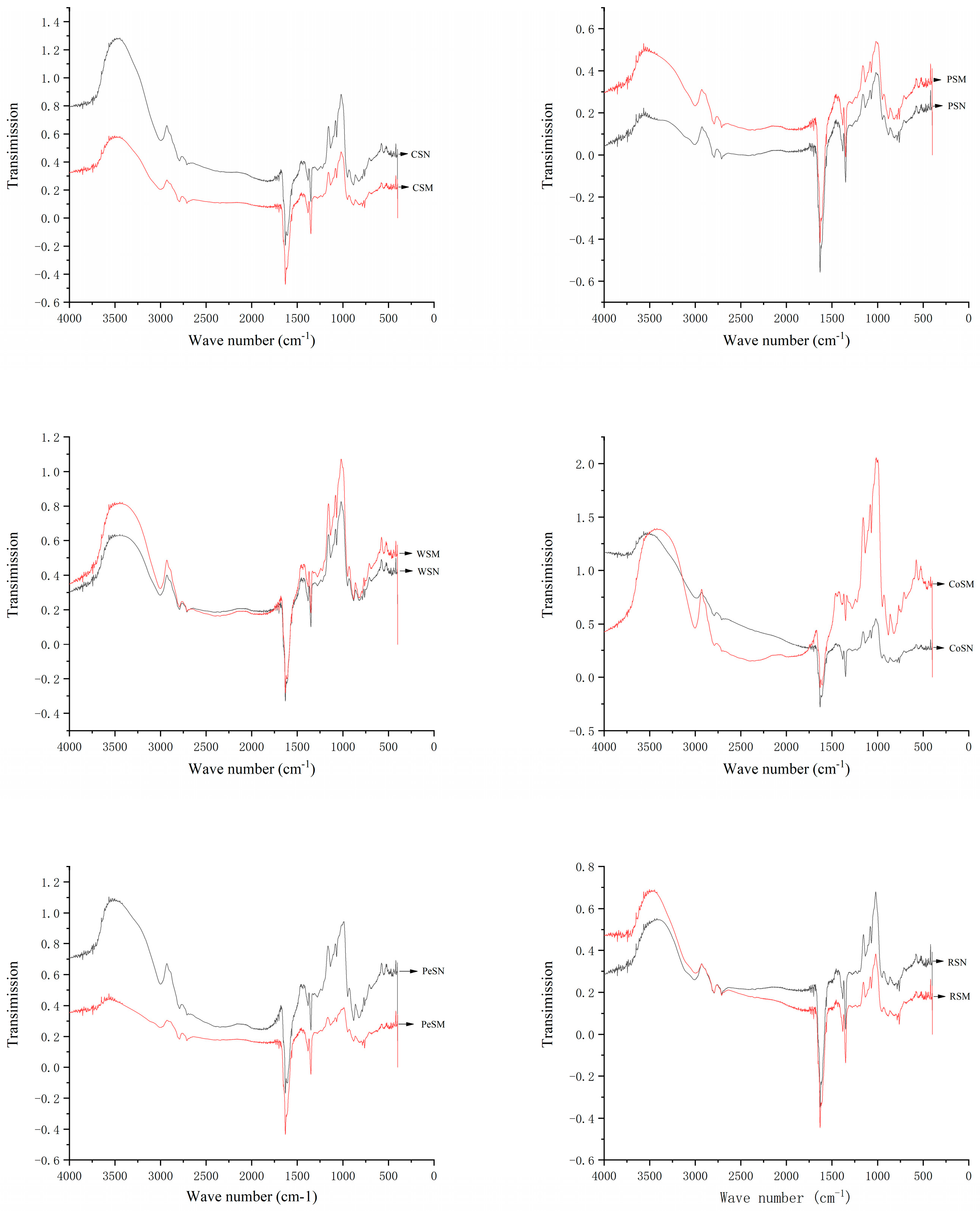
2.3. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
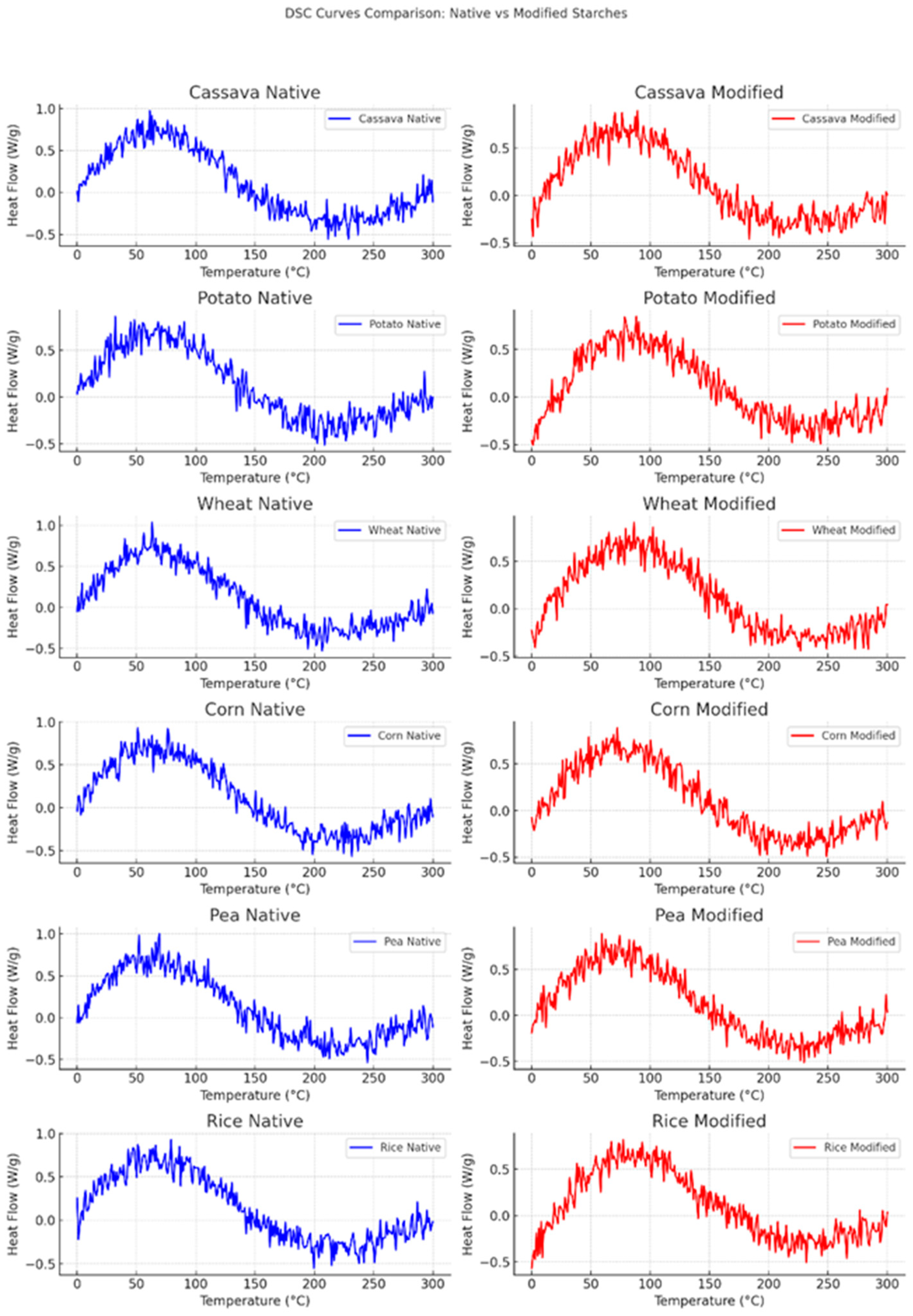
2.4. Determination of Thermal Properties with DSC
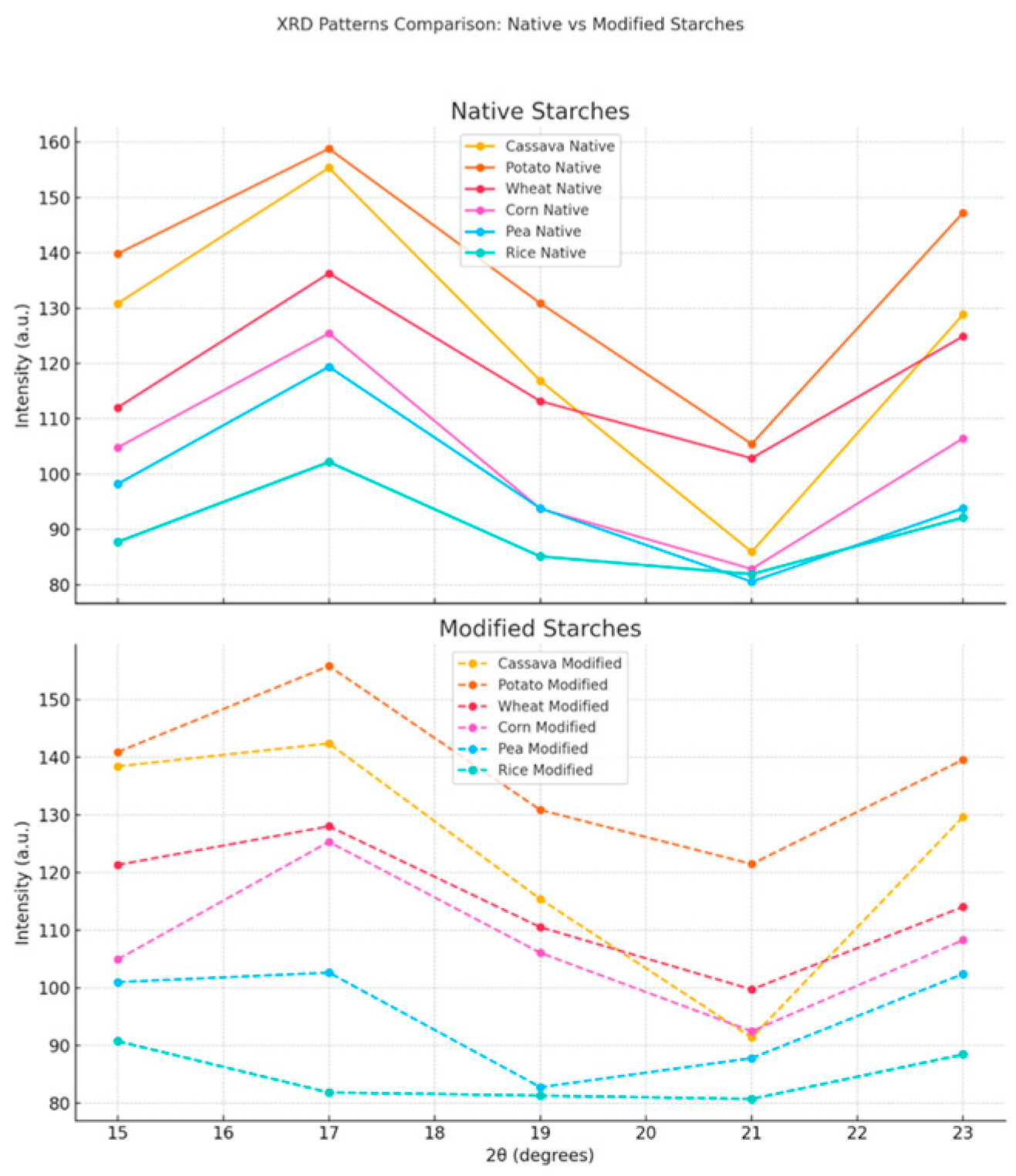
2.5. X-Ray Diffraction (XRD) Analysis
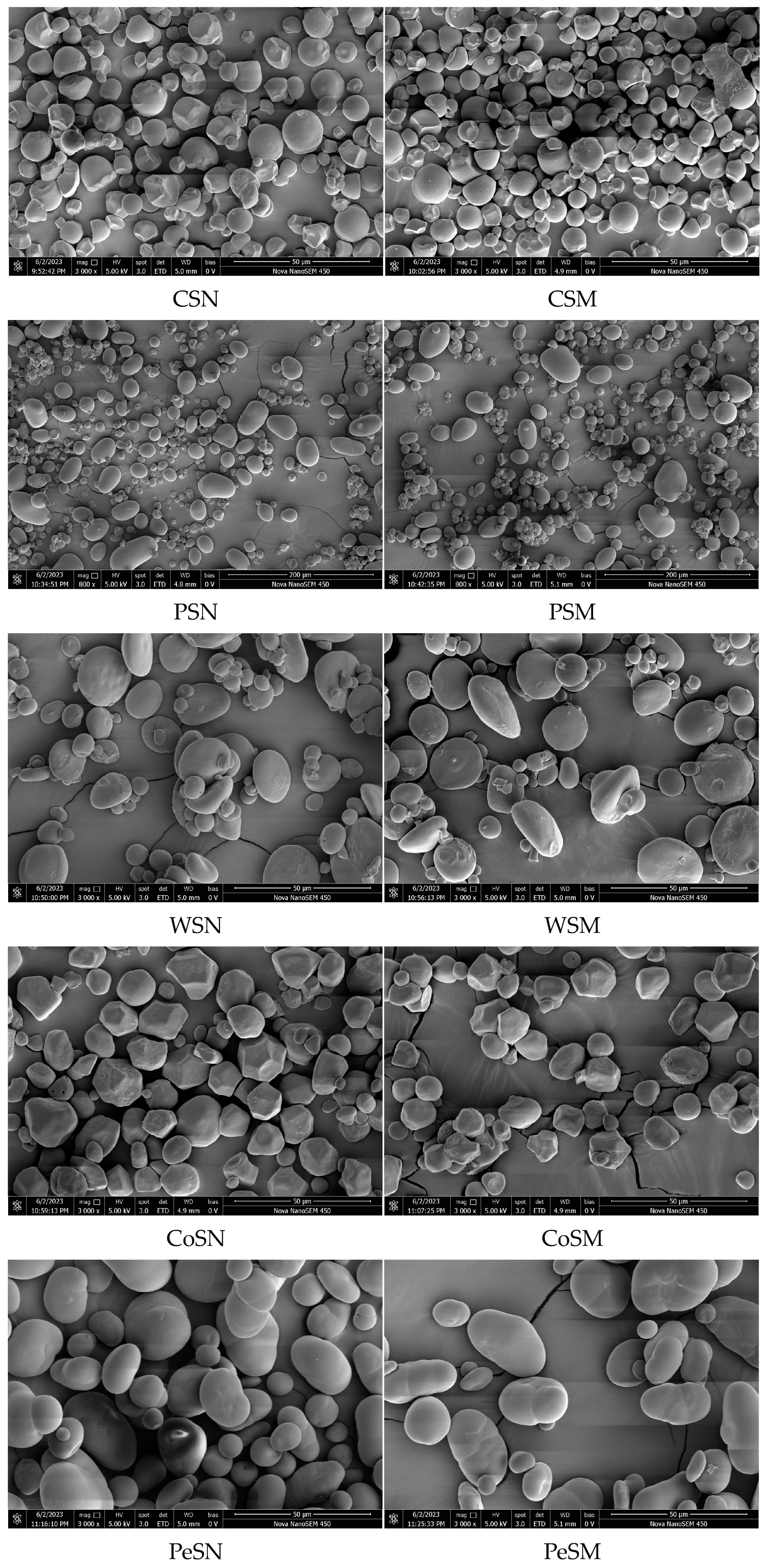
2.6. Scanning Electron Microscopy (SEM) Analysis
2.7. Viscosity Analysis (RVA)
2.8. Molecular Weight (MW) Analysis
2.9. Statistical Analysis
3. Results
3.1. Physicochemical Properties
3.2. FT-IR Analysis
3.3. Thermal Properties with DSC
3.4. X-Ray Diffraction (XRD)
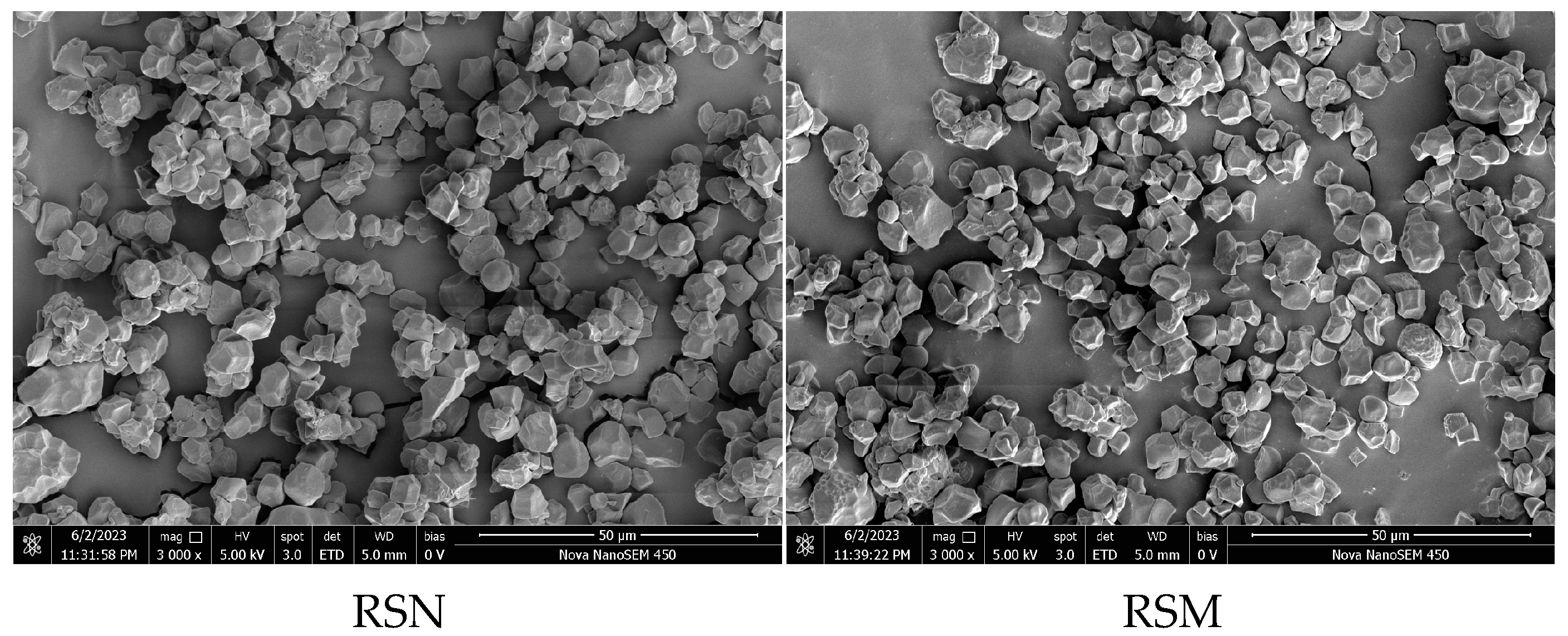
3.5. Scanning Electron Microscopy (SEM)
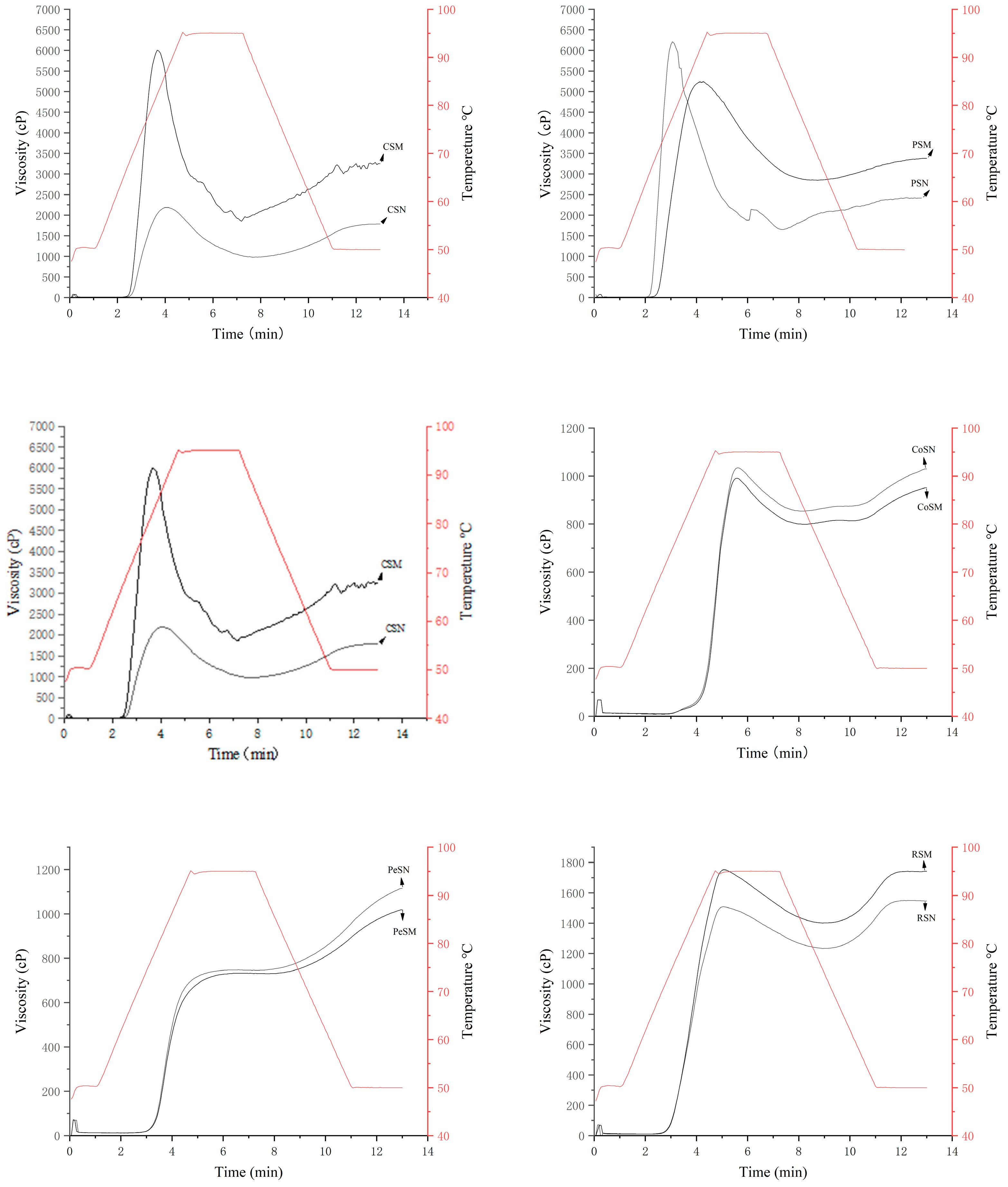
3.6. Viscosity Analysis (RVA)
3.7. Molecular Weight (MW)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Assessment of Agricultural Plastics and Their Sustainability: A Call for Action; FAO: Rome, Italy, 2021. [Google Scholar]
- Adhikari, R.; Bristow, K.L.; Casey, P.S.; Freischmidt, G.; Hornbuckle, J.W.; Adhikari, B. Preformed and sprayable polymeric mulch film to improve agricultural water use efficiency. Agric. Water Manag. 2016, 169, 1–13. [Google Scholar] [CrossRef]
