A Review on Flavonoids as Anti-Helicobacter pylori Agents
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Review on H. pylori Infection and Treatment
3.1.1. Infection
3.1.2. Diagnosis and Treatment
3.1.3. Genome and Markers
3.2. Medicinal Plants and Their Potential Application
3.3. Flavonoids Usefulness in Treating H. pylori
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roszczenko-Jasinska, P.; Wojtys, M.I.; Jagusztyn-Krynicka, E.K. Helicobacter pylori treatment in the post-antibiotics era-searching for new drug targets. Appl. Microbiol. Biotechnol. 2020, 104, 9891–9905. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yuan, C.; Zhou, S.; Lu, J.; Zeng, M.; Cai, X.; Song, H. Helicobacter pylori infection: A dynamic process from diagnosis to treatment. Front. Cell. Infect. Microbiol. 2023, 13, 1257817. [Google Scholar] [CrossRef] [PubMed]
- Mišak, Z.; Hojsak, I.; Homan, M. Review: Helicobacter pylori in pediatrics. Helicobacter 2019, 24 (Suppl. S1), e12639. [Google Scholar] [CrossRef]
- Fatema, J.; Khan, A.H.; Uddin, M.J.; Rahman, M.H.; Saha, M.; Safwath, S.A.; Alam, M.J.; Mamun, M.A. Chronic Gastritis and its Association with H. Pylori Infection. Mymensingh Med. J. 2015, 24, 717–722. [Google Scholar]
- Howden, C.W.; Sheldon, K.L.; Almenoff, J.S.; Chey, W.D. Pitfalls of Physician-Directed Treatment of Helicobacter pylori: Results from Two Phase 3 Clinical Trials and Real-World Prescribing Data. Dig. Dis. Sci. 2022, 67, 4382–4386. [Google Scholar] [CrossRef]
- Smith, S.; Jolaiya, T.; Fowora, M.; Palamides, P.; Ngoka, F.; Bamidele, M.; Lesi, O.; Onyekwere, C.; Ugiagbe, R.; Agbo, I.; et al. Clinical and Socio- Demographic Risk Factors for Acquisition of Helicobacter pylori Infection in Nigeria. Asian Pac. J. Cancer Prev. 2018, 19, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Wang, T.C. Helicobacter pylori and Gastric Cancer. Gastrointest. Endosc. Clin. N. Am. 2021, 31, 451–465. [Google Scholar] [CrossRef]
- Atrisco-Morales, J.; Martinez-Santos, V.I.; Roman-Roman, A.; Alarcon-Millan, J.; De Sampedro-Reyes, J.; Cruz-Del Carmen, I.; Martinez-Carrillo, D.N.; Fernandez-Tilapa, G. vacA s1m1 genotype and cagA EPIYA-ABC pattern are predominant among Helicobacter pylori strains isolated from Mexican patients with chronic gastritis. J. Med. Microbiol. 2018, 67, 314–324. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, R.; Smith, S.M. An Overview of Helicobacter pylori Infection. In Helicobacter Pylori; Smith, S.M., Ed.; Springer: New York, NY, USA, 2021; pp. 1–14. [Google Scholar]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef]
- Matos, J.I.; de Sousa, H.A.C.; Marcos-Pinto, R.; Dinis-Ribeiro, M. Helicobacter pylori CagA and VacA genotypes and gastric phenotype: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2013, 25, 1431–1441. [Google Scholar] [CrossRef]
- Salimzadeh, L.; Bagheri, N.; Zamanzad, B.; Azadegan-Dehkordi, F.; Rahimian, G.; Hashemzadeh-Chaleshtori, M.; Rafieian-Kopaei, M.; Sanei, M.H.; Shirzad, H. Frequency of virulence factors in Helicobacter pylori-infected patients with gastritis. Microb. Pathog. 2015, 80, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Umit, H.; Tezel, A.; Bukavaz, S.; Unsal, G.; Otkun, M.; Soylu, A.R.; Tucer, D.; Otkun, M.; Bilgi, S. The relationship between virulence factors of Helicobacter pylori and severity of gastritis in infected patients. Dig. Dis. Sci. 2009, 54, 103–110. [Google Scholar] [CrossRef]
- Guo, C.G.; Zhang, F.; Jiang, F.; Wang, L.; Chen, Y.; Zhang, W.; Zhou, A.; Zhang, S.; Leung, W.K. Long-term effect of Helicobacter pylori eradication on colorectal cancer incidences. Ther. Adv. Gastroenterol. 2023, 16, 17562848231170943. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Beydoun, H.A.; Elbejjani, M.; Dore, G.A.; Zonderman, A.B. Helicobacter pylori seropositivity and its association with incident all-cause and Alzheimer’s disease dementia in large national surveys. Alzheimer’s Dement. 2018, 14, 1148–1158. [Google Scholar] [CrossRef]
- Dardiotis, E.; Tsouris, Z.; Mentis, A.A.; Siokas, V.; Michalopoulou, A.; Sokratous, M.; Dastamani, M.; Bogdanos, D.P.; Deretzi, G.; Kountouras, J.H. pylori and Parkinson’s disease: Meta-analyses including clinical severity. Clin. Neurol. Neurosurg. 2018, 175, 16–24. [Google Scholar] [CrossRef] [PubMed]
- AlBalbeesi, A.; Alsalman, H.; Alotaibi, H.; Halawani, M.; Almukhadeb, E.; Alsaif, F.; Azzam, N.; AlKaff, T.; Aldosari, M.; Shadid, A. Prevalence of Helicobacter pylori Infection Among Rosacea and Chronic Spontaneous Urticaria Patients in a Tertiary Hospital in Riyadh, Saudi Arabia. Cureus 2021, 13, e17617. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Beydoun, H.A.; Weiss, J.; Hossain, S.; El-Hajj, Z.W.; Zonderman, A.B. Helicobacter pylori, periodontal pathogens, and their interactive association with incident all-cause and Alzheimer’s disease dementia in a large national survey. Mol. Psychiatry 2021, 26, 6038–6053. [Google Scholar] [CrossRef]
- World Health Organization. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis. Available online: https://www.who.int/publications/i/item/WHO-EMP-IAU-2017.12 (accessed on 4 June 2024).
- Liou, J.M.; Lee, Y.C.; Wu, M.S. Treatment of Helicobacter pylori infection and its long-term impacts on gut microbiota. J. Gastroenterol. Hepatol. 2020, 35, 1107–1116. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Report on Traditional and Complementary Medicine 2019. Available online: https://www.who.int/publications/i/item/978924151536 (accessed on 5 June 2024).
