Structural, Morphological and Thermoluminescence Properties of Mn-Doped Zinc Zirconate (ZnZrO3) Phosphors
Abstract
1. Introduction
2. Experimental Details
2.1. Procedure
2.2. Characterization
3. Results and Discussion
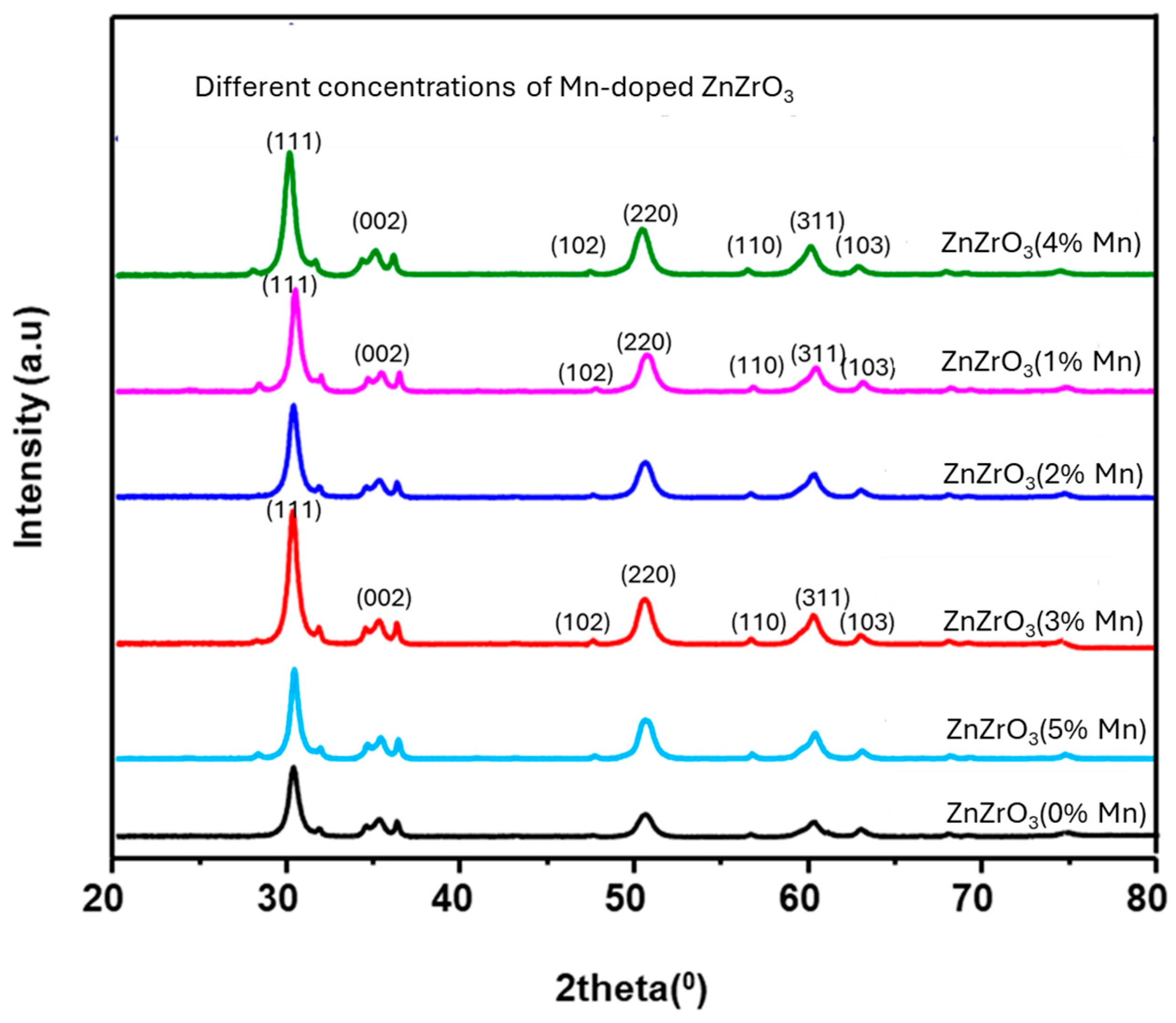
3.1. Morphological and Structural Properties
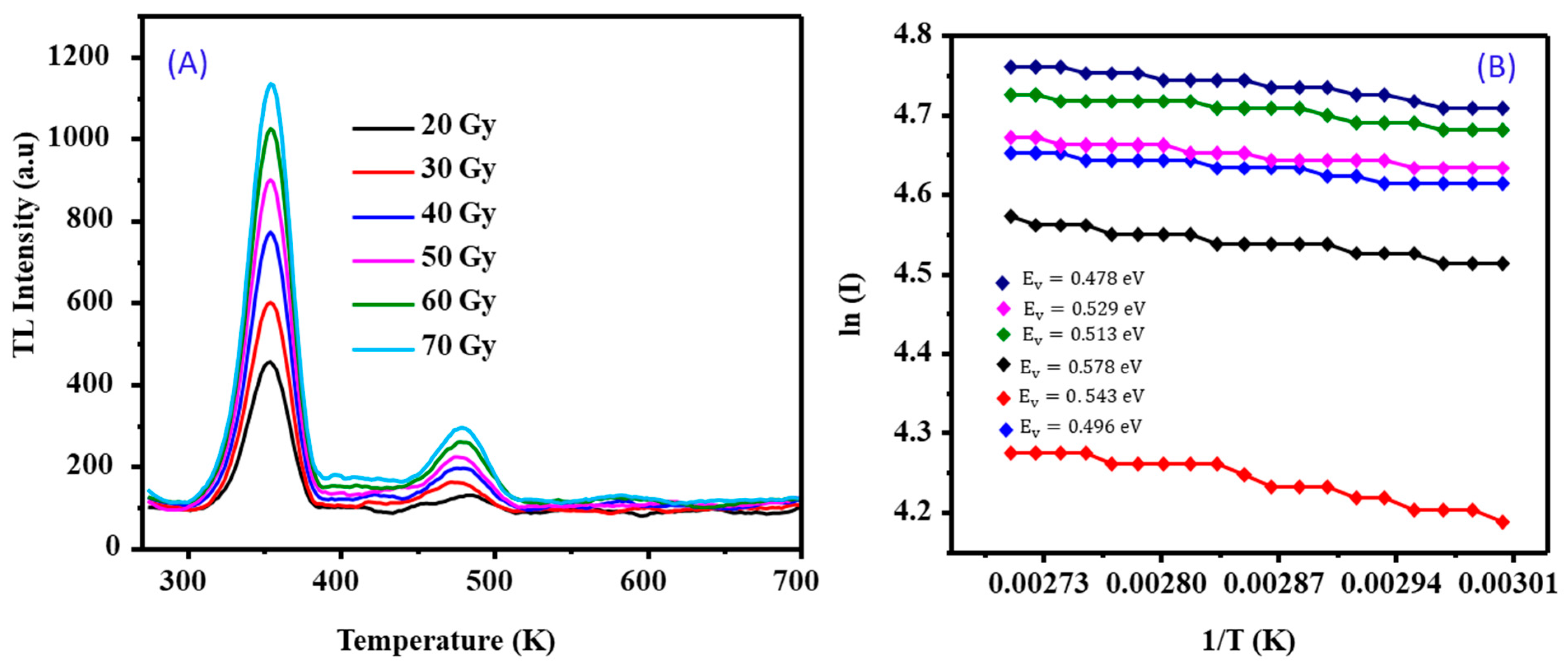
3.2. Analysis of Dose−Response Graphs
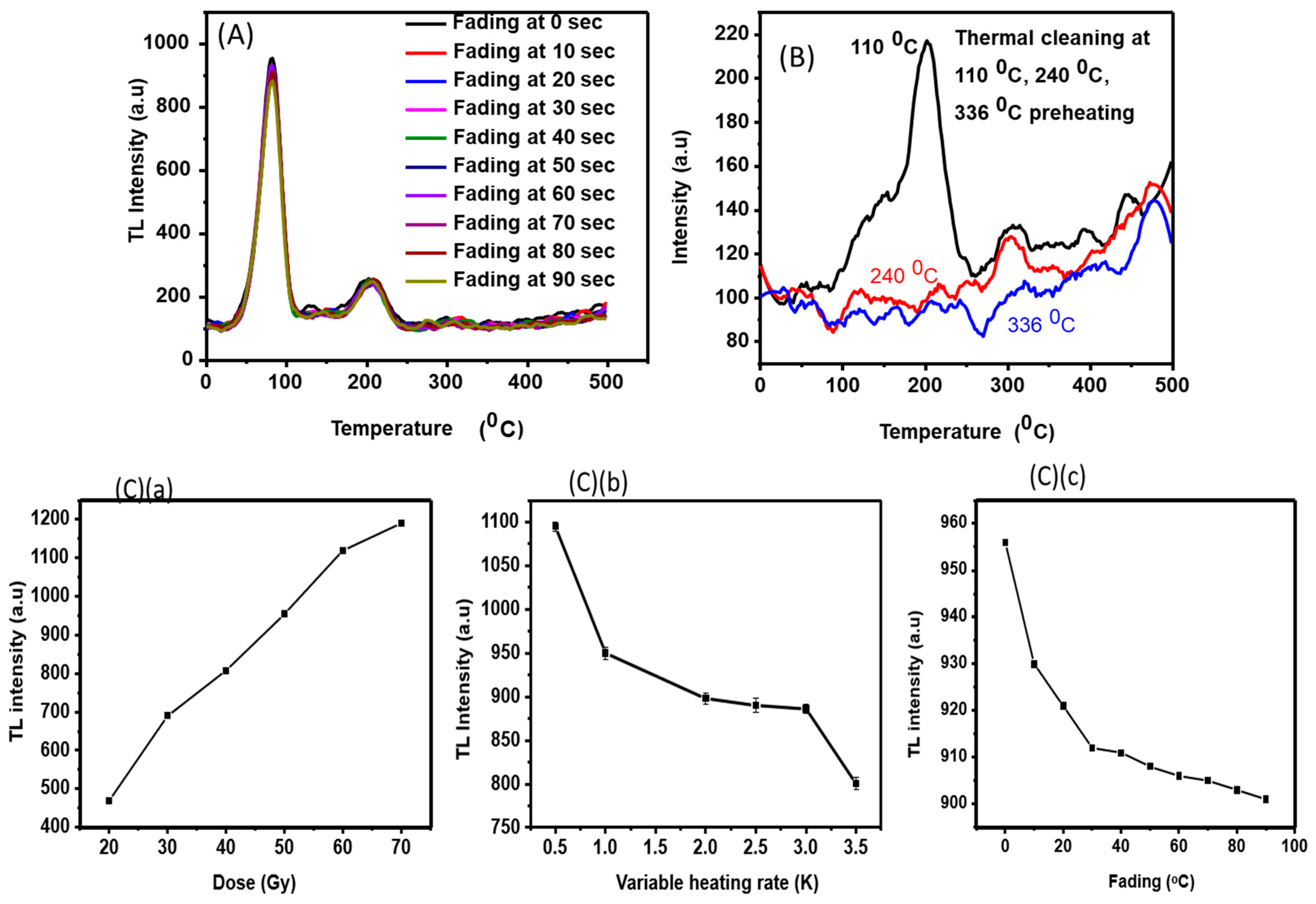
3.2.1. Stability Study
3.2.2. Dose−Response
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, O.M.; Spooner, N.A. Defect pair mechanism for quartz intermediate temperature thermoluminescence bands. Radiat. Meas. 2018, 108, 41–44. [Google Scholar]
- Chen, R.; Lawless, J.; Pagonis, V. Thermoluminescence associated with two-electron traps. Radiat. Meas. 2017, 99, 10–17. [Google Scholar]
- Gaber, A.; Abd El-Hamid, H.; Ngida, R.E.; Sadek, H.; Khattab, R. Synthesis, characterization and corrosive resistance of ZnO and ZrO2 coated TiO2 substrate prepared via polymeric method and microwave combustion. Ceram. Int. 2024, 50, 38917–38932. [Google Scholar]
- Kothiyal, G.; Ananthanarayanan, A.; Dey, G. Glass and glass-ceramics. In Functional Materials Preparation, Processing and Applications; Elsevier: Amsterdam, The Netherlands, 2012; pp. 323–386. [Google Scholar]
- Shinde, K.N.; Dhoble, S.; Swart, H.; Park, K. Phosphate Phosphors for Solid-State Lighting; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Murthy, K. Phosphor for Optoelectronic Devices: A Review. J. Innov. Electron. Commun. Eng. 2013, 3, 21–28. [Google Scholar]
