Effects of Frost Mulberry Leaf Superfine Powder on the Hypoglycemic and Gut Microbiota of High-Fat Diet/Streptozotocin-Induced Type 2 Diabetes Mellitus Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of FMLSP
2.2. Determination of Physicochemical Properties and Components of FMLSP
2.2.1. Particle Size
2.2.2. Structural Characterization Analysis
2.2.3. Determination of Basic Nutrients
2.2.4. Analysis of Bioactive Substances
Flavonoid and Polyphenol
Polysaccharides
1-Deoxynojirimycin (1-DNJ)
2.3. Determination of FMLSP Functional Properties
2.3.1. In Vitro Antioxidant Activity
2.3.2. In Vitro Digestive Simulation
2.3.3. In Vitro Hypoglycemic Analysis
2.4. Animal Experimental
2.5. Biochemical Analysis
2.6. Analysis of Tissue Sections
2.7. Examination of the Gut Microbiota
2.8. Data Analysis
3. Results
3.1. Physicochemical Properties and Compositional Analysis of FMLSP
3.2. Functional Properties of FMLSP
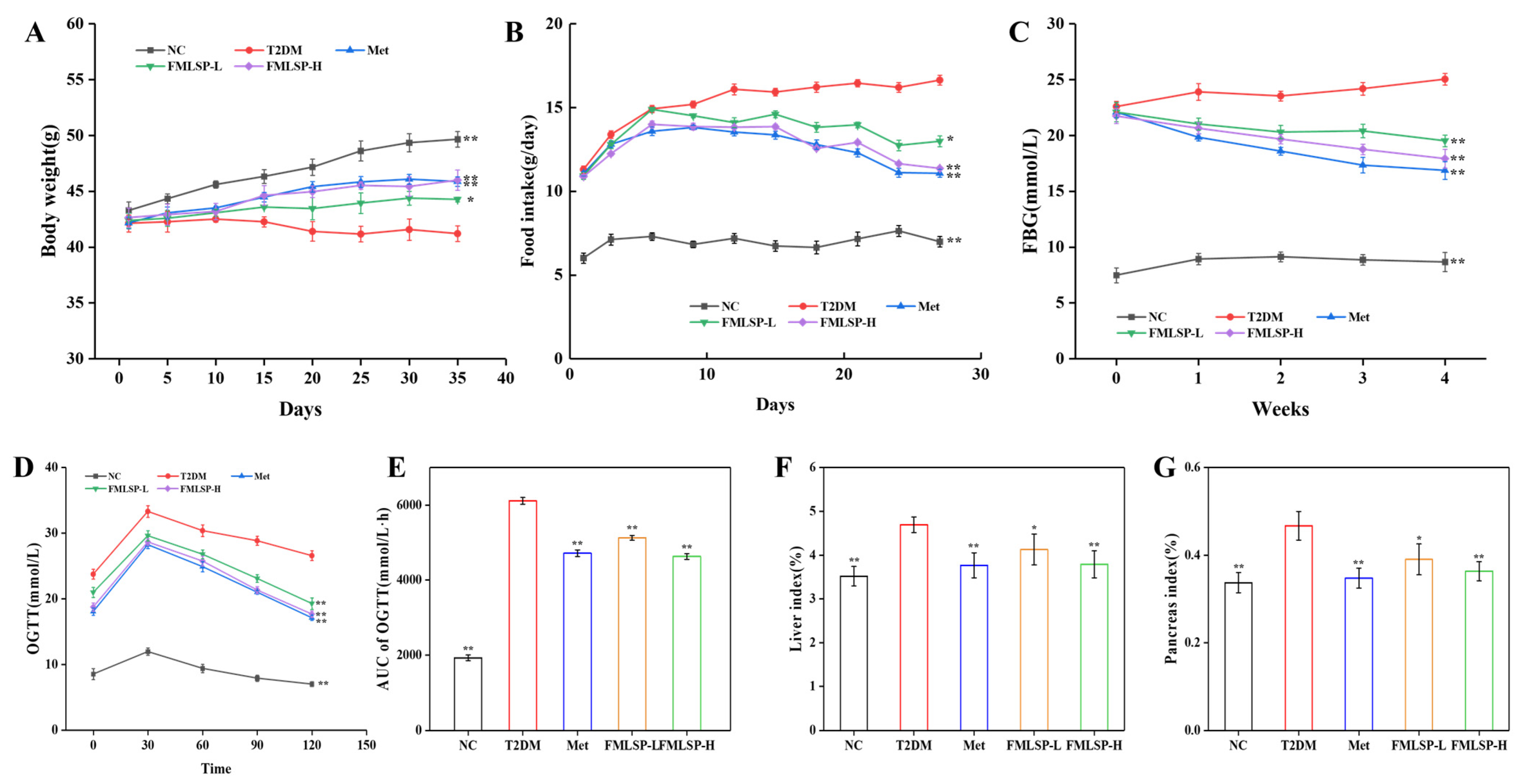
3.3. Effect of FMLSP on Body Weight, FBG, and Organ Index in T2DM Mice
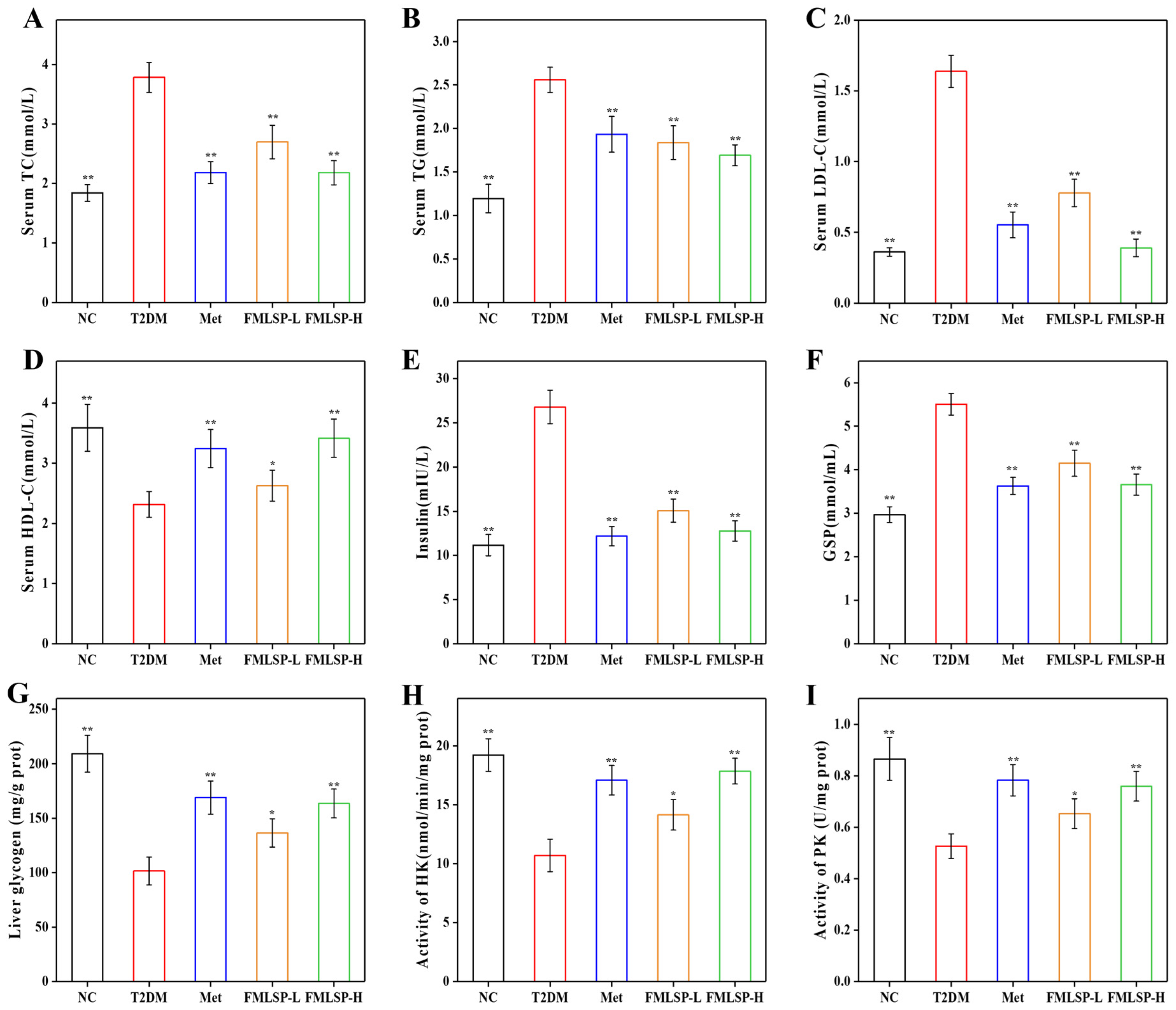
3.4. Effect of FMLSP on Lipid Metabolism
3.5. Effect of FMLSP on Glucose Metabolism in Mice
3.6. Impact of FMLSP on the Mice’s Antioxidant System
3.7. Protective Effect of FMLSP on Mouse Liver Tissues
3.8. Protective Effect of FMLSP on Mouse Pancreatic Tissues
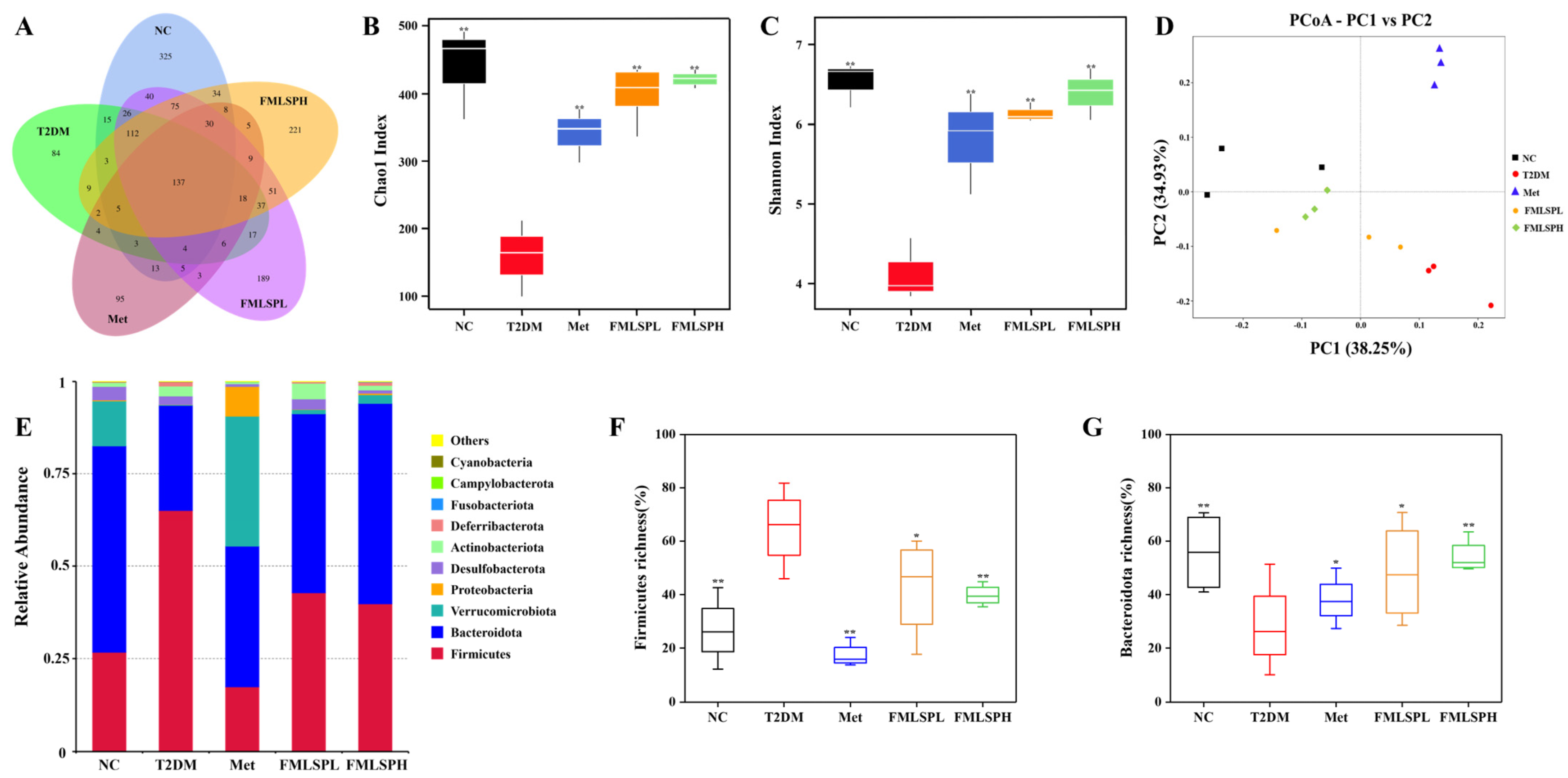
3.9. FMLSP’s Impact on the Taxonomic Diversity of Mice’s Gut Microbiota
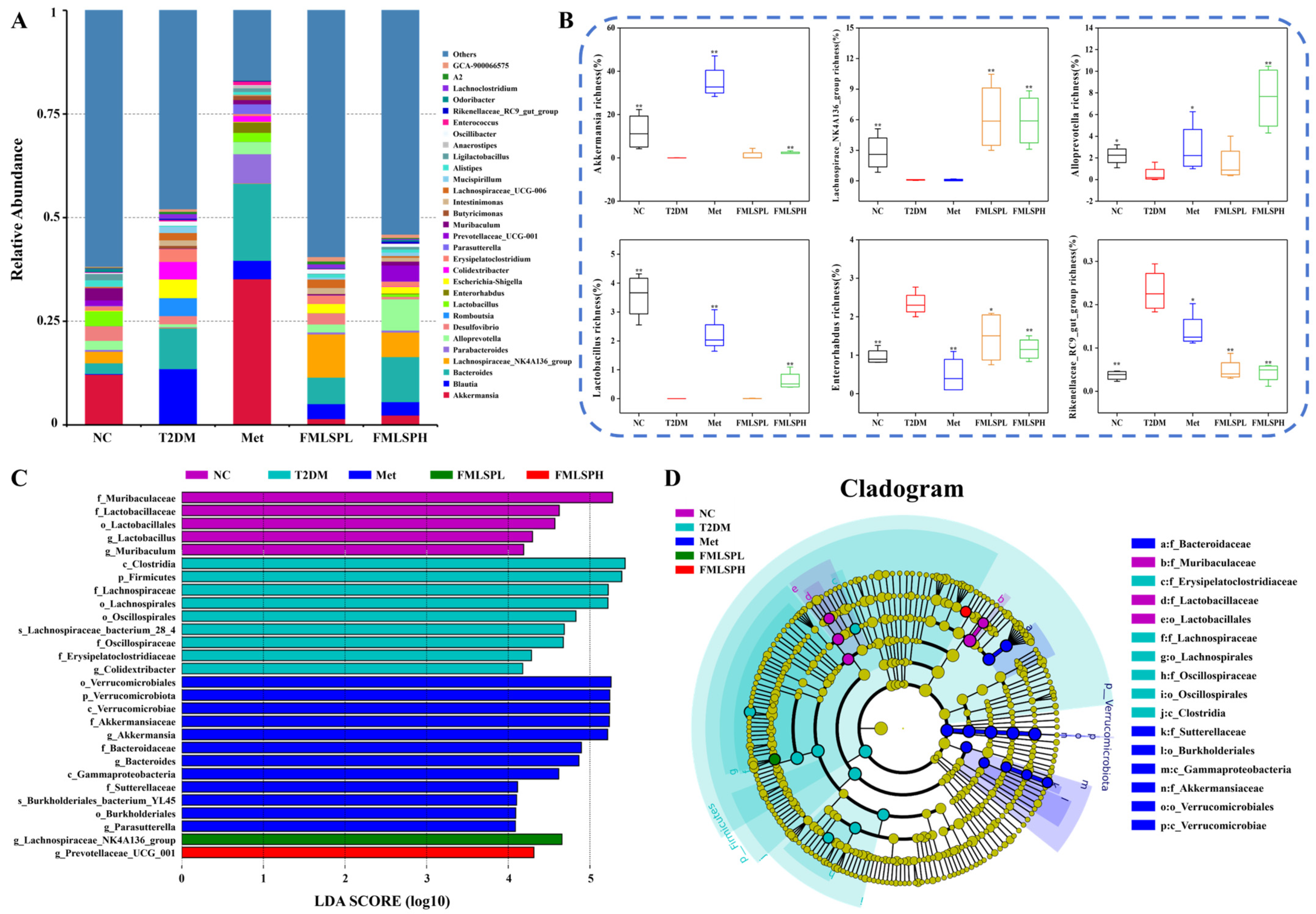
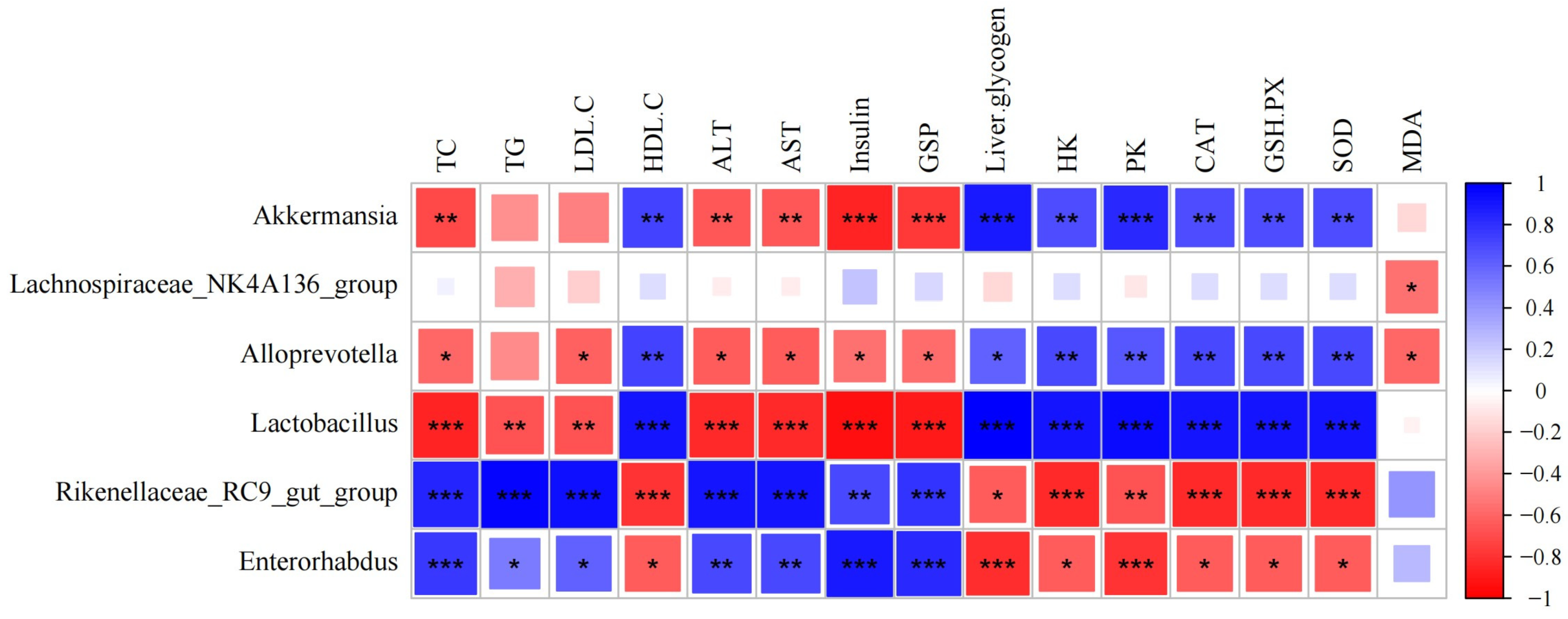
3.10. FMLSP Restores Gut Microbial Composition in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taheri, S. Defining type 2 diabetes remission: KISS goodbye to confusion? Lancet Diabetes Endocrinol. 2021, 9, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Rachdaoui, N. Insulin: The Friend and the Foe in the Development of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 1770. [Google Scholar] [CrossRef]
- Atlas, D. International Diabetes Federation, 7th ed.; International Diabetes Federation: Brussels, Belgium, 2015; Volume 33. [Google Scholar]
- Jouad, H.; Haloui, M.; Rhiouani, H.; El Hilaly, J.; Eddouks, M. Ethnobotanical survey of medicinal plants used for the treatment of diabetes, cardiac and renal diseases in the North centre region of Morocco (Fez-Boulemane). J. Ethnopharmacol. 2001, 77, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Zheng, S.; Ma, T.; Zhang, C.; Ou, X.; He, X.; Xu, W.; Huang, K. Mulberry leaf alleviates streptozotocin-induced diabetic rats by attenuating NEFA signaling and modulating intestinal microflora. Sci. Rep. 2017, 7, 12041. [Google Scholar] [CrossRef]
