Magnetism and Low-Temperature Magnetocaloric Effect in Gd7(BO3)(PO4)2O6 Compound with Monoclinic Lattice
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Crystal Structure
3.2. Magnetic Properties
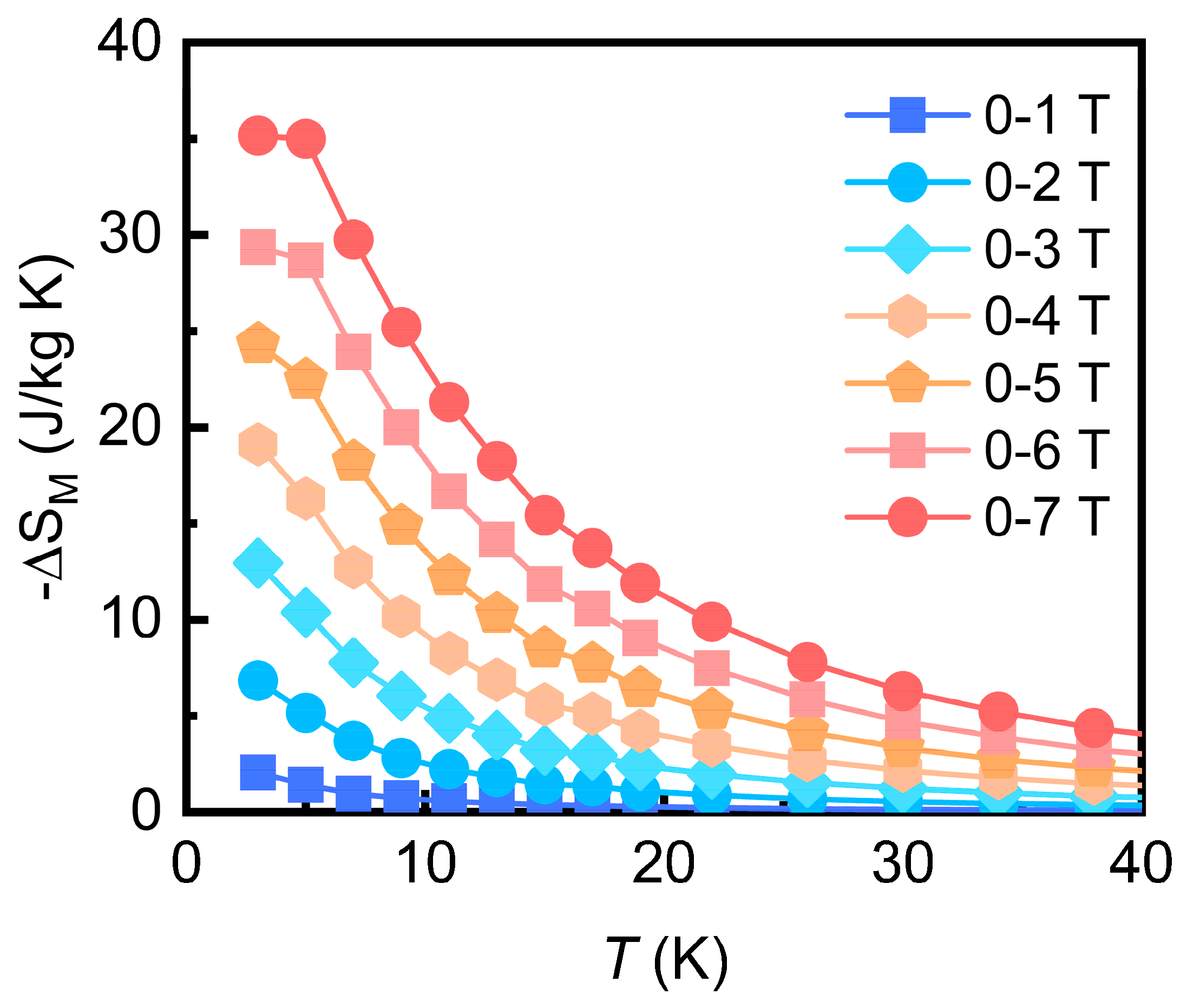
3.3. Magnetocaloric Effect
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lucas, M.; Danilov, A.V.; Levitin, L.V.; Jayaraman, A.; Casey, A.J.; Faoro, L.; Tzalenchuk, A.Y.; Kubatkin, S.E.; Saunders, J.; de Graaf, S.E. Quantum bath suppression in a superconducting circuit by immersion cooling. Nat. Commun. 2023, 14, 3522. [Google Scholar] [PubMed]
- Zu, H.; Dai, W.; de Waele, A.T.A.M. Development of dilution refrigerators—A review. Cryogenics 2021, 121, 103390. [Google Scholar]
- Zheng, N.W.; Quan, J.; Wang, N.L.; Li, C.Z.; Zhao, M.G.; Wei, L.J.; Liang, J.T. A Brief Review of Dilution Refrigerator Development for Space Applications. J. Low Temp. Phys. 2019, 197, 1–9. [Google Scholar]
- Wang, Y.P.; Xiang, J.S.; Zhang, L.; Gong, J.J.; Li, W.; Mo, Z.J.; Shen, J. Giant Low-Field Cryogenic Magnetocaloric Effect in a Polycrystalline EuB4O7 Compound. J. Am. Chem. Soc. 2024, 146, 3315–3322. [Google Scholar] [PubMed]