- Hussain, N.; Ayoub, I.; Mushtaq, U.; Sehgal, R.; Rubab, S.; Sehgal, R.; Swart, H.C.; Kumar, V. Introduction to phosphors and luminescence. In Rare-Earth-Activated Phosphors; Elsevier: Amsterdam, The Netherlands, 2022; pp. 3–41. [Google Scholar]
- Matinise, N.; Mayedwa, N.; Kaviyarasu, K.; Nuru, Z.; Madiba, I.; Mongwaketsi, N.; Fuku, X.; Maaza, M. Zinc zirconate (ZnZrO3) nanocomposites bimetallic designed by green synthesis via Moringa Olefeira extract for high-performance electrochemical applications. Mater. Today Proc. 2021, 36, 401–407. [Google Scholar] [CrossRef]
- Ramos-Garcés, M.V.; Colón, J.L. Preparation of zirconium phosphate nanomaterials and their applications as inorganic supports for the oxygen evolution reaction. Nanomaterials 2020, 10, 822. [Google Scholar] [CrossRef]
- Martínez-Martínez, R.; García-Hipólito, M.; Juárez-López, G.; González, J.Z.; Brito, F.R.; Olvera, R.C.; Mendoza, J.G.; Falcony, C. Synthesis, structural and optical studies for ZnO-ZrO2-ZnZrO3: Tb3+ luminescent coatings deposited by Ultrasonic Spray Pyrolysis method. Ceram. Int. 2024, 50, 54885–54896. [Google Scholar] [CrossRef]
- Kafadar, V.E. The Effect of Various Experimental Parameters on Glow Peaks and TL Kinetics of Some Dosimetric Materials. Ph.D. Thesis, National Thesis Center of Turkey (Ulusal Tez Merkezi), Ankara, Turkey, 2009. [Google Scholar]
- Bakr, M.; Omer, M. Determination of thermoluminescence kinetic parameters of La2O3 doped with Dy3+ and Eu3+. Materials 2020, 13, 1047. [Google Scholar] [CrossRef]
- Yazan, H.; Portakal-Uçar, Z.; Akça, S.; Topaksu, M.; Townsend, P.; Can, N. Thermoluminescence of Ce and Nd co-doped CaF2 phosphors after beta irradiation. J. Lumin. 2021, 234, 117949. [Google Scholar]
