Abstract
Paint loss is one of the main forms of deterioration in historical wall paintings, generally restored by the application of chromatic reintegration. In the specific case of outdoor exposed paintings, it is essential to find a binder that will withstand diverse weather conditions. Since chromatic reintegrations have to be compatible with the original painting, fresco paint mock-ups were manufactured and compared to chromatic reintegrations made with an aqueous colloidal dispersion of silica nanoparticles as binder. The physical compatibility was studied by colour spectrophotometry and measurements of static contact angle, gloss, and roughness values, together with a peeling test, stereomicroscopy, and polarised light microscopy. They were also characterised from a mineralogical, chemical, and molecular point of view using X-ray diffraction, X-ray fluorescence and Fourier-transform infrared spectroscopy. The microtexture was studied by scanning electron microscopy with energy-dispersive X-ray spectroscopy. Chromatic reintegrations showed similar roughness and lower gloss values than frescoes, and the nano-silica binder ensured the natural breathability of the wall. Overall, the chemical nature of pigments was highly influential. The reintegrations with silicate-based pigments were more homogenous, with hardly any fissures, while those carried out with sulphide- or oxide-based pigments were severely cracked. The use of verdigris is discouraged due to the lack of affinity between the binder and the pigment.
1. Introduction
Historic wall paintings are highly appreciated not only for their unquestionable aesthetic value, but also because they provide insights into the art, history, architecture, and customs of the time [1]. As they are frequently outdoor-exposed, their conservation is highly dependent on their surrounding environment, among other factors. They can suffer alteration, decay, and even destruction due to the action of—and fluctuations in—various different environmental agents (solar irradiation, humidity, soluble salts, etc.) [2,3,4,5]. This often manifests itself in the detachment and consequent loss of the painted surface and the appearance of lacunae (meaning cavity or lack) [2,3,6]. When this happens, professional restorers are engaged to repair these lacunae in a process known as chromatic reintegration or inpainting [2,3]. The most widely reported inpainting techniques involve water-based (pigments generally dispersed in gum Arabic) and varnish-based (pigments dispersed in natural and synthetic resins) procedures [2,7]. However, they only appear to be effective in indoor settings where environmental conditions can be controlled [3,8]. Ropret et al. [9] evaluated different inpainting techniques, both natural and synthetic, and reported positive results in terms of colour stability with ammonium caseinate after accelerated ageing. However, cracks were observed after temperature and relative humidity fluctuations. Therefore, it is paramount to find an inpainting technique that can withstand the significant environmental fluctuations to which many wall paintings are partially or completely exposed [8]. This requires the use of a weather-resistant binder, which must also ensure the natural breathability of the wall, i.e., by creating a layer that allows the exchange of moisture between the wall and the environment. Despite this, little research has been conducted into chromatic reintegration in terms of the stability of the restored lacunae against environmental factors, their long-term durability and behaviour, and their visual compatibility with the original painting [8].
In this context, the use of silica-based paints has become more popular in recent decades [10,11,12], although little is known about their compatibility, durability, and reversibility when used in chromatic reintegrations [8]. Silica was first used as a binder in paintings at the end of the 19th century, in a technique known as ‘stereochrome painting’ (from the Greek stéreos, meaning solid, and cromía, meaning colour), also referred to as ‘mineral painting’ [12,13,14]. Soluble silicates (sodium and potassium) were first used by von Fuchs (1825) and later by Keim (1881), who improved the method for obtaining these paints and helped disseminate the ‘mineral painting’ technique [11]. From then on, technological advances in the manufacture of silica-based paints led to binders of this kind being used in the construction and artistic field [10,11,12,15]. They seem to be particularly suitable for outdoor settings due to their water-repellent properties and their high resistance to harsh weather conditions [5,16]. Various silicates that are widely used in cultural heritage restoration, such as sodium (Na) and potassium (K) silicates, appear to be unsuitable as binders for outdoor-exposed paintings. Na-silicates do not provide a water-resistant coating [15] and K-silicates have shown poor resistance to freeze–thaw cycles, leading to a loss of adhesion of the paint layer [16]. In this situation, nano-silicates, with their apparent chemical inertness and durability, could be considered a viable, environmentally friendly alternative.
Cultural heritage restoration work such as chromatic reintegration must always meet certain requirements. According to [17], these are recognisability (i.e., ensuring that the inpainted area is visually distinguishable from the original painting), reversibility (i.e., it can be removed without damaging the original paintwork) and compatibility. To meet this last requirement, bearing in mind that the choice of the binder can have a determining impact on colour and gloss [18], this study aims to compare the historical fresco wall painting technique with nano-size silica-based chromatic reintegrations. The fresco technique was selected as it is considered one of the earliest, with examples dating back to Ancient Greece in the 2nd Millennium BC [19]. In this technique, pigments are dispersed in water and applied onto a wet lime-based mortar, bonding to the surface via the reaction of calcium hydroxide (Ca(OH)2) with environmental carbon dioxide (CO2), forming calcium carbonate (CaCO3) [2,13]. Secondly, chromatic reintegrations must also be chemically and mineralogically compatible so as not to cause damage to the original paintwork. It is well known that some pigments are less stable and more prone to chemical modifications than others [20]. Therefore, as this will undoubtedly affect the long-term durability of the chromatic reintegrations, origin (natural or synthetic), nature (inorganic or organic), and mineralogical composition (silicates, oxides, etc.) of the pigments were considered in this study.
In all, the aim of this study was to assess the physical and chemical compatibility between a historical painting (fresco wall painting mock-up) and its nano-silica-based chromatic reintegration. Pigments with diverse mineralogical compositions were selected to compare the affinity of each pigment with the nano-silica binder: inorganic silicates (Egyptian blue, lapis lazuli, ultramarine blue, and green earth), oxides (mars red and chromium green) and sulphides (cinnabar and vermilion), and an organic acetate (verdigris). Bearing in mind that the materials used in the restoration of cultural heritage must ensure optimum performance [21], a preliminary physical, mineralogical, and chemical characterisation of the raw materials was carried out. We also analysed the particle size range and morphology of the pigment particles as this can influence the optical properties (colour, gloss and hiding power) [22]. To evaluate compatibility, the colour, gloss, roughness, and wettability of the paint layer were evaluated, as was the cohesion of the pigment particles within the paint layer and its adhesion to the surface of the mock-ups. We also used optical (stereomicroscopy and polarised light microscopy) and scanning electron microscopy modalities to evaluate the pigment–binder distribution and the degree of adhesion of the paint layer to the intonaco.
2. Materials and Methods
2.1. Materials
The materials used for the manufacture of the mock-ups are presented in Table 1. Specifications provided by the supplier are compared with those obtained from the preliminary characterisation of the materials conducted by the authors.

Table 1.
Raw materials used in the study. Data provided by the supplier as compared to the authors’ characterisation. Particle size was assessed by laser diffraction (LD), mineralogical composition by X-ray diffraction (XRD) and chemical composition by X-ray fluorescence (XRF).
In the preparation of the mortar, a calcitic lime paste, stored under water for more than 20 years, was used as the binder. This lime is composed mainly of portlandite (Ca(OH)2), and small amounts of calcite (CaCO3) due to partial carbonation during handling. The lime paste was mixed with three aggregates with different particle sizes: (i) coarse silica; (ii) fine silica; and (iii) Carrara marble powder. Coarse and fine silica aggregates, composed of quartz (SiO2), sodium/potassium feldspars, and rutile (TiO2), were supplied by a local hardware store (Granada, Spain). The marble powder, which had irregular particles in terms of size and shape, was composed of calcite, quartz, and dolomite (CaMg(CO3)2) and was supplied by CTS S.L. (Madrid, Spain).
The following pigments were selected for the study: Egyptian blue (EG-B), lapis lazuli (LA-B), ultramarine blue (UL-B), green earth (GE-G), mars red (MAR-R), chromium green (CHR-G), cinnabar (CIN-R), vermilion (VER-R), and verdigris (VER-G). They were all supplied by Kremer Pigments GmbH & Co. KG (Aichstetten, Germany). The origin and main characteristics of these pigments are shown in Table 1.
Chromatic reintegrations were carried out using Nano Estel® [23], also supplied by CTS S.L. (Madrid, Spain), as the binder (NE). This product consists of an aqueous colloidal dispersion of SiO2 nanoparticles (around 10–20 nm), with alkaline pH (9.8–10.4). It is a non-toxic and non-flammable product, which lowers the risk factors for the restorer and the artwork. The nano-size silica particles bind together forming a silica gel, similar to that obtained with ethyl-silicate consolidants [23]. The ambient temperature must be between 5 °C and 35 °C, as must be the temperature of the surface to which the product is applied.
