Effect of Ultrasound on Fermentation of Thick Molasses Worts by Distiller’s Yeast
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Biological Material
2.3. pH and Total Extract Content
2.4. Preparation of Molasses Worts for the Fermentation Process
2.5. Fermentation Process
2.6. Ultrasound Treatment
2.7. Options of the Experiments and Sample Abbreviations
2.8. HPLC Analysis of Molasses Wort Before and After Fermentation
2.9. Scanning Electron Microscopy (SEM)
2.10. The Number of Replications and the Statistical Treatment Applied
3. Results and Discussion
3.1. Wort Analysis
3.2. The Influence of Ultrasound Treatment and Wort Density on the Fermentation Dynamics and Yields of the Selected Products
3.2.1. The Influence of Ultrasound Treatment on Sugar Utilisation
3.2.2. The Influence of Ultrasound Treatment During Fermentation on Selected Acids and Glycerol Concentrations
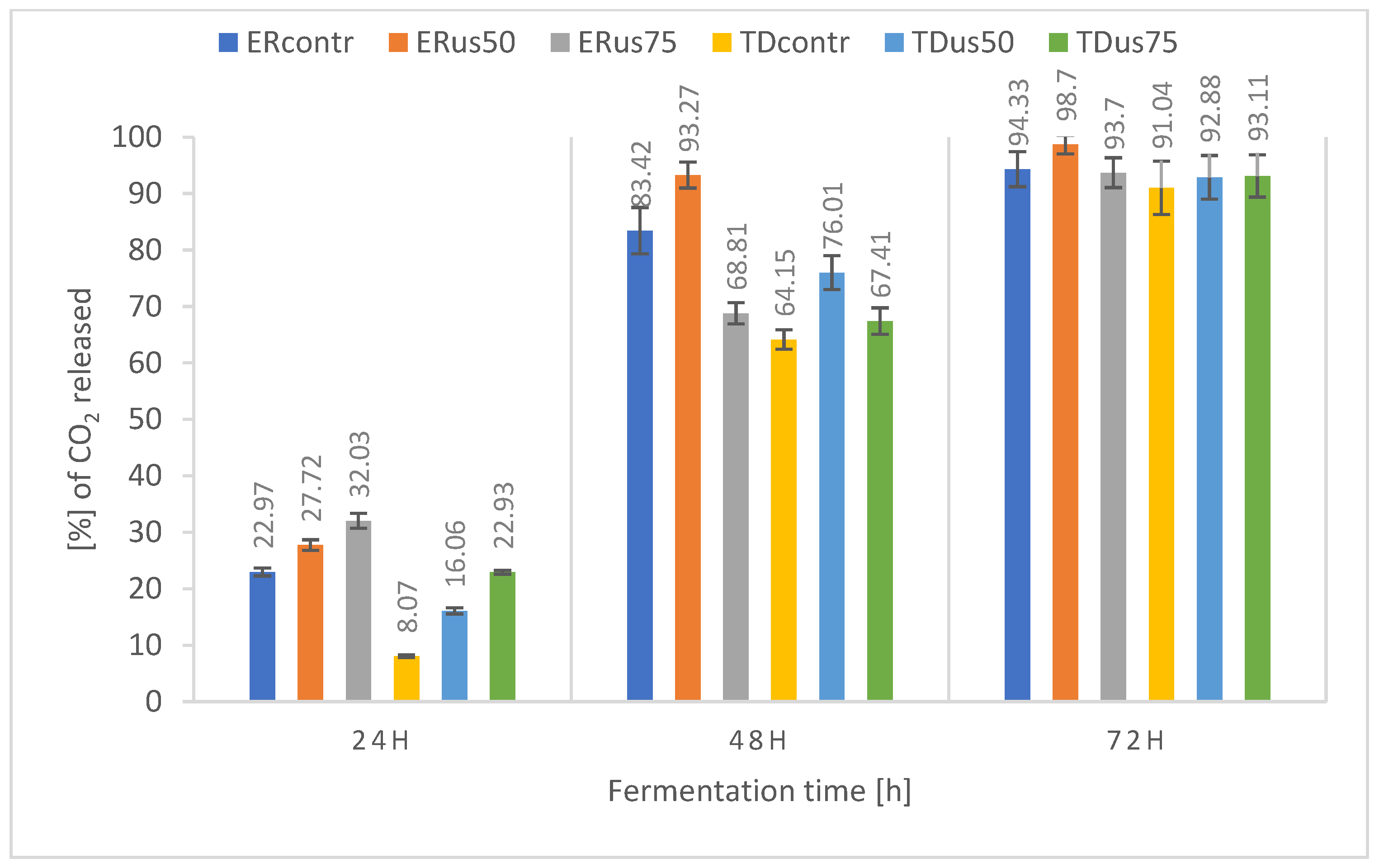
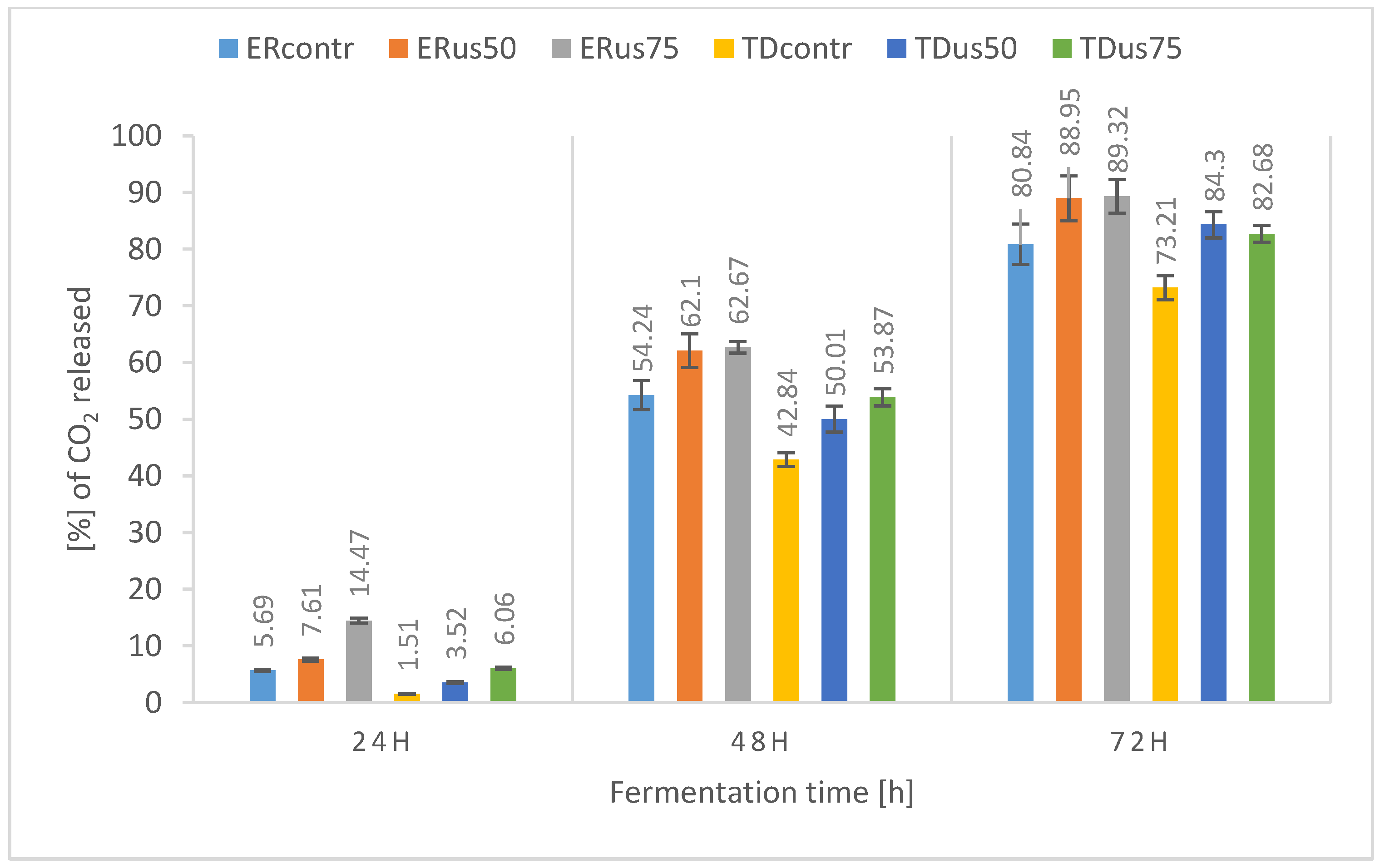
3.2.3. Influence of Ultrasound Treatment on Fermentation Dynamics
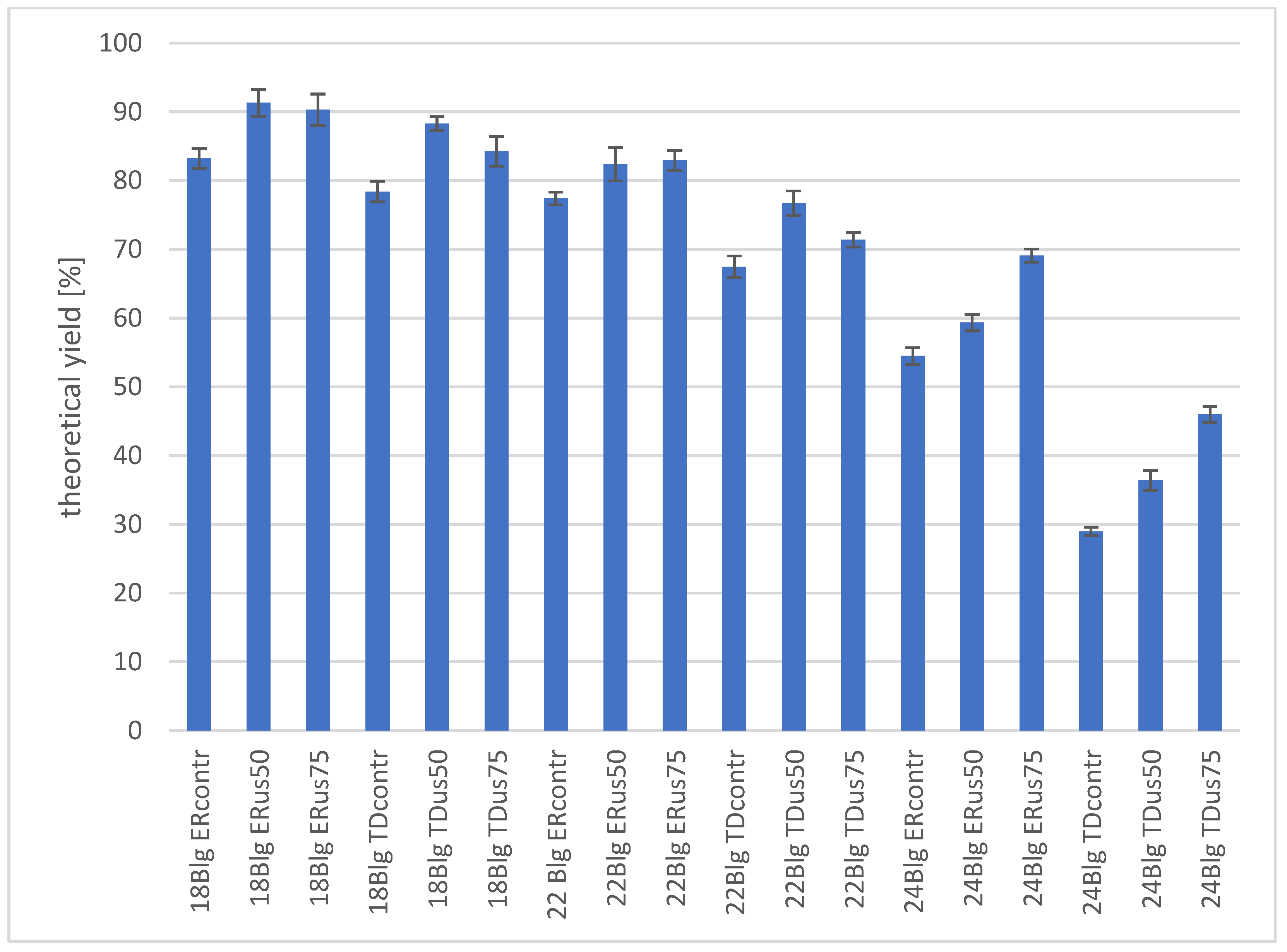
3.2.4. The Influence of Ultrasound Treatment on Ethanol Formation in Molasses Wort
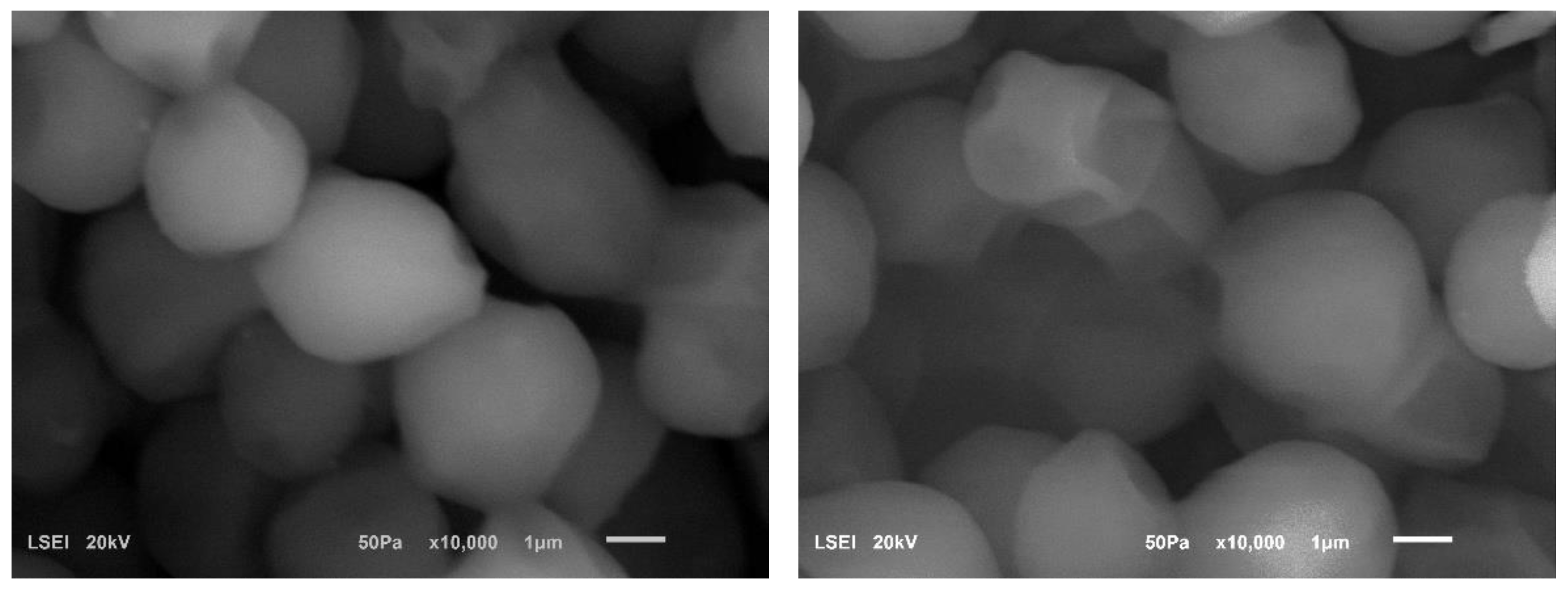
3.3. Scanning Electron Microscopy of Ultrasound-Treated Distillers Yeast
4. Summary and Conclusions
- In the case of thick wort (22 and 24 °Blg), a positive effect of sonification on fermentation was observed. Therefore, it seems advisable to conduct further research to determine the optimal combination of exposure time, frequency, and, above all, ultrasound amplitude for fermentation.
- Ultrasonic wort treatment with a density greater than 18 °Blg improves the final ethanol yield by 5 to 20%, depending on the strain used.
- The higher the density of the wort, the more pronounced the positive effect of ultrasound on the dynamics and yield of fermentation.
- Ultrasound causes visible indentations in the cell walls of Saccharomyces cerevisiae yeast and stimulates the cells to clump together.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajarajan, G.; Irshad, A.; Raghunath, B.V.; Mahesh Kumar, G.; Punnagaiarasi, A. Utilization of Cheese Industry Whey for Biofuel–Ethanol Production. In Integrated Waste Management in India. Environmental Science and Engineering; Prashanthi, M., Sundaram, R., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 59–64. ISBN 978-3-319-27226-9. [Google Scholar]
- World Ethanol Production to Rebound in 2021. Available online: https://informaconnect.com/world-ethanol-production-to-rebound-in-2021/ (accessed on 11 February 2025).