- Siritapetawee, J.; Limphirat, W.; Wongviriya, W.; Maneesan, J.; Samosornsuk, W. Isolation and characterization of a galactose-specific lectin (EantH) with antimicrobial activity from Euphorbia antiquorum L. latex. Int. J. Biol. Macromol. 2018, 120, 1846–1854. [Google Scholar] [CrossRef]
- Bahar, E.; Siddika, M.S.; Nath, B.; Yoon, H. Evaluation of In vitro Antioxidant and In vivo Antihyperlipidemic Activities of Methanol Extract of Aerial Part of Crassocephalum crepidioides (Asteraceae) Benth S Moore. Trop. J. Pharm. Res. 2016, 15, 481. [Google Scholar] [CrossRef]
- Gao, T.; Zhu, Z.Y.; Zhou, X.; Xie, M.L. Chrysanthemum morifolium extract improves hypertension-induced cardiac hypertrophy in rats by reduction of blood pressure and inhibition of myocardial hypoxia inducible factor-1alpha expression. Pharm. Biol. 2016, 54, 2895–2900. [Google Scholar] [CrossRef] [PubMed]
- de Souza, W.F.M.; Mariano, X.M.; Isnard, J.L.; de Souza, G.S.; de Souza Gomes, A.L.; de Carvalho, R.J.T.; Rocha, C.B.; Junior, C.L.S.; Moreira, R.F.A. Evaluation of the volatile composition, toxicological and antioxidant potentials of the essential oils and teas of commercial Chilean boldo samples. Food Res. Int. 2019, 124, 27–33. [Google Scholar] [CrossRef]
- Uberti, A.F.; Callai-Silva, N.; Grahl, M.V.C.; Piovesan, A.R.; Nachtigall, E.G.; Furini, C.R.G.; Carlini, C.R. Helicobacter pylori Urease: Potential Contributions to Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 3091. [Google Scholar] [CrossRef]
- Kato, T.; Yagi, N.; Kamada, T.; Shimbo, T.; Watanabe, H.; Ida, K.; Study Group for Establishing Endoscopic Diagnosis of Chronic Gastritis. Diagnosis of Helicobacter pylori infection in gastric mucosa by endoscopic features: A multicenter prospective study. Dig. Endosc. 2013, 25, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Alkim, H.; Koksal, A.R.; Boga, S.; Sen, I.; Alkim, C. Role of Bismuth in the Eradication of Helicobacter pylori. Am. J. Ther. 2017, 24, e751–e757. [Google Scholar] [PubMed]
- Suzuki, S.; Kusano, C.; Horii, T.; Ichijima, R.; Ikehara, H. The Ideal Helicobacter pylori Treatment for the Present and the Future. Digestion 2022, 103, 62–68. [Google Scholar] [CrossRef]
- Ji, J.; Yang, H. Using Probiotics as Supplementation for Helicobacter pylori Antibiotic Therapy. Int. J. Mol. Sci. 2020, 21, 1136. [Google Scholar] [CrossRef]
- Gonzalez, A.; Salillas, S.; Velazquez-Campoy, A.; Espinosa Angarica, V.; Fillat, M.F.; Sancho, J.; Lanas, A. Identifying potential novel drugs against Helicobacter pylori by targeting the essential response regulator HsrA. Sci. Rep. 2019, 9, 11294. [Google Scholar] [CrossRef]
- Sierra, J.M.; Fuste, E.; Rabanal, F.; Vinuesa, T.; Vinas, M. An overview of antimicrobial peptides and the latest advances in their development. Expert Opin. Biol. Ther. 2017, 17, 663–676. [Google Scholar] [CrossRef]
- Xu, L.; Shao, C.; Li, G.; Shan, A.; Chou, S.; Wang, J.; Ma, Q.; Dong, N. Conversion of Broad-Spectrum Antimicrobial Peptides into Species-Specific Antimicrobials Capable of Precisely Targeting Pathogenic Bacteria. Sci. Rep. 2020, 10, 944. [Google Scholar] [CrossRef]
- Zharkova, M.S.; Orlov, D.S.; Golubeva, O.Y.; Chakchir, O.B.; Eliseev, I.E.; Grinchuk, T.M.; Shamova, O.V. Application of Antimicrobial Peptides of the Innate Immune System in Combination with Conventional Antibiotics-A Novel Way to Combat Antibiotic Resistance? Front. Cell. Infect. Microbiol. 2019, 9, 128. [Google Scholar] [CrossRef]
- Marshall, B.J.; Warren, J.R. Unidentified Curved Bacilli in the Stomach of Patients with Gastrities and Peptic Ulceration. Lancet 1984, 1, 1311–1315. [Google Scholar] [CrossRef]
- Tomb, J.-F.; White, O.; Kerlavage, A.R.; Clayton, R.A.; Sutton, G.G.; Fleischmann, R.D.; Ketchum, K.A.; Klenk, H.P.; Gill, S.; Dougherty, B.A.; et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 1997, 388, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Alm, R.A.; Ling, L.-S.L.; Moir, D.T.; King, B.L.; Brown, E.D.; Doig, P.C.; Smith, D.R.; Noonan, B.; Guild, B.C.; deJonge, B.L.; et al. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 1999, 397, 176–180. [Google Scholar] [CrossRef]
- Mobley, H.L.T.; Mendz, G.L.; Hazell, S.L. Helicobacter pylori: Physiology and Genetics; ASM Press: Washington, DC, USA, 2001. [Google Scholar]
- Wattam, A.R.; Gabbard, J.L.; Shukla, M.; Sobral, B.W. Comparative genomic analysis at the PATRIC, a bioinformatic resource center. Methods Mol. Biol. 2014, 1197, 287–308. [Google Scholar] [CrossRef]