2.2. Mock-Up Preparation
In order to simulate the traditional structure of historical wall paintings, we prepared mock-ups composed of two layers of mortar that set, forming a rigid material. The layers are generally referred to by their Italian names, arriccio (the inner layer) and intonaco (the outermost layer, where the paint is applied) [13,19]. The first layer or arriccio was thicker (around 2 cm) and was made with a 1:3 L:S (lime–sand) ratio in volume, where the lime was mixed with 2 parts of coarse silica and 1 part of fine silica. Four days later, the outer layer or intonaco was applied, thinner (around 1 cm), made with a 1:2 L:S ratio: the lime was mixed with 1 part of fine silica and 1 part of marble powder. As shown in Figure 1, each mock-up was then divided into two parts: the bottom part was painted a fresco (hereinafter F) (10 cm × 10 cm), while the top part was painted as a nano-silica-based chromatic reintegration (hereinafter SB) (10 cm × 5 cm). In the F mock-ups, pigments mixed with demineralised water were applied by paintbrush during the ‘golden hour’ (i.e., around 4 h after applying the intonaco), when the lime mortar still had a high degree of humidity [24]. Later, they were left to carbonate (harden) for two months under laboratory conditions (18 ± 5 °C and 50 ± 10% RH). In the SB mock-ups, as NE is a concentrated product with 30% dry residue, it was diluted with demineralised water in a 1:1 ratio, as recommended by the supplier [23]. The pigments were then dispersed in the resulting solution and the mixture was applied on the mortar surface after two months of carbonation. The paint was applied in line with the ‘neutral tone’ inpainting technique, known in Italian as reintegrato neutro, i.e., with the same degree of opacity as the fresco mock-ups. They were then left for two weeks under the same laboratory conditions. In total, nine 10 cm × 15 cm × 3 cm painted mock-ups were made (F and SB), as shown in Figure 1a–d. Two additional sets of unpainted mock-ups of 10 × 10 cm2 (Figure 1e) were also prepared: one without NE (FR-F) and the other with (FR-SB).
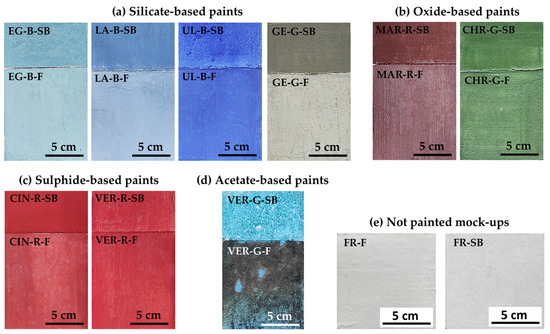
Figure 1.
Fresco (F) and nano-silica-based chromatic reintegration (SB) mock-ups painted with (a) silicate-, (b) oxide-, (c) sulphide-, and (d) acetate-based pigment. (e) Not painted mock-ups without NE (FR-F) and with NE (FR-SB). See Table 1 for an explanation of the identification codes.
2.3. Analytical Techniques
The pigment particle size was analysed using the Mastersizer 2000LF (Malvern Instruments, Worcestershire, UK) laser particle size analyser. The samples were previously dispersed in alcohol. As for the aggregates, grain size distribution was determined using the dry sieving method with different metal mesh opening sizes (0.063, 0.125, 0.250, 0.500, 0.800, 1, 1.6, 2, 3.15, and 4 mm) according to European standards [25].
The mineralogical composition of the pigments, aggregates and lime was determined using X-ray diffraction (XRD, XPert PRO PANalytical B.V., Almelo, The Netherlands) according to the random-powder method. Analyses were performed using Cu-Kα radiation, Ni filter, 45 kV voltage, and 40 mA intensity. The exploration range was 3° to 60° 2θ and the goniometer speed was 0.05° 2θ s−1. The oriented aggregate method was also used to properly identify the phyllosilicates in the GE-G sample [26,27]. The mock-up surfaces were characterised by XRD grazing incidence (XPert PRO PANalytical B.V.) under the same conditions. The mineral phases were identified using the X’Pert HighScore software (version 4.9.0.27512).
The molecular compositions of the raw materials (lime, aggregates, NE, and pigments) and the mock-ups were obtained by Attenuated Transmittance Reflectance Fourier–Transform Infrared Spectroscopy (ATR-FTIR) using a Thermo Nicolet 6700 (Thermo Fisher, Waltham, MA, USA) at a 2 cm−1 resolution over 100 scans in the mid-infrared spectral region (400–4000 cm−1). For the test, samples were pressed with the plunger against a diamond crystal. For CIN-R, VER-R, and MAR-R (pigments and mock-ups), FTIR spectra were also taken in the far (50–400 cm−1) infrared spectral region where IR bands characteristic of sulphur and oxides appear.
The elemental composition of the pigments was determined by X-ray fluorescence (XRF) with an Olympus Vanta C Handheld XRF analyser (Hamburg, Germany) in ‘GeoChem’ mode, using 3 beams working at 40, 10, and 50 kV, respectively. The total measurement time was 60 s: 20 s for each beam. Element recognition was obtained by means of the supplier’s database. The equipment was used to identify chemical elements of atomic number greater than 12. As no specific calibration was applied apart from the default calibration of the equipment, the composition obtained is semi-quantitative. Aggregate elemental characterisation was also carried out by XRF using a S4 PIONEER-BRUKER (Billerica, MA, USA) wavelength dispersive X-ray fluorescence spectrometer, with a Rh tube (60 kV; 150 mA), LIF200/PET/OVO-55 analyser crystals and gas-flow proportional and scintillation detectors. The samples were ground and dispersed in potassium bromide tablets before the analysis. Measurements were taken in a vacuum, with the samples in rotation, in order to obtain the content in major elements (expressed as oxides), (Zetium, PANalytical).
The pigment and aggregate microtexture and elemental composition were studied using a FEI Quanta400 environmental scanning electron microscopy (Hillsboro, OR, USA) with energy-dispersive X-ray spectroscopy (EDS) in both secondary (SE) and backscattered electron (BSE) detection modes. Observation conditions included a working distance of ~10 mm, an accelerating potential of 20 kV and a specimen current of ~60 mA. The microtexture and elemental composition of the mock-ups were studied with a JEOL JSM 6010LA microscope (Tokyo, Japan) under the same conditions. The samples were studied by observing the surface directly, and by studying the paint layer in cross-sections. For this, the specimens were embedded in epoxy resin (EpoThin 2 Epoxy Resin and EpoThin 2 Epoxy Hardener) and then cut and polished to a mirror finish.
The colour of the painted mock-ups was characterised using CIELAB and CIELCH colour spaces [28,29], measuring L* (lightness), a* and b* (colour coordinates), and C*ab (chroma) values using a Konica Minolta CM-700d spectrophotometer (Tokyo, Japan). L* represents lightness, varying from 0 (black) to 100 (white). The other two parameters are chromaticity coordinates: a* goes from red to green (where +a* is red and −a* is green) and b* from yellow to blue (+b* is yellow and −b* is blue). C*ab is calculated according to the following formula: C*ab = (a2 + b2)1/2. The measurements were made in the specular component excluded (SCE) mode, for a spot diameter of 8 mm, using illuminant D65 at an observer angle of 10°. A total of fifteen measurements were made for each mock-up and their average values and standard deviations (STDs) were computed. Colour parameters were also measured for the pigment powders after first flattening them against a glass support to obtain a smooth surface. ΔL*, Δa*, Δb*, and ΔC*ab colour differences and the total colour difference (ΔE*ab) [28,29] were calculated in order to compare the colour of each pigment with (i) its corresponding fresco paint colour (F) and (ii) its corresponding chromatic reintegration (SB). The colour differences between F and SB were also calculated.
The gloss of the mock-ups was measured with a Novo-Gloss Lite 20/60/85° glossmeter (Neurtek, Basque Country, Spain), with a reflection angle of 60°, following the relevant Spanish standard [30]. The gloss parameter expresses the way the mock-ups reflect the light and, in particular, the way light is reflected by the painted surface at and near the specular direction [31]. Five measurements were taken per sample, and their average value and standard deviation (STD) were calculated.
The surface hydrophobicity of the mock-ups was evaluated using a Phoenix-300 Touch SEO goniometer (KROMTEK, London, UK), with an outer diameter of 0.012″ (0.3048 mm) and an inner diameter needle of 0.005″ (0.127 mm). Measurements were carried out with 3 drops of 8 µL per mock-up following the relevant Spanish standard [32]. Average values and standard deviations (STDs) were computed.
The roughness of the mock-ups was measured with a Mitutoyo SJ400 (Mitutoyo, Kanagawa, Japan) profilometer that measures the arithmetic average roughness (Ra, μm) [33] over a distance of 2 cm. For each sample, 3 profiles were obtained, and the Ra average values were calculated, together with their corresponding standard deviations (STDs).