- Nawi, S.N.M.; Khandaker, M.U.; Bradley, D.; Sani, S.A.; Al-mugren, K. Characterization of a promising luminescence-based graphite radiation dosimeter. Radiat. Phys. Chem. 2021, 188, 109663. [Google Scholar]
- Yang, L.; Gai, S.; Ding, H.; Yang, D.; Feng, L.; Yang, P. Recent progress in inorganic afterglow materials: Mechanisms, persistent luminescent properties, modulating methods, and bioimaging applications. Adv. Opt. Mater. 2023, 11, 2202382. [Google Scholar] [CrossRef]
- Gaggero, E.; Calza, P.; Cerrato, E.; Paganini, M.C. Cerium-, europium-and erbium-modified ZnO and ZrO2 for photocatalytic water treatment applications: A review. Catalysts 2021, 11, 1520. [Google Scholar] [CrossRef]
- Dogan, T.; Tormo, L.; Akca, S.; Kucuk, N.; Guinea, J.G.; Karabulut, Y.; Ayvacikli, M.; Oglakci, M.; Topaksu, M.; Can, N. Cathodoluminescence and thermoluminescence of ZnB2O4: Eu3+ phosphors prepared via wet-chemical synthesis. Ceram. Int. 2019, 45, 4918–4925. [Google Scholar] [CrossRef]
- Dejene, F.B.; Etefa, H.F. Thermoluminescence (TL) properties of Eu3+ incorporated with CaB4O7 phosphors prepared by solution-combustion process. Appl. Radiat. Isot. 2024, 214, 111522. [Google Scholar] [CrossRef]
- Correcher Delgado, V.; Benavente Cuevas, J.F. Thermoluminescence glow curve analysis and kinetic parameters of Eu doped Li2MoO4 ceramic phosphors. Ceram. Int. 2023, 48, 19258–19265. [Google Scholar]
- Horowitz, Y.; Oster, L.; Eliyahu, I. The saga of the thermoluminescence (TL) mechanisms and dosimetric characteristics of LiF: Mg, Ti (TLD-100). J. Lumin. 2019, 214, 116527. [Google Scholar] [CrossRef]