- Maixner, F.; Krause-Kyora, B.; Turaev, D.; Herbig, A.; Hoopmann, M.R.; Hallows, J.L.; Kusebauch, U.; Vigl, E.E.; Malfertheiner, P.; Megraud, F.; et al. The 5300-year-old Helicobacter pylori genome of the Iceman. Science 2016, 351, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Uotani, T.; Miftahussurur, M.; Yamaoka, Y. Effect of bacterial and host factors on Helicobacter pylori eradication therapy. Expert Opin. Ther. Targets 2015, 19, 1637–1650. [Google Scholar] [CrossRef]
- van Tonder, A.J.; Mistry, S.; Bray, J.E.; Hill, D.M.; Cody, A.J.; Farmer, C.L.; Klugman, K.P.; von Gottberg, A.; Bentley, S.D.; Parkhill, J.; et al. Defining the estimated core genome of bacterial populations using a Bayesian decision model. PLoS Comput. Biol. 2014, 10, e1003788. [Google Scholar] [CrossRef]
- Alvarez, M.C.; Fernandes, J.; Michel, V.; Touati, E.; Ribeiro, M.L. Effect of Helicobacter pylori Infection on GATA-5 and TFF1 Regulation, Comparison Between Pediatric and Adult Patients. Dig. Dis. Sci. 2018, 63, 2889–2897. [Google Scholar] [CrossRef]
- Teng, K.W.; Hsieh, K.S.; Hung, J.S.; Wang, C.J.; Liao, E.C.; Chen, P.C.; Lin, Y.H.; Wu, D.C.; Lin, C.H.; Wang, W.C.; et al. Helicobacter pylori employs a general protein glycosylation system for the modification of outer membrane adhesins. Gut Microbes 2022, 14, 2130650. [Google Scholar] [CrossRef]
- Salillas, S.; Sancho, J. Flavodoxins as Novel Therapeutic Targets against Helicobacter pylori and Other Gastric Pathogens. Int. J. Mol. Sci. 2020, 21, 1881. [Google Scholar] [CrossRef] [PubMed]
- Cover, T.L.; Lacy, D.B.; Ohi, M.D. The Helicobacter pylori Cag Type IV Secretion System. Trends Microbiol. 2020, 28, 682–695. [Google Scholar] [CrossRef] [PubMed]
- Lertsethtakarn, P.; Ottemann, K.M.; Hendrixson, D.R. Motility and chemotaxis in Campylobacter and Helicobacter. Annu. Rev. Microbiol. 2011, 65, 389–410. [Google Scholar] [CrossRef] [PubMed]
- Sycuro, L.K.; Wyckoff, T.J.; Biboy, J.; Born, P.; Pincus, Z.; Vollmer, W.; Salama, N.R. Multiple peptidoglycan modification networks modulate Helicobacter pylori’s cell shape, motility, and colonization potential. PLoS Pathog. 2012, 8, e1002603. [Google Scholar] [CrossRef]
- Benavides-Ward, A.; Vasquez-Achaya, F.; Silva-Caso, W.; Aguilar-Luis, M.A.; Mazulis, F.; Urteaga, N.; Del Valle-Mendoza, J. Helicobacter pylori and its relationship with variations of gut microbiota in asymptomatic children between 6 and 12 years. BMC Res. Notes 2018, 11, 468. [Google Scholar] [CrossRef]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef]
- Rajoo, K.S.; Lepun, P.; Alan, R.; Karam, D.S.; Abdu, A.; Rosli, Z.; Izani, N.; Gerusu, G.J. Ethnobotanical study of medicinal plants used by the Kenyah community of Borneo. J. Ethnopharmacol. 2023, 301, 115780. [Google Scholar] [CrossRef]
- Richard, K.; Andrae-Marobela, K.; Tietjen, I. An ethnopharmacological survey of medicinal plants traditionally used by the BaKalanga people of the Tutume subdistrict in Central Botswana to manage HIV/AIDS, HIV-associated conditions, and other health conditions. J. Ethnopharmacol. 2023, 316, 116759. [Google Scholar] [CrossRef]
- Mon, A.M.; Hein, P.P.; Zaw, M.; Kyaw, M.T.; Yang, Y.; Yang, X.; Shi, Y. Ethnobotanical surveys reveal the crucial role of medicinal plants in the primary healthcare system of the Shan people in Myanmar. J. Ethnopharmacol. 2024, 327, 117875. [Google Scholar] [CrossRef]
- Valli, M.; Bolzani, V.S. Natural Products: Perspectives and Challenges for use of Brazilian Plant Species in the Bioeconomy. Acad. Bras. Cienc. 2019, 91, e20190208. [Google Scholar] [CrossRef]
- Calixto, J.B. The role of natural products in modern drug discovery. Acad. Bras. Cienc. 2019, 91 (Suppl. S3), e20190105. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E.J. What is hidden in the biodiversity? The role of natural products and medicinal chemistry in the drug discovery process. Acad. Bras. Cienc. 2019, 91, e20190306. [Google Scholar] [CrossRef]
- Yang, C.S.; Chen, G.; Wu, Q. Recent scientific studies of a traditional chinese medicine, tea, on prevention of chronic diseases. J. Tradit. Complement. Med. 2014, 4, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Welz, A.N.; Emberger-Klein, A.; Menrad, K. Why people use herbal medicine: Insights from a focus-group study in Germany. BMC Complement. Altern. Med. 2018, 18, 92. [Google Scholar] [CrossRef] [PubMed]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Spencer, J.P.; Abd El Mohsen, M.M.; Minihane, A.M.; Mathers, J.C. Biomarkers of the intake of dietary polyphenols: Strengths, limitations and application in nutrition research. Br. J. Nutr. 2008, 99, 12–22. [Google Scholar] [CrossRef]
- Cassidy, A.; Minihane, A.M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef]
- Beslo, D.; Golubic, N.; Rastija, V.; Agic, D.; Karnas, M.; Subaric, D.; Lucic, B. Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants 2023, 12, 1141. [Google Scholar] [CrossRef]
