Physico-Chemical Compatibility of an Aqueous Colloidal Dispersion of Silica Nano-Particles as Binder for Chromatic Reintegration in Wall Paintings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mock-Up Preparation
2.3. Analytical Techniques
3. Results
| Pigment ID | Bond/Group Type and Vibration | Wavenumber (cm−1) and Relative Intensity * | Assignment |
|---|---|---|---|
| EG-B | Si–O antisymmetrical stretching | 1230 (w), 1159 (m), 1050 (vs), 997 (vs) | Cuprorivaite [37,38] |
| Si–O symmetrical stretching | ≈800 (vw) | Quartz [36,39] | |
| Si–O symmetrical stretching | 755 (w), 662 (s) | Cuprorivaite [37,38] | |
| Si–O antisymmetrical bending | ≈518 (m) | Quartz [36,39] | |
| Si–O bending | 480 (vs) | Cuprorivaite [37,38] | |
| Si,Al–O | ≈420 (vs) | Feldspars [40] | |
| LA-B | –OH stretching | 3580–3000 (b) | Muscovite (?) |
| Si,Al–O | 1430 (vw), 709 (w), 620 (sh) | Lazurite [41,42] | |
| Si,Al–O stretching | ≈965 (vs) | Sodalite [36,43,44] | |
| CO32− antisymmetrical bending | ≈875 (sh) | Calcite [36,45] | |
| Al–O–Si in-plane vibration | ≈750 (vw) | Muscovite [46] | |
| –SO42− | 665 (m) | Lazurite [42] | |
| UL-B | –OH stretching | 3688 (w), 3621 (w) | Kaolinite [47] |
| Si,Al–O stretching | 980 (vs) | Sodalite [43,44] | |
| –SO42− | 665 (m) | Lazurite [42] | |
| Al,Si–O | 797 (vw), 752 (vw), 420 (vs) | Kaolinite [40] | |
| GE-G | –OH stretching | 3600–3200 (b) | Phyllosilicates [40,48,49] |
| Si–O | ≈1630 (w), 970 (vs) | Glauconite/Celadonite [40,48,49] | |
| CO32− antisymmetrical bending | 875 (sh) | Calcite [36,45] | |
| CO32− symmetrical bending | 711 (sh) | Calcite [36,45] | |
| MAR-R | Fe–O stretching | 535 (m), 510 (s), 465 (m), 425 (s), 385 (w), 325 (w) | Iron oxide [50,51,52] |
| CHR-G | Cr–O tension | ≈607 (s)5, 577 (vw), 497 (sh), 474 (vs), 460 (vs), 450 (vs) | Chromium oxide [53,54] |
| CIN-R | Si–O antisymmetrical stretching | 1210–1030 (br) | Quartz [55] |
| Si–O symmetrical stretching | 750–600 (br) | Quartz [55] | |
| Hg–S tension | 345 (m), 283 (m) | Cinnabar [56] | |
| VER-R | Si–O antisymmetrical stretching | 1210–1030 (br) | Quartz [55] |
| Si–O symmetrical stretching | 750–600 (br) | Quartz [55] | |
| Hg–S tension | 344 (m), 284 (m) | Cinnabar [56] | |
| VER-G | –OH stretching | 3450 (s), 3365 (s), 3270 (s) | Copper acetate [57,58,59] |
| COO– symmetric stretching | ≈1594 (vs) | Copper acetate [57,58,59] | |
| COO– antisymmetric stretching | 1418 (vs) | Copper acetate [57,58,59] | |
| C–H related to CH3 group | 1355 (m), 1049 (m), 1033 (m) | Copper acetate [57,58,59] | |
| C=O symmetric stretching | 1260 (vw) | Copper acetate [57,58,59] | |
| O–C–O deformation mode | 690 (m), 626 (m) | Copper acetate [57,58,59] |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ICOMOS. Principles for the preservation, conservation and restoration of wall paintings. In Proceedings of the 14th ICOMOS General Assembly, Victoria Falls, Zimbabue, 27–31 October 2003. [Google Scholar]
- Mora, L.; Mora, P.; Philippot, P. La Conservazione Delle Pitture Murali; Editrice Compositori: Bologna, Italy, 1999. [Google Scholar]
- Venegas García, C.; Barrainkua Legid, A. La Conservación de Pintura Mural; Síntesis: Madrid, Spain, 2021. [Google Scholar]
- Matteini, M.; Moles, A. La Química en la Restauración: Los Materiales del Arte Pictórico; Editorial Nerea: Guipúzcua, Spain, 2001. [Google Scholar]