- Lakhiar, I.A.; Yan, H.; Zhang, J.; Wang, G.; Deng, S.; Bao, R.; Zhang, C.; Syed, T.N.; Wang, B.; Zhou, R.; et al. Plastic pollution in agriculture as a threat to food security, the ecosystem, and the environment: An overview. Agronomy 2024, 14, 548. [Google Scholar] [CrossRef]
- Chen, X.; Hong, D.; Hou, Y. Analysis of the eco-environmental condition of Kazakhstan and its impact factors using remote sensing data. J. Geo-Inf. Sci. 2016, 18, 1000–1008. [Google Scholar]
- Yang, L.; Heng, T.; He, X.; Yang, G.; Zhao, L.; Li, Y.; Xu, Y. Spatial-temporal distribution and accumulation characteristics of residual plastic film in cotton fields in arid oasis area and the effects on soil salt transport and crop growth. Soil Tillage Res. 2023, 231, 105737. [Google Scholar] [CrossRef]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-based biodegradable materials: Challenges and opportunities. Adv. Ind. Eng. Polym. Res. 2020, 3, 8–18. [Google Scholar] [CrossRef]
- Dias, F.; Souza, R.; Lucas, E. Influence of modified starches composition on their performance as fluid loss additives in invert-emulsion drilling fluids. Fuel 2015, 140, 711–716. [Google Scholar] [CrossRef]
- Putri, T.R.; Adhitasari, A.; Paramita, V.; Yulianto, M.E.; Ariyanto, H.D. Effect of different starch on the characteristics of edible film as functional packaging in fresh meat or meat products: A review. Mater. Today Proc. 2023, 87, 192–199. [Google Scholar] [CrossRef]
- Fredriksson, H.; Silverio, J.; Andersson, R.; Eliasson, A.-C.; Åman, P. The influence of amylose and amylopectin characteristics on gelatinization and retrogradation properties of different starches. Carbohydr. Polym. 1998, 35, 119–134. [Google Scholar] [CrossRef]
- Persin, Z.; Stana-Kleinschek, K.; Foster, T.J.; Van Dam, J.E.; Boeriu, C.G.; Navard, P. Challenges and opportunities in polysaccharides research and technology: The EPNOE views for the next decade in the areas of materials, food and health care. Carbohydr. Polym. 2011, 84, 22–32. [Google Scholar] [CrossRef]
- da Rosa Zavareze, E.; Dias, A.R.G. Impact of heat-moisture treatment and annealing in starches: A review. Carbohydr. Polym. 2011, 83, 317–328. [Google Scholar] [CrossRef]