- Zhang, W.; Qi, S.; Xue, X.; Al Naggar, Y.; Wu, L.; Wang, K. Understanding the Gastrointestinal Protective Effects of Polyphenols using Foodomics-Based Approaches. Front. Immunol. 2021, 12, 671150. [Google Scholar] [CrossRef]
- Man, A.W.C.; Zhou, Y.; Xia, N.; Li, H. Involvement of Gut Microbiota, Microbial Metabolites and Interaction with Polyphenol in Host Immunometabolism. Nutrients 2020, 12, 3054. [Google Scholar] [CrossRef]
- Makarewicz, M.; Drozdz, I.; Tarko, T.; Duda-Chodak, A. The Interactions between Polyphenols and Microorganisms, Especially Gut Microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef]
- Rego, R.I.d.A.; Silvestre, G.F.G.; de Melo, D.F.; Albino, S.L.; Pimentel, M.M.; Cruz, S.B.S.C.; Wurzba, S.D.S.; Rodrigues, W.F.; de Lima Damasceno, B.P.G.; Castellano, L.R.C. Flavonoids-Rich Plant Extracts Against Helicobacter pylori Infection as Prevention to Gastric Cancer. Front. Pharmacol. 2022, 13, 951125. [Google Scholar] [CrossRef]
- de Paula, M.N.; Ribeiro, T.; Isolani, R.; de Medeiros Araujo, D.C.; Borges, A.S.; Philippsen, G.S.; de Cassia Ribeiro Goncalves, R.; Kitagawa, R.R.; Seixas, F.A.V.; de Mello, J.C.P. An In Vitro and In Silico Investigation about Monteverdia ilicifolia Activity against Helicobacter pylori. Antioxidants 2022, 12, 46. [Google Scholar] [CrossRef]
- Krzyzek, P.; Migdal, P.; Paluch, E.; Karwanska, M.; Wieliczko, A.; Gosciniak, G. Myricetin as an Antivirulence Compound Interfering with a Morphological Transformation into Coccoid Forms and Potentiating Activity of Antibiotics against Helicobacter pylori. Int. J. Mol. Sci. 2021, 22, 2695. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Tran Trung, H.; Truong Thi Huynh, H.; Nguyen Thi Thuy, L.; Nguyen Van Minh, H.; Thi Nguyen, M.N.; Luong Thi, M.N. Growth-Inhibiting, Bactericidal, Antibiofilm, and Urease Inhibitory Activities of Hibiscus rosa sinensis L. Flower Constituents toward Antibiotic Sensitive- and Resistant-Strains of Helicobacter pylori. ACS Omega 2020, 5, 20080–20089. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Gao, H.; Miao, J.; Zhang, Z.; Zheng, L.; Li, F.; Zhou, S.; Zhang, Z.; Li, S.; Liu, H.; et al. Helicobacter pylori infection in humans and phytotherapy, probiotics, and emerging therapeutic interventions: A review. Front. Microbiol. 2024, 14, 1330029. [Google Scholar] [CrossRef]
- Bisignano, C.; Filocarno, A.; La Camera, E.; Zummo, S.; Fera, M.T.; Mandalari, G. Antibacterial activities of almond skins on cagA-positive and-negative clinical isolates of Helicobacter pylori. BMC Microbiol. 2013, 13, 103. [Google Scholar]
- Zengin, G.; Menghini, L.; Di Sotto, A.; Mancinelli, R.; Sisto, F.; Carradori, S.; Cesa, S.; Fraschetti, C.; Filippi, A.; Angiolella, L.; et al. Chromatographic Analyses, In Vitro Biological Activities, and Cytotoxicity of Cannabis sativa L. Essential Oil: A Multidisciplinary Study. Molecules 2018, 23, 3266. [Google Scholar] [CrossRef]
- Moon, S.H.; Lee, J.H.; Kim, K.T.; Park, Y.S.; Nah, S.Y.; Ahn, D.U.; Paik, H.D. Antimicrobial effect of 7-O-butylnaringenin, a novel flavonoid, and various natural flavonoids against Helicobacter pylori strains. Int. J. Environ. Res. Public Health 2013, 10, 5459–5469. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Huh, J.Y.; Nam, S.H.; Moon, S.K.; Lee, S.B. Enzymatic bioconversion of citrus hesperidin by Aspergillus sojae naringinase: Enhanced solubility of hesperetin-7-O-glucoside with in vitro inhibition of human intestinal maltase, HMG-CoA reductase, and growth of Helicobacter pylori. Food Chem. 2012, 135, 2253–2259. [Google Scholar] [CrossRef]
- de Cassia Dos Santos, R.; Bonamin, F.; Perico, L.L.; Rodrigues, V.P.; Zanatta, A.C.; Rodrigues, C.M.; Sannomiya, M.; Dos Santos Ramos, M.A.; Bonifacio, B.V.; Bauab, T.M.; et al. Byrsonima intermedia A. Juss partitions promote gastroprotection against peptic ulcers and improve healing through antioxidant and anti-inflammatory activities. Biomed. Pharmacother. 2019, 111, 1112–1123. [Google Scholar] [CrossRef]
- Salehi, B.; Sharopov, F.; Martorell, M.; Rajkovic, J.; Ademiluyi, A.O.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Iriti, M.; Sharifi-Rad, J. Phytochemicals in Helicobacter pylori Infections: What Are We Doing Now? Int. J. Mol. Sci. 2018, 19, 2361. [Google Scholar] [CrossRef] [PubMed]
- Abulizi, P.; Cong, Y.; Yakupu, M.; Wahepu, A. Chemical Constituents of Euphorbia sororia. Chem. Nat. Compd. 2014, 50, 908–909. [Google Scholar] [CrossRef]
- Novello, C.R.; Marques, L.C.; Pires, M.E.; Kutschenco, A.P.; Nakamura, C.V.; Nocchi, S.; Sarragiotto, M.H.; Mello, J.C.P. Bioactive Indole Alkaloids from Croton echioides. J. Braz. Chem. Soc. 2016, 27, 2203–2209. [Google Scholar] [CrossRef]
