Self-Esteem Differentiates the Dietary Behaviours and Adipose Tissue Distribution in Women with Menstrual Bleeding Disorders—Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
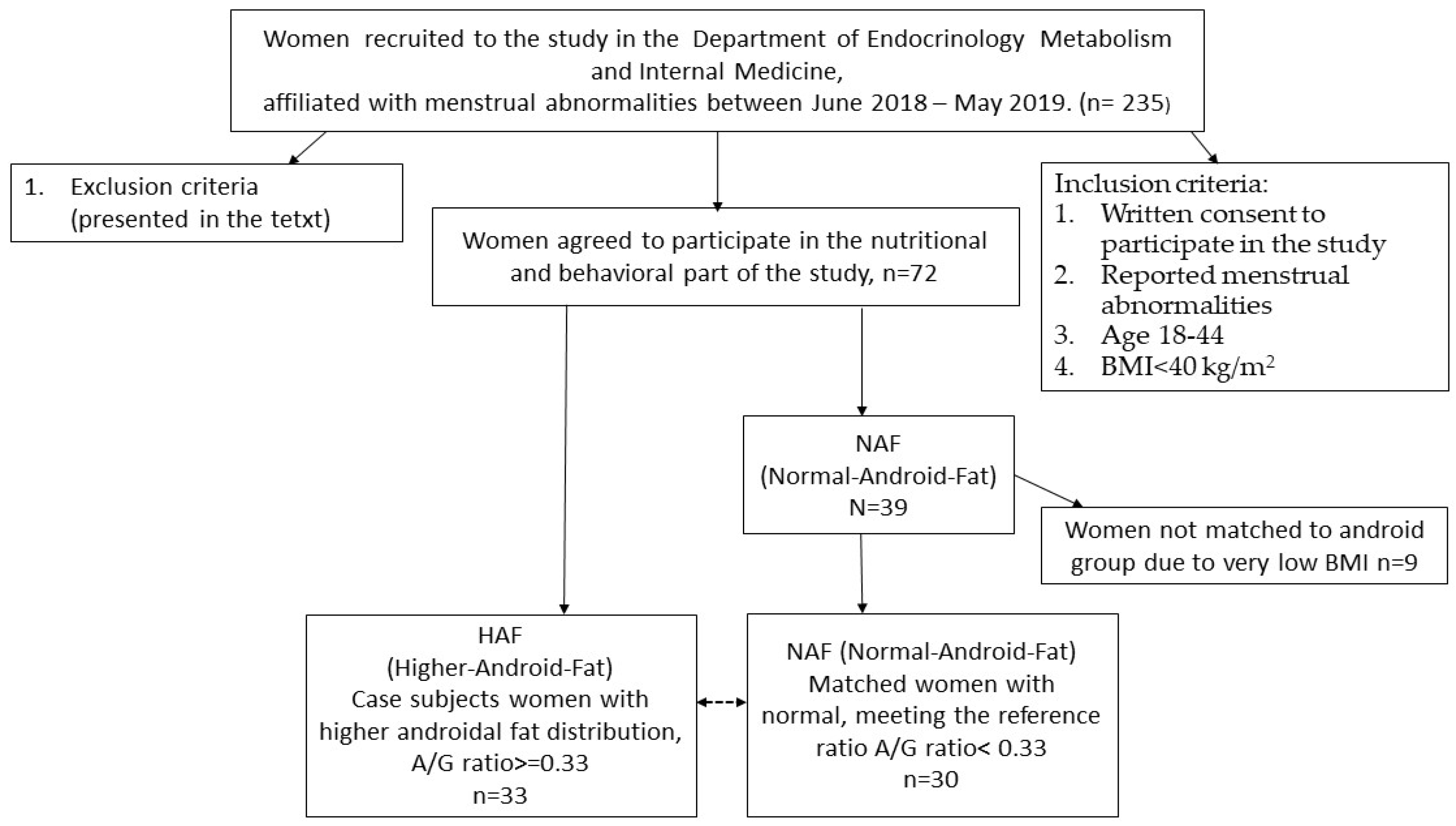
2.2. Participants
2.3. The Self-Esteem
2.4. Dietary Behaviours
2.5. Body Composition, Fat Distribution and Anthropometrics
2.6. Statistics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Govorov, I.; Ekelund, L.; Chaireti, R.; Elfvinge, P.; Holmström, M.; Bremme, K.; Mints, M. Heavy Menstrual Bleeding and Health-Associated Quality of Life in Women with von Willebrand’s Disease. Exp. Ther. Med. 2016, 11, 1923–1929. [Google Scholar] [CrossRef] [PubMed]
- Shimamoto, K.; Hirano, M.; Wada-Hiraike, O.; Goto, R.; Osuga, Y. Examining the Association between Menstrual Symptoms and Health-Related Quality of Life among Working Women in Japan Using the EQ-5D. BMC Womens Health 2021, 21, 325. [Google Scholar] [CrossRef]
- Weyand, A.C.; Fitzgerald, K.D.; McGrath, M.; Gupta, V.; Braun, T.M.; Quint, E.H.; Choi, S.W. Depression in Female Adolescents with Heavy Menstrual Bleeding. J. Pediatr. 2022, 240, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Friberg, B.; Kristin Örnö, A.; Lindgren, A.; Lethagen, S. Bleeding Disorders among Young Women: A Population-Based Prevalence Study. Acta Obstet. Gynecol. Scand. 2006, 85, 200–206. [Google Scholar] [CrossRef]
- Skierska, E.; Leszczyńska-bystrzanowska, J.; Gajewski, A.K.; Występowania, A.R.; Wielkomiejskiej, Z.P. Risk Analysis of Menstrual Disorders in Young Women from Urban Population. Przegląd Epidemilogiczny 1996, 50, 467. [Google Scholar]
- Comishen, K.J.; Bhatt, M.; Yeung, K.; Irfan, J.; Zia, A.; Sidonio, R.F.; James, P. Etiology and Diagnosis of Heavy Menstrual Bleeding among Adolescent and Adult Patients: A Systematic Review and Meta-Analysis of the Literature. J. Thromb. Haemost. 2024, 23, 863–876. [Google Scholar] [CrossRef]
- Fielder, S.; Nickkho-Amiry, M.; Seif, M.W. Obesity and Menstrual Disorders. Best. Pract. Res. Clin. Obstet. Gynaecol. 2023, 89, 102343. [Google Scholar] [CrossRef]
- Sinharoy, S.S.; Chery, L.; Patrick, M.; Conrad, A.; Ramaswamy, A.; Stephen, A.; Chipungu, J.; Reddy, Y.M.; Doma, R.; Pasricha, S.R.; et al. Prevalence of Heavy Menstrual Bleeding and Associations with Physical Health and Wellbeing in Low-Income and Middle-Income Countries: A Multinational Cross-Sectional Study. Lancet Glob. Health 2023, 11, e1775–e1784. [Google Scholar] [CrossRef]
- Lethaby, A.; Irvine, G.; Cameron, I. Cyclical Progestogens for Heavy Menstrual Bleeding. Cochrane Database Syst. Rev. 2008, 8, CD001016. [Google Scholar] [CrossRef]
- Bykowska-Derda, A.; Kolay, E.; Kaluzna, M.; Czlapka-Matyasik, M. Emerging Trends in Research on Food Compounds and Women’s Fertility: A Systematic Review. Appl. Sci. 2020, 10, 4518. [Google Scholar] [CrossRef]
- Dutkowska, A.; Konieczna, A.; Breska-Kruszewska, J.; Sendrakowska, M.; Kowalska, I.; Rachoń, D. Recomendations on Non-Pharmacological Interventions in Women with PCOS to Reduce Body Weight and Improve Metabolic Disorders. Endokrynol. Pol. 2019, 70, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Bykowska-Derda, A.; Czlapka-Matyasik, M.; Kaluzna, M.; Ruchala, M.; Ziemnicka, K. Diet Quality Scores in Relation to Fatness and Nutritional Knowledge in Women with Polycystic Ovary Syndrome: Case–Control Study. Public Health Nutr. 2020, 24, 3389–3398. [Google Scholar] [CrossRef] [PubMed]
- Bebelska, K.P.; Ehmke vel Emczyńska, E.; Gmoch-Gajzlerska, E. Otyłość Jako Czynnik Zaburzający Procesy Rozrodcze. Now. Lek. 2011, 80, 499–507. [Google Scholar]