The adhesion of the paint layer was studied by carrying out peeling tests using a double-sided Tesa Powerbond adhesive tape, which was applied and removed 3 times consecutively on each paint mock-up (fresco and reintegration). The weight of the peeled-off material was determined by calculating the difference in the weight (mg) of the tape before and after application, in line with [34].
The texture and appearance of the mock-ups were studied with a Nikon SMZ 1000 microscope (Melville, NY, USA). Cross-sections were studied under Polarised-Light Microscopy (PLM), in both plane-polarised (PPL) and cross-polarised (XPL) light modes.
3. Results
The preliminary particle size (LD), mineralogical (XRD), and chemical (XRF) characterisation of the raw pigments, presented in Table 1, proved that the information provided by the supplier was not always precise as impurities were present. The molecular characterisation of the pigments by FTIR, presented in Table 2, complemented the previous characterisation and suggested the presence of other compounds. For instance, Si,Al–O bands were identified in EG-B. This, together with XRF identification of aluminium (Al), titanium (Ti), and iron (Fe), could be related to the presence of feldspars and pyroxenes related to the manufacturing process of the pigment [35]. Also, sulphide pigments (CIN-R and VER-R) presented silica (Si) by XRF, assigned to the presence of quartz (SiO2) as an impurity, also identified through FTIR Si–O characteristic bands [36].

Table 2.
Molecular composition of the raw pigments by Attenuated Total Reflection Fourier-transform infrared spectroscopy (ATR-FTIR). See Table 1 for an explanation of the pigment identification codes (ID).
Table 2.
Molecular composition of the raw pigments by Attenuated Total Reflection Fourier-transform infrared spectroscopy (ATR-FTIR). See Table 1 for an explanation of the pigment identification codes (ID).
| Pigment ID | Bond/Group Type and Vibration | Wavenumber (cm−1) and Relative Intensity * | Assignment |
|---|---|---|---|
| EG-B | Si–O antisymmetrical stretching | 1230 (w), 1159 (m), 1050 (vs), 997 (vs) | Cuprorivaite [37,38] |
| Si–O symmetrical stretching | ≈800 (vw) | Quartz [36,39] | |
| Si–O symmetrical stretching | 755 (w), 662 (s) | Cuprorivaite [37,38] | |
| Si–O antisymmetrical bending | ≈518 (m) | Quartz [36,39] | |
| Si–O bending | 480 (vs) | Cuprorivaite [37,38] | |
| Si,Al–O | ≈420 (vs) | Feldspars [40] | |
| LA-B | –OH stretching | 3580–3000 (b) | Muscovite (?) |
| Si,Al–O | 1430 (vw), 709 (w), 620 (sh) | Lazurite [41,42] | |
| Si,Al–O stretching | ≈965 (vs) | Sodalite [36,43,44] | |
| CO32− antisymmetrical bending | ≈875 (sh) | Calcite [36,45] | |
| Al–O–Si in-plane vibration | ≈750 (vw) | Muscovite [46] | |
| –SO42− | 665 (m) | Lazurite [42] | |
| UL-B | –OH stretching | 3688 (w), 3621 (w) | Kaolinite [47] |
| Si,Al–O stretching | 980 (vs) | Sodalite [43,44] | |
| –SO42− | 665 (m) | Lazurite [42] | |
| Al,Si–O | 797 (vw), 752 (vw), 420 (vs) | Kaolinite [40] | |
| GE-G | –OH stretching | 3600–3200 (b) | Phyllosilicates [40,48,49] |
| Si–O | ≈1630 (w), 970 (vs) | Glauconite/Celadonite [40,48,49] | |
| CO32− antisymmetrical bending | 875 (sh) | Calcite [36,45] | |
| CO32− symmetrical bending | 711 (sh) | Calcite [36,45] | |
| MAR-R | Fe–O stretching | 535 (m), 510 (s), 465 (m), 425 (s), 385 (w), 325 (w) | Iron oxide [50,51,52] |
| CHR-G | Cr–O tension | ≈607 (s)5, 577 (vw), 497 (sh), 474 (vs), 460 (vs), 450 (vs) | Chromium oxide [53,54] |
| CIN-R | Si–O antisymmetrical stretching | 1210–1030 (br) | Quartz [55] |
| Si–O symmetrical stretching | 750–600 (br) | Quartz [55] | |
| Hg–S tension | 345 (m), 283 (m) | Cinnabar [56] | |
| VER-R | Si–O antisymmetrical stretching | 1210–1030 (br) | Quartz [55] |
| Si–O symmetrical stretching | 750–600 (br) | Quartz [55] | |
| Hg–S tension | 344 (m), 284 (m) | Cinnabar [56] | |
| VER-G | –OH stretching | 3450 (s), 3365 (s), 3270 (s) | Copper acetate [57,58,59] |
| COO– symmetric stretching | ≈1594 (vs) | Copper acetate [57,58,59] | |
| COO– antisymmetric stretching | 1418 (vs) | Copper acetate [57,58,59] | |
| C–H related to CH3 group | 1355 (m), 1049 (m), 1033 (m) | Copper acetate [57,58,59] | |
| C=O symmetric stretching | 1260 (vw) | Copper acetate [57,58,59] | |
| O–C–O deformation mode | 690 (m), 626 (m) | Copper acetate [57,58,59] |
(*) relative intensity vs (very strong); s (strong); m (medium); w (weak); vw (very weak); sh (shoulder); br (broad).
Figure 2 shows micrographs of the powdered pigment (left column), the F sample (central column), and the SB sample (right column). It was observed that the powder pigment and the inpainting (SB) were similar in appearance, as opposed to F paints which generally manifested a whitish appearance (especially LA-B-F and GE-G-F, Figure 2b,d, respectively). MAR-R-F also differed as it showed a darker hue. Synthetic pigments EG-B, UL-B, CHR-G, and VER-R exhibited lower hiding powers in SB paints since whitish areas ascribable to the intonaco were sporadically glimpsed throughout the surface. As for verdigris paints (Figure 2i), different coloured alterations were observed in the F paint (VER-G-F) and, in the chromatic reintegration (VER-G-SB), the paint layer did not bond to the intonaco and eventually fell off. For this reason, no further physical properties (gloss, roughness, etc.) were evaluated on the paints manufactured with this semi-organic pigment as it proved to be incompatible with both the F and SB procedures.
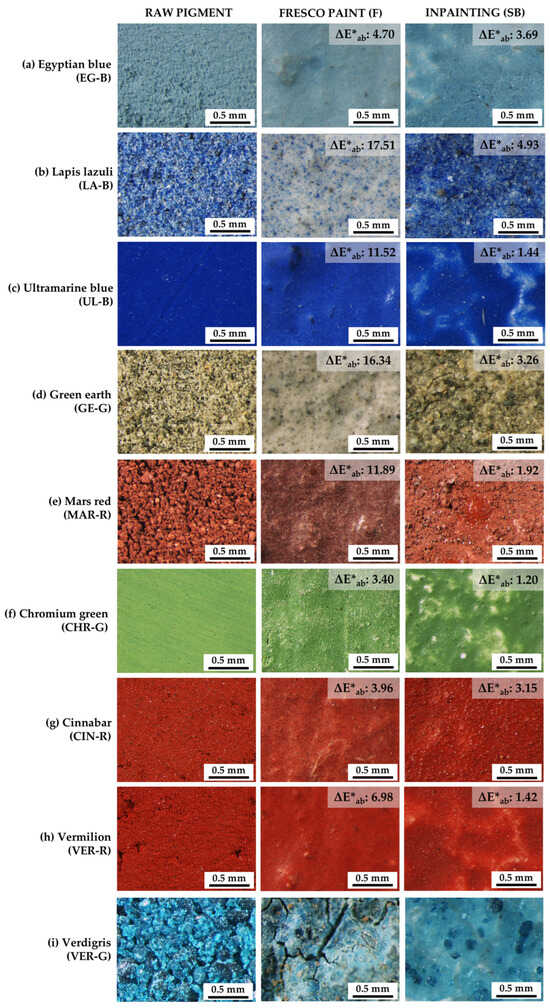
Figure 2.
Micrographs of the raw pigment (left column), fresco paints (-F, central column), and the nano-silica-based reintegrations (-SB, right column). The colour difference (ΔE*ab, in CIELAB units) between the raw pigment and F paints, and between the raw pigment and SB paints is included in each figure. See Table 1 for an explanation of the pigment identification codes (ID).