- Mora, P. Causes of Deterioration of Mural Painting; DAPCO: Rome, Italy, 1974. [Google Scholar]
- Giannini, C. Dizionario del Restauro. In Techniche Diagnostica, Convervazione; Nardini Editore: Perugia, Italy, 2010. [Google Scholar]
- Mercado Hervás, M.S. Técnicas y procedimientos de reintegración cromática. Cuad. Restaurac. 2009, 7, 5–12. [Google Scholar]
- Jiménez-Desmond, D.; Pozo-Antonio, J.S.; Arizzi, A. Present and future of chromatic reintegrations of wall paintings. J. Cult. Herit. 2024, 67, 237–247. [Google Scholar] [CrossRef]
- Ropret, P.; Zoubek, R.; Škapin, A.S.; Bukovec, P. Effects of ageing on different binders for retouching and on some binder–pigment combinations used for restoration of wall paintings. Mater. Charac. 2007, 58, 1148–1159. [Google Scholar] [CrossRef]
- Baldó, J.M. Reintegración de Pinturas Murales Exteriores: Estudio y Valoración de Sistemas y Materiales. Ph.D. Thesis, Polytechnic University, Valencia, Spain, 2015. [Google Scholar]
- Sánchez Pons, M.; Sanesi Bigagli, D. Los silicatos como aglutinantes pictóricos de pinturas murales en los siglos XX y XXI: Caracterización de las principales tipologías. Ge-Conserv. 2021, 20, 318–336. [Google Scholar]
- Miller, B.F. Painting materials research in Munich from 1825 to 1937. Stud. Conserv. 1998, 43, 246–248. [Google Scholar] [CrossRef]
- Mayer, R. Materiales y Técnicas del Arte; Hermann Blume Ediciones: Madrid, Spain, 2005. [Google Scholar]
- Pedrola, A. Materiales, Procedimientos y Técnicas Pictóricas; Editorial Ariel: Barcelona, Spain, 2019. [Google Scholar]
- Loganina, V.I.; Kislitsyna, S.N.; Mazhitov, Y.B. Development of sol-silicate composition for decoration of building walls. CSCM 2018, 9, e00173. [Google Scholar] [CrossRef]
- Gridchin, A.M.; Loganina, V.I.; Mazhitov, E.B.; Ryapukhin, A.N. Assessment of the Durability of Coatings Based on Sol Silicate Paint. In Proceedings of the Innovations and Technologies in Construction, BUILDINTECH BIT 2020, Belgorod, Russia, 8–9 October 2020. [Google Scholar] [CrossRef]
- Brandi, C. Teoria del Restauro; Nardini Editore: Florence, Italy, 2011. [Google Scholar]
- Magnain, C.; Elias, M.; Frigerio, J.-M. Influence of the artistic techniques on the visual appearance of complexions in art. In O3A: Optics for Arts, Architecture, and Archaeology II; SPIE: Bellingham, WA, USA, 2009; Volume 7391, pp. 72–84. [Google Scholar] [CrossRef]
- Jiménez-Desmond, D.; Pozo-Antonio, J.S.; Arizzi, A. The fresco wall painting techniques in the Mediterranean area from Antiquity to the present: A review. J. Cult. Herit. 2024, 66, 166–186. [Google Scholar] [CrossRef]
- Nevin, A. Pigment Alteration. In The Encyclopedia of Archaeological Sciences; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2025. [Google Scholar] [CrossRef]
- Napoleone, L. Integrazione pittorica. Acqua sporca, sottotono, tinta neutra, rigatino. Progett. Color. 2008, 2, 21–22. [Google Scholar]
- Merkus, H.G. Pigments and Paint Dispersions. In Particulate Products; Merkus, H., Meesters, G., Eds.; Springer International Publishing: New York, NY, USA, 2013; Volume 19, pp. 343–370. [Google Scholar] [CrossRef]
- Data Sheet; Nano ESTEL. Consolidant and Fixativ Product for Natural Stone, Brick, Terracotta, Mortars and Plasters. CTS SL n.d.:1–3. Available online: https://shop-espana.ctseurope.com/174-nano-estel-consolidante-a-base-de-nanosilice (accessed on 25 May 2023).