- Hoover, R. The impact of heat-moisture treatment on molecular structures and properties of starches isolated from different botanical sources. Crit. Rev. Food Sci. Nutr. 2010, 50, 835–847. [Google Scholar] [PubMed]
- Wang, S.; Wang, J.; Liu, Y.; Liu, X. Starch modification and application. In Starch Structure, Functionality and Application in Foods; Springer: Singapore, 2020; pp. 131–149. [Google Scholar]
- Zia-ud-Din; Xiong, H.; Fei, P. Physical and chemical modification of starches: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2691–2705. [Google Scholar] [PubMed]
- Velazquez, G.; Mendez-Montealvo, G.; Flores-Silva, P.C.; Soler, A. Multi-Scale Structures, Functional Properties, and Applications of Starch Modified by Dry Heat Treatment. Biopolymers 2025, 116, e70000. [Google Scholar]
- Liu, S.; Liu, H.; Gao, S.; Guo, S.; Zhang, C. Dry heating affects the multi-structures, physicochemical properties, and in vitro digestibility of blue highland barley starch. Front. Nutr. 2023, 10, 1191391. [Google Scholar]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch retrogradation: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar]
- Jenkins, P.J.; Donald, A.M. Gelatinisation of starch: A combined SAXS/WAXS/DSC and SANS study. Carbohydr. Res. 1998, 308, 133–147. [Google Scholar]
- WHO. Regional Overview of Food Security and Nutrition in Europe and Central Asia 2020: Affordable Healthy Diets to Address All Forms of Malnutrition for Better Health; Food & Agriculture Org.: Rome, Italy, 2021. [Google Scholar]
- Liang, W.; Lin, Q.; Zeng, J.; Gao, H.; Muratkhan, M.; Li, W. Understanding the improvement of sorghum starch acid hydrolysis modification by E-beam irradiation: A supramolecular structure perspective. Food Chem. 2024, 437, 137820. [Google Scholar] [CrossRef]
- Liang, W.; Ge, X.; Lin, Q.; Niu, L.; Zhao, W.; Liu, X.; Guo, S.; Muratkhan, M.; Li, W. pH-responsive PVA/konjac glucomannan/functional liposome terpolymer composites: Spotlight on its structure-performance, functionality, barrier properties, controlled-release behavior, biodegradability, and its intelligent preservation role for food. Food Packag. Shelf Life 2024, 42, 101250. [Google Scholar] [CrossRef]