- Allin, K.H.; Tremaroli, V.; Caesar, R.; Jensen, B.A.H.; Damgaard, M.T.F.; Bahl, M.I.; Licht, T.R.; Hansen, T.H.; Nielsen, T.; Dantoft, T.M.; et al. Aberrant intestinal microbiota in individuals with prediabetes. Diabetologia 2018, 61, 810–820. [Google Scholar] [CrossRef]
- Navab-Moghadam, F.; Sedighi, M.; Khamseh, M.E.; Alaei-Shahmiri, F.; Talebi, M.; Razavi, S.; Amirmozafari, N. The association of type II diabetes with gut microbiota composition. Microb. Pathog. 2017, 110, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.L.; Stephens, J.W.; Harris, D.A. Gut microbiota influence in type 2 diabetes mellitus (T2DM). Gut Pathog. 2021, 13, 50. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing A Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef]
- Ling, X.; Hu, Y.; Hu, Y.; Meng, J. Analysis of chlorogenic acid and two flavonoids in mulberry leaves of different harvest periods and origins and HPLC fingerprint study for quality control. J. Food Compos. Anal. 2024, 132, 106284. [Google Scholar] [CrossRef]
- Chan, E.W.; Lye, P.Y.; Wong, S.K. Phytochemistry, pharmacology, and clinical trials of Morus alba. Chin. J. Nat. Med. 2016, 14, 17–30. [Google Scholar] [CrossRef]
- Ma, G.; Chai, X.; Hou, G.; Zhao, F.; Meng, Q. Phytochemistry, bioactivities and future prospects of mulberry leaves: A review. Food Chem 2022, 372, 131335. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Katsube, T.; Koyama, A.; Itamura, H. Seasonal Changes in Functional Component Contents in Mulberry (Morus alba L.) Leaves. Hortic. J. 2017, 86, 534–542. [Google Scholar] [CrossRef]
- Xu, D.-Q.; Cheng, S.-Y.; Zhang, J.-Q.; Lin, H.-F.; Chen, Y.-Y.; Yue, S.-J.; Tian, M.; Tang, Y.-P.; Zhao, Y.-C. Morus alba L. Leaves—Integration of Their Transcriptome and Metabolomics Dataset: Investigating Potential Genes Involved in Flavonoid Biosynthesis at Different Harvest Times. Front. Plant Sci. 2021, 12, 736332. [Google Scholar]
- Yu, X.; Zhu, Y.; Fan, J.; Wang, D.; Gong, X.; Ouyang, Z. Accumulation of Flavonoid Glycosides and UFGT Gene Expression in Mulberry Leaves (Morus alba L.) before and after Frost. Chem. Biodivers. 2017, 14, e1600496. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Liu, F.; Xiong, L. Medicinal parts of mulberry (leaf, twig, root bark, and fruit) and compounds thereof are excellent traditional Chinese medicines and foods for diabetes mellitus. J. Funct. Foods 2023, 106, 105619. [Google Scholar] [CrossRef]
- Bae, U.J.; Jung, E.S.; Jung, S.J.; Chae, S.W.; Park, B.H. Mulberry leaf extract displays antidiabetic activity in db/db mice via Akt and AMP-activated protein kinase phosphorylation. Food Nutr. Res. 2018, 62, 1473. [Google Scholar] [CrossRef]