- Itriyeva, K. The Effects of Obesity on the Menstrual Cycle. Curr. Probl. Pediatr. Adolesc. Health Care 2022, 52, 101241. [Google Scholar] [CrossRef]
- Ahmed, G.S.; Lotfy, A.M.M. Dietary Pattern and Menstrual Disorders among Female University Students. Int. J. Adolesc. Med. Health 2024, 36, 497–504. [Google Scholar] [CrossRef]
- Fujiwara, T.; Sato, N.; Awaji, H.; Nakata, R. Adverse Effects of Dietary Habits on Menstrual Disorders in Young Women. Open Food Sci. J. 2007, 1, 24–30. [Google Scholar] [CrossRef]
- Bazarganipour, F.; Ziaei, S.; Montazeri, A.; Foroozanfard, F.; Kazemnejad, A.; Faghihzadeh, S. Body Image Satisfaction and Self-Esteem Status among the Patients with Polycystic Ovary Syndrome. Iran. J. Reprod. Med. 2013, 11, 829. [Google Scholar]
- Korkmaz, N.; Çetin, S. Investigation of Self-Esteem and Sexual Function Levels of Patients Who Diagnosed with Polycystic Ovary Syndrome: A Prospective Study. J. Med. Palliat. Care 2022, 3, 169–174. [Google Scholar] [CrossRef]
- Zachurzok, A.; Pasztak-Opilka, A.; Gawlik, A.M. Depression, Anxiety and Self-Esteem in Adolescent Girls with Polycystic Ovary Syndrome. Ginekol. Pol. 2021, 92, 399–405. [Google Scholar] [CrossRef]
- Drosdzol, A.; Skrzypulec, V.; Plinta, R. Quality of Life, Mental Health and Self-Esteem in Hirsute Adolescent Females. J. Psychosom. Obstet. Gynecol. 2010, 31, 168–175. [Google Scholar] [CrossRef]
- Supriya, R.; Tam, B.T.; Yu, A.P.; Lee, P.H.; Lai, C.W.; Cheng, K.K.; Yau, S.Y.; Chan, L.W.; Yung, B.Y.; Sheridan, S.; et al. Adipokines Demonstrate the Interacting Influence of Central Obesity with Other Cardiometabolic Risk Factors of Metabolic Syndrome in Hong Kong Chinese Adults. PLoS ONE 2018, 13, e0201585. [Google Scholar] [CrossRef] [PubMed]
- Łagowska, K.; Kazmierczak, D.; Szymczak, K. Comparison of Anthropometrical Parameters and Dietary Habits of Young Women with and without Menstrual Disorders. Nutr. Diet. 2018, 75, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, K.R.; Blue, M.N.M.; Trexler, E.T.; Smith-Ryan, A.E. Visceral Adipose Tissue Normative Values in Adults from the United States Using GE Lunar IDXA. Clin. Physiol. Funct. Imaging 2019, 39, 407–414. [Google Scholar] [CrossRef]
- Rogowicz-Frontczak, A.; Majchrzak, A.; Zozuliska-Ziolkiewicz, D. Insulin Resistance in Endocrine Disorders-Treatment Options. Endokrynol. Pol. 2017, 68, 334–350. [Google Scholar] [CrossRef]
- Wei, S.; Schmidt, M.D.; Dwyer, T.; Norman, R.J.; Venn, A.J. Obesity and Menstrual Irregularity: Associations with SHBG, Testosterone, and Insulin. Obesity 2009, 17, 1070–1076. [Google Scholar] [CrossRef]
- West, S.; Lashen, H.; Bloigu, A.; Franks, S.; Puukka, K.; Ruokonen, A.; Järvelin, M.R.; Tapanainen, J.S.; Morin-Papunen, L. Irregular Menstruation and Hyperandrogenaemia in Adolescence Are Associated with Polycystic Ovary Syndrome and Infertility in Later Life: Northern Finland Birth Cohort 1986 Study. Hum. Reprod. 2014, 29, 2339–2351. [Google Scholar] [CrossRef]
- Zheng, L.; Yang, L.; Guo, Z.; Yao, N.; Zhang, S.; Pu, P. Obesity and Its Impact on Female Reproductive Health: Unraveling the Connections. Front. Endocrinol. 2024, 14, 1326546. [Google Scholar] [CrossRef]
- Seif, M.W.; Diamond, K.; Nickkho-Amiry, M. Obesity and Menstrual Disorders. Best. Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 516–527. [Google Scholar] [CrossRef]
- Chammas, N.; Brytek-Matera, A.; Tornquist, D.; Barreto Schuch, F.; Bitar, Z.; Malaeb, D.; Fawaz, M.; Fekih-Romdhane, F.; Hallit, S.; Obeid, S.; et al. Profiles of Intuitive Eating in Adults: The Role of Self-Esteem, Interoceptive Awareness, and Motivation for Healthy Eating. BMC Psychiatry 2024, 24, 288. [Google Scholar] [CrossRef]