- Halefoglu, Y.; Oglakci, M.; Portakal, Z.; Akca, S.; Souadi, G.; Canimoglu, A.; Topaksu, M.; Can, N. A study on thermoluminescence behaviour of Eu doped LaB3O6 irradiated with beta particles. Radiat. Phys. Chem. 2020, 168, 108571. [Google Scholar] [CrossRef]
- Halefoglu, Y.Z.; Oglakci, M.; Yuksel, M.; Canimoglu, A.; Topaksu, M.; Can, N. Structural and spectroscopic properties of LaAlBO3 doped with Eu3+ ions. Appl. Radiat. Isot. 2019, 154, 108876. [Google Scholar]
- Lakshmanan, A. Photoluminescence and thermostimulated luminescence processes in rare-earth-doped CaSO4 phosphors. Prog. Mater. Sci. 1999, 44, 1–187. [Google Scholar] [CrossRef]
- Silva, A.M.; Rodrigues, D.S.; Guedes, B.D.; Silveira, I.S.; Antonio, P.L.; Junot, D.O.; Caldas, L.V.; Souza, D.N. Exploring the luminescence properties and dosimetric characteristics of CaSO4: Tb, CaSO4: Mn, and CaSO4: Mn, Tb phosphors synthesized by slow evaporation route. Radiat. Meas. 2024, 177, 107261. [Google Scholar] [CrossRef]
- Ma, Y.; Linghu, C.; Yang, M.; Yu, H.; Dong, X.; Xia, L. A porous Eu-PTA/Tb-SSA/ZrO2/ZnZrO3 light color adjustable phosphor: Luminescent property and application of warm white LED. J. Rare Earths 2024, 42, 1421–1428. [Google Scholar] [CrossRef]
- Sherly, E.; Vijaya, J.J.; Selvam, N.C.S.; Kennedy, L.J. Microwave assisted combustion synthesis of coupled ZnO–ZrO2 nanoparticles and their role in the photocatalytic degradation of 2, 4-dichlorophenol. Ceram. Int. 2014, 40, 5681–5691. [Google Scholar]