- Jung, S.-H.; Han, J.-H.; Park, H.-S.; Lee, D.-H.; Kim, S.J.; Cho, H.S.; Kang, J.S.; Myung, C.-S. Effects of unaltered and bioconverted mulberry leaf extracts on cellular glucose uptake and antidiabetic action in animals. BMC Complement. Altern. Med. 2019, 19, 55. [Google Scholar] [CrossRef]
- Meng, Q.; Qi, X.; Fu, Y.; Chen, Q.; Cheng, P.; Yu, X.; Sun, X.; Wu, J.; Li, W.; Zhang, Q.; et al. Flavonoids extracted from mulberry (Morus alba L.) leaf improve skeletal muscle mitochondrial function by activating AMPK in type 2 diabetes. J. Ethnopharmacol. 2020, 248, 112326. [Google Scholar] [CrossRef]
- Usman, I.; Muzzamal, H.; Ali, I.; Muhammad, A.; Farhan, S.; Mehak, J.; Atka, A.; Iqra, A.; Entessar, A.J.; Saewan, S.A. Traditional and innovative approaches for the extraction of bioactive compounds. Int. J. Food Prop. 2022, 25, 1215–1233. [Google Scholar] [CrossRef]
- Huang, X.; Liang, K.-h.; Liu, Q.; Qiu, J.; Wang, J.; Zhu, H. Superfine grinding affects physicochemical, thermal and structural properties of Moringa Oleifera leaf powders. Ind. Crops Prod. 2020, 151, 112472. [Google Scholar] [CrossRef]
- Gao, W.; Chen, F.; Wang, X.; Meng, Q. Recent advances in processing food powders by using superfine grinding techniques: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2222–2255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dong, Y.; Nisar, T.; Fang, Z.; Wang, Z.-C.; Guo, Y. Effect of superfine-grinding on the physicochemical and antioxidant properties of Lycium ruthenicum Murray powders. Powder Technol. 2020, 372, 68–75. [Google Scholar] [CrossRef]
- Palavecino, P.M.; Penci, M.C.; Ribotta, P.D. Effect of planetary ball milling on physicochemical and morphological properties of sorghum flour. J. Food Eng. 2019, 262, 22–28. [Google Scholar] [CrossRef]
- Wang, F.; Zeng, J.; Gao, H.; Sukmanov, V. Effects of different physical technology on compositions and characteristics of bean dregs. Innov. Food Sci. Emerg. Technol. 2021, 73, 102789. [Google Scholar] [CrossRef]
- Ma, X.; Sang, X.; Yan, C.; Zhang, Y.; Bi, J.; Zhang, G.; Hao, H.; Hou, H. Dynamics of Bacterial Composition and Association with Quality Formation and Biogenic Amines Accumulation during Fish Sauce Spontaneous Fermentation. Appl. Environ. Microbiol. 2022, 88, e00690-00622. [Google Scholar] [CrossRef]
- Tu, J.; Liu, G.; Jin, Y.; Tang, C.; Yao, T.; Zhuo, J.; Li, Q.; Liu, L.; Wang, J. Enrichment of γ-aminobutyric acid in mulberry leaves and the inhibitory effects of the water extract on ACE and α-glucosidase activity. Ind. Crops Prod. 2022, 177, 114485. [Google Scholar] [CrossRef]