- Kapoor, A.; Upadhyay, M.K.; Saini, N.K. Relationship of Eating Behavior and Self-Esteem with Body Image Perception and Other Factors among Female College Students of University of Delhi. J. Educ. Health Promot. 2022, 11, 80. [Google Scholar] [CrossRef]
- Ilić, A.; Rumbak, I.; Dizdarić, D.; Matek Sarić, M.; Colić Barić, I.; Guiné, R.P.F. Motivations Associated with Food Choices among Adults from Urban Setting. Foods 2023, 12, 3546. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Chen, T.; Li, Z.; Yu, Z.; Liu, X.; Li, J.; Guo, Y.; Xu, D.; Wang, X. Association between Dietary Related Factors and Central Obesity among Married Women: China Health and Nutrition Survey. Appetite 2022, 168, 105785. [Google Scholar] [CrossRef] [PubMed]
- Crosignani, P.G.; Colombo, M.; Vegetti, W.; Somigliana, E.; Gessati, A.; Ragni, G. Overweight and Obese Anovulatory Patients with Polycystic Ovaries: Parallel Improvements in Anthropometric Indices, Ovarian Physiology and Fertility Rate Induced by Diet. Human Reprod. 2003, 18, 1928–1932. [Google Scholar] [CrossRef] [PubMed]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. Dietary Fatty Acid Intakes and the Risk of Ovulatory Infertility. Am. J. Clin. Nutr. 2007, 85, 231–237. [Google Scholar] [CrossRef]
- Shishehgar, F.; Ramezani Tehrani, F.; Mirmiran, P.; Hajian, S.; Baghestani, A.R.; Moslehi, N. Comparison of Dietary Intake between Polycystic Ovary Syndrome Women and Controls. Glob. J. Health Sci. 2016, 8, 302. [Google Scholar] [CrossRef]
- Löffler, A.; Luck, T.; Then, F.S.; Sikorski, C.; Kovacs, P.; Böttcher, Y.; Breitfeld, J.; Tönjes, A.; Horstmann, A.; Löffler, M.; et al. Eating Behaviour in the General Population: An Analysis of the Factor Structure of the German Version of the Three-Factor-Eating-Questionnaire (TFEQ) and Its Association with the Body Mass Index. PLoS ONE 2015, 10, e0133977. [Google Scholar] [CrossRef]
- Polivy, J.; Heatherton, T.F.; Herman, C.P. Self-Esteem, Restraint, and Eating Behavior. J. Abnorm. Psychol. 1988, 97, 354–356. [Google Scholar]
- Obara-Gołębiowska, M. Forum Medycyny Rodzinnej: Czasopismo Polskiego Towarzystwa Medycyny Rodzinnej. Forum Med. Rodz. 2007, 9, 106–108. [Google Scholar]
- Mallaram, G.K.; Sharma, P.; Kattula, D.; Singh, S.; Pavuluru, P. Body Image Perception, Eating Disorder Behavior, Self-Esteem and Quality of Life: A Cross-Sectional Study among Female Medical Students. J. Eat. Disord. 2023, 11, 225. [Google Scholar] [CrossRef]
- Fraser, I.S.; Critchley, H.O.D.; Broder, M.; Munro, M.G. The FIGO Recommendations on Terminologies and Definitions for Normal and Abnormal Uterine Bleeding. Semin. Reprod. Med. 2011, 29, 383–390. [Google Scholar] [CrossRef]
- Mihajlovic, J.; Leutner, M.; Hausmann, B.; Kohl, G.; Schwarz, J.; Röver, H.; Stimakovits, N.; Wolf, P.; Maruszczak, K.; Bastian, M.; et al. Combined Hormonal Contraceptives Are Associated with Minor Changes in Composition and Diversity in Gut Microbiota of Healthy Women. Environ. Microbiol. 2021, 23, 3037–3047. [Google Scholar] [CrossRef] [PubMed]
- Lopez, L.M.; Ramesh, S.; Chen, M.; Edelman, A.; Otterness, C.; Trussell, J.; Helmerhorst, F.M. Progestin-Only Contraceptives: Effects on Weight. Cochrane Database Syst. Rev. 2016, 2016, CD008815. [Google Scholar] [CrossRef] [PubMed]
- Sitruk-Ware, R.; Nath, A. Characteristics and Metabolic Effects of Estrogen and Progestins Contained in Oral Contraceptive Pills. Best. Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Imboden, M.T.; Welch, W.A.; Swartz, A.M.; Montoye, A.H.K.; Finch, H.W.; Harber, M.P.; Kaminsky, L.A. Reference Standards for Body Fat Measures Using GE Dual Energy X-Ray Absorptiometry in Caucasian Adults. PLoS ONE 2017, 12, e0175110. [Google Scholar] [CrossRef]