- Xu, Q.F.; Liu, B.L.; Ye, M.Y.; Zhuang, G.L.; Long, L.S.; Zheng, L.S. Gd(OH)F2: A Promising Cryogenic Magnetic Refrigerant. J. Am. Chem. Soc. 2022, 144, 13787–13793. [Google Scholar]
- Wang, H.J.; Mo, Z.J.; Gong, J.J.; Tu, H.; Zhang, G.C.; Shen, J. Large low-field reversible magnetocaloric effect in K3Gd(PO4)2 at sub-Kelvin temperature. J. Rare Earths 2024, 42, 1087–1092. [Google Scholar]
- Yang, Y.; Zhang, Q.C.; Pan, Y.Y.; Long, L.S.; Zheng, L.S. Magnetocaloric effect and thermal conductivity of Gd(OH)3 and Gd2O(OH)4(H2O)2. Chem. Commun. 2015, 51, 7317–7320. [Google Scholar]
- Chen, Y.C.; Qin, L.; Meng, Z.S.; Yang, D.F.; Wu, C.; Fu, Z.D.; Zheng, Y.Z.; Liu, J.L.; Tarasenko, R.; Orendác, M.; et al. Study of a magnetic-cooling material Gd(OH)CO3. J. Mater. Chem. A 2014, 2, 9851–9858. [Google Scholar]
- Liu, J.L.; Chen, Y.C.; Guo, F.S.; Tong, M.L. Recent advances in the design of magnetic molecules for use as cryogenic magnetic coolants. Coord. Chem. Rev. 2014, 281, 26–49. [Google Scholar]
- Palacios, E.; Rodriguez-Velamazan, J.A.; Evangelisti, M.; Mclntyre, G.J.; Lorusso, G.; Visser, D.; de Jongh, L.J.; Boatner, L.A. Magnetic structure and magnetocalorics of GdPO4. Phys. Rev. B 2014, 90, 214423. [Google Scholar]