- Cibis, E.; Krzywonos, M.; Miśkiewicz, T. Etanol w Świecie—Kierunki Użytkowania, Surowce i Produkty Uboczne. Przemysl Chem. 2006, 85, 1263–1267. [Google Scholar]
- Rendleman, C.; Shapouri, H. New Technologies in Ethanol Production; Agricultural Economic Reports 308483; United States Department of Agriculture, Economic Research Service: Washington, DC, USA, 2007. [CrossRef]
- Galbe, M.; Sassner, P.; Wingren, A.; Zacchi, G. Process Engineering Economics of Bioethanol Production. In Biofuels. Advances in Biochemical Engineering/Biotechnology; Olsson, L., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 108, pp. 303–327. ISBN 978-3-540-73650-9. [Google Scholar]
- Ceaser, R.; Montané, D.; Constantí, M.; Medina, F. Current Progress on Lignocellulosic Bioethanol Including a Technological and Economical Perspective. Environ. Dev. Sustain. 2024. [Google Scholar] [CrossRef]
- Duque, A.; Álvarez, C.; Doménech, P.; Manzanares, P.; Moreno, A.D. Advanced Bioethanol Production: From Novel Raw Materials to Integrated Biorefineries. Processes 2021, 9, 206. [Google Scholar] [CrossRef]
- Chandel, A.K.; Kapoor, R.K.; Narasu, M.L.; Viswadevan, V.; Kumaran, S.G.S.; Rudravaram, R.; Rao, L.V.; Tripathi, K.K.; Lal, B.; Kuhad, R.C. Economic Evaluation and Environmental Benefits of Biofuel: An Indian Perspective. Int. J. Glob. Energy Issues 2007, 28, 357–381. [Google Scholar] [CrossRef]
- Rosales-Calderon, O.; Arantes, V. A Review on Commercial-Scale High-Value Products That Can Be Produced alongside Cellulosic Ethanol. Biotechnol. Biofuels 2019, 12, 240. [Google Scholar] [CrossRef] [PubMed]
- Bio Ethanol Production—Raw Materials. Available online: https://www.engineeringtoolbox.com/ethanol-bio-fuel-d_1357.html (accessed on 11 February 2025).
- Cardona, C.A.; Sanchez, O.J.; Gutierrez, L.F. Process Synthesis for Fuel Ethanol Production; CRC Press: Boca Raton, FL, USA, 2009; ISBN 978-0-429-13056-4. [Google Scholar]
- Roehr, M. (Ed.) The Biotechnology of Ethanol: Classical and FutureApplications; Wiley-VCH: Weinheim, Germany, 2001; ISBN 3-527-30199-2-PMC. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC6236454/ (accessed on 11 February 2025).
- Boulton, C.; Quain, D. Brewing Yeast and Fermentation; Wiley-Blackwell: Hoboken, NJ, USA, 2006; p. 468. [Google Scholar]
- Claassen, P.A.M.; Van Lier, J.B.; Lopez Contreras, A.M.; Van Niel, E.W.J.; Sijtsma, L.; Stams, A.J.M.; De Vries, S.S.; Weusthuis, R.A. Utilisation of Biomass for the Supply of Energy Carriers. Appl. Microbiol. Biotechnol. 1999, 52, 741–755. [Google Scholar] [CrossRef]
- Mousdale, D.M. Biofuels: Biotechnology, Chemistry, and Sustainable Development; CRC Press: Boca Raton, FL, USA, 2008; ISBN 978-0-429-13756-3. [Google Scholar]
- Walker, G.M.; Stewart, G.G. Saccharomyces cerevisiae in the Production of Fermented Beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- Walker, G.M. Metals in Yeast Fermentation Processes. Adv. Appl. Microbiol. 2004, 54, 197–229. [Google Scholar] [CrossRef]
- Soccol, C.R.; Pandey, A.; Larroche, C. (Eds.) Fermentation Processes Engineering in the Food Industry; CRC Press: Boca Raton, FL, USA, 2013; ISBN 978-0-429-11200-3. [Google Scholar]
- Busturia, A.; Lagunas, R. Catabolite Inactivation of the Glucose Transport System in Saccharomyces cerevisiae. J. Gen. Microbiol. 1986, 132, 379–385. [Google Scholar] [CrossRef]
- Fermentation Assisted by Pulsed Electric Field and Ultrasound: A Review. Available online: https://www.mdpi.com/2311-5637/4/1/1 (accessed on 11 February 2025).
- Wang, Z.; Huang, J.; Ma, S.; Wang, X.; Sun, B.; Wang, F.; Li, L.; Bao, Q. Novel Heating Technologies to Improve Fermentation Efficiency and Quality in Wheat Products: A Short Review. Grain Oil Sci. Technol. 2021, 4, 81–87. [Google Scholar] [CrossRef]
- Metaxas, A. Microwave Heating. Power Eng. J. 1991, 5, 237–247. [Google Scholar] [CrossRef]
- Kapcsándi, V.; Neményi, M.; Lakatos, E. Effect of Microwave Treatment of the Grape Must Fermentation Process. 2013. Available online: http://real.mtak.hu/16643/2/FoodScienceConference2013.pdf (accessed on 10 January 2025).