- Deb, S.; Austin, P.C.; Tu, J.V.; Ko, D.T.; Mazer, C.D.; Kiss, A.; Fremes, S.E. A Review of Propensity-Score Methods and Their Use in Cardiovascular Research. Can. J. Cardiol. 2016, 32, 259–265. [Google Scholar] [CrossRef]
- Austin, P.C.; Stuart, E.A. The Performance of Inverse Probability of Treatment Weighting and Full Matching on the Propensity Score in the Presence of Model Misspecification When Estimating the Effect of Treatment on Survival Outcomes. Stat. Methods Med. Res. 2017, 26, 1654–1670. [Google Scholar] [CrossRef]
- Dzwonkowska, I.; Lachowicz-Tabaczek, K.; Łaguna, M. Samoocena-i-Jej-Pomiar-SES. Polska Adaptacja Skali SES M. Rosenberga. Psychol. Społeczna 2008, 2, 164–176. [Google Scholar]
- Rosenberg, M. Society and the Adolescent Self-Image; Princeton University Press: Princeton, NJ, USA, 1965; 326p. [Google Scholar]
- Kowalkowska, J.; Wadolowska, L.; Czarnocinska, J.; Czlapka-Matyasik, M.; Galinski, G.; Jezewska-Zychowicz, M.; Bronkowska, M.; Dlugosz, A.; Loboda, D.; Wyka, J. Reproducibility of a Questionnaire for Dietary Habits, Lifestyle and Nutrition Knowledge Assessment (KomPAN) in Polish Adolescents and Adults. Nutrients 2018, 10, 1845. [Google Scholar] [CrossRef]
- Garbacz, A.; Stelcer, B.; Wielgosik, M.; Czlapka-Matyasik, M. Assessment of Sugar-Related Dietary Patterns to Personality Traits and Cognitive–Behavioural and Emotional Functioning in Working-Age Women. Appl. Sci. 2024, 14, 3176. [Google Scholar] [CrossRef]
- Czlapka-Matyasik, M.; Gut, P. A Preliminary Study Investigating the Effects of Elevated Antioxidant Capacity of Daily Snacks on the Body’s Antioxidant Defences in Patients with CVD. Appl. Sci. 2023, 13, 5863. [Google Scholar] [CrossRef]
- Czlapka-Matyasik, M.; Ast, K. Total Antioxidant Capacity and Its Dietary Sources and Seasonal Variability in Diets of Women with Different Physical Activity Levels. Pol. J. Food Nutr. Sci. 2014, 64, 267–276. [Google Scholar] [CrossRef]
- Stults-Kolehmainen, M.A.; Stanforth, P.R.; Bartholomew, J.B.; Lu, T.; Abolt, C.J.; Sinha, R. DXA Estimates of Fat in Abdominal, Trunk and Hip Regions Varies by Ethnicity in Men. Nutr. Diabetes 2013, 3, e64. [Google Scholar] [CrossRef] [PubMed]
- Imboden, M.T.; Swartz, A.M.; Finch, H.W.; Harber, M.P.; Kaminsky, L.A. Reference Standards for Lean Mass Measures Using GE Dual Energy X-Ray Absorptiometry in Caucasian Adults. PLoS ONE 2017, 12, e0176161. [Google Scholar] [CrossRef]
- World Health Organisation (WHO). Waist Circumference and Waist–Hip Ratio: Report of a WHO Expert Consultation; WHO: Geneva, Switzerland, 2008; pp. 8–11. [Google Scholar]
- Nieśpiałowski, A.; Terelak, J. Obesity-Related Self-Esteem and Its Relationship To Coping with Stress. Pol. J. Aviat. Med. Bioeng. Psychol. 2017, 22, 18–26. [Google Scholar] [CrossRef]
- Pinto, V.R.A.; Campos, R.F.d.A.; Rocha, F.; Emmendoerfer, M.L.; Vidigal, M.C.T.R.; da Rocha, S.J.S.S.; Della Lucia, S.M.; Cabral, L.F.M.; de Carvalho, A.F.; Perrone, Í.T. Perceived Healthiness of Foods: A Systematic Review of Qualitative Studies. Future Foods 2021, 4, 100056. [Google Scholar] [CrossRef]
- Collins, N.; Lalor, F. Consumer Behaviour towards Milk and Dairy Yoghurt Products Carrying Nutrition and Health Claims: A Qualitative Study. Nutr. Food Sci. 2024, 54, 56–70. [Google Scholar] [CrossRef]
- Jakubowska, D.; Abrowska, A.Z.; Staniewska, K.; Kiełczewska, K.; Przybyłowicz, K.E.; Zulewska, J.; Jakubowska, D.; Zofia, A.; Abrowska, D.; Staniewska, K.; et al. Health Benefits of Dairy Products’ Consumption—Consumer Point of View. Foods 2024, 13, 3925. [Google Scholar] [CrossRef]