The colour data for the non-painted mock-ups, the raw pigments, and the F and SB paint mock-ups are presented in Table 3. On the one hand, it was observed that in the unpainted mock-ups, regardless of whether NE was applied or not (FR-SB and FR-F, respectively), the values were very similar to each other. In fact, the colour difference (ΔE*ab) between FR-F and FR-SB was 0.81 CIELAB units, therefore, an unnoticeable difference according to [60]. This means that the use of NE by itself did not lead to a difference in colour when applied to the lime-based intonaco [60]. On the other hand, ΔE*ab was also calculated between the raw pigment and F paints, and between the raw pigment and SB inpaintings. The results are included in Figure 2, where it can be seen that the ΔE*ab between the raw pigment and SB mock-ups was below 5 CIELAB units in all the paints, a value above which two different colours would be observed [60]. Among all, LA-B-SB was the paint with the highest variation (ΔE*ab: 4.93) and, in general terms, synthetic pigments (UL-B, MAR-R, CHR-G, and VER-R) showed values below 2 CIELAB units when used in SB paintings. Regarding the ΔE*ab between the raw pigments and the F paints, the values were generally well above 5 CIELAB units, except for CHR-G-F and CIN-R-F, as expected. In the fresco technique, the pigments are immersed in a calcium carbonate matrix, which makes the colour much less intense, as opposed to SB paints where pigments are mixed with a transparent dispersion (NE). Of the various colour parameters measured (Table 3), the SB mock-ups showed lower L* than their F counterparts and higher C*ab.

Table 3.
L*, a*, b*, and C*ab of the non-painted paint mock-ups (FR-F and FR-SB), the raw pigments and the fresco (-F) and nano-silica-based (-SB) paint mock-ups. Standard deviations (STDs) are also shown. See Table 1 for an explanation of the pigment identification codes (ID).
Among other physical properties, the gloss (G) for the F and SB mock-ups was evaluated, represented in Figure 3a. The differences between FR-F and FR-SB were particularly high (3.67 and 7.05 G units, respectively), which suggested that NE made the surface glossier. However, when mixed with the pigments, the gloss values were lower than for their F equivalents (except for CHR-G). Regarding surface roughness (Figure 3b), the F and SB mock-ups showed similar Ra values. The only SB mock-ups that showed higher roughness than their F equivalents were LA-B, GE-G, and MAR-R. Moreover, the degree of adhesion of the paint layer was evaluated by a peeling test (Figure 3c). The results showed that in both situations (F and SB), the tape peeled off almost all the paint layers, indicating that the degree of adhesion was very low. Still, larger amounts (in mg) of paint were extracted from the F mock-ups. Lastly, static contact angle (θ°) results are shown in Figure 3d. When the θ° is below 90°, the surface is considered hydrophilic (as opposed to hydrophobic, i.e., water-repellent) [61,62]. In this regard, both the F and SB mock-ups had values below 90° and were, therefore, hydrophilic.
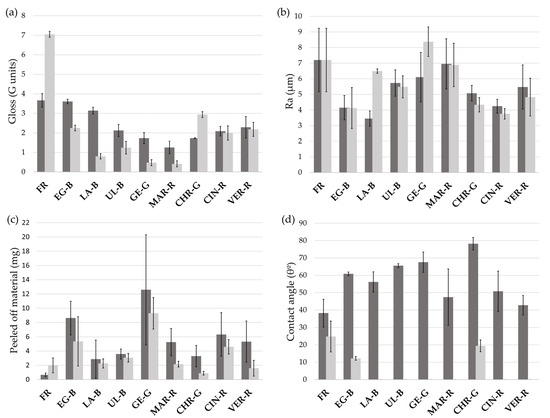
Figure 3.
(a) Average gloss (G, G units) values for all the mock-ups with their standard deviations; (b) average roughness (Ra, µm) values for all the mock-ups with their standard deviations; (c) average weight in mg of peeled-off material from all the mock-ups with their standard deviations; and (d) static contact angle (θ°) values for all the mock-ups with their standard deviations. Dark grey bars represent F paints and light grey bars SB paints. See Table 1 for an explanation of the pigment identification codes (ID).
The mineralogical characterisation of the paint mock-ups by XRD, regardless of the paint (F or SB), remained in line with the earlier characterisation of the raw pigments (Table 1) with the difference that calcite (CaCO3) appeared in all the paintings: in F due to the painting technique itself, and in the SB mock-ups due to the underlying lime-based mortar. The only painting that showed differences in mineralogical composition was VER-G-F. Verdigris pigment corresponds chemically and structurally with the mineral hoganite (Cu(CH3COO)2·H2O), as detected by XRD (Table 1). This phase disappeared in the fresco paint and other compounds were identified instead: a mixed calcium and copper acetate known as paceite (CaCu(CH3COO)4·6(H2O)) and a hydrated copper silicate known as apachite (Cu9Si10O29·11(H2O)).
The FTIR analysis of each paint mock-up (F and SB) showed the characteristic bands previously identified in each pigment, shown in Table 2, except for VER-G-F. In addition, bands assigned to calcium carbonate (i.e., the lime-based mortar, marked in Figure 4a) were observed in both paints (F and SB) regardless of the pigment used: at 1795 cm−1 (C=O) and 875 cm−1 (CO32− antisymmetrical bending) [36,45]. These bands were generally more intense on F paints. An additional band around 1400 cm−1 (CO32− stretching), also associated with calcite [36,45], was exclusively present in the F paint spectra. In relation to NE as the binder, Figure 4a shows the FTIR spectra for the colloidal solution NE (after polymerisation) and is compared to EG-B-SB (silicate), CHR-G-SB (oxide), and VER-R-SB (sulphide) paint mock-ups. Bands around 1065, 1045, and 445 cm−1 (Si–O) were present in the spectra of all the SB mock-ups, assigned to the formation of silica gel [63,64]. In silicate pigments, e.g., EG-B-SB; however, the bands were covered by the characteristic Si–O bands of the pigments, marked with a black rectangle in Figure 4a (in this case assigned to cuprorivaite, see Table 2). In VER-R-SB (sulphide), the silica gel band could also be ascribable to SiO2 present as an impurity. Regarding the silanol bands from NE, the broad band at 3700–2800 cm−1 (Si–OH stretching) decreased in intensity, and the bands at 1645 cm−1 (Si–OH bending) and 960 cm−1 (Si–OH stretching) disappeared, overall suggesting the good polymerisation of NE [63,65,66]. Lastly, regarding the alteration observed in VER-G-F (Figure 2i), Figure 4b shows the FTIR spectra for the raw pigment compared to that of the fresco. The characteristic bands assigned to the acetate changed considerably in the F paint. The three large characteristic bands related to the water molecules associated with the acetate ion (Table 2), were no longer visible in the F paint. A broad band between 3600 and 2700 cm−1 appeared in its place, also assigned to –OH stretching [55] (marked with a black rectangle in Figure 4b). Regarding copper carboxylate groups (COO−), the band present in the powder pigment at 1600 cm−1, shifted to 1536 cm−1 in the F paint (marked with a black arrow in Figure 4b). Lastly, the bands assigned to O–C–O bonds shifted to lower wavenumbers (670 and 620 cm−1, respectively) and lost intensity in the F paint (marked with a black rectangle in Figure 4b).
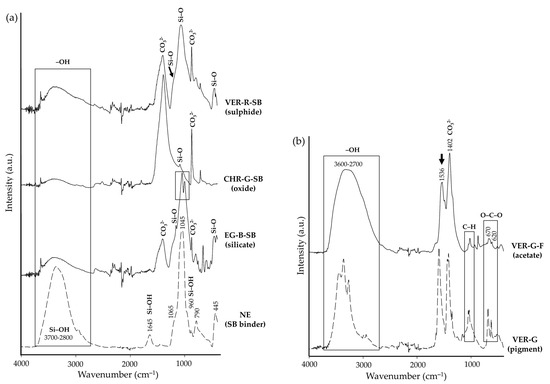
Figure 4.
FTIR spectra of (a) NE, EG-B-SB, CHR-G-SB, and VER-R-SB paint mock-ups; and (b) VER-G and VER-G-F. See Table 1 for an explanation of the identification codes (ID).
The morphological and textural study of the surface by SEM revealed that in some cases the NE binder did not fully penetrate the intonaco, remaining in part on the surface. A cracked xerogel appeared on the surface of the SB mock-ups (marked with white arrows in Figure 5a). The presence of pigment particles on the surface was also observed to a greater or lesser extent in all the SB mock-ups (Figure 5b), regardless of the pigment composition. The study of cross-sections of the SB mock-up by SEM revealed fissures which in places penetrated up to the intonaco, and even within the SB layer itself (Figure 5c).