- ISO 6647-1:2020; Rice–Determination of amylose content—Part 1: Reference Method. ISO: Geneva, Switzerland, 2020.
- ISO 712:2009; Cereals and Cereal Products—Determination of Moisture Content—Reference Method. ISO: Geneva, Switzerland, 2009.
- ISO 2171:2023; Cereals, Pulses and by-Products—Determination of Ash Yield. ISO: Geneva, Switzerland, 2023.
- ISO 17294-2:2016; Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of Selected Elements. ISO: Geneva, Switzerland, 2016.
- ISO 6869:2000; Animal Feeding Stuffs—Determination of the Contents of Calcium, Copper, Iron, Magnesium, Manganese, Potassium, Sodium and Zinc—Method Using Atomic Absorption Spectrometry. ISO: Geneva, Switzerland, 2000.
- ISO 2590:1973; General Method for the Determination of Oxidizing Substances—Iodometric Method. ISO: Geneva, Switzerland, 1973.
- ISO 6491:1998; Animal Feeding Stuffs—Determination of Phosphorus Content—Spectrometric Method. ISO: Geneva, Switzerland, 1998.
- ISO 20649:2015; Infant Formula and Adult Nutritionals—Determination of the Content of Galacto-Oligosaccharides (GOS) and Fructo-Oligosaccharides (FOS). ISO: Geneva, Switzerland, 2015.