- Giacone, L.; Siegrist, M.; Stadelmann, A.; Hartmann, C. Consumers’ Perceptions of Healthiness and Environmental Friendliness of Plant-Based and Dairy Product Concepts. Food Humanit. 2024, 2, 100288. [Google Scholar] [CrossRef]
- Farvid, M.S.; Malekshah, A.F.; Pourshams, A.; Poustchi, H.; Sepanlou, S.G.; Sharafkhah, M.; Khoshnia, M.; Farvid, M.; Abnet, C.C.; Kamangar, F.; et al. Dairy Food Intake and All-Cause, Cardiovascular Disease, and Cancer Mortality: The Golestan Cohort Study. Am. J. Epidemiol. 2017, 185, 697–711. [Google Scholar] [CrossRef]
- Wadolowska, L.; Ulewicz, N.; Sobas, K.; Wuenstel, J.W.; Slowinska, M.A.; Niedzwiedzka, E.; Czlapka-Matyasik, M. Dairy-Related Dietary Patterns, Dietary Calcium, Body Weight and Composition: A Study of Obesity in Polish Mothers and Daughters, the MODAF Project. Nutrients 2018, 10, 90. [Google Scholar] [CrossRef]
- Sobas, K.; Wadolowska, L.; Slowinska, M.A.; Czlapka-Matyasik, M.; Wuenstel, J.; Niedzwiedzka, E. Like Mother, Like Daughter? Dietary and Non-Dietary Bone Fracture Risk Factors in Mothers and Their Daughters. Iran. J. Public Health 2015, 44, 939–952. [Google Scholar] [PubMed]
- Aparicio, A.; Rodríguez-Rodríguez, E.; Lorenzo-Mora, A.M.; Sánchez-Rodríguez, P.; Ortega, R.M.; López-Sobaler, A.M. Myths and Fallacies in Relation to the Consumption of Dairy Products. Nutr. Hosp. 2019, 36, 20–24. [Google Scholar] [CrossRef]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.; Willett, W.C. A Prospective Study of Dairy Foods Intake and Anovulatory Infertility. Human Reprod. 2007, 22, 1340–1347. [Google Scholar] [CrossRef]
- Kim, K.; Wactawski-Wende, J.; Michels, K.A.; Plowden, T.C.; Chaljub, E.N.; Sjaarda, L.A.; Mumford, S.L. Dairy Food Intake Is Associated with Reproductive Hormones and Sporadic Anovulation among Healthy Premenopausal Women. J. Nutr. 2017, 147, 218–226. [Google Scholar] [CrossRef]
- Albert, K.; Pruessner, J.; Newhouse, P. Estradiol Levels Modulate Brain Activity and Negative Responses to Psychosocial Stress across the Menstrual Cycle. Psychoneuroendocrinology 2015, 59, 14–24. [Google Scholar] [CrossRef]
- Blouin, K.; Boivin, A.; Tchernof, A. Androgens and Body Fat Distribution. J. Steroid Biochem. Mol. Biol. 2008, 108, 272–280. [Google Scholar] [CrossRef]
- Goldbohm, R.A.; Chorus, A.M.J.; Garre, F.G.; Schouten, L.J.; Van Den Brandt, P.A. Dairy Consumption and 10-y Total and Cardiovascular Mortality: A Prospective Cohort Study in the Netherlands. Am. J. Clin. Nutr. 2011, 93, 615–627. [Google Scholar] [CrossRef]
- Wise, L.A.; Wesselink, A.K.; Mikkelsen, E.M.; Cueto, H.; Hahn, K.A.; Rothman, K.J.; Tucker, K.L.; Sorensen, H.T.; Hatch, E.E. Dairy Intake and Fecundability in 2 Preconception Cohort Studies. Am. J. Clin. Nutr. 2017, 105, 100–110. [Google Scholar] [CrossRef]
- Carwile, J.L.; Willett, W.C.; Michels, K.B. Consumption of Low-Fat Dairy Products May Delay Natural Menopause. J. Nutr. 2013, 143, 1642–1650. [Google Scholar] [CrossRef]
- Wirt, A.; Collins, C.E. Diet Quality—What Is It and Does It Matter? Public Health Nutr. 2009, 12, 2473–2492. [Google Scholar] [CrossRef]
- Specjalski, R. Psychosexual Disorders in Women with Polycystic Ovary Syndrome. Pielęgniarstwo Pol. 2013, 3, 230–234. [Google Scholar]