- Yang, Z.Y.; Zhang, H.H.; Bai, M.J.; Li, W.; Huang, S.L.; Ruan, S.C.; Zeng, Y.J. Large magnetocaloric effect in gadolinium borotungstate Gd3BWO9. J. Mater. Chem. C 2020, 8, 11866–11873. [Google Scholar]
- Xia, M.J.; Shen, S.P.; Lu, J.; Sun, Y.; Li, R.K. K3Li3Gd7(BO3)9: A New Gadolinium-Rich Orthoborate for Cryogenic Magnetic Cooling. Chem. Eur. J. 2018, 24, 3147–3150. [Google Scholar] [PubMed]
- Xie, Y.; Na, Y.Z.; Chen, F.Y.; Zhang, Y.K. Cryogenic magnetic properties and magnetocaloric responses in Gd11GeP3O26 oxide. Inorg. Chem. Commun. 2025, 177, 114395. [Google Scholar]
- Chen, Y.W.; Liu, W.; Feng, J.C.; Guo, R.X.; Fan, F.D.; Shen, J.; Zhang, G.C.; Tu, H. Magnetocaloric effect in LiLn6O5(BO3)3 (Ln = Gd, Tb, Dy, and Ho). Cryogenics 2022, 124, 103476. [Google Scholar]
- Yang, Z.W.; Qin, S.J.; Zhang, J.; Lu, D.B.; Zhao, H.T.; Kang, C.X.; Cui, H.Z.; Long, Y.W.; Zeng, Y.J. Gadolinium oxyorthogermanate Gd2GeO5: An efficient solid refrigerant material for magnetic cryocoolers. Mater. Today Phys. 2022, 27, 100810. [Google Scholar]