- Melikoglu, M. Solid-State Fermentation of Wheat Pieces by Aspergillus Oryzae: Effects of Microwave Pretreatment on Enzyme Production in a Biorefinery. Int. J. Green Energy 2012, 9, 529–539. [Google Scholar] [CrossRef]
- Nowicka, A.; Zieliński, M.; Dębowski, M.; Dudek, M.R. Wykorzystanie Promieniowania Mikrofalowego do Wspomagania Procesu Fermentacji Alkoholowej Biomasy. Ecol. Eng. Environ. Technol. 2017, 18, 109–116. [Google Scholar] [CrossRef]
- Cho, H.Y.; Yousef, A.E.; Sastry, S.K. Growth Kinetics of Lactobacillus Acidophilus under Ohmic Heating. Biotechnol. Bioeng. 1996, 49, 334–340. [Google Scholar] [CrossRef]
- Lebovka, N. Enhanced Extraction from Solid Foods and Biosuspensions by Pulsed Electrical Energy. Food Eng. Rev. 2010, 2, 95–108. [Google Scholar]
- Lebovka, N.; Vorobiev, E. Techniques to Detect Electroporation in Food Tissues. In Handbook of Electroporation; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- Koubaa, M.; Roselló-Soto, E.; Šic Žlabur, J.; Režek Jambrak, A.; Brnčić, M.; Grimi, N.; Boussetta, N.; Barba, F.J. Current and New Insights in the Sustainable and Green Recovery of Nutritionally Valuable Compounds from Stevia Rebaudiana Bertoni. J. Agric. Food Chem. 2015, 63, 6835–6846. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, A.Z.; Ajit, A.; Yunus, R.; Chisti, Y. Ultrasound-Assisted Fermentation Enhances Bioethanol Productivity. Biochem. Eng. J. 2011, 54, 141–150. [Google Scholar] [CrossRef]
- Hauff, P.; Reinhardt, M.; Foster, S. Ultrasound Basics. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 91–107. [Google Scholar] [CrossRef]
- Ojha, K.S.; Mason, T.J.; O’Donnell, C.P.; Kerry, J.P.; Tiwari, B.K. Ultrasound Technology for Food Fermentation Applications. Ultrason. Sonochem. 2017, 34, 410–417. [Google Scholar] [CrossRef]
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of Ultrasound in Food Technology: Processing, Preservation and Extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Tsukamoto, I.; Yim, B.; Stavarache, C.E.; Furuta, M.; Hashiba, K.; Maeda, Y. Inactivation of Saccharomyces cerevisiae by Ultrasonic Irradiation. Ultrason. Sonochem. 2004, 11, 61–65. [Google Scholar] [CrossRef]
- Wood, B.E.; Aldrich, H.C.; Ingram, L.O. Ultrasound Stimulates Ethanol Production during the Simultaneous Saccharification and Fermentation of Mixed Waste Office Paper. Biotechnol. Prog. 1997, 13, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Chuanyun, D.; Bochu, W.; Chuanren, D.; Sakanishi, A. Low Ultrasonic Stimulates Fermentation of Riboflavin Producing Strain Ecemothecium Ashbyii. Colloids Surf. B Biointerfaces 2003, 30, 37–41. [Google Scholar] [CrossRef]
- Matsuura, K.; Hirotsune, M.; Nunokawa, Y.; Satoh, M.; Honda, K. Acceleration of Cell Growth and Ester Formation by Ultrasonic Wave Irradiation. J. Ferment. Bioeng. 1994, 77, 36–40. [Google Scholar] [CrossRef]
- Schläfer, O.; Sievers, M.; Klotzbücher, H.; Onyeche, T.I. Improvement of Biological Activity by Low Energy Ultrasound Assisted Bioreactors. Ultrasonics 2000, 38, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y.; Moo-Young, M. Disruption of Microbial Cells for Intracellular Products. Enzyme Microb. Technol. 1986, 8, 194–204. [Google Scholar] [CrossRef]
- Lanchun, S.; Bochu, W.; Zhiming, L.; Chuanren, D.; Chuanyun, D.; Sakanishi, A. The Research into the Influence of Low-Intensity Ultrasonic on the Growth of S. Cerevisiaes. Colloids Surf. B Biointerfaces 2003, 30, 43–49. [Google Scholar] [CrossRef]
- Michel, F.C., Jr.; Grulke, E.A.; Reddy, C.A. A Kinetic Model for the Fungal Pellet Lifecycle. AIChE J. 1992, 38, 1449–1460. [Google Scholar] [CrossRef]
- Stewart, G.G.; Hill, A.E.; Russell, I. 125th Anniversary Review: Developments in Brewing and Distilling Yeast Strains: Developments in Brewing and Distilling Yeast Strains. J. Inst. Brew. 2013, 119, 202–220. [Google Scholar] [CrossRef]
- Pátková, J.; Šmogrovičová, D.; Dömény, Z.; Bafrncová, P. Very High Gravity Wort Fermentation by Immobilised Yeast. Biotechnol. Lett. 2000, 22, 1173–1177. [Google Scholar] [CrossRef]
- Dziugan, P.; Balcerek, M.; Pielech-Przybylska, K.; Patelski, P. Evaluation of the Fermentation of High Gravity Thick Sugar Beet Juice Worts for Efficient Bioethanol Production. Biotechnol. Biofuels 2013, 6, 158. [Google Scholar] [CrossRef]
- Walker, G.; Hill, A. Saccharomyces cerevisiae in the Production of Whisk(e)y. Beverages 2016, 2, 38. [Google Scholar] [CrossRef]
- Arshad, M.; Hussain, T.; Iqbal, M.; Abbas, M. Enhanced Ethanol Production at Commercial Scale from Molasses Using High Gravity Technology by Mutant S. Cerevisiae. Braz. J. Microbiol. 2017, 48, 403–409. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Ren, W.; Xiang, J.; Dabbour, M.; Kumah Mintah, B.; Li, Y.; Ma, H. Fermentation of Saccharomyces cerevisiae in a 7.5 L Ultrasound-Enhanced Fermenter: Effect of Sonication Conditions on Ethanol Production, Intracellular Ca2+ Concentration and Key Regulating Enzyme Activity in Glycolysis. Ultrason. Sonochem. 2021, 76, 105624. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xiang, J.; Zhang, Z.; Umego, E.C.; Huang, G.; He, R.; Ma, H. Stimulation of in Situ Low Intensity Ultrasound on Batch Fermentation of to Enhance the GSH Yield. J. Food Process Eng. 2020, 43, e13489. [Google Scholar] [CrossRef]
- Hao, J.; Xu, H.; Yan, P.; Yang, M.; Mintah, B.K.; Dai, C.; Zhang, R.; Ma, H.; He, R. Application of Fixed-Frequency Ultrasound in the Cultivation of Saccharomyces cerevisiae for Rice Wine Fermentation. J. Sci. Food Agric. 2024, 104, 6417–6430. [Google Scholar] [CrossRef]
- Available online: https://leaf-lesaffre.com/wp-content/uploads/2022/07/er_en_th_v3.pdf (accessed on 10 January 2025).