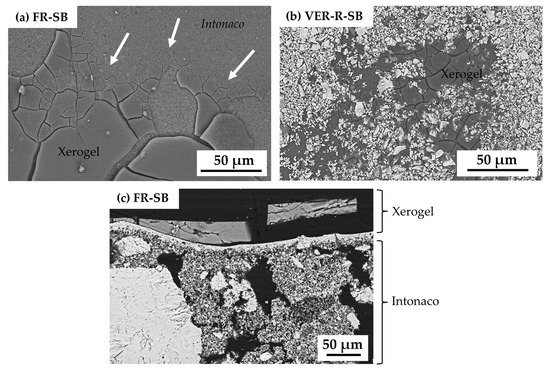
Figure 5.
SEM micrographs of (a) the surface of FR-SB, (b) the surface of VER-R-SB, and (c) the cross-section of FR-SB. White arrows indicate the presence of xerogel on the surface. See Table 1 for an explanation of the pigment identification codes (ID).
The study of the SB paint mock-up cross-sections by PLM and SEM allowed us to evaluate the distribution of pigment particles within the paint layer and the degree of adhesion of the paint to the intonaco. Silicate-based SB paints presented a homogeneous distribution of the pigment particles, especially synthetic EG-B-SB (Figure 6a) and UL-B-SB (Figure 6c). The others (natural LA-B-SB and GE-G-SB, Figure 6b,d, respectively) were more heterogeneous, with pigment particles protruding from the paint layer, especially in GE-G-SB. All the paints showed small fissures to a greater or lesser extent, though they were much more evident and pronounced in GE-G-SB (marked with a white arrow in Figure 6d), in addition to a slight detachment of the paint layer from the intonaco.
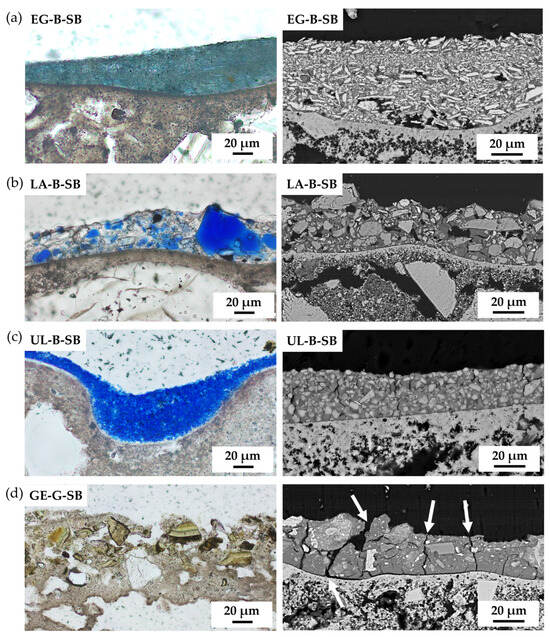
Figure 6.
Plane-polarised transmitted-light (PPL-PLM) and SEM micrograph of silicate-based paints: (a) EG-B-SB, (b) LA-B-SB, (c) UL-B-SB, and (d) GE-G-SB. White arrows indicate the presence of fissures in the SB paint layer. See Table 1 for an explanation of the pigment identification codes (ID).
Oxide-based SB paints MAR-R and CHR-G showed a homogeneous distribution of the pigment particles throughout the paint layer. However, lack of adhesion was observed in both paints (marked with white arrows in Figure 7a,b, respectively). In addition, SEM highlighted an irregular surface on MAR-R-SB (marked with a black rectangle in Figure 7a), different from all the other SB mock-ups.
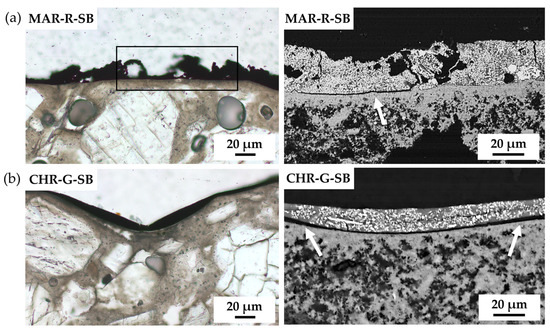
Figure 7.
Plane-polarised transmitted-light (PPL-PLM) and SEM micrograph of oxide-based paints: (a) MAR-R-SB and (b) CHR-G-SB. The black rectangle indicates irregularities in the SB paint layer; white arrows indicate detachment of the SB paint layer from the substrate. See Table 1 for an explanation of the pigment identification codes (ID).
Lastly, for sulphide-based SB paints, the situation was similar to that of oxides. Although both sulphide pigments showed a homogeneous distribution of the pigment particles, natural CIN-R-SB presented great irregularities (marked with a black rectangle in Figure 8a) and lack of adhesion (marked with a white arrow in Figure 8a) compared with its artificial analogue (VER-R-SB).
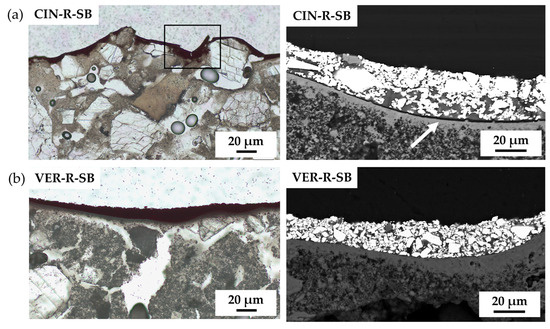
Figure 8.
Plane-polarised transmitted-light (PPL-PLM) and SEM micrograph of sulphide-based paints: (a) CIN-R-SB and (b) VER-R-SB. The black rectangle indicates irregularities in the SB paint layer; the white arrow indicates detachment of the SB paint layer from the substrate. See Table 1 for an explanation of the pigment identification codes (ID).
4. Discussion
The chemical, mineralogical, and molecular characterisation of the pigments proved to be useful as impurities were identified. These impurities, whether related to their provenance, manufacturing process or added as inert fillers, could determine the durability of the inpainting in the long term. For instance, high relative humidity environments, in the presence of expandable clays such as kaolinite–Al2Si2O5(OH)4– or montmorillonite–(Na,Ca)0,3(Al,Mg)2Si4O10(OH)2 ·nH2O–, found in UL-B or GE-G (see Table 1), could lead to swelling processes [67]. Thus, variable moisture conditions could provoke swellable clays to shrink and expand, leading to paint loss. This was observed in a similar study which evaluated the durability of this same inpainting procedure [68]. The authors reported that the presence of expandable clays did indeed lead to paint loss in green earth-based inpaintings [68]. Moreover, the presence of sulphides such as pyrite–FeS2–, identified in LA-B, in oxidising environments could favour acidic microenvironments, reacting with the calcium present in the intonaco, leading to the formation of calcium sulphates and sulphites [69].
Regarding colour compatibility, the fact that the raw pigment colour varied little once the inpainting layer was dry is of great interest to professional restorers. This would enable them to know approximately what colour will be obtained when mixing the nano-silica with the pigment, as the inpainting did not differ much from the original colour. In fact, considering pigment nature, synthetic pigments generally showed the least colour variation when mixed with the NE binder. The colour difference (ΔE*ab) between frescoes and inpaintings when using the same pigment was very high. The colour parameters showed that colour saturation (C*ab) was always higher in the inpainting. In order to avoid the inpainting work having a strong visual impact, applying the paint a sottotono, i.e., ‘in a lower tone’, following traditional inpainting techniques [2], would reduce the C*ab values and would make sure that the chromatic reintegration would not stand out from the original.
The other physical properties evaluated in this study showed a good compatibility between frescoes (F) and this novel inpainting procedure (SB). Inpaintings (except for CHR-G-SB) showed slightly lower gloss values than their fresco counterparts. This meets the compatibility criterion established by Cesare Brandi in the 20th century [17] as both the fresco and the chromatic reintegration have a matte appearance (ergo compatible) though slightly different (ergo recognisable). The roughness values were also similar, except for LA-B and GE-G paints where it was higher. This was directly related to their pigment particle size since both showed higher size ranges (0.3–100 µm and 0.3–125 µm, respectively). Nevertheless, we should also bear in mind that roughness could also be affected by the brushstrokes and the irregular surface of the intonaco itself. Moreover, regarding wettability, even though contact angle results (shown in Figure 3d) were different between F and SB paints, they both showed θ° below 90°, which means that all the surfaces are hydrophilic [61,62]. Hence, the use of NE as a binder would not influence the wettability properties and would show similar behaviour to water as fresco paintings, thus ensuring the natural breathability of the wall, allowing the exchange of moisture between the wall and the environment. Lastly, regarding the peeling test, the SB layer showed less loss of material compared to the F paints. This may be because the tape removed more grains of sand from the intonaco (i.e., so increasing the weight) or because the paint layer was thicker in frescoes.