- Cayona, R.; Creencia, E. Phytochemicals of Euphorbia hirta L. and Their Inhibitory Potential Against SARS-CoV-2 Main Protease. Front. Mol. Biosci. 2021, 8, 801401. [Google Scholar] [CrossRef]
- Cardenas, D.M.; Gomez Rave, L.J.; Soto, J.A. Biological Activity of Sacha Inchi (Plukenetia volubilis Linneo) and Potential Uses in Human Health: A Review. Food Technol. Biotechnol. 2021, 59, 253–266. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, F.; Kakuda, Y. Sacha inchi (Plukenetia volubilis L.): Nutritional composition, biological activity, and uses. Food Chem. 2018, 265, 316–328. [Google Scholar] [CrossRef]
- Goyal, A.; Tanwar, B.; Kumar Sihag, M.; Sharma, V. Sacha inchi (Plukenetia volubilis L.): An emerging source of nutrients, omega-3 fatty acid and phytochemicals. Food Chem. 2022, 373, 131459. [Google Scholar] [CrossRef]
- Kittibunchakul, S.; Kemsawasd, V.; Hudthagosol, C.; Sanporkha, P.; Sapwarobol, S.; Suttisansanee, U. The Effects of Different Roasting Methods on the Phenolic Contents, Antioxidant Potential, and In Vitro Inhibitory Activities of Sacha Inchi Seeds. Foods 2023, 12, 4178. [Google Scholar] [CrossRef] [PubMed]
- Mhd Rodzi, N.A.R.; Lee, L.K. Sacha Inchi (Plukenetia volubilis L.): Recent insight on phytochemistry, pharmacology, organoleptic, safety and toxicity perspectives. Heliyon 2022, 8, e10572. [Google Scholar] [CrossRef]
- Abd Rahman, I.Z.; Nor Hisam, N.S.; Aminuddin, A.; Hamid, A.A.; Kumar, J.; Ugusman, A. Evaluating the Potential of Plukenetia volubilis Linneo (Sacha Inchi) in Alleviating Cardiovascular Disease Risk Factors: A Mini Review. Pharmaceuticals 2023, 16, 1588. [Google Scholar] [CrossRef]
- Tan, A.; Scortecci, K.C.; Cabral De Medeiros, N.M.; Kukula-Koch, W.; Butler, T.J.; Smith, S.M.; Boylan, F. Plukenetia volubilis leaves as source of anti-Helicobacter pylori agents. Front. Pharmacol. 2024, 15, 1461447. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Casado, J.; Lanas, A. Fighting the Antibiotic Crisis: Flavonoids as Promising Antibacterial Drugs Against Helicobacter pylori Infection. Front. Cell. Infect. Microbiol. 2021, 11, 709749. [Google Scholar] [CrossRef]
- Hasnat, H.; Shompa, S.A.; Islam, M.M.; Alam, S.; Richi, F.T.; Emon, N.U.; Ashrafi, S.; Ahmed, N.U.; Chowdhury, M.N.R.; Fatema, N.; et al. Flavonoids: A treasure house of prospective pharmacological potentials. Heliyon 2024, 10, e27533. [Google Scholar] [CrossRef]
- Biglar, M.; Soltani, K.; Nabati, F.; Bazl, R.; Mojab, F.; Amanlou, M. A preliminary investigation of the jack-bean urease inhibition by randomly selected traditionally used herbal medicine. Iran. J. Pharm. Res. 2012, 11, 831–837. [Google Scholar] [PubMed]
- Uddin, G.; Ismail; Rauf, A.; Raza, M.; Khan, H.; Nasruddin; Khan, M.; Farooq, U.; Khan, A.; Arifullah. Urease inhibitory profile of extracts and chemical constituents of Pistacia atlantica ssp. cabulica Stocks. Nat. Prod. Res. 2016, 30, 1411–1416. [Google Scholar] [CrossRef]
- Xiao, Z.P.; Wang, X.D.; Peng, Z.Y.; Huang, S.; Yang, P.; Li, Q.S.; Zhou, L.H.; Hu, X.J.; Wu, L.J.; Zhou, Y.; et al. Molecular docking, kinetics study, and structure-activity relationship analysis of quercetin and its analogous as Helicobacter pylori urease inhibitors. J. Agric. Food Chem. 2012, 60, 10572–10577. [Google Scholar] [CrossRef]
- Egas, V.; Salazar-Cervantes, G.; Romero, I.; Mendez-Cuesta, C.A.; Rodriguez-Chavez, J.L.; Delgado, G. Anti-Helicobacter pylori metabolites from Heterotheca inuloides (Mexican arnica). Fitoterapia 2018, 127, 314–321. [Google Scholar] [CrossRef]
- Wu, D.; Kong, Y.; Han, C.; Chen, J.; Hu, L.; Jiang, H.; Shen, X. D-Alanine:D-alanine ligase as a new target for the flavonoids quercetin and apigenin. Int. J. Antimicrob. Agents 2008, 32, 421–426. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, J.; Xie, X.; He, Y.; Mo, F.; Luo, Z. Quercetin from Polygonum capitatum Protects against Gastric Inflammation and Apoptosis Associated with Helicobacter pylori Infection by Affecting the Levels of p38MAPK, BCL-2 and BAX. Molecules 2017, 22, 744. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Jiang, X. Activities of muscadine grape skin and polyphenolic constituents against Helicobacter pylori. J. Appl. Microbiol. 2013, 114, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Qing, L.; Li, S.; Yan, S.; Wu, C.; Yan, X.; He, Z.; Chen, Q.; Huang, M.; Shen, C.; Wang, S.; et al. Anti-Helicobacter pylori activity of Fagopyrum tataricum (L.) Gaertn. Bran flavonoids extract and its effect on Helicobacter pylori-induced inflammatory response. Food Sci. Nutr. 2023, 11, 3394–3403. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, P.; Navarro-Herrera, D.; Romo-Hualde, A.; Zabala, M.; López-Yoldi, M.; González-Ferrero, C.; Gil, A.G.; Martinez, J.A.; Vizmanos, J.L.; Milagro, F.I.; et al. Broccoli extract improves high fat diet-induced obesity, hepatic steatosis and glucose intolerance in Wistar rats. J. Funct. Foods 2019, 59, 319–328. [Google Scholar] [CrossRef]