- Kitzinger, C.; Willmott, J. “The Thief of Womanhood”: Women’s Experience of Polycystic Ovarian Syndrome. Soc. Sci. Med. 2002, 54, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Daneshzad, E.; Askari, M.; Moradi, M.; Ghorabi, S.; Rouzitalab, T.; Heshmati, J.; Azadbakht, L. Red Meat, Overweight and Obesity: A Systematic Review and Meta-Analysis of Observational Studies. Clin. Nutr. ESPEN 2021, 45, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Rasmussen, S.I.; Hjorth, M.F.; Asping, S.; Rosenkrans, M.I.; Sjödin, A.M.; Astrup, A.V.; Geiker, N.R. Unprocessed Red Meat in the Dietary Treatment of Obesity: A Randomized Controlled Trial of Beef Supplementation during Weight Maintenance after Successful Weight Loss. Am. J. Clin. Nutr. 2022, 116, 1820–1830. [Google Scholar] [CrossRef]
- Mazidi, M.; Kengne, A.P.; George, E.S.; Siervo, M. The Association of Red Meat Intake with Inflammation and Circulating Intermediate Biomarkers of Type 2 Diabetes Is Mediated by Central Adiposity. Br. J. Nutr. 2021, 125, 1043–1050. [Google Scholar] [CrossRef]
- Gaesser, G.A. Perspective: Refined Grains and Health: Genuine Risk, or Guilt by Association? Adv. Nutr. 2019, 10, 361. [Google Scholar] [CrossRef]
- Gaesser, G.A. Refined Grain Intake and Cardiovascular Disease: Meta-Analyses of Prospective Cohort Studies. Trends Cardiovasc. Med. 2024, 34, 59–68. [Google Scholar] [CrossRef]
- Liu, S.; Willett, W.C.; Manson, J.A.E.; Hu, F.B.; Rosner, B.; Colditz, G. Relation between Changes in Intakes of Dietary Fiber and Grain Products and Changes in Weight and Development of Obesity among Middle-Aged Women. Am. J. Clin. Nutr. 2003, 78, 920–927. [Google Scholar] [CrossRef]
- Pinho, M.G.M.; Mackenbach, J.D.; Charreire, H.; Oppert, J.-M.; Bardos, H.; Glonti, K.; Rutter, H.; Compernolle, S.; De Bourdeaudhuij, I.; Beulens, J.W.J.; et al. Exploring the Relationship between Perceived Barriers to Healthy Eating and Dietary Behaviours in European Adults. Eur. J. Nutr. 2018, 57, 1761–1770. [Google Scholar] [CrossRef]
- Erata, M.C.; Eroğlu, S.; Özkul, B.; Uslu, Ö.; Erdoğan, Y.; Kitiş, Ö.; Gönül, A.S. The Reflection of Self-Esteem on the Brain Structure: A Voxel Based Morphometry Study in Healthy Young Adults. Arch. Neuropsychiatry 2023, 60, 202. [Google Scholar] [CrossRef]
- Veit, R.; Schag, K.; Schopf, E.; Borutta, M.; Kreutzer, J.; Ehlis, A.C.; Zipfel, S.; Giel, K.E.; Preissl, H.; Kullmann, S. Diminished Prefrontal Cortex Activation in Patients with Binge Eating Disorder Associates with Trait Impulsivity and Improves after Impulsivity-Focused Treatment Based on a Randomized Controlled IMPULS Trial. Neuroimage Clin. 2021, 30, 102679. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.A. Role of Brain Dopamine in Food Reward and Reinforcement. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1149. [Google Scholar] [CrossRef]
- Bakshi, A.; Tadi, P. Biochemistry, Serotonin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lewis, R.G.; Florio, E.; Punzo, D.; Borrelli, E. The Brain’s Reward System in Health and Disease. Adv. Exp. Med. Biol. 2021, 1344, 57. [Google Scholar] [CrossRef]
- Cay, M.; Ucar, C.; Senol, D.; Cevirgen, F.; Ozbag, D.; Altay, Z.; Yildiz, S. Effect of Increase in Cortisol Level Due to Stress in Healthy Young Individuals on Dynamic and Static Balance Scores. North. Clin. Istanb. 2018, 5, 295. [Google Scholar] [CrossRef]
- Epel, E.; Lapidus, R.; McEwen, B.; Brownell, K. Stress May Add Bite to Appetite in Women: A Laboratory Study of Stress-Induced Cortisol and Eating Behavior. Psychoneuroendocrinology 2001, 26, 37–49. [Google Scholar] [CrossRef]
- Skoracka, K.; Hryhorowicz, S.; Schulz, P.; Zawada, A.; Ratajczak-Pawłowska, A.E.; Rychter, A.M.; Słomski, R.; Dobrowolska, A.; Krela-Kaźmierczak, I. The Role of Leptin and Ghrelin in the Regulation of Appetite in Obesity. Peptides 2025, 186, 171367. [Google Scholar] [CrossRef]