- Zeng, C.; Ye, G.; Li, G.; Cao, H.; Wang, Z.; Ji, S. RID serve as a more appropriate measure than phenol sulfuric acid method for natural water-soluble polysaccharides quantification. Carbohydr. Polym. 2022, 278, 118928. [Google Scholar] [CrossRef] [PubMed]
- Vichasilp, C.; Nakagawa, K.; Sookwong, P.; Higuchi, O.; Luemunkong, S.; Miyazawa, T. Development of high 1-deoxynojirimycin (DNJ) content mulberry tea and use of response surface methodology to optimize tea-making conditions for highest DNJ extraction. LWT—Food Sci. Technol. 2012, 45, 226–232. [Google Scholar] [CrossRef]
- Faidi, A.; Becheikh, M.E.H.; Lassoued, M.A.; Stumbé, J.F.; Safta, F.; Sfar, S. Isolation of sodium alginate-like polysaccharide from Padina pavonica: Optimization, characterization and antioxidant properties. J. Mol. Struct. 2025, 1321, 139737. [Google Scholar] [CrossRef]
- Yu, W.; Wang, J.; Xiong, Y.; Liu, J.; Baranenko, D.; Zhang, Y.; Lu, W. In vivo absorption, in vitro simulated digestion, and fecal fermentation properties of Imperata cylindrica polysaccharides and their effects on gut microbiota. Food Chem. 2024, 461, 140773. [Google Scholar] [CrossRef]
- Awosika, T.O.; Aluko, R.E. Inhibition of the in vitro activities of α-amylase, α-glucosidase and pancreatic lipase by yellow field pea (Pisum sativum L.) protein hydrolysates. Int. J. Food Sci. Technol. 2019, 54, 2021–2034. [Google Scholar] [CrossRef]
- Yang, M.; Lv, J.; Yang, J.; Yang, S.; Wang, F.; Wang, Y.; Zhang, C. Effects of Codonopsis pilosula crude polysaccharides by hypoglycemic and modulating gut microbiome in a high-fat diet and streptozotocin-induced mouse model of T2DM. J. Funct. Foods 2023, 111, 105893. [Google Scholar] [CrossRef]
- Lin, S.; Jin, X.; Gao, J.; Qiu, Z.; Ying, J.; Wang, Y.; Dong, Z.; Zhou, W. Impact of wheat bran micronization on dough properties and bread quality: Part I—Bran functionality and dough properties. Food Chem. 2021, 353, 129407. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, M.; Sang, Y.; Sun, J. Effect of ball-milling treatment on the structure, physicochemical properties and allergenicity of proteins from oyster (Crassostrea gigas). LWT 2022, 166, 113803. [Google Scholar] [CrossRef]
- Xu, S.; Li, X.; Podio, N.S.; Han, Y.; Wang, X.-Y.; Gong, E.S. Black rice phenolics alleviate T2DM through comprehensive regulation of oxidative stress, metabolic pathways and gut microbiota. Food Biosci. 2024, 62, 105494. [Google Scholar] [CrossRef]
- Yang, C.; Ma, L.; Ma, J.; Liu, S.; Fu, J.; Fan, Y.; Liu, Y. Lycium barbarum leaf flavonoids ameliorate high fructose induced insulin resistance in mice by regulating blood glucose and gut microbiota composition. Food Biosci. 2024, 62, 105087. [Google Scholar] [CrossRef]