The presence of fissures observed by SEM on the SB layer should not be considered as a reason to refuse this inpainting procedure. These fissures were reported in other studies [70,71,72,73] in which NE was used as a consolidant product, being caused by polymerisation, after the evaporation of the solvent. Some of these authors claimed that this could affect the durability of the consolidation treatment [72]. Nevertheless, in [68] it was identified that NE as a binder for inpainting did not influence the overall durability of the inpainting after natural ageing tests were carried out. It is also important to highlight that the nano-silica binder did not always form a homogeneous layer including the pigment particles. Some particles were observed protruding from the surface of LA-B-SB and GE-G-SB inpaintings. In [68], where these paintings were exposed for one year to natural environments, LA-B-SB remained stable, while GE-G-SB did not. Lastly, the fact that slight detachment was observed in sulphide- and oxide-based inpaintings could be assigned to sample extraction or sample preparation since in [68] no detachments were reported after natural exposure for 1 year.
As for the chemical compatibility between the tested pigments and the NE binder, the blue silicate-based pigments (EG-B, LA-B, and UL-B) showed higher affinity. Observations of the cross-sections revealed fewer fissures, with outstanding adhesion to the intonaco, and a more homogeneous dispersion of the pigment particles within the paint layer when compared to the other pigments. Due to their similarity with the binder in terms of chemical composition (both silicate-based), the blue pigment particles bound better with the colloidal aqueous solution of silica nanoparticles, leading to the formation of a solid product of hydrated amorphous silica that behaved in a similar way to a silica gel [74]. On the other hand, despite GE-G being primarily composed of silicates, it also contained many impurities which surely influenced compatibility leading to major fissures and lack of adhesion. As for oxide- (MAR-R and CHR-G) and sulphide-based pigments (CIN-R and VER-R), even though they all showed excellent pigment distribution, fissures and lack of adhesion were observed. This was likely related to their chemical nature as oxides and sulphides.
Finally, semi-organic verdigris proved to be unsuitable, regardless of the painting technique (F or SB). When a fresco was applied, the paint layer began to deteriorate 24 h after application. Though the instability of verdigris has been stated [75,76], it has also been reported to be found in historical frescoes [77,78]. Therefore, an inaccurate attribution may have been made whereas another painting technique (e.g., tempera) may have been used instead. Regarding the alteration compounds identified by XRD, Ca-Cu acetate paceite forms due to the reaction of acetate salts and calcium carbonate [79]. As for hydrated Cu-silicate apachite, this compound might have been formed by the reaction between the silicic acid from Si-compounds (feldspars from the aggregates) and Cu2+ ions, under alkaline pH and in the presence of water, as reported by Toupance et al. [80]. This possible formation needs further research. Other compounds, present in low percentages and below the detection limit of XRD, cannot be discounted. Indeed, the FTIR results suggested the formation of hydrated compounds due to the presence of a broad hydroxyl band, while at the same time, shifts in the carboxylate groups indicated the formation of a mixture of compounds [57]. In short, the stability of verdigris in fresco paintings should be evaluated in depth as it is clearly not suitable for fresco painting, despite being reported in historical frescoes.
5. Conclusions
This study has shown promising results regarding the use of an aqueous colloidal dispersion of nano-sized silica as a binder in chromatic reintegrations for wall paintings. Given that professional restorers must ensure the compatibility of the inpainting with the original historical paintwork, this research showed, on the one hand, that the chromatic reintegration had slightly lower gloss values than the fresco technique (except for chromium green) and could be easily distinguished from the ‘historical’ fresco technique. Wettability was unaffected, and both techniques (fresco and inpainting) retained their hydrophilic character. Another interesting finding was that the chromatic reintegrations had similar colour values to the raw pigment. This will enable professionals to know approximately what colour will be obtained.
The chemical nature of pigments and the presence of impurities were determined in the compatibility with the nano-silica binder. Even though all the inorganic pigments provided promising results in terms of physical compatibility (colour, gloss, roughness, and wettability), blue silicate-based pigments showed better pigment distribution within the paint layer and outstanding adhesion to the mortar substrate compared to oxide- and sulphide-based pigments. This was likely related to the fact that silicates were more compatible with the silica-based binder.
Finally, this study has also shown that despite its use in ancient and medieval wall paintings, the semi-organic pigment verdigris is not suitable for use in either the fresco technique or in chromatic reintegrations with NE, mainly due to the poor adhesion of the paint layer to the intonaco surface. In order to understand the decay processes to which verdigris is subjected when applied to a fresco, more research is required into the chemical–mineralogical transformations it undergoes.
Author Contributions
Conceptualisation, D.J.-D., J.S.P.-A. and A.A.; methodology, D.J.-D., J.S.P.-A. and A.A.; software, D.J.-D., J.S.P.-A. and A.A.; validation, J.S.P.-A., A.A. and T.L.-M.; formal analysis, D.J.-D., J.S.P.-A. and A.A.; investigation, D.J.-D.; resources, D.J.-D., J.S.P.-A. and A.A.; data curation, D.J.-D., J.S.P.-A. and A.A.; writing—original draft preparation, D.J.-D.; writing—review and editing, J.S.P.-A., A.A. and T.L.-M.; visualisation, J.S.P.-A. and A.A.; supervision, J.S.P.-A., A.A. and T.L.-M.; project administration, J.S.P.-A.; funding acquisition, J.S.P.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Research Projects PID2020-119838RA-I00 and PID2023-146405OBI00 (funded by MICIU/AEI/10.13039/501100011033 and FEDER, UE), and ED431F 2022/07.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed at the corresponding author.
Acknowledgments
Daniel Jiménez-Desmond was supported by the ED481A-2023/086 predoctoral contract through ‘Programa de axudas á etapa predoutoral da Xunta de Galicia’ co-financed by the European Union within the framework of the FSE+ Galicia 2021–2027 programme and partially financed by the Xunta de Galicia project Limpeza sostible do patrimonio pictorico: optimizacion dos procesos de ablacion laser (ED431F 2022/07). J.S. Pozo-Antonio was supported by the RYC2020-028902-I project funded by MICIU/AEI/10.13039/501100011033 and, by ‘ESF Investing in your future’. Anna Arizzi was funded by the State Research Agency (SRA) and the Spanish Ministry of Innovation and Science, as part of the projects PID2020-119838RA-100 (2021–2024) and PID2023-146405OB-100 (2024–2027), within the Junta de Andalucía Research Group RNM179. Teresa López-Martínez was funded by Grant C-HUM-109-UGR23 funded by Consejería de Universidad, Investigación e Innovación and by ERDF Andalusia Program 2021–2027 and pre-competitive project PP2024.PP-02 of the University of Granada.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- ICOMOS. Principles for the preservation, conservation and restoration of wall paintings. In Proceedings of the 14th ICOMOS General Assembly, Victoria Falls, Zimbabue, 27–31 October 2003. [Google Scholar]