- Ibrahim, M. Preparation and characterization of mesoporous ZnO-ZrO2 doped by Cr, Nd and Dy as a catalyst for conversion of coumarin using ultrasensitive fluorometric method. Solid State Sci. 2017, 66, 23–30. [Google Scholar] [CrossRef]
- Dodson, J.J.; Neal, L.M.; Hagelin-Weaver, H.E. The influence of ZnO, CeO2 and ZrO2 on nanoparticle-oxide-supported palladium oxide catalysts for the oxidative coupling of 4-methylpyridine. J. Mol. Catal. A Chem. 2011, 341, 42–50. [Google Scholar]
- Fuku, X.; Matinise, N.; Masikini, M.; Kasinathan, K.; Maaza, M. An electrochemically active green synthesized polycrystalline NiO/MgO catalyst: Use in photo-catalytic applications. Mater. Res. Bull. 2018, 97, 457–465. [Google Scholar]
- Kennedy, J.; Maaza, M.; Kaviyarasu, K.; Manikandan, E. Synthesis and analytical applications of photoluminescent carbon nanosheet by exfoliation of graphite oxide without purification. J. Mater. Sci. Mater. Electron. 2016, 27, 13080–13085. [Google Scholar]
- Musembi, M.; Dejene, F. Investigation of the effect of precursor ratios on the solution combustion synthesis of zinc zirconate nanocomposite. Heliyon 2019, 5, e03028. [Google Scholar]
- Musembi, M.K.; Dejene, F.B.; Ahemen, I. Structural and optical characteristics of dysprosium-doped zinc zirconate nanocomposites. OSA Contin. 2020, 3, 2332–2346. [Google Scholar] [CrossRef]
- Siyalo, S.; Etefa, H.F.; Dejene, F.B. Enhancing Structural and Optical Properties of CuO Thin Films through Gallium Doping: A Pathway to Enhanced Photoluminescence and Predict for Solar Cells Applications. Chem. Phys. Impact 2025, 10, 100832. [Google Scholar]