- Yang, H.-B.; Song, J.-Y.; Xu, C.; Li, J.; Zhang, C.; Xie, S.; Teng, C.-l. Interventional effects of Pueraria oral liquid on T2DM rats and metabolomics analysis. Biomed. Pharmacother. 2024, 175, 116780. [Google Scholar] [CrossRef]
- Semaming, Y.; Kukongviriyapan, U.; Kongyingyoes, B.; Thukhammee, W.; Pannangpetch, P. Protocatechuic Acid Restores Vascular Responses in Rats With Chronic Diabetes Induced by Streptozotocin. Phytother. Res. 2016, 30, 227–233. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef]
- Huang, W.; Huang, G.-P.; Zhang, L.-X.; da Yu, E.; Yang, W.-K.; Ye, M.; Zou, S.-Q.; Ni, L.; He, H.-Q. Lignan-rich extract from Cinnamomum camphora leaf attenuates metabolic syndrome by modulating glycolipid metabolism and gut microbiota in T2DM mice. Phytomedicine 2024, 135, 156118. [Google Scholar] [CrossRef]
- Zaheer, R.; Noyes, N.; Ortega Polo, R.; Cook, S.R.; Marinier, E.; Van Domselaar, G.; Belk, K.E.; Morley, P.S.; McAllister, T.A. Impact of sequencing depth on the characterization of the microbiome and resistome. Sci. Rep. 2018, 8, 5890. [Google Scholar] [CrossRef]
- Zhao, L.; Lou, H.; Peng, Y.; Chen, S.; Zhang, Y.; Li, X. Comprehensive relationships between gut microbiome and faecal metabolome in individuals with type 2 diabetes and its complications. Endocrine 2019, 66, 526–537. [Google Scholar] [CrossRef]
- Hao, J.-Y.; Wan, Y.; Yao, X.-H.; Zhao, W.-G.; Hu, R.-Z.; Chen, C.; Li, L.; Zhang, D.-Y.; Wu, G.-H. Effect of different planting areas on the chemical compositions and hypoglycemic and antioxidant activities of mulberry leaf extracts in Southern China. PLoS ONE 2018, 13, e0198072. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, S.; Yu, J.; Sun, Y.; Zhu, J.; Ji, D.; Wu, C. The mulberry-derived 1-deoxynojirimycin (DNJ) inhibits high-fat diet (HFD)-induced hypercholesteremia and modulates the gut microbiota in a gender-specific manner. J. Funct. Foods 2019, 52, 63–72. [Google Scholar] [CrossRef]
- He, X.; Wang, C.e.; Zhu, Y.; Jiang, X.; Qiu, Y.; Yin, F.; Xiong, W.; Liu, B.; Huang, Y. Spirulina compounds show hypoglycemic activity and intestinal flora regulation in type 2 diabetes mellitus mice. Algal Res. 2022, 66, 102791. [Google Scholar] [CrossRef]
- Han, C.; Kong, X.; Xia, X.; Huang, X.; Mao, Z.; Han, J.; Shi, F.; Liang, Y.; Wang, A.; Zhang, F. Effects of ginseng peptides on the hypoglycemic activity and gut microbiota of a type 2 diabetes mellitus mice model. J. Funct. Foods 2023, 111, 105897. [Google Scholar] [CrossRef]
- Virtue, S.; Vidal-Puig, A. GTTs and ITTs in mice: Simple tests, complex answers. Nat. Metab. 2021, 3, 883–886. [Google Scholar] [CrossRef]