- Available online: http://www.lallemandbds.com/wp-content/uploads/2012/12/2013_LBDS_Data-Sheet_Thermosacc-Dry_10080_Data_Rev.00.01.01.2013.pdf (accessed on 10 January 2025).
- Da Silva Fernandes, F.; De Souza, É.S.; Carneiro, L.M.; Alves Silva, J.P.; De Souza, J.V.B.; Da Silva Batista, J. Current Ethanol Production Requirements for the Yeast Saccharomyces cerevisiae. Int. J. Microbiol. 2022, 2022, 7878830. [Google Scholar] [CrossRef]
- Cagnin, L.; Gronchi, N.; Basaglia, M.; Favaro, L.; Casella, S. Selection of Superior Yeast Strains for the Fermentation of Lignocellulosic Steam-Exploded Residues. Front. Microbiol. 2021, 12, 756032. [Google Scholar] [CrossRef]
- Pielech-Przybylska, K.; Balcerek, M.; Klebeko, M.; Dziekońska-Kubczak, U.; Hebdzyński, M. Ethanolic Fermentation of Rye Mashes: Factors Influencing the Formation of Aldehydes and Process Efficiency. Biomolecules 2022, 12, 1085. [Google Scholar] [CrossRef]
- Beigbeder, J.-B.; De Medeiros Dantas, J.M.; Lavoie, J.-M. Optimization of Yeast, Sugar and Nutrient Concentrations for High Ethanol Production Rate Using Industrial Sugar Beet Molasses and Response Surface Methodology. Fermentation 2021, 7, 86. [Google Scholar] [CrossRef]
- Barbosa, H.S.; Silveira, E.D.A.; Miranda, M.; Ernandes, J.R. Efficient Very-High-Gravity Fermentation of Sugarcane Molasses by Industrial Yeast Strains: Bioethanol or Very-High-Gravity Fermentation. J. Inst. Brew. 2016, 122, 329–333. [Google Scholar] [CrossRef]
- A New Model of Alcoholic Fermentation Under a Byproduct Inhibitory Effect|ACS Omega. Available online: https://pubs.acs.org/doi/10.1021/acsomega.0c04025 (accessed on 19 February 2025).
- Lino, F.S.D.O.; Basso, T.O.; Sommer, M.O.A. A Synthetic Medium to Simulate Sugarcane Molasses. Biotechnol. Biofuels 2018, 11, 221. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.C.; Hynes, S.H.; Ingledew, W.M. Influence of Medium Buffering Capacity on Inhibition of Saccharomyces cerevisiae Growth by Acetic and Lactic Acids. Appl. Environ. Microbiol. 2002, 68, 1616–1623. [Google Scholar] [CrossRef]
- Yang, P.; Jiang, S.; Lu, S.; Jiang, S.; Jiang, S.; Deng, Y.; Lu, J.; Wang, H.; Zhou, Y. Ethanol Yield Improvement in Saccharomyces cerevisiae GPD2 Delta FPS1 Delta ADH2 Delta DLD3 Delta Mutant and Molecular Mechanism Exploration Based on the Metabolic Flux and Transcriptomics Approaches. Microb. Cell Factories 2022, 21, 160. [Google Scholar] [CrossRef]
- Pielech-Przybylska, K.; Balcerek, M.; Ciepielowski, G.; Pacholczyk-Sienicka, B.; Albrecht, Ł.; Dziekońska-Kubczak, U.; Bonikowski, R.; Patelski, P. Effect of Co-Inoculation with Saccharomyces cerevisiae and Lactic Acid Bacteria on the Content of Propan-2-Ol, Acetaldehyde and Weak Acids in Fermented Distillery Mashes. Int. J. Mol. Sci. 2019, 20, 1659. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, W.; Xu, H.; Cheng, L.; Dapaah, M.F.; He, R.; Ma, H. Incorporating Transcriptomic-Metabolomic Analysis Reveal the Effect of Ultrasound on Ethanol Production in Saccharomyces cerevisiae. Ultrason. Sonochem. 2021, 79, 105791. [Google Scholar] [CrossRef] [PubMed]
- Hoang Nguyen Tran, P.; Ko, J.K.; Gong, G.; Um, Y.; Lee, S.-M. Improved Simultaneous Co-Fermentation of Glucose and Xylose by Saccharomyces cerevisiae for Efficient Lignocellulosic Biorefinery. Biotechnol. Biofuels 2020, 13, 12. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, J.; Yuan, Z.; Zhang, Y.; Liang, C.; Liu, Y. Ethanol Production from High Solids Loading of Alkali-Pretreated Sugarcane Bagasse with an SSF Process. BioResources 2014, 9, 3466–3479. [Google Scholar] [CrossRef]