- Segal, Y.; Gunturu, S. Psychological Issues Associated with Obesity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
| Characteristics | HAF (n = 33) | NAF (n = 30) | p |
|---|---|---|---|
| Age (years) | 31 ± 6 | 28 ± 7 | 0.06 |
| Body mass (kg) | 70 ± 16 | 65 ± 14 | 0.22 |
| Height (cm) | 166 ± 6 | 166 ± 8 | 0.91 |
| BMI (kg/m2) | 23.7 ± 5.0 | 25.2 ± 5.0 | 0.23 |
| Fat (%) | 41 ± 5 | 29 ± 7 | 0.00 * |
| A/G (-) | 0.46 ± 0.07 | 0.25 ± 0.05 | 0.00 * |
| Visceral adipose tissue VAT (g) | 474 ± 431 | 262 ± 322 | 0.03 * |
| WHR (-) | 0.81 ± 0.07 | 0.80 ± 0.06 | 0.49 |
| WHtR (-) | 0.49 ± 0.07 | 0.45 ± 0.07 | 0.05 |
| Waist circumference (cm) | 82 ± 13 | 75 ± 12 | 0.04 * |
| Hip circumference (cm) | 100 ± 11 | 94 ± 9 | 0.02 * |
| Total lean mass (kg) | 41.15 ± 5.70 | 39.90 ± 5.37 | 0.37 |
| Nutrition knowledge score (points) | 15.8 ± 5.7 | 14.6 ± 6.0 | 0.42 |
| Rosenberg Self-esteem (RSES) scale 2 | %(n) | %(n) | 0.00 * |
| Low (<27 points) | 18(6) | 43(13) | |
| Medium (27–32 points) | 49(16) | 40(12) | |
| High (>32 points) | 33(11) | 17(5) | |
| Mean value of RSES | 30.3 ± 4.0 | 27.2 ± 3.8 | 0.05 |
| Occurrence (%)/N | OR (95% CI 1) Crude HAF | OR (95%CI) Age-Adjusted HAF | |
|---|---|---|---|
| Healthy food intake (pHDI-10) | |||
| ≥7 a day | (30)/10 | 2.8 (0.8; 10.5) p = 0.11 | 2.5 (0.7; 9.7) p = 0.17 |
| ≥5 a day | (48)/16 | 0.8 (0.3; 2.3) p = 0.70 | 0.7 (0.2; 2.0) p = 0.46 |
| ≥3 a day | (88)/29 | 1.4 (0.3; 6.2) p = 0.60 | 0.9 (0.2; 4.2) p = 0.86 |
| Yoghurt and fermented drinks | |||
| ≥1 a day | (24)/8 | 1.6 (0.4; 5.7) p = 0.46 | 1.2 (0.3; 4.7) p = 0.76 |
| ≥Every other day | (58)/19 | 1.4 (0.5; 3.7) p = 0.55 | 1.2 (0.4; 3.4) p = 0.72 |
| Fresh cheese curd products | |||
| ≥every other day | (55)/18 | 3.3 (1.1; 9.7) p = 0.02 * | 2.6 (0.8; 8.2) p = 0.09 |
| ≥once a week | (76)/25 | 2.1 (0.7; 6.3) p = 0.18 | 1.6 (0.5; 5.2) p = 0.39 |
| Milk product intake | |||
| ≥1 a day | (51)/17 | 3.5 (1.2; 10.5) p = 0.02 * | 3.2 (1.0; 9.9) p = 0.04 * |
| ≥every other day | (73)/24 | 3.5 (1.2; 10.2) p = 0.02 * | 3.9 (1.3; 12.3) p = 0.01 * |
| Fish | |||
| ≥once a week | (48)/16 | 1.4 (0.5; 3.9) p = 0.49 | 1.4 (0.5; 3.9) p = 0.54 |
| White meat | |||
| ≥every other day | (73)/24 | 1.1 (0.4; 3.5) p = 0.81 | 0.9 (0.3; 3.0) p = 0.96 |
| ≥once a week | (88)/29 | 1.0 (0.2; 5.1) p = 0.88 | 0.8 (0.2; 4.2) p = 0.84 |
| Wholegrain bread | |||
| ≥1 a day | (18)/6 | 1.4 (0.4; 5.9) p = 0.60 | 1.3 (0.3; 5.7) p = 0.71 |
| ≥every other day | (61)/20 | 1.5 (0.6; 4.3) p = 0.39 | 1.3 (0.4; 3.7) p = 0.65 |
| Wholegrain pasta and rice | |||
| ≥every other day | (61)/20 | 1.02 (0.4; 2.9) p = 0.96 | 1.1 (0.4; 3.1) p = 0.60 |
| ≥once a week | (73)/24 | 0.7 (0.2; 2.2) p = 0.49 | 0.6 (0.2; 2.2) p = 0.46 |
| Fruits | |||
| at least twice a day | (18)/6 | 0.7 (0.2; 2.5) p = 0.61 | 0.7 (0.2; 2.7) p = 0.61 |
| ≥2 a day | (61)/20 | 1.2 (0.4; 3.3) p = 0.75 | 1.1 (0.4; 3.2) p = 0.83 |
| Vegetables | |||
| ≥2 a day | (48)/16 | 1.2 (0.4; 3.4) p = 0.68 | 1.2 (0.4; 3.5) p = 0.70 |
| ≥1 a day | (61)/20 | 0.8 (0.3; 2.2) p = 0.62 | 0.7 (0.2; 2.0) p = 0.49 |
| Legumes | |||
| ≥every other day | (12)/4 | 0.7 (0.2; 2.9) p = 0.61 | 0.7 (0.1; 3.0) p = 0.60 |
| Occurrence (%)/N | OR (95% CI 1) Crude HAF | OR (95%CI) Age-Adjusted HAF | |
|---|---|---|---|
| Unhealthy food Intake (nHDI14) | |||
| ≥3 a day | (61)/20 | 0.9 (0.3; 2.5) p = 0.82 | 0.8 (0.3; 2.4) p = 0.73 |
| ≥1 a day | (91)/30 | 0.7 (0.1; 4.8) p = 0.72 | 0.8 (0.1; 5.6) p = 0.83 |
| Cold cuts | |||
| ≥1 a day | (12)/4 | 0.4 (0.1; 1.5) p = 0.15 | 0.3 (0.1; 1.3) p = 0.10 |
| ≥every other day | (58)/19 | 1.4 (0.5; 3.7) p = 0.58 | |
| Red meat | |||