- Li, M.; Ma, S. A review of healthy role of dietary fiber in modulating chronic diseases. Food Res. Int. 2024, 191, 114682. [Google Scholar] [CrossRef]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef]
- Mo, Z.; Zhan, M.; Yang, X.; Xie, P.; Xiao, J.; Cao, Y.; Xiao, H.; Song, M. Fermented dietary fiber from soy sauce residue exerts antidiabetic effects through regulating the PI3K/AKT signaling pathway and gut microbiota-SCFAs-GPRs axis in type 2 diabetic mellitus mice. Int. J. Biol. Macromol. 2024, 270, 132251. [Google Scholar] [CrossRef]
- Jiang, S.; Young, J.L.; Wang, K.; Qian, Y.; Cai, L. Diabetic-induced alterations in hepatic glucose and lipid metabolism: The role of type 1 and type 2 diabetes mellitus (Review). Mol. Med. Rep. 2020, 22, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Enkhmaa, B.; Shiwaku, K.; Katsube, T.; Kitajima, K.; Anuurad, E.; Yamasaki, M.; Yamane, Y. Mulberry (Morus alba L.) Leaves and Their Major Flavonol Quercetin 3-(6-Malonylglucoside) Attenuate Atherosclerotic Lesion Development in LDL Receptor-Deficient Mice1. J. Nutr. 2005, 135, 729–734. [Google Scholar] [CrossRef]
- Chen, J.; Meng, X. Aronia melanocarpa Anthocyanin Extracts Improve Hepatic Structure and Function in High-Fat Diet-/Streptozotocin-Induced T2DM Mice. J. Agric. Food Chem. 2022, 70, 11531–11543. [Google Scholar] [CrossRef]
- Zhang, L.; Su, S.; Zhu, Y.; Guo, J.; Guo, S.; Qian, D.; Ouyang, Z.; Duan, J.-A. Mulberry leaf active components alleviate type 2 diabetes and its liver and kidney injury in db/db mice through insulin receptor and TGF-β/Smads signaling pathway. Biomed. Pharmacother. 2019, 112, 108675. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.Y.; Wang, X.T.; Xu, H.L.; Yang, Z.L.; Cheng, Y.; Zhou, B. Protective effect of ferulic acid on STZ-induced diabetic nephropathy in rats. Food Funct. 2020, 11, 3706–3718. [Google Scholar] [CrossRef]
- Fatima, F.; Zakaria, M. Chapter 8—Oxidative stress and insulin resistance. In Fundamental Principles of Oxidative Stress in Metabolism and Reproduction; Alam, F., Rehman, R., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 123–137. [Google Scholar]
- Zhang, Y.; Li, L.; Chai, T.; Xu, H.; Du, H.-y.; Jiang, Y. Mulberry leaf multi-components exert hypoglycemic effects through regulation of the PI-3K/Akt insulin signaling pathway in type 2 diabetic rats. J. Ethnopharmacol. 2024, 319, 117307. [Google Scholar] [CrossRef]
- Hu, T.-G.; Wen, P.; Liu, J.; Long, X.-S.; Liao, S.-T.; Wu, H.; Zou, Y.-X. Combination of mulberry leaf and oat bran possessed greater hypoglycemic effect on diabetic mice than mulberry leaf or oat bran alone. J. Funct. Foods 2019, 61, 103503. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; Wu, H.; Zhang, L.; Hu, X.; Li, C.; Liu, S. Noni (Morinda citrifolia L.) fruit phenolic extract supplementation ameliorates NAFLD by modulating insulin resistance, oxidative stress, inflammation, liver metabolism and gut microbiota. Food Res. Int. 2022, 160, 111732. [Google Scholar] [CrossRef]