- Gupta, R.K.; Guha, P.; Srivastav, P.P. Physical action of nonthermal cold plasma technology for starch modification. Food Phys. 2024, 1, 100011. [Google Scholar]
- Bonilla, J.; Fortunati, E.; Atarés, L.; Chiralt, A.; Kenny, J.M. Physical, structural and antimicrobial properties of poly vinyl alcohol–chitosan biodegradable films. Food Hydrocoll. 2014, 35, 463–470. [Google Scholar] [CrossRef]
- Ge, Y.; Cai, Q.; Wang, Y.; Gao, J.; Chi, Y.; Dai, S. Synthesis of high-molecular-weight branched polyethylene using a hybrid “sandwich” pyridine-imine Ni (II) catalyst. Front. Chem. 2022, 10, 886888. [Google Scholar] [CrossRef] [PubMed]
- Firouz, M.S.; Mohi-Alden, K.; Omid, M. A critical review on intelligent and active packaging in the food industry: Research and development. Food Res. Int. 2021, 141, 110113. [Google Scholar] [CrossRef]
- Shanmugavel, D.; Ramadoss, R. Unveiling the potential of viscosity-modifying amylopectin for enhancing hydraulic lime mortar performance. Eur. Phys. J. Plus 2024, 139, 739. [Google Scholar] [CrossRef]
- Channab, B.-E.; El Idrissi, A.; Essamlali, Y.; Zahouily, M. Nanocellulose: Structure, modification, biodegradation and applications in agriculture as slow/controlled release fertilizer, superabsorbent, and crop protection: A review. J. Environ. Manag. 2024, 352, 119928. [Google Scholar] [CrossRef]
- Pei, J.; Palanisamy, C.P.; Srinivasan, G.P.; Panagal, M.; Kumar, S.S.D.; Mironescu, M. A comprehensive review on starch-based sustainable edible films loaded with bioactive components for food packaging. Int. J. Biol. Macromol. 2024, 274 Pt 1, 133332. [Google Scholar] [CrossRef]
- Wu, X.; Yan, X.; Zhang, B.; Zhang, Q.; Zhang, X.; Zhang, J.; Wu, X. Effect of strengthening agents on properties of dual-modified cassava starch-based degradable films. Int. J. Biol. Macromol. 2025, 291, 139142. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wang, Y.; Yang, H. Effects of cation valence on swelling power, solubility, pasting, gel strength characteristics of potato starch. Food Chem. 2024, 434, 137510. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Manrique, J.A.; Salcedo-Mendoza, J.; Chávez-Salazar, A.; Cortés-Rodríguez, M.; Castellanos-Galeano, F.J. Isolation, Modification, and Characterization of Starches from Hawthorn yam (Dioscorea rotundata) Grown in Colombia: Bromatological Composition and Structural Analysis. Starch-Stärke 2024, 77, 2400052. [Google Scholar] [CrossRef]
- Xie, S.; Li, Z.; Duan, Q.; Huang, W.; Huang, W.; Deng, Y.; Chen, P.; Xie, F. Reducing oil absorption in pea starch through two-step annealing with varying temperatures. Food Hydrocoll. 2024, 150, 109701. [Google Scholar] [CrossRef]
- Huang, X.; Chen, L.; Liu, Y. Effects of ultrasonic and ozone modification on the morphology, mechanical, thermal and barrier properties of corn starch films. Food Hydrocoll. 2024, 147, 109376. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, X.; Al-Maqtari, Q.A.; He, H.-J.; Othman, N. Evaluation of amylose content: Structural and functional properties, analytical techniques, and future prospects. Food Chem. X 2024, 24, 101830. [Google Scholar] [PubMed]
- Gu, W.; Tan, Y.; Pang, H.; Ye, Q.; Li, X.; Li, J. Recent progress in robust regenerated soy protein film. Macromol. Mater. Eng. 2024, 309, 2300224. [Google Scholar] [CrossRef]
- Almeida, R.L.J.; Santos, N.C.; Monteiro, S.S.; Rios, N.S.; dos Santos, E.S. Exploring the potential of native and modified starch and starch nanocrystals in Pickering emulsions: Current advances, future perspectives, and challenges. Food Biosci. 2024, 61, 104675. [Google Scholar]
- Dimonie, D.; Grigorescu, R.M.; Trică, B.; Damian, C.-M.; Vasile, E.; Trusca, R.; Nicolae, C.-A.; Constantinescu-Aruxandei, D.; Oancea, F. Increasing the Efficiency of Multilayered Silicate Melt Incorporation into Starch-Based Polymeric Matrices. J. Compos. Sci. 2024, 8, 72. [Google Scholar] [CrossRef]
- Ranade, T.; Sati, A.; Pratap, A.; Mali, S.N. Curcumin-integrated biopolymer films for active packaging: Current trends and future directions. Chem. Pap. 2025, 79, 1303–1334. [Google Scholar]
- Shrivastava, A.; Gupta, R.K.; Srivastav, P.P. Exploring novel frontiers of advancements in purple yam (Dioscorea alata L.) starch extraction, modification, characterization, applications in food and other industries. Meas. Food 2024, 15, 100196. [Google Scholar]
- Rostamabadi, H.; Yildirim-Yalcin, M.; Demirkesen, I.; Toker, O.S.; Colussia, R.; do Nascimentob, L.Á.; Şahin, S.; Falsafi, S.R. Improving physicochemical and nutritional attributes of rice starch through green modification techniques. Food Chem. 2024, 458, 140212. [Google Scholar]