- Yu, X.D.; Zheng, R.B.; Xie, J.H.; Su, J.Y.; Huang, X.Q.; Wang, Y.H.; Zheng, Y.F.; Mo, Z.Z.; Wu, X.L.; Wu, D.W.; et al. Biological evaluation and molecular docking of baicalin and scutellarin as Helicobacter pylori urease inhibitors. J. Ethnopharmacol. 2015, 162, 69–78. [Google Scholar] [CrossRef]
- Chen, M.E.; Su, C.H.; Yang, J.S.; Lu, C.C.; Hou, Y.C.; Wu, J.B.; Hsu, Y.M. Baicalin, Baicalein, and Lactobacillus Rhamnosus JB3 Alleviated Helicobacter pylori Infections in Vitro and in Vivo. J. Food Sci. 2018, 83, 3118–3125. [Google Scholar] [CrossRef]
- Dmitrieva, A.; Kozlova, O.; Atuchin, V.; Milentieva, I.; Vesnina, A.; Ivanova, S.; Asyakina, L.; Prosekov, A. Study of the Effect of Baicalin from Scutellaria baicalensis on the Gastrointestinal Tract Normoflora and Helicobacter pylori. Int. J. Mol. Sci. 2023, 24, 11906. [Google Scholar] [CrossRef]
- Kim, H.W.; Woo, H.J.; Yang, J.Y.; Kim, J.B.; Kim, S.H. Hesperetin Inhibits Expression of Virulence Factors and Growth of Helicobacter pylori. Int. J. Mol. Sci. 2021, 22, 10035. [Google Scholar] [CrossRef]
- Krzyzek, P.; Grande, R. Transformation of Helicobacter pylori into Coccoid Forms as a Challenge for Research Determining Activity of Antimicrobial Substances. Pathogens 2020, 9, 184. [Google Scholar] [CrossRef]
- Qing, W.; Haibing, H.; Na, Z.; Meitian, X.; Jing, Y. Exogenous spraying of sucrose improves sensory quality, bioactive components content and anti-obesity activity of Tieguanyin oolong tea. Ind. Crops Prod. 2023, 193, 116146. [Google Scholar] [CrossRef]
- Garro, M.F.; Salinas Ibanez, A.G.; Vega, A.E.; Arismendi Sosa, A.C.; Pelzer, L.; Saad, J.R.; Maria, A.O. Gastroprotective effects and antimicrobial activity of Lithraea molleoides and isolated compounds against Helicobacter pylori. J. Ethnopharmacol. 2015, 176, 469–474. [Google Scholar] [CrossRef]
- Abdel-Baki, P.M.; El-Sherei, M.M.; Khaleel, A.E.; Abdel-Aziz, M.M.; Okba, M.M. Irigenin, a novel lead from Iris confusa for management of Helicobacter pylori infection with selective COX-2 and HpIMPDH inhibitory potential. Sci. Rep. 2022, 12, 11457. [Google Scholar] [CrossRef]
- Ankolekar, C.; Johnson, D.; Pinto Mda, S.; Johnson, K.; Labbe, R.; Shetty, K. Inhibitory potential of tea polyphenolics and influence of extraction time against Helicobacter pylori and lack of inhibition of beneficial lactic acid bacteria. J. Med. Food 2011, 14, 1321–1329. [Google Scholar] [CrossRef]
- Díaz-Gómez, R.; López-Solís, R.; Obreque-Slier, E.; Toledo-Araya, H. Comparative antibacterial effect of gallic acid and catechin against Helicobacter pylori. LWT Food Sci. Technol. 2013, 54, 331–335. [Google Scholar] [CrossRef]
- Escandón, R.A.; del Campo, M.; López-Solis, R.; Obreque-Slier, E.; Toledo, H. Antibacterial effect of kaempferol and (−)-epicatechin on Helicobacter pylori. Eur. Food Res. Technol. 2016, 242, 1495–1502. [Google Scholar] [CrossRef]
- Pastene, E.; Parada, V.; Avello, M.; Ruiz, A.; Garcia, A. Catechin-based procyanidins from Peumus boldus Mol. aqueous extract inhibit Helicobacter pylori urease and adherence to adenocarcinoma gastric cells. Phytother. Res. 2014, 28, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.C.; Kushima, H.; Rodrigues, C.M.; Sannomiya, M.; Rocha, L.R.; Bauab, T.M.; Tamashiro, J.; Vilegas, W.; Hiruma-Lima, C.A. Byrsonima intermedia A. Juss.: Gastric and duodenal anti-ulcer, antimicrobial and antidiarrheal effects in experimental rodent models. J. Ethnopharmacol. 2012, 140, 203–212. [Google Scholar] [CrossRef]
- Lee, B.E.; Park, S.J.; Kim, G.H.; Joo, D.C.; Lee, M.W. Anti-inflammatory effects of eupatilin on Helicobacter pylori CagA-induced gastric inflammation. PLoS ONE 2024, 19, e0313251. [Google Scholar] [CrossRef]
- Palacios-Espinosa, J.F.; Nunez-Aragon, P.N.; Gomez-Chang, E.; Linares, E.; Bye, R.; Romero, I. Anti-Helicobacter pylori Activity of Artemisia ludoviciana subsp. mexicana and Two of Its Bioactive Components, Estafiatin and Eupatilin. Molecules 2021, 26, 3654. [Google Scholar] [CrossRef]
- Qanash, H.; Al-Rajhi, A.M.H.; Almashjary, M.N.; Basabrain, A.A.; Hazzazi, M.S.; Abdelghany, T.M. Inhibitory potential of rutin and rutin nano-crystals against Helicobacter pylori, colon cancer, hemolysis and Butyrylcholinesterase in vitro and in silico. Appl. Biol. Chem. 2023, 66, 79. [Google Scholar] [CrossRef]