- Mora, L.; Mora, P.; Philippot, P. La Conservazione Delle Pitture Murali; Editrice Compositori: Bologna, Italy, 1999. [Google Scholar]
- Venegas García, C.; Barrainkua Legid, A. La Conservación de Pintura Mural; Síntesis: Madrid, Spain, 2021. [Google Scholar]
- Matteini, M.; Moles, A. La Química en la Restauración: Los Materiales del Arte Pictórico; Editorial Nerea: Guipúzcua, Spain, 2001. [Google Scholar]
- Mora, P. Causes of Deterioration of Mural Painting; DAPCO: Rome, Italy, 1974. [Google Scholar]
- Giannini, C. Dizionario del Restauro. In Techniche Diagnostica, Convervazione; Nardini Editore: Perugia, Italy, 2010. [Google Scholar]
- Mercado Hervás, M.S. Técnicas y procedimientos de reintegración cromática. Cuad. Restaurac. 2009, 7, 5–12. [Google Scholar]
- Jiménez-Desmond, D.; Pozo-Antonio, J.S.; Arizzi, A. Present and future of chromatic reintegrations of wall paintings. J. Cult. Herit. 2024, 67, 237–247. [Google Scholar] [CrossRef]
- Ropret, P.; Zoubek, R.; Škapin, A.S.; Bukovec, P. Effects of ageing on different binders for retouching and on some binder–pigment combinations used for restoration of wall paintings. Mater. Charac. 2007, 58, 1148–1159. [Google Scholar] [CrossRef]
- Baldó, J.M. Reintegración de Pinturas Murales Exteriores: Estudio y Valoración de Sistemas y Materiales. Ph.D. Thesis, Polytechnic University, Valencia, Spain, 2015. [Google Scholar]
- Sánchez Pons, M.; Sanesi Bigagli, D. Los silicatos como aglutinantes pictóricos de pinturas murales en los siglos XX y XXI: Caracterización de las principales tipologías. Ge-Conserv. 2021, 20, 318–336. [Google Scholar]
- Miller, B.F. Painting materials research in Munich from 1825 to 1937. Stud. Conserv. 1998, 43, 246–248. [Google Scholar] [CrossRef]
- Mayer, R. Materiales y Técnicas del Arte; Hermann Blume Ediciones: Madrid, Spain, 2005. [Google Scholar]
- Pedrola, A. Materiales, Procedimientos y Técnicas Pictóricas; Editorial Ariel: Barcelona, Spain, 2019. [Google Scholar]
- Loganina, V.I.; Kislitsyna, S.N.; Mazhitov, Y.B. Development of sol-silicate composition for decoration of building walls. CSCM 2018, 9, e00173. [Google Scholar] [CrossRef]
- Gridchin, A.M.; Loganina, V.I.; Mazhitov, E.B.; Ryapukhin, A.N. Assessment of the Durability of Coatings Based on Sol Silicate Paint. In Proceedings of the Innovations and Technologies in Construction, BUILDINTECH BIT 2020, Belgorod, Russia, 8–9 October 2020. [Google Scholar] [CrossRef]
- Brandi, C. Teoria del Restauro; Nardini Editore: Florence, Italy, 2011. [Google Scholar]
- Magnain, C.; Elias, M.; Frigerio, J.-M. Influence of the artistic techniques on the visual appearance of complexions in art. In O3A: Optics for Arts, Architecture, and Archaeology II; SPIE: Bellingham, WA, USA, 2009; Volume 7391, pp. 72–84. [Google Scholar] [CrossRef]
- Jiménez-Desmond, D.; Pozo-Antonio, J.S.; Arizzi, A. The fresco wall painting techniques in the Mediterranean area from Antiquity to the present: A review. J. Cult. Herit. 2024, 66, 166–186. [Google Scholar] [CrossRef]
- Nevin, A. Pigment Alteration. In The Encyclopedia of Archaeological Sciences; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2025. [Google Scholar] [CrossRef]
- Napoleone, L. Integrazione pittorica. Acqua sporca, sottotono, tinta neutra, rigatino. Progett. Color. 2008, 2, 21–22. [Google Scholar]
- Merkus, H.G. Pigments and Paint Dispersions. In Particulate Products; Merkus, H., Meesters, G., Eds.; Springer International Publishing: New York, NY, USA, 2013; Volume 19, pp. 343–370. [Google Scholar] [CrossRef]
- Data Sheet; Nano ESTEL. Consolidant and Fixativ Product for Natural Stone, Brick, Terracotta, Mortars and Plasters. CTS SL n.d.:1–3. Available online: https://shop-espana.ctseurope.com/174-nano-estel-consolidante-a-base-de-nanosilice (accessed on 25 May 2023).
- Piovesan, R.; Mazzoli, C.; Maritan, L.; Cornale, P. Fresco and lime-paint: An experimental study and objective criteria for distinguishing between these painting techniques. Archaeometry 2012, 54, 723–736. [Google Scholar] [CrossRef]
- UNE-EN 933-1; Ensayos Para Determinar las Propiedades Geométricas de Los Áridos. Parte 1: Determinación de la Granulometría de las Partículas. Método de Tamizado. AENOR: Madrid, Spain, 2012.
- Brindley, G.W.; Brown, G. Crystal Structures of Clay Minerals and Their X-Ray Identification; Mineralogical Society: London, UK, 1982. [Google Scholar]
- Thorez, J. Phyllosilicates and Clay Minerals: A Laboratory Handbook for Their X-Ray Diffraction Analysis; Lelotte: Dison, Belgium, 1975. [Google Scholar]
- CIE S014-4/E:2007; Colorimetry-Part 4: CIE 1976 L* a* b* Colour Space. International Standard. International Organization for Standardization: Geneva, Switzerland, 2007.
- CIE Publication 15-2; Colorimetry. CIE Central Bureau: Vienna, Austria, 1986.
- UNE-EN ISO 2813; Pinturas y Barnices. Determinación del Índice de Brillo Especular a 20°, 60° y 85°. AENOR: Madrid, Spain, 2015.
- Bodart, M.; de Peñaranda, R.; Deneyer, A.; Flamant, G. Photometry and colorimetry characterisation of materials in daylighting evaluation tools. Build. Environ. 2008, 43, 2046–2058. [Google Scholar] [CrossRef]
- UNE-EN 828; Adhesivos. Mojabilidad. Determinación por Medida de Ángulo de Contacto y de la Tensión Superficial Crítica de la Superficie Sólida. AENOR: Madrid, Spain, 2013.
- UNE-EN ISO 21920-3; Geometrical Product Specifications (GPS)—Surface Texture: Profile—Part 3: Specification Operators. AENOR: Madrid, Spain, 2023.
- UNE-EN ISO 29862; Cintas Autoadhesivas. Determinación de las Propiedades Adhesivas de Pelado. AENOR: Madrid, Spain, 2020.
- Dariz, P.; Schmid, T. Trace compounds in Early Medieval Egyptian blue carry information on provenance, manufacture, application, and ageing. Sci. Rep. 2021, 11, 11296. [Google Scholar] [CrossRef]
- Derrick, M.R.; Stulik, D.; Landry, J.M. Scientific Tools for Conservation. In Infrared Spectroscopy in Conservation Science; The Getty Conservation Institute: Los Angeles, CA, USA, 1999. [Google Scholar]
- Mirtit, P.; Appolonia, L.; Casoli, A.; Ferrari, R.P.; Laurenti, E.; Amisano Canesi, A.; Chiari, G. Spectrochemical and structural studies on a Roman sample of Egyptian blue. Spectrochim. Acta Part A 1995, 51, 437–446. [Google Scholar] [CrossRef]
- Coimbra, M.M.; Martins, I.; Bruno, S.M.; Vaz, P.D.; Ribeiro-Claro, P.J.A.; Rudić, S.; Nolasco, M.M. Shedding Light on Cuprorivaite, the Egyptian Blue Pigment: Joining Neutrons and Photons for a Computational Spectroscopy Study. Cryst. Growth Des. 2023, 23, 4961–4969. [Google Scholar] [CrossRef]
- Anbalagan, G.; Prabakaran, A.R.; Gunasekaran, S. Spectroscopic characterization of Indian standard sand. J. Appl. Spectrosc. 2010, 77, 86–94. [Google Scholar] [CrossRef]
- Bishop, J.L.; Lane, M.D.; Dyar, M.D.; Brown, A.J. Reflectance and emission spectroscopy study of four groups of phyllosilicates: Smectites, kaolinite-serpentines, chlorites and micas. Clay Miner. 2008, 43, 35–54. [Google Scholar] [CrossRef]
- Silva, C.E.; Silva, L.P.; Edwards, H.G.M.; De Oliveira, L.F.C. Diffuse reflection FTIR spectral database of dyes and pigments. Anal. Bioanal. Chem. 2006, 386, 2183–2191. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Chervonnyi, A.D. Infrared Spectroscopy of Minerals and Related Compounds; Springer International Publishing: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Zilio, S.C.; Bagnato, V.S. Infrared Spectra of Natural Sodalite. J. Phys. Chem. 1984, 88, 1373–1376. [Google Scholar]
- Mofrad, A.M.; Peixoto, C.; Blumeyer, J.; Liu, J.; Hunt, H.K.; Hammond, K.D. Vibrational Spectroscopy of Sodalite: Theory and Experiments. J. Phys. Chem. C 2018, 122, 24765–24779. [Google Scholar] [CrossRef]
- Fernández-Carrasco, L.; Torrens-Martín, D.; Morales, L.M.; Martínez-Ramírez, S. Infrared spectroscopy in the analysis of building and construction materials. In Infrared Spectroscopy: Materials Science, Engineering and Technology; Theophanides, T., Ed.; InTech: Rijeka, Croatia, 2012; pp. 369–381. [Google Scholar]