- Carobene, A.; Braga, F.; Roraas, T.; Sandberg, S.; Bartlett, W.A. A systematic review of data on biological variation for alanine aminotransferase, aspartate aminotransferase and γ-glutamyl transferase. Clin. Chem. Lab. Med. 2013, 51, 1997–2007. [Google Scholar] [CrossRef]
- Khajuria, P.; Raghuwanshi, P.; Rastogi, A.; Koul, A.L.; Zargar, R.; Kour, S. Hepatoprotective effect of seabuckthorn leaf extract in streptozotocin induced diabetes mellitus in Wistar rats. Indian J. Anim. Res. 2018, 52, 1745–1750. [Google Scholar] [CrossRef]
- Zhong, R.-F.; Liu, C.-J.; Hao, K.-X.; Fan, X.-D.; Jiang, J.-G. Polysaccharides from Flos Sophorae Immaturus ameliorates insulin resistance in IR-HepG2 cells by co-regulating signaling pathways of AMPK and IRS-1/PI3K/AKT. Int. J. Biol. Macromol. 2024, 280, 136088. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Neculae, E.; Costea, C.F.; Ciocoiu, M.; Hurjui, L.L.; Tarniceriu, C.C.; Maranduca, M.A.; Lacatusu, C.M.; Floria, M.; et al. Role of Gut Microbiota on Onset and Progression of Microvascular Complications of Type 2 Diabetes (T2DM). Nutrients 2020, 12, 3719. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zheng, Y.; Zhou, J.; Geng, Y.; Zou, P.; Li, Y.; Zhang, C. Polyphenol-Rich Extracts from Brown Macroalgae Lessonia trabeculate Attenuate Hyperglycemia and Modulate Gut Microbiota in High-Fat Diet and Streptozotocin-Induced Diabetic Rats. J. Agric. Food Chem. 2019, 67, 12472–12480. [Google Scholar] [CrossRef]
- Rodrigues, V.F.; Elias-Oliveira, J.; Pereira, Í.S.; Pereira, J.A.; Barbosa, S.C.; Machado, M.S.G.; Carlos, D. Akkermansia muciniphila and Gut Immune System: A Good Friendship That Attenuates Inflammatory Bowel Disease, Obesity, and Diabetes. Front. Immunol. 2022, 13, 934695. [Google Scholar] [CrossRef]
- Guo, P.; Zeng, M.; Zhang, Y.; Zhang, Z.; Wu, Y.; Ye, K.; Chang, F.; Wang, Y.; Zheng, X.; Feng, W. Integration strategies involving 16S rDNA sequencing combined with untargeted metabolomics revealed the mechanism of Selaginella tamariscina (Beauv.) Spring in db/db diabetic mice. Biomed. Pharmacother. 2024, 180, 117546. [Google Scholar] [CrossRef]