- Lawless, J.; Chen, R.; Lo, D.; Pagonis, V. A model for non-monotonic dose dependence of thermoluminescence (TL). J. Phys. Condens. Matter 2005, 17, 737. [Google Scholar] [CrossRef]
- Chen, R. On the calculation of activation energies and frequency factors from glow curves. J. Appl. Phys. 1969, 40, 570–585. [Google Scholar] [CrossRef]
- Dejene, F. Thermoluminescence study of beta irradiated Sr2CeO4: Eu3+ phosphor synthesized using solution-combustion process. J. Lumin. 2018, 199, 433–441. [Google Scholar] [CrossRef]
- Sankara, R.; Rao, G.S. Eu3+ Luminescence, Ce4+ → Eu3+ Energy Transfer, and White-Red Light Generation in Sr2CeO4. J. Electrochem. Soc. 2000, 147, 2773. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Etefa, H.F.; Dejene, F.B. Structural, Morphological and Thermoluminescence Properties of Mn-Doped Zinc Zirconate (ZnZrO3) Phosphors. Appl. Sci. 2025, 15, 3761. https://doi.org/10.3390/app15073761
Etefa HF, Dejene FB. Structural, Morphological and Thermoluminescence Properties of Mn-Doped Zinc Zirconate (ZnZrO3) Phosphors. Applied Sciences. 2025; 15(7):3761. https://doi.org/10.3390/app15073761
Chicago/Turabian StyleEtefa, Habtamu F., and Francis B. Dejene. 2025. "Structural, Morphological and Thermoluminescence Properties of Mn-Doped Zinc Zirconate (ZnZrO3) Phosphors" Applied Sciences 15, no. 7: 3761. https://doi.org/10.3390/app15073761
APA StyleEtefa, H. F., & Dejene, F. B. (2025). Structural, Morphological and Thermoluminescence Properties of Mn-Doped Zinc Zirconate (ZnZrO3) Phosphors. Applied Sciences, 15(7), 3761. https://doi.org/10.3390/app15073761