- Krivokuća, M.; Niketić, M.; Milenković, M.; Golić, N.; Masia, C.; Scaltrito, M.M.; Sisto, F.; Kundaković, T. Anti-Helicobacter pylori Activity of Four Alchemilla Species (Rosaceae). Nat. Prod. Commun. 2015, 10, 1369–1371. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.; Yin, C.; Hang, X.; Bai, Y.; Zhang, C.; Xu, J.; Huang, Y.; Ge, Y.; Chen, T.; Zeng, L.; et al. Loureirin A is a narrow-spectrum antimicrobial agent against Helicobacter pylori. Antimicrob. Agents Chemother. 2024, 68. [Google Scholar] [CrossRef]
- Yeon, M.J.; Lee, M.H.; Kim, D.H.; Yang, J.Y.; Woo, H.J.; Kwon, H.J.; Moon, C.; Kim, S.H.; Kim, J.B. Anti-inflammatory effects of Kaempferol on Helicobacter pylori-induced inflammation. Biosci. Biotechnol. Biochem. 2019, 83, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, M.; Arif, M.; Hamouda, H.I.; Khan, S.; Abdalla, M.; Shabana, S.; Rozan, H.E.; Khan, T.U.; Chi, Z.; Liu, C. Preparation, urease inhibition mechanisms, and anti-Helicobacter pylori activities of hesperetin-7-rhamnoglucoside. Curr. Res. Microb. Sci. 2022, 3, 100103. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Chellia, R.; Hu, X.; Kathiresan, K.; Oh, D.H.; Wang, M.H. Eradication of Helicobacter pylori through the inhibition of urease and peptide deformylase: Computational and biological studies. Microb. Pathog. 2019, 128, 236–244. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2018, 18, 241–272. [Google Scholar] [CrossRef]
- Tarahovsky, Y.S.; Kim, Y.A.; Yagolnik, E.A.; Muzafarov, E.N. Flavonoid-membrane interactions: Involvement of flavonoid-metal complexes in raft signaling. Biochim. Biophys. Acta 2014, 1838, 1235–1246. [Google Scholar] [CrossRef]
- Zhang, L.; Kong, Y.; Wu, D.; Zhang, H.; Wu, J.; Chen, J.; Ding, J.; Hu, L.; Jiang, H.; Shen, X. Three flavonoids targeting the beta-hydroxyacyl-acyl carrier protein dehydratase from Helicobacter pylori: Crystal structure characterization with enzymatic inhibition assay. Protein Sci. 2008, 17, 1971–1978. [Google Scholar] [CrossRef]
- Oliveira, D.M.; Melo, F.G.; Balogun, S.O.; Flach, A.; de Souza, E.C.; de Souza, G.P.; Rocha Ido, N.; da Costa, L.A.; Soares, I.M.; da Silva, L.I.; et al. Antibacterial mode of action of the hydroethanolic extract of Leonotis nepetifolia (L.) R. Br. involves bacterial membrane perturbations. J. Ethnopharmacol. 2015, 172, 356–363. [Google Scholar] [CrossRef]
- Qiu, T.; Wu, D.; Yang, L.; Ye, H.; Wang, Q.; Cao, Z.; Tang, K. Exploring the Mechanism of Flavonoids Through Systematic Bioinformatics Analysis. Front. Pharmacol. 2018, 9, 918. [Google Scholar] [CrossRef]
- Gonzalez-Aspajo, G.; Belkhelfa, H.; Haddioui-Hbabi, L.; Bourdy, G.; Deharo, E. Sacha Inchi Oil (Plukenetia volubilis L.), effect on adherence of Staphylococus aureus to human skin explant and keratinocytes in vitro. J. Ethnopharmacol. 2015, 171, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Akash, S.R.; Tabassum, A.; Aditee, L.M.; Rahman, A.; Hossain, M.I.; Hannan, M.A.; Uddin, M.J. Pharmacological insight of rutin as a potential candidate against peptic ulcer. Biomed. Pharmacother. 2024, 177, 116961. [Google Scholar] [CrossRef]
- Jeong, C.-S. Evaluation for Protective Effect of Rutin, a Natural Flavonoid, against HCl/Ethanol-Induced Gastric Lesions. Biomol. Ther. 2009, 17, 199–204. [Google Scholar] [CrossRef]
- Narayanan, L.; Sr, S. Pharmacological insights into Ipomoea staphylina: Therapeutic activities and the isolated bioactive metabolic compounds. Mong. J. Chem. 2024, 25, 1–9. [Google Scholar] [CrossRef]
- Martinelli, G.; Fumagalli, M.; Pozzoli, C.; Nicotra, G.; Vicentini, S.F.; Maranta, N.; Sangiovanni, E.; Dell’Agli, M.; Piazza, S. Exploring In Vitro the Combination of Cistus x incanus L. and Castanea sativa Mill. Extracts as Food Supplement Ingredients against H. pylori Infection. Foods 2023, 13, 40. [Google Scholar] [CrossRef]
- Thanh, D.T.; Tan, M.T.; Thu, N.T.M.; Trinh, P.N.P.; Thuong, P.T.H.; Tuyet, P.T.G.; Ngan, L.T.M.; Hieu, T.T. Phytochemical Composition, Antioxidant, Anti-Helicobacter pylori, and Enzyme Inhibitory Evaluations of Cleistocalyx operculatus Flower Bud and Leaf Fractions. BioTech 2024, 13, 42. [Google Scholar] [CrossRef]
- Park, W.S.; Bae, J.-Y.; Kim, H.J.; Kim, M.G.; Lee, W.-K.; Kang, H.-L.; Baik, S.-C.; Lim, K.M.; Lee, M.K.; Ahn, M.J. Anti-Helicobacter pylori Compounds from Maackia amurensis. Nat. Prod. Sci. 2015, 21, 49–53. [Google Scholar]
- Fiore, C.; Antoniciello, F.; Roncarati, D.; Scarlato, V.; Grepioni, F.; Braga, D. Levofloxacin and Ciprofloxacin Co-Crystals with Flavonoids: Solid-State Investigation for a Multitarget Strategy against Helicobacter pylori. Pharmaceutics 2024, 16, 203. [Google Scholar] [CrossRef]


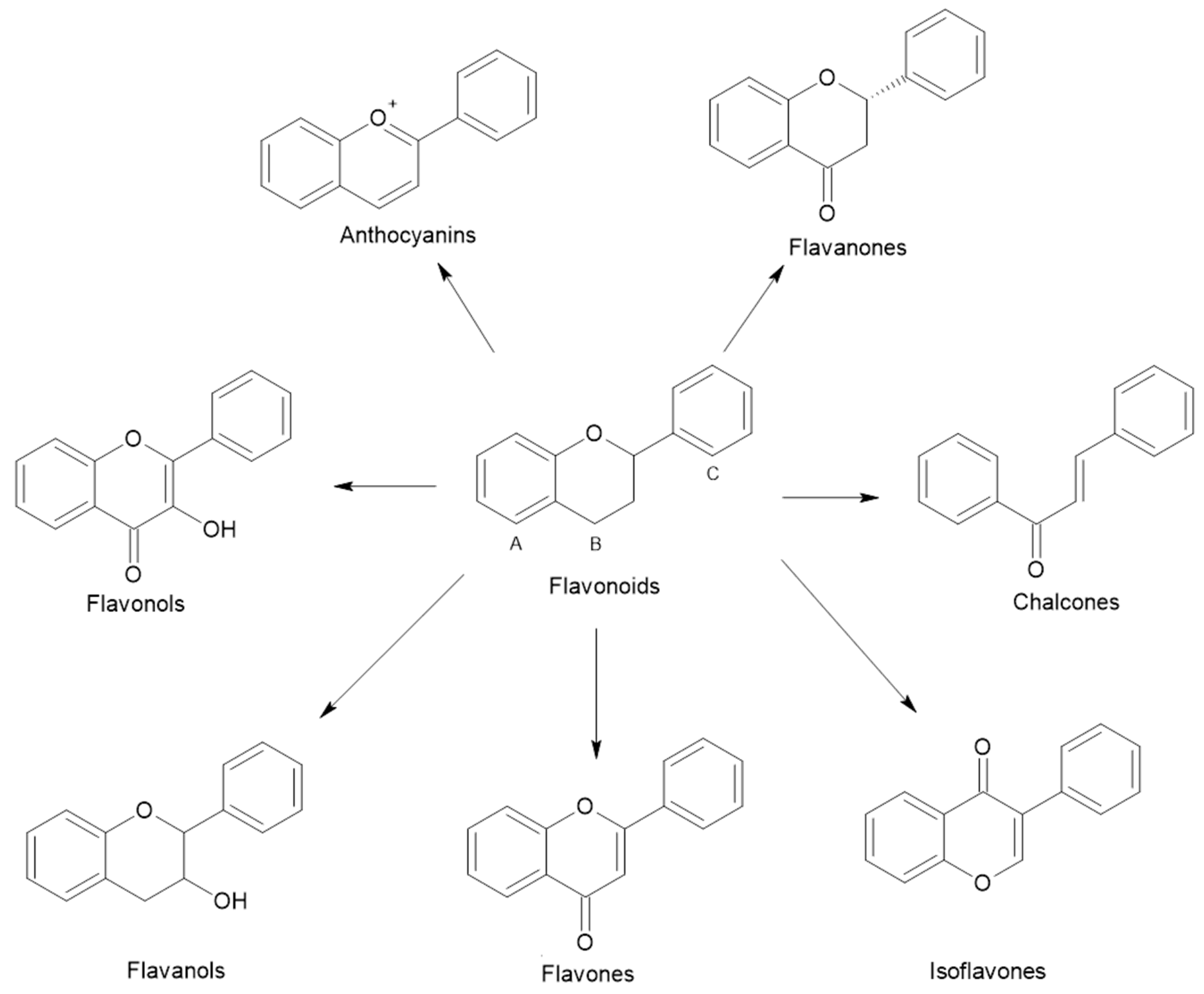
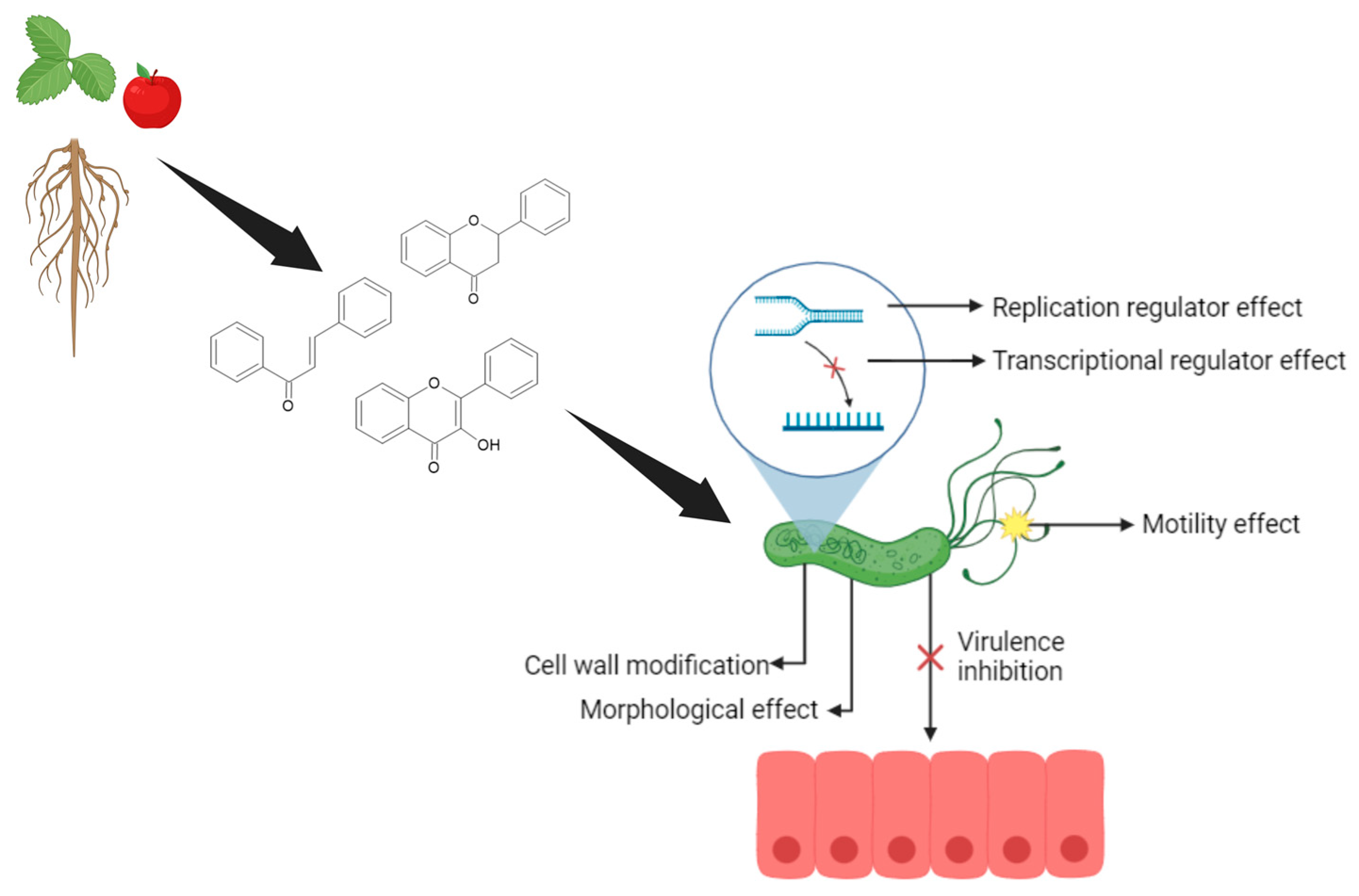
| Gene/Protein | Genome | Function | Reference |
|---|---|---|---|