- Chen, Y.C.; Prokleska, J.; Xu, W.J.; Liu, J.L.; Liu, J.; Zhang, W.X.; Jia, J.H.; Sechovsky, V.; Tong, M.L. A brilliant cryogenic magnetic coolant: Magnetic and magnetocaloric study of ferromagnetically coupled GdF3. J. Mater. Chem. C 2015, 3, 12206–12211. [Google Scholar]
- Zhang, Y.K.; Na, Y.Z.; Hao, W.X.; Gottschall, T.; Li, L.W. Enhanced Cryogenic Magnetocaloric Effect from 4f-3d Exchange Interaction in B-Site Ordered Gd2CuTiO6 Double Perovskite Oxide. Adv. Funct. Mater. 2024, 34, 2409061. [Google Scholar]
- Chen, Z.H.; Zhang, C.L.; Zhang, Z.M.; Yu, S.L.; Zhang, G.C.; Tu, H.; Wang, D.H.; Shen, J. Large magnetocaloric effect in gadolinium-rich silicate NaGd9(SiO4)6O2. J. Alloys Compd. 2024, 976, 173351. [Google Scholar] [CrossRef]
- Pakhira, S.; Mazumdar, C.; Ranganathan, R.; Avdeev, M. Magnetic frustration induced large magnetocaloric effect in the absence of long range magnetic order. Sci. Rep. 2017, 7, 7367. [Google Scholar]
- Das, M.; Roy, S.; Khan, N.; Mandal, P. Giant magnetocaloric effect in an exchange-frustrated GdCrTiO5 antiferromagnet. Phys. Rev. B 2018, 98, 104420. [Google Scholar]