- Mohd, H.; Kamarudin, N.; Nadir, M.; Mel, M.; Ismail, A.; Karim, M.I.A. Comparison of Sago and Sweet Sorghum for Ethanol Production Using Saccharomyces cerevisiae. In Proceedings of the Malaysian International Conference on Trends in Bioprocess Engineering (MICOTriBE), Langkawi, Malaysia, 3–7 July 2012. [Google Scholar]
- Mojovic, L.; Rakin, M.; Vukasinovic, M.; Nikolic, S.; Pejin, J.; Pejin, D. Production of Bioethanol by Simultaneous Saccharification and Fermentation of Corn Meal by Immobilized Yeast. Chem. Eng. Trans. 2010, 21, 1333–1338. [Google Scholar] [CrossRef]
- Vučurović, V.M.; Puškaš, V.S.; Miljić, U.D. Bioethanol Production from Sugar Beet Molasses and Thick Juice by Free and Immobilised Saccharomyces cerevisiae. J. Inst. Brew. 2019, 125, 134–142. [Google Scholar] [CrossRef]
- Kartini, A.M.; Dhokhikah, Y. Bioethanol Production from Sugarcane Molasses with Simultaneous Saccharification and Fermentation (SSF) Method Using Saccaromyces Cerevisiae-Pichia Stipitis Consortium. IOP Conf. Ser. Earth Environ. Sci. 2018, 207, 012061. [Google Scholar] [CrossRef]
- Osmolak, K.; Mikulski, D.; Kłosowski, G. Efficient Production of Fuel Ethanol via the Simultaneous Use of Distillery Stillage Biomass and Beet Molasses. Energies 2025, 18, 312. [Google Scholar] [CrossRef]
- Vučurović, V.; Katanski, A.; Vučurović, D.; Bajić, B.; Dodić, S. Simultaneous Saccharification and Fermentation of Wheat Starch for Bioethanol Production. Fermentation 2025, 11, 80. [Google Scholar] [CrossRef]
- Dai, C.; Xiong, F.; He, R.; Zhang, W.; Ma, H. Effects of Low-Intensity Ultrasound on the Growth, Cell Membrane Permeability and Ethanol Tolerance of Saccharomyces cerevisiae. Ultrason. Sonochem. 2017, 36, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Subhedar, P.B.; Gogate, P.R. Ultrasound-Assisted Bioethanol Production from Waste Newspaper. Ultrason. Sonochem. 2015, 27, 37–45. [Google Scholar] [CrossRef]
- Shaheen, M.; Choi, M.; Ang, W.; Zhao, Y.; Xing, J.; Yang, R.; Xing, J.; Zhang, J.; Chen, J. Application of Low-Intensity Pulsed Ultrasound to Increase Bio-Ethanol Production. Renew. Energy 2013, 57, 462–468. [Google Scholar] [CrossRef]
- Huezo, L.; Shah, A.; Michel, F., Jr. Effects of Ultrasound on Fermentation of Glucose to Ethanol by Saccharomyces cerevisiae. Fermentation 2019, 5, 16. [Google Scholar] [CrossRef]

| Raw Material | Theoretical Ethanol Yield [L/100 kg] |
|---|---|
| Corn grain | 400 |
| Sugarcane juice | 70 |
| Sugarcane molasses | 205 |
| Cassava roots | 180 |
| Sweet potato | 160 |
| Wort Extract [°Blg] | Yeast Strain | Ultrasound Treatment |
|---|---|---|
| 18 | Ethanol Red or Thermosacc Dry | no sonification |
| 22 | 24 kHz; 0.5 pulse, 50% amplitude | |
| 24 | 24 kHz; 0.5 pulse, 75% amplitude |
| ER contr | Ethanol Red yeast, no sonification |
| ER u50 | Ethanol Red yeast, sonification at 50% amplitude |
| ER u75 | Ethanol Red yeast, sonification at 75% amplitude |
| TD contr | Thermosacc Dry yeast, no sonification |
| TD u50 | Thermosacc Dry yeast, sonification at 50% amplitude |
| TD u75 | Thermosacc Dry yeast, sonification at 75% amplitude |
| Compound | Glucose | Fructose | Succinic Acid | Lactic Acid | Acetic Acid |
|---|---|---|---|---|---|
| extract | g/L | ||||
| 18 °Blg | 61.08 ± 0.87 | 60.67 ± 2.64 | 1.04 ± 0.02 | 3.45 ± 0.14 | 1.41 ± 0.04 |
| 22 °Blg | 76.98 ± 3.09 | 78.85 ± 3.51 | 1.75 ± 0.07 | 4.23 ± 0.16 | 1.81 ± 0.08 |
| 24 °Blg | 79.80 ± 3.70 | 79.14 ± 2.84 | 1.82 ± 0.02 | 4.46 ± 0.13 | 1.83 ± 0.04 |
| Extract | 18 °Blg | 22 °Blg | 24 °Blg | |||