- Robles-Vera, I.; Toral, M.; de la Visitación, N.; Sánchez, M.; Romero, M.; Olivares, M.; Jiménez, R.; Duarte, J. The Probiotic Lactobacillus fermentum Prevents Dysbiosis and Vascular Oxidative Stress in Rats with Hypertension Induced by Chronic Nitric Oxide Blockade. Mol. Nutr. Food Res. 2018, 62, 1800298. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Zhang, M.; Ren, F.; Ren, Y.; Li, Y.; Liu, N.; Zhang, Y.; Zhang, Q.; Wang, R. Effects of Fermented Milk Containing Lacticaseibacillus paracasei Strain Shirota on Constipation in Patients with Depression: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 2238. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, Z.; Zhao, Y.; Kang, C.; Zhang, X.; Tian, Y.; Yu, J.; Cao, H.; Wang, M. The anti-diabetic activity of polyphenols-rich vinegar extract in mice via regulating gut microbiota and liver inflammation. Food Chem. 2022, 393, 133443. [Google Scholar] [CrossRef]



| Group | Feed Type | Gavage Drug | Dose |
|---|---|---|---|
| NC | Normal diet | Sterile water | 0.4 mL |
| T2DM | HFD | Sterile water | 0.4 mL |
| Met | HFD | Metformin hydrochloride | 0.5 g/kg |
| FMLSPL | HFD | FMLSP | 1.0 g/kg |
| FMLSPH | HFD | FMLSP | 2.0 g/kg |
| D10%/μm | D50%/μm | D90%/μm | D [4,3]/μm |
|---|---|---|---|
| 4.21 ± 0.16 | 8.09 ± 0.20 | 18.52 ± 0.57 | 8.52 ± 0.33 |
| Compound | Content (mg/g) | Compound | Content (mg GAE/g dw) |
|---|---|---|---|
| Ash | 82.28 ± 0.04 | Polyphenol | 50.41 ± 1.04 |
| Protein | 227.52 ± 0.07 | Flavonoid | 91.30 ± 2.92 |
| Fat | 31.93 ± 0.08 | Polysaccharides | 58.41 ± 1.39 |
| Dietary fiber | 305.18 ± 5.28 | 1-DNJ | 0.89 ± 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Wu, Q.; Zhao, G.; Liang, J.; Sun, L.; Jia, M.; Sun, R.; Yang, M. Effects of Frost Mulberry Leaf Superfine Powder on the Hypoglycemic and Gut Microbiota of High-Fat Diet/Streptozotocin-Induced Type 2 Diabetes Mellitus Mice. Appl. Sci. 2025, 15, 3766. https://doi.org/10.3390/app15073766
Wu J, Wu Q, Zhao G, Liang J, Sun L, Jia M, Sun R, Yang M. Effects of Frost Mulberry Leaf Superfine Powder on the Hypoglycemic and Gut Microbiota of High-Fat Diet/Streptozotocin-Induced Type 2 Diabetes Mellitus Mice. Applied Sciences. 2025; 15(7):3766. https://doi.org/10.3390/app15073766
Chicago/Turabian StyleWu, Jingya, Qiu Wu, Guojian Zhao, Jing Liang, Lei Sun, Ming Jia, Rui Sun, and Mingguan Yang. 2025. "Effects of Frost Mulberry Leaf Superfine Powder on the Hypoglycemic and Gut Microbiota of High-Fat Diet/Streptozotocin-Induced Type 2 Diabetes Mellitus Mice" Applied Sciences 15, no. 7: 3766. https://doi.org/10.3390/app15073766
APA StyleWu, J., Wu, Q., Zhao, G., Liang, J., Sun, L., Jia, M., Sun, R., & Yang, M. (2025). Effects of Frost Mulberry Leaf Superfine Powder on the Hypoglycemic and Gut Microbiota of High-Fat Diet/Streptozotocin-Induced Type 2 Diabetes Mellitus Mice. Applied Sciences, 15(7), 3766. https://doi.org/10.3390/app15073766