- Koskelo, E.C.; Kelly, N.D.; Nagle, L.A.V.; Bocarsly, J.D.; Mukherjee, P.; Liu, C.; Zhang, Q.; Dutton, S.E. Magnetic and Magnetocaloric Properties of the A2LnSbO6 Lanthanide Oxides on the Frustrated fcc Lattice. Inorg. Chem. 2023, 62, 10317–10328. [Google Scholar] [CrossRef] [PubMed]
- Midya, A.; Khan, N.; Bhoi, D.; Mandal, P. Giant magnetocaloric effect in magnetically frustrated EuHo2O4 and EuDy2O4 compounds. Appl. Phys. Lett. 2012, 101, 132415. [Google Scholar] [CrossRef]
- Liu, A.D.; Zhou, J.; Wang, L.; Cao, Y.T.; Song, F.Y.; Han, Y.Y.; Li, J.X.; Tong, W.; Xia, Z.Z.C.; Ouyang, Z.W.; et al. Large magnetocaloric effect in the Shastry-Sutherland lattice compound Yb2Be2GeO7 with spin-disordered ground state. Phys. Rev. B 2024, 110, 144445. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Zhu, J.; Li, S.; Zhang, Z.Q.; Wang, J.; Ren, Z.M. Magnetic properties and promising magnetocaloric performances in the antiferromagnetic GdFe2Si2 compound. Sci. China Mater. 2022, 65, 1345–1352. [Google Scholar] [CrossRef]
- Gschneidner, K.A.; Pecharsky, V.K.; Tsoko, A.O. Recent developments in magnetocaloric materials. Rep. Prog. Phys. 2005, 68, 1479. [Google Scholar] [CrossRef]