| ≥every other day or more | (21)/7 | 0.3 (0.1; 0.9) p = 0.03 * | 0.2 (0.0; 0.7) p = 0.01 * |
| ≥once a week | (45)/15 | 0.5 (0.2; 1.4) p = 0.15 | 0.4 (0.1; 1.2) p = 0.09 |
| White bread | |||
| ≥1 a day | (33)/11 | 1 (0.3; 2.9) p = 1 | 1.0 (0.3; 2.9) p = 0.95 |
| ≥every other day | (33)/11 | 1 (0.3; 2.9) p = 1 | 1.0 (0.3; 2.9) p = 0.95 |
| White grains | |||
| ≥every other day | (33)/11 | 0.4 (0.1; 1.1) p = 0.07 | 0.4 (0.1; 1.3) p = 0.13 |
| Hard cheese | |||
| ≥every other day | (48)/16 | 1.4 (0.5; 3.9) p = 0.49 | 1.7 (0.6; 4.9) p = 0.34 |
| Butter | |||
| ≥1 a day | (36)/12 | 1.0 (0.3; 2.8) p = 0.98 | 0.9 (0.3; 2.7) p = 0.88 |
| Sweets | |||
| ≥at least once a day | (21)/7 | 0.6 (0.2; 2.0) p = 0.42 | 0.7 (0.2; 2.5) p = 0.62 |
| ≥every other day | (54)/18 | 1.2 (0.4; 3.3) p = 0.72 | 1.5 (0.5; 4.6) p = 0.42 |
| Sweetened drinks | |||
| ≥once a week | (18)/6 | 0.5 (0.2; 1.7) p = 0.27 | 0.6 (0.2; 2.2) p = 0.45 |
| Fast food | |||
| ≥once a week | (9)/3 | 0.3 (0.1:1.4) p = 0.13 | 0.4 (0.1; 1.7) p = 0.19 |
| Fried food | |||
| ≥once a week | (64)/21 | 0.8 (0.3; 2.2) p = 0.59 | 1.1 (0.3; 3.6) p = 0.87 |
| ≥every other day | (42)/14 | 1.1 (0.4; 3.1) p = 0.84 | 1.3 (0.4; 3.9) p = 0.61 |
| ≥every day | (3)/1 | 0.4 (0.0; 5.3) p = 0.51 | 0.7 (0.0; 8.9) p = 0.76 |
| Alcohol ≥ once a week | (33)/11 | 1.6 (0.5; 5.1) p = 0.38 | 1.3 (0.4; 4.3) p = 0.61 |
| Cigarette smoking | (42)/14 | 2.4 (0.8; 7.37) p = 0.10 | 2.0 (0.6; 6.3) p = 0.23 |
| Self-reported physical activity in leisure time: | |||
| -High | (15)/5 | 0.5 (0.1; 1.8) p = 0.26 | 0.4 (0.1; 1.6) p = 0.19 |
| -Medium and high | (63)/21 | 0.5 (0.2; 1.6) p = 0.26 | 0.4 (0.1; 1.3) p = 0.12 |
| Self-reported physical activity at work: | |||
| -High | (12)/4 | 1.2 (0.2; 6.3) p = 0.79 | 1.5 (0.3; 8.2) p = 0.62 |
| -Medium and high | (36)/12 | 0.7 (0.3; 2.1) p = 0.57 | 0.8 (0.3; 2.4) p = 0.70 |
| Medium or high self-esteem | (82)/27 | 3.4 (1.0; 11.0) p = 0.03 * | 3.1 (1.0; 10.4) p = 0.05 * |
| Nutrition knowledge: | |||
| -Above upper tertile | (42)/14 | 1.3 (0.5; 4.0) p = 0.52 | 1.4 (0.5; 4.3) p = 0.51 |
| -Below lowest tertile | (30)/10 | 0.5 (0.2,1.6) p = 0.24 | 0.6 (0.2; 1.8) p = 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czlapka-Matyasik, M.; Bykowska-Derda, A.; Stelcer, B.; Nowicka, A.; Piasecka, A.; Kałużna, M.; Ruchała, M.; Ziemnicka, K. Self-Esteem Differentiates the Dietary Behaviours and Adipose Tissue Distribution in Women with Menstrual Bleeding Disorders—Pilot Study. Appl. Sci. 2025, 15, 3701. https://doi.org/10.3390/app15073701
Czlapka-Matyasik M, Bykowska-Derda A, Stelcer B, Nowicka A, Piasecka A, Kałużna M, Ruchała M, Ziemnicka K. Self-Esteem Differentiates the Dietary Behaviours and Adipose Tissue Distribution in Women with Menstrual Bleeding Disorders—Pilot Study. Applied Sciences. 2025; 15(7):3701. https://doi.org/10.3390/app15073701
Chicago/Turabian StyleCzlapka-Matyasik, Magdalena, Aleksandra Bykowska-Derda, Bogusław Stelcer, Aleksandra Nowicka, Aleksandra Piasecka, Małgorzata Kałużna, Marek Ruchała, and Katarzyna Ziemnicka. 2025. "Self-Esteem Differentiates the Dietary Behaviours and Adipose Tissue Distribution in Women with Menstrual Bleeding Disorders—Pilot Study" Applied Sciences 15, no. 7: 3701. https://doi.org/10.3390/app15073701
APA StyleCzlapka-Matyasik, M., Bykowska-Derda, A., Stelcer, B., Nowicka, A., Piasecka, A., Kałużna, M., Ruchała, M., & Ziemnicka, K. (2025). Self-Esteem Differentiates the Dietary Behaviours and Adipose Tissue Distribution in Women with Menstrual Bleeding Disorders—Pilot Study. Applied Sciences, 15(7), 3701. https://doi.org/10.3390/app15073701