| CYP2C19 | Host | Drug metabolisation | [1,41] |
| NikR | Bacteria | Nickel-responsive regulator | [45] |
| HsrA | Bacteria | Metabolism and stress defence | [45] |
| OipA | Bacteria | Outer membrane protein | [1,45] |
| CagA | Bacteria | Cell transformation to cancer cells | [1,45] |
| BabA | Bacteria | Adhesion to mucosa cells | [1,45] |
| HopQ | Bacteria | Adhesion to CEACAMs | [1,45] |
| Urease | Bacteria | Protein involved in helping the colonization in the acid pH in stomach | [45,48] |
| Flavodoxin | Bacteria | Small electron transfer protein involved in pyruvate metabolism | [45] |
| Flavonoid | Action Mechanism | MIC | In Vitro Data | In Vivo Data | Synergistic Effect | Scientific Reference |
|---|---|---|---|---|---|---|
| Quercetin | Urease inhibition via binding with zinc cation; binding to Ddl; lipid peroxidation of bacterial membrane. | 64–128 µg/mL; 100–200 µg/mL | Molecular docking; inhibits urease by forming ionic bonds with zinc; binding to Ddl. | Prevents inflammation in gastric cells, affects p38MAPK, BCL-2, and BAX protein levels in mouse animal model. | Co-crystallisation with antibiotics enhances efficacy. | [31,67,71,93,94,95,96,97,98,99] |
| Baicalin and Baicalein | Binding to urease active site reducing virulence by decreasing vacA gene expression. | ≥1 mM (Baicalin); 0.125–1 mM (Baicalein) | Reduces virulence by lowering vacA expression. | Baicalein has enhanced bactericidal effects. | N/A | [100,101,102] |
| Hesperetin | Downregulates virulence factors UreA and UreB; prevents bacterial infection by gene expression reduction. | 8 mg/L | Reduces gene expression essential for H. pylori development, inhibition of urease. | N/A | Synergistic with metronidazole and clarithromycin. | [31,75,103] |
| Chrysin | Forms a stable HsrA-flavonoid complex, inhibiting HsrA. | ≤8 mg/L | Inhibits HsrA in H. pylori. | N/A | N/A | [31] |
| Myricetin | Disrupts transcription of virulence-associated genes; delays spiral-to-coccid transformation. | 160 µg/mL | Affects gene expression related to muropeptide dimers and monomers. | Delays morphological transformation, improving antimicrobial potential. | Synergistic effects with antibiotics (FICI = 0.31–0.5). | [69,104] |
| Naringenin | Urease inhibition | N/A | Inhibitis effect of urease | N/A | N/A | [75] |
| Rutin | Impairs biofilm formation; moderate antibacterial activity. | 8–125 µg/mL | Biofilm formation inhibition. | N/A | N/A | [105,106] |
| Apigenin | Binds to Ddl as competitive and non-competitive inhibitor; inhibits DNA-binding activity of HsrA. | 8 mg/L | Inhibits HsrA and Ddl activities. | N/A | N/A | [31,95] |
| Luteolin | Inhibits H. pylori strains; possible action on adhesion to host cells. | 125 mg/L | Inhibition and bactericidal action. | N/A | N/A | [71,107] |
| Catechins and Epicatechin | Binding to cell membranes; urease inhibition and anti-adherent activities. | N/A | Growth inhibition at low concentrations; potential gastroprotective effects. | N/A | Synergistic activity with antibiotics likely present but not fully understood. | [67,71,108,109,110,111,112] |
| Eupatilin | Inhibits NF-κB inflammatory pathway; suppresses CagA gene. | 125–250 µg/mL | Anti-inflammatory effects; modulation of NF-κB and IL-6 transcription. | Clinical trial show high cure rates in erosive gastritis patients. | N/A | [113,114] |
| Orientin | Bacteriostatic action | 15.53 µg/mL | Bacteriostatic effect | N/A | N/A | [115] |
| Isoquercetin(quercetin-3-O-β-D-glucoside) | Inhibitis H. pylori growth | 480 μg/mL | N/A | N/A | N/A | [116] |
| Loureirin A (from Sanguis Draconis) | bactericidal effect | 4 mg/mL | N/A | N/A | N/A | [117] |
| Kaempferol | Prevents secretion of virulence factors; prevention of transcription; forms complexes with the cell wall. | N/A | Decreases mRNA levels of T4SS components; modifies modulation of cagA and vacA genes; prevention of transcription fractors binding. | N/A | N/A | [31,98,118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, A.; Scortecci, K.C.; Boylan, F. A Review on Flavonoids as Anti-Helicobacter pylori Agents. Appl. Sci. 2025, 15, 3936. https://doi.org/10.3390/app15073936
Tan A, Scortecci KC, Boylan F. A Review on Flavonoids as Anti-Helicobacter pylori Agents. Applied Sciences. 2025; 15(7):3936. https://doi.org/10.3390/app15073936
Chicago/Turabian StyleTan, Aditya, Katia Castanho Scortecci, and Fabio Boylan. 2025. "A Review on Flavonoids as Anti-Helicobacter pylori Agents" Applied Sciences 15, no. 7: 3936. https://doi.org/10.3390/app15073936
APA StyleTan, A., Scortecci, K. C., & Boylan, F. (2025). A Review on Flavonoids as Anti-Helicobacter pylori Agents. Applied Sciences, 15(7), 3936. https://doi.org/10.3390/app15073936