- Airinei, D.N.; Modrogan, C.; Orbuleț, O.D.; Dǎncilǎ, A.M.; Boşomoiu, M.; Matei, C. Biodegradable Thermoplastic Materials with Application in the Manufacture of Bags Without Synthetic Polymers. Polymers 2025, 17, 356. [Google Scholar] [CrossRef]
- da Silva, T.N.; Cardoso, S.A.; da Silva, F.F.F.; de Carvalho Patricio, B.F.; Gonçalves, R.P.; Weissmuller, G.; El-cheikh, M.C.; Carneiro, K.; Barradas, T.N. Chitosan-based films filled with nanoencapsulated essential oil: Physical-chemical characterization and enhanced wound healing activity. Int. J. Biol. Macromol. 2024, 261, 129049. [Google Scholar]
| Raw Material | Variety | Production Region |
|---|---|---|
| Potato | Gala | East Kazakhstan |
| Wheat | Steklovidnaya-24 | North Kazakhstan |
| Corn | Altyn Dan | Almaty Region |
| Pea | Zhambyl 8 | Zhambyl Region |
| Rice | Marzhan | Kyzylorda Region |
| Cassava | Cassava 531 | South Kazakhstan |
| Starch | Amylose Content | Amylopectin Content | Water Content | Mineral Content |
|---|---|---|---|---|
| Potato | 20–30 | 70–80 | 12–14 | 0.2–0.3 |
| Wheat | 25–28 | 72–75 | 11–13 | 0.3–0.4 |
| Corn | 24–28 | 72–76 | 10–12 | 0.1–0.2 |
| Pea | 30–35 | 65–70 | 11–13 | 0.2–0.3 |
| Rice | 18–20 | 80–82 | 12–14 | 0.1–0.2 |
| Cassava | 17–24 | 76–83 | 10–12 | 0.1–0.2 |
| Mineral | Cassava Starch | Potato Starch | Wheat Starch | Corn Starch | Pea Starch | Rice Starch |
|---|---|---|---|---|---|---|
| Zn | 0.48 ± 0.04 | 2.6 ± 0.08 | 2.63 ± 1.66 | 1.3 ± 0.06 | 2.3 ± 0.09 | 1.0 ± 0.16 |
| Mg | 4307 ± 39 | 2107 ± 44 | 1390 ± 99 | 867 ± 42.4 | 1757 ± 26 | 722.5 ± 52.1 |
| Fe | 12.6 ± 0.2 | 16.5 ± 0.4 | 2.65 ± 2.0 | 5.5 ± 0.08 | 22.8 ± 1.1 | 4.6 ± 0.18 |
| Ca | 25.9 ± 2.2 | 300 ± 5.3 | 299.8 ± 8.7 | 210.6 ± 3.1 | 250 ± 3.3 | 160.6 ± 1.1 |
| Se | 12.1 ± 0.6 | 3.3 ± 0.06 | - | 1.2 ± 0.03 | 2.75 ± 0.16 | 1.0 ± 0.5 |
| Starch | Average Molecular Weight (kDa) | Polydispersity Index (PDI) | Retention Time of Main Peak (min) | Observations |
|---|---|---|---|---|
| Cassava, natural | 500 | 1.5 | 12.5 | High amylopectin content; large molecules indicated by an early peak. |
| Cassava, modified | 480 | 1.4 | 12.3 | Stable molecular structure with improved humidity resistance. |
| Potato, natural | 450 | 1.4 | 13.0 | Medium molecular weight with dense distribution. |
| Potato, modified | 430 | 1.3 | 13.2 | Lower PDI enhances stability and mechanical properties. |
| Wheat, natural | 320 | 2.0 | 15.2 | Broad distribution, likely related to the ratio of amylose and amylopectin. |
| Wheat, modified | 310 | 1.8 | 15.0 | Moderate reduction in PDI improves stability and biodegradability. |
| Corn, natural | 470 | 1.3 | 12.8 | Consistent molecular size with low PDI, suitable for strong film formation. |
| Corn, modified | 455 | 1.2 | 12.6 | Low PDI and high molecular weight provide excellent mechanical properties. |
| Pea, natural | 350 | 1.7 | 14.5 | Medium molecular size with moderate polydispersity. |
| Pea, modified | 340 | 1.6 | 14.3 | Lower PDI enhances resistance to external factors. |
| Rice, natural | 300 | 2.1 | 15.5 | High polydispersity; smaller molecules ideal for rapid degradation. |
| Rice, modified | 290 | 1.9 | 15.4 | Moderate polydispersity supports high biodegradation rates. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muratkhan, M.; Zhainagul, K.; Yermekov, Y.; Svetlana, K.; Toimbayeva, D.; Temirova, I.; Amirsana, K.; Khamitova, D.; Zharykbasov, Y.; Sugirbay, A.; et al. Comparable Analysis of Natural and Modified Starches from Kazakhstan: Physicochemical Properties, Applications, and Insights on Biodegradable Films. Appl. Sci. 2025, 15, 3938. https://doi.org/10.3390/app15073938
Muratkhan M, Zhainagul K, Yermekov Y, Svetlana K, Toimbayeva D, Temirova I, Amirsana K, Khamitova D, Zharykbasov Y, Sugirbay A, et al. Comparable Analysis of Natural and Modified Starches from Kazakhstan: Physicochemical Properties, Applications, and Insights on Biodegradable Films. Applied Sciences. 2025; 15(7):3938. https://doi.org/10.3390/app15073938
Chicago/Turabian StyleMuratkhan, Marat, Kakimova Zhainagul, Yernaz Yermekov, Kamanova Svetlana, Dana Toimbayeva, Indira Temirova, Kiykbay Amirsana, Dina Khamitova, Yerlan Zharykbasov, Adilet Sugirbay, and et al. 2025. "Comparable Analysis of Natural and Modified Starches from Kazakhstan: Physicochemical Properties, Applications, and Insights on Biodegradable Films" Applied Sciences 15, no. 7: 3938. https://doi.org/10.3390/app15073938
APA StyleMuratkhan, M., Zhainagul, K., Yermekov, Y., Svetlana, K., Toimbayeva, D., Temirova, I., Amirsana, K., Khamitova, D., Zharykbasov, Y., Sugirbay, A., Saule, S., & Ospankulova, G. (2025). Comparable Analysis of Natural and Modified Starches from Kazakhstan: Physicochemical Properties, Applications, and Insights on Biodegradable Films. Applied Sciences, 15(7), 3938. https://doi.org/10.3390/app15073938