- Piovesan, R.; Mazzoli, C.; Maritan, L.; Cornale, P. Fresco and lime-paint: An experimental study and objective criteria for distinguishing between these painting techniques. Archaeometry 2012, 54, 723–736. [Google Scholar] [CrossRef]
- UNE-EN 933-1; Ensayos Para Determinar las Propiedades Geométricas de Los Áridos. Parte 1: Determinación de la Granulometría de las Partículas. Método de Tamizado. AENOR: Madrid, Spain, 2012.
- Brindley, G.W.; Brown, G. Crystal Structures of Clay Minerals and Their X-Ray Identification; Mineralogical Society: London, UK, 1982. [Google Scholar]
- Thorez, J. Phyllosilicates and Clay Minerals: A Laboratory Handbook for Their X-Ray Diffraction Analysis; Lelotte: Dison, Belgium, 1975. [Google Scholar]
- CIE S014-4/E:2007; Colorimetry-Part 4: CIE 1976 L* a* b* Colour Space. International Standard. International Organization for Standardization: Geneva, Switzerland, 2007.
- CIE Publication 15-2; Colorimetry. CIE Central Bureau: Vienna, Austria, 1986.
- UNE-EN ISO 2813; Pinturas y Barnices. Determinación del Índice de Brillo Especular a 20°, 60° y 85°. AENOR: Madrid, Spain, 2015.
- Bodart, M.; de Peñaranda, R.; Deneyer, A.; Flamant, G. Photometry and colorimetry characterisation of materials in daylighting evaluation tools. Build. Environ. 2008, 43, 2046–2058. [Google Scholar] [CrossRef]
- UNE-EN 828; Adhesivos. Mojabilidad. Determinación por Medida de Ángulo de Contacto y de la Tensión Superficial Crítica de la Superficie Sólida. AENOR: Madrid, Spain, 2013.
- UNE-EN ISO 21920-3; Geometrical Product Specifications (GPS)—Surface Texture: Profile—Part 3: Specification Operators. AENOR: Madrid, Spain, 2023.
- UNE-EN ISO 29862; Cintas Autoadhesivas. Determinación de las Propiedades Adhesivas de Pelado. AENOR: Madrid, Spain, 2020.
- Dariz, P.; Schmid, T. Trace compounds in Early Medieval Egyptian blue carry information on provenance, manufacture, application, and ageing. Sci. Rep. 2021, 11, 11296. [Google Scholar] [CrossRef]
- Derrick, M.R.; Stulik, D.; Landry, J.M. Scientific Tools for Conservation. In Infrared Spectroscopy in Conservation Science; The Getty Conservation Institute: Los Angeles, CA, USA, 1999. [Google Scholar]
- Mirtit, P.; Appolonia, L.; Casoli, A.; Ferrari, R.P.; Laurenti, E.; Amisano Canesi, A.; Chiari, G. Spectrochemical and structural studies on a Roman sample of Egyptian blue. Spectrochim. Acta Part A 1995, 51, 437–446. [Google Scholar] [CrossRef]
- Coimbra, M.M.; Martins, I.; Bruno, S.M.; Vaz, P.D.; Ribeiro-Claro, P.J.A.; Rudić, S.; Nolasco