- Friedrich, F.; Heissler, S.; Faubel, W.; Nüesch, R.; Weidler, P.G. Cu(II)-intercalated muscovite: An infrared spectroscopic study. Vib. Spectrosc. 2007, 43, 427–434. [Google Scholar] [CrossRef]
- Helwig, K. The Characterisation of Iron Earth Pigments Using Infrared Spectroscopy; Canadian Conservation Institute: Ottawa, ON, Canada, 1998. [Google Scholar]
- Moretto, L.M.; Orsega, E.F.; Mazzocchin, G.A. Spectroscopic methods for the analysis of celadonite and glauconite in Roman green wall paintings. J. Cult. Herit. 2011, 12, 384–391. [Google Scholar] [CrossRef]
- Fanost, A.; Gimat, A.; de Viguerie, L.; Martinetto, P.; Giot, A.C.; Clémancey, M.; Blondin, G.; Gaslain, F.; Glanville, H.; Walter, P.; et al. Revisiting the identification of commercial and historical green earth pigments. Colloids Surf. A Physicochem. Eng. Asp. 2020, 584, 124035. [Google Scholar] [CrossRef]
- Wang, F.; Qin, X.F.; Meng, Y.F.; Guo, Z.L.; Yang, L.X.; Ming, Y.F. Hydrothermal synthesis and characterization of α-Fe2O3 nanoparticles. Mater. Sci. Semicond. Process 2013, 16, 802–806. [Google Scholar] [CrossRef]
- Xiao, W.; Jones, A.M.; Collins, R.N.; Bligh, M.W.; David Waite, T. Use of fourier transform infrared spectroscopy to examine the Fe(II)-Catalyzed transformation of ferrihydrite. Talanta 2017, 175, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Salama, W.; El Aref, M.; Gaupp, R. Spectroscopic characterization of iron ores formed in different geological environments using FTIR, XPS, Mössbauer spectroscopy and thermoanalyses. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136, 1816–1826. [Google Scholar] [CrossRef] [PubMed]
- Wiesinger, R.; Pagnin, L.; Anghelone, M.; Moretto, L.M.; Orsega, E.F.; Schreiner, M. Pigment and Binder Concentrations in Modern Paint Samples Determined by IR and Raman Spectroscopy. Angew. Chem. Int. Ed. 2018, 57, 7401–7407. [Google Scholar] [CrossRef]
- Bai, Y.L.; Xu, H.B.; Zhang, Y.; Li, Z.H. Reductive conversion of hexavalent chromium in the preparation of ultra-fine chromia powder. J. Phys. Chem. Sol. 2006, 67, 2589–2595. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequenciues: Tables and Charts; John Wiley & Sons Ltd.: New York, NY, USA, 2004. [Google Scholar]
- Vahur, S.; Knuutinen, U.; Leito, I. ATR-FT-IR spectroscopy in the region of 500–230 cm−1 for identification of inorganic red pigments. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 73, 764–771. [Google Scholar] [CrossRef]
- Salvadó, N.; Butí, S.; Cotte, M.; Cinque, G.; Pradell, T. Shades of green in 15th century paintings: Combined microanalysis of the materials using synchrotron radiation XRD, FTIR and XRF. Appl. Phys. A 2013, 111, 47–57. [Google Scholar] [CrossRef]
- Prati, S.; Bonacini, I.; Sciutto, G.; Genty-Vincent, A.; Cotte, M.; Eveno, M.; Menu, M.; Mazzeo, R. ATR-FTIR microscopy in mapping mode for the study of verdigris and its secondary products. Appl. Phys. A 2016, 122, 10. [Google Scholar] [CrossRef]
- San Andrés, M.; De La Roja, J.M.; Baonza, V.G.; Sancho, N. Verdigris pigment: A mixture of compounds. Input from Raman spectroscopy. J. Raman Spectrosc. 2010, 41, 1468–1476. [Google Scholar] [CrossRef]
- Mokrzycki, W.; Tatol, M. Color difference Delta E-A survey Colour difference ∆E-A survey. MG&V 2011, 20, 383–411. [Google Scholar]
- Gomes, D.J.C.; De Souza, N.C.; Silva, J.R. Using a monocular optical microscope to assemble a wetting contact angle analyser. Measurement 2013, 46, 3623–3627. [Google Scholar] [CrossRef]
- Bico, J.; Thiele, U.; Quéré, D. Wetting of textured surfaces. Colloids Surf. A Physicochem. Eng. 2022, 206, 41–46. [Google Scholar] [CrossRef]
- Rubio, F.; Rubio, J.; Oteo, J.L. A FT-IR study of the hydrolysis of Tetraethylorthoselicate (TEOS). Spectrosc. Lett. 1998, 31, 199–219. [Google Scholar] [CrossRef]
- De Ferri, L.; Lottici, P.P.; Lorenzi, A.; Montenero, A.; Salvioli-Mariani, E. Study of silica nanoparticles-polysiloxane hydrophobic treatments for stone-based monument protection. J. Cult. Herit. 2011, 12, 356–363. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; Otero, J.; Alonso, P.; Mas i Barberà, X. Nanolime- and nanosilica-based consolidants applied on heated granite and limestone: Effectiveness and durability. Constr. Build. Mater. 2019, 201, 852–870. [Google Scholar] [CrossRef]
- Barberena-Fernández, A.M.; Carmona-Quiroga, P.M.; Blanco-Varela, M.T. Interaction of TEOS with cementitious materials: Chemical and physical effects. Cem. Concr. Compos. 2015, 55, 145–152. [Google Scholar] [CrossRef]
- Paineau, E.; Bihannic, I.; Baravian, C.; Philippe, A.M.; Davidson, P.; Levitz, P.; Funari, S.S.; Rochas, C.; Michot, L.J. Aqueous suspensions of natural swelling clay minerals. 1. structure and electrostatic interactions. Langmuir 2011, 27, 5562–5573. [Google Scholar] [CrossRef]
- Jiménez-Desmond, D.; Pozo-Antonio, J.S.; Arizzi, A. Outdoor durability of nano-sized silica-based chromatic reintegrations. Influence of exposure conditions and pigment composition. Dyes Pig. 2025, 235, 112651. [Google Scholar] [CrossRef]
- Cockell, C.S.; Pybus, D.; Olsson-Francis, K.; Kelly, L.; Petley, D.; Rosser, N.; Howard, K.; Mosselmans, F. Molecular Characterization and Geological Microenvironment of a Microbial Community Inhabiting Weathered Receding Shale Cliffs. Environ. Microbiol. 2011, 61, 166–181. [Google Scholar] [CrossRef]
- Licchelli, M.; Malagodi, M.; Weththimuni, M.; Zanchi, C. Nanoparticles for conservation of bio-calcarenite stone. Appl. Phys. A 2014, 114, 673–683. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; Noya, D.; Montojo, C. Aesthetic Effects on Granite of Adding Nanoparticle TiO2 to Si-Based Consolidants (Ethyl Silicate or Nano-Sized Silica). Coatings 2020, 10, 215. [Google Scholar] [CrossRef]
- Borsoi, G.; Veiga, R.; Santos Silva, A. Effect of nanostructured lime-based and silica-based products on the consolidation of historical renders. In Proceedings of the 3rd Historic Mortars Conference, Glasgow, Scotland, 11–13 September 2013. [Google Scholar]
- López-Martínez, T.; Collado-Montero, F.J.; García-Bueno, A. Consolidation tests in archaeological wall painting: Comparing treatments depending on the painting technique. Conserv. Património 2022, 39, 33–44. [Google Scholar]
- Zhuravlev, L.T. The surface chemistry of amorphous silica. Zhuravlev model. Colloids Surf. A Physicochem. Eng. Asp. 2000, 173, 1–38. [Google Scholar] [CrossRef]
- Hofmann, C.; Hartl, A.; Ahn, K.; Druceikaite, K.; Henniges, U.; Potthast, A. Stabilization of Verdigris. J. Pap. Conserv. 2016, 17, 88–99. [Google Scholar] [CrossRef]
- Brostoff, L.B.; Connelly Ryan, C. Tracing the Alteration of Verdigris Pigment through Combined Raman Spectroscopy and X-ray Diffraction, Part I. Restaurator 2020, 4, 3–30. [Google Scholar] [CrossRef]
- Damiani, D.; Gliozzo, E.; Memmi, I.T. The ‘Madonna and Child Enthroned with Saints’ of Ambrogio Lorenzetti in the St. Augustine Church (Siena, Italy): Raman microspectroscopy and SEM-EDS characterisation of the pigments. Archaeol. Anthropol. Sci. 2014, 6, 363–371. [Google Scholar] [CrossRef]
- Asensi Muñoz, R. Documentación y Análisis del Tríptico Ejecutado por Matarana en la Capilla del Colegio Corpus Christi. Ph.D. Thesis, Polytechnic University, Valencia, Spain, 2014. [Google Scholar]
- Bette, S.; Eggert, G.; Emmerling, S.; Etter, M.; Schleid, T.; Dinnebier, R.E. Crystal Structure, Polymorphism, and Anisotropic Thermal Expansion of α-Ca(CH3COO)2. Cryst. Growth. Des. 2020, 20, 5346–5355. [Google Scholar] [CrossRef]
- Toupance, T.; Kermarec, M.; Lambert, J.F.; Louis, C. Conditions of formation of copper phyllosilicates in silica-supported copper catalysts prepared by selective adsorption. J. Phys. Chem. B 2002, 106, 2277–2286. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).