- Tian, L.; Xu, B.; Chen, H.; Mo, Z.J.; Li, Z.X.; Liu, G.D.; Shen, J. Unstable antiferromagnetism and large reversible magnetocaloric effect in TmNi2Si2. Sci. China Mater. 2023, 66, 3984–3990. [Google Scholar] [CrossRef]
- Griffith, L.D.; Mudryk, Y.; Slaughter, J.; Pecharsky, V.K. Material-based figure of merit for caloric materials. J. Appl. Phys. 2018, 123, 034902. [Google Scholar] [CrossRef]
- Gschneidner, K.A.; Pecharsky, V.K. Magnetocaloric materials. Annu. Rev. Mater. Sci. 2000, 30, 387–429. [Google Scholar] [CrossRef]
- Kang, B.Y.; Kim, J.Y.; Choi, H.Y.; Cho, B.K. Anomalous weak ferromagnetism in the magnetically frustrated system R1−xYxB4 (R=Tb and Dy). Phys. Rev. B 2015, 91, 024414. [Google Scholar] [CrossRef]
- Mahana, S.; Manju, U.; Topwal, D. GdCrO3: A potential candidate for low temperature magnetic refrigeration. J. Phys. D Appl. Phys. 2018, 51, 305002. [Google Scholar] [CrossRef]
- Chen, Z.H.; Wang, D.H.; Zhang, C.L.; Zhang, Z.M.; Zhang, G.C.; Tu, H.; Shen, J. Cryogenic magnetocaloric effect in R2GeMoO8 (R = Gd and Dy) compounds. J. Appl. Phys. 2023, 133, 143904. [Google Scholar]
- Dey, K.; Indra, A.; Majumdar, S.; Giri, S. Cryogenic magnetocaloric effect in zircon-type RVO4 (R = Gd, Ho, Er, and Yb). J. Mater. Chem. C 2017, 5, 1646–1650. [Google Scholar]