|---|---|---|---|---|---|---|
| Glucose | Fructose | Glucose | Fructose | Glucose | Fructose | |
| % of Utilised | ||||||
| ER contr | 99.56 ± 3.17 | 93.75 ± 2.43 | 98.27 ± 1.23 | 81.49 ± 0.57 | 67.42 ± 2.51 | 27.04 ± 0.65 |
| ER u50 | 99.36 ± 3.15 | 92.67 ± 2.69 | 98.60 ± 4.02 | 87.33 ± 3.71 | 81.13 ± 3.17 | 41.03 ± 0.98 |
| ER u75 | 99.05 ± 3.25 | 94.89 ± 1.99 | 98.82 ± 3.35 | 83.89 ± 2.06 | 81.57 ± 1.08 | 47.89 ± 1.64 |
| TD contr | 98.33 ± 3.07 | 86.79 ± 4.23 | 96.68 ± 2.70 | 70.03 ± 1.45 | 58.05 ± 2.46 | 24.45 ± 0.42 |
| TD u50 | 99.97 ± 3.32 | 92.40 ± 2.64 | 96.98 ± 4.49 | 76.06 ± 2.64 | 66.91 ± 2.94 | 37.37 ± 1.14 |
| TD u75 | 98.95 ± 3.36 | 93.96 ± 1.98 | 97.02 ± 2.49 | 73.67 ± 3.60 | 78.30 ± 2.51 | 72.58 ± 2.54 |
| g/L | SucA | LacA | AceA | GlycOH |
|---|---|---|---|---|
| 18 °Blg | ||||
| ER contr | 1.56 ± 0.08 | 2.03 ± 0.08 | 1.87 ± 0.04 | 7.92 ± 0.21 |
| ER u50 | 1.76 ± 0.04 | 2.12 ± 0.06 | 2.10 ± 0.08 | 8.74 ± 0.26 |
| ER u75 | 1.59 ± 0.05 | 2.25 ± 0.04 | 1.98 ± 0.11 | 8.51 ± 0.21 |
| TD contr | 1.60 ± 0.03 | 2.10 ± 0.06 | 1.96 ± 0.06 | 7.83 ± 0.16 |
| TD u50 | 1.62 ± 0.06 | 2.00 ± 0.06 | 1.92 ± 0.07 | 8.17 ± 0.08 |
| TD u75 | 1.56 ± 0.05 | 1.86 ± 0.06 | 1.59 ± 0.04 | 6.96 ± 0.09 |
| 22 °Blg | ||||
| ER contr | 1.92 ± 0.04 | 3.03 ± 0.03 | 2.51 ± 0.08 | 9.80 ± 0.46 |
| ER u50 | 1.94 ± 0.05 | 2.75 ± 0.10 | 2.52 ± 0.05 | 10.29 ± 0.29 |
| ER u75 | 2.05 ± 0.07 | 3.01 ± 0.06 | 2.54 ± 0.09 | 10.17 ± 0.29 |
| TD contr | 1.67 ± 0.08 | 2.89 ± 0.08 | 2.33 ± 0.08 | 9.27 ± 0.35 |
| TD u50 | 1.61 ± 0.06 | 2.71 ± 0.11 | 2.47 ± 0.04 | 9.76 ± 0.20 |
| TD u75 | 1.52 ± 0.04 | 2.71 ± 0.07 | 2.16 ± 0.07 | 8.52 ± 0.14 |
| 24 °Blg | ||||
| ER contr | 1.46 ± 0.05 | 3.56 ± 0.13 | 2.62 ± 0.09 | 8.36 ± 0.21 |
| ER u50 | 1.59 ± 0.05 | 3.46 ± 0.12 | 2.56 ± 0.06 | 8.44 ± 0.20 |
| ER u75 | 1.52 ± 0.07 | 3.19 ± 0.15 | 2.31 ± 0.05 | 8.19 ± 0.23 |
| TD contr | 1.26 ± 0.06 | 3.30 ± 0.12 | 2.04 ± 0.07 | 5.06 ± 0.10 |
| TD u50 | 1.35 ± 0.05 | 3.60 ± 0.06 | 2.25 ± 0.08 | 5.74 ± 0.19 |
| TD u75 | 1.21 ± 0.04 | 3.53 ± 0.10 | 2.02 ± 0.09 | 5.37 ± 0.14 |
| Initial Extract | 18 °Blg | 22 °Blg | 24 °Blg |
|---|---|---|---|
| g/L | |||
| ER contr | 51.82 ± 0.91 | 61.67 ± 0.74 | 44.28 ± 1.00 |
| ER u50 | 56.85 ± 1.22 | 65.64 ± 1.92 | 48.23 ± 0.98 |
| ER u75 | 56.22 ± 1.43 | 66.11 ± 1.16 | 56.15 ± 0.77 |
| TD contr | 48.81 ± 0.93 | 53.76 ± 1.26 | 23.54 ± 0.49 |
| TD u50 | 54.97 ± 0.63 | 61.12 ± 1.42 | 29.57 ± 1.20 |
| TD u75 | 52.45 ± 1.35 | 56.89 ± 0.85 | 37.38 ± 0.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patelski, A.M.; Kobalczyk, A.; Dziekońska-Kubczak, U.; Januszewicz, B.; Domański, J. Effect of Ultrasound on Fermentation of Thick Molasses Worts by Distiller’s Yeast. Appl. Sci. 2025, 15, 3811. https://doi.org/10.3390/app15073811
Patelski AM, Kobalczyk A, Dziekońska-Kubczak U, Januszewicz B, Domański J. Effect of Ultrasound on Fermentation of Thick Molasses Worts by Distiller’s Yeast. Applied Sciences. 2025; 15(7):3811. https://doi.org/10.3390/app15073811
Chicago/Turabian StylePatelski, Andrea Maria, Aleksandra Kobalczyk, Urszula Dziekońska-Kubczak, Bartłomiej Januszewicz, and Jarosław Domański. 2025. "Effect of Ultrasound on Fermentation of Thick Molasses Worts by Distiller’s Yeast" Applied Sciences 15, no. 7: 3811. https://doi.org/10.3390/app15073811
APA StylePatelski, A. M., Kobalczyk, A., Dziekońska-Kubczak, U., Januszewicz, B., & Domański, J. (2025). Effect of Ultrasound on Fermentation of Thick Molasses Worts by Distiller’s Yeast. Applied Sciences, 15(7), 3811. https://doi.org/10.3390/app15073811