- Mahana, S.; Manju, U.; Topwal, D. Giant magnetocaloric effect in GdAlO3 and a comparative study with GdMnO3. J. Phys. D Appl. Phys. 2016, 50, 035002. [Google Scholar]
- Wang, P.Y.; Zhang, Z.Q.; Su, W.T.; Li, L.W. Structural and cryogenic magnetic properties in AGd(MoO4)2 (A = Li, Na and K) compounds. Ceram. Int. 2019, 45, 21735–21741. [Google Scholar]
- Mukherjee, P.; Wu, Y.; Lampronti, G.I.; Dutton, S.E. Magnetic properties of monoclinic lanthanide orthoborates, LnBO3, Ln = Gd, Tb, Dy, Ho, Er, Yb. Mater. Res. Bull. 2018, 98, 173–179. [Google Scholar]
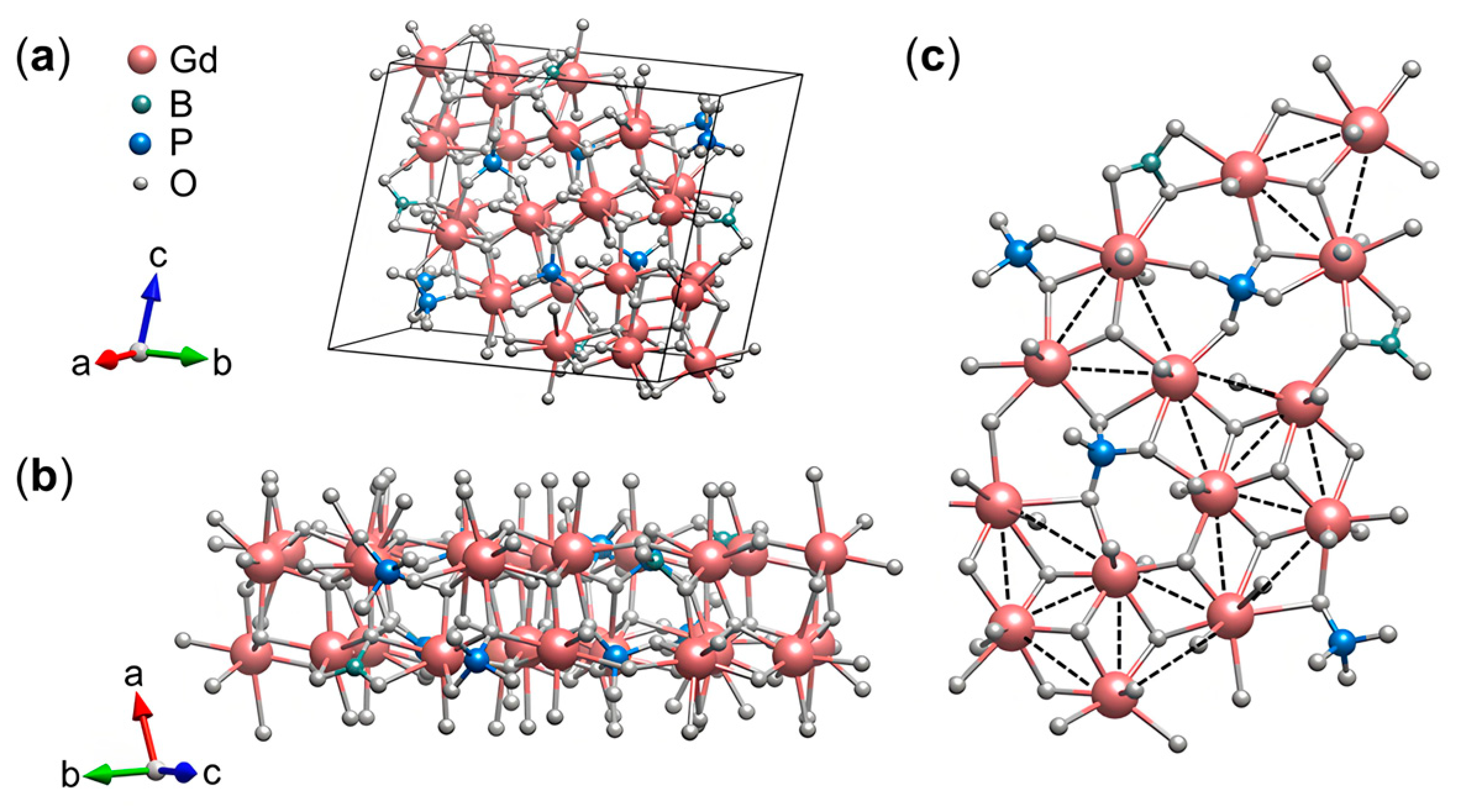
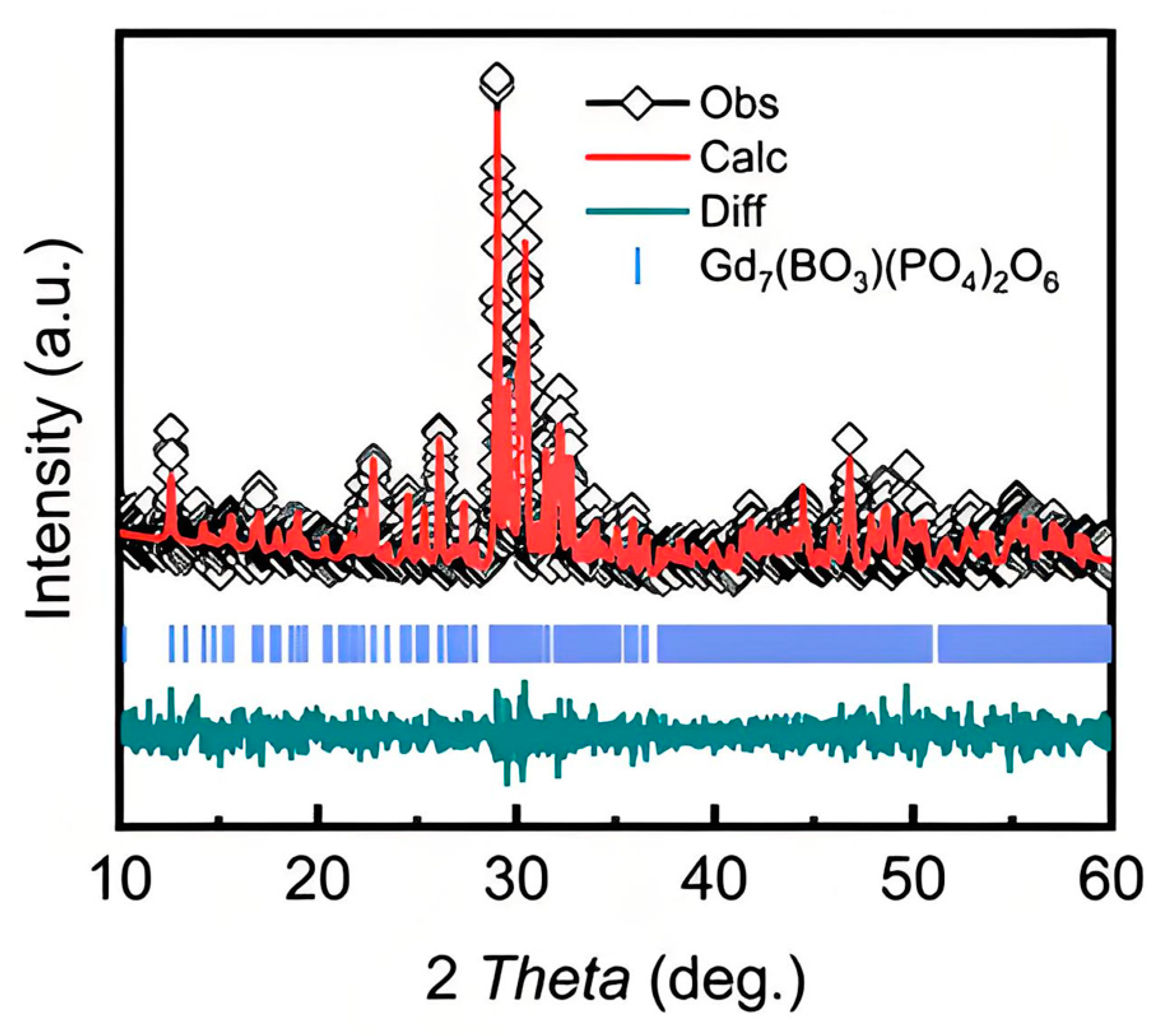
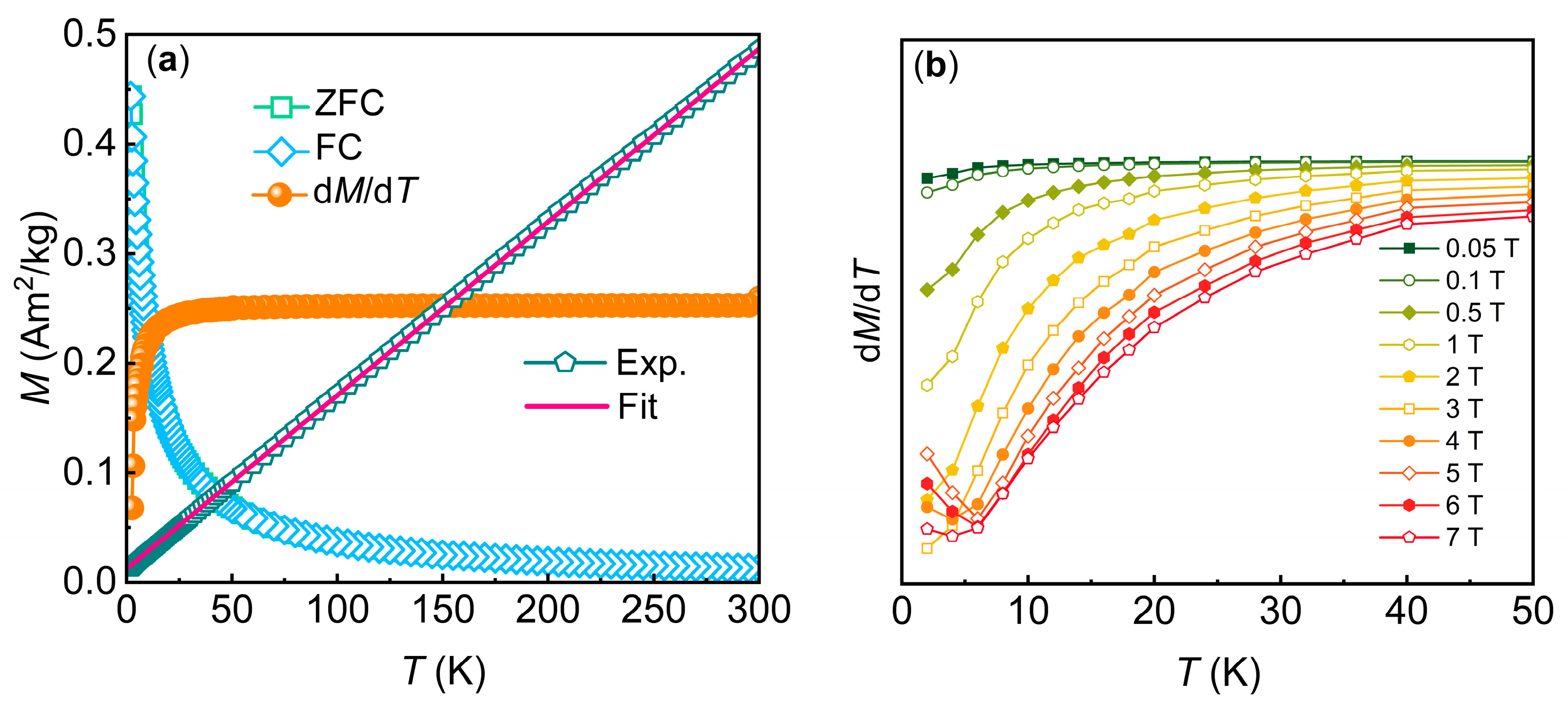
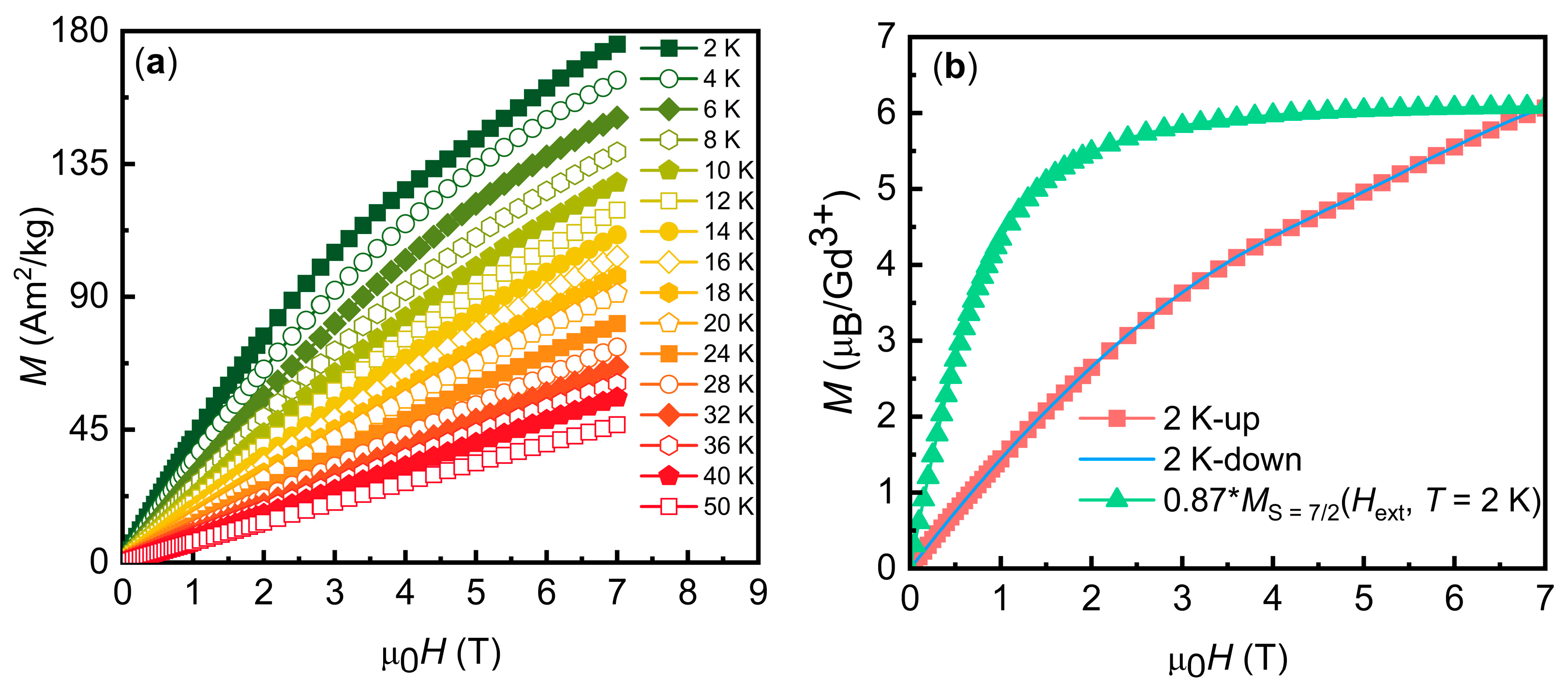
| Compound | Gd7(BO3)(PO4)2O6 |
|---|---|
| Space group | P21/c (No. 14) |
| a (Å) | 6.6924 |
| b (Å) | 17.2453 |
| c (Å) | 12.1073 |
| V (Å3) | 1285.0881 |
| RP (%) | 13.07 |
| RWP (%) | 14.699 |
| RF (%) | 0.886 |
| Compounds | (K) | ∆H (T) | (J/kg K) | RC (J/kg) | Reference |
|---|---|---|---|---|---|
| Gd7(BO3)(PO4)2O6 | 2 | 7 | 35.2 | 288.6 | This work |
| GdCrO3 | 2.3 | 7 | 37.0 | 542.0 | [30] |
| Gd2GeMoO8 | 1.4 | 7 | 41.2 | 257.4 | [31] |
| GdVO4 | 2.4 | 5 | 41.1 | * | [32] |
| GdAlO3 | 3.9 | 9 | 40.9 | 271.0 | [33] |
| LiGd(MoO4)2 | 2 | 7 | 33.6 | * | [34] |
| DyBO3 | 1.01 | 5 | 22.0 | * | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, L.; He, X.; Shen, Z.; Gao, X.; Mo, Z. Magnetism and Low-Temperature Magnetocaloric Effect in Gd7(BO3)(PO4)2O6 Compound with Monoclinic Lattice. Appl. Sci. 2025, 15, 3802. https://doi.org/10.3390/app15073802
Tian L, He X, Shen Z, Gao X, Mo Z. Magnetism and Low-Temperature Magnetocaloric Effect in Gd7(BO3)(PO4)2O6 Compound with Monoclinic Lattice. Applied Sciences. 2025; 15(7):3802. https://doi.org/10.3390/app15073802
Chicago/Turabian StyleTian, Lu, Xuetong He, Zhiwen Shen, Xinqiang Gao, and Zhaojun Mo. 2025. "Magnetism and Low-Temperature Magnetocaloric Effect in Gd7(BO3)(PO4)2O6 Compound with Monoclinic Lattice" Applied Sciences 15, no. 7: 3802. https://doi.org/10.3390/app15073802
APA StyleTian, L., He, X., Shen, Z., Gao, X., & Mo, Z. (2025). Magnetism and Low-Temperature Magnetocaloric Effect in Gd7(BO3)(PO4)2O6 Compound with Monoclinic Lattice. Applied Sciences, 15(7), 3802. https://doi.org/10.3390/app15073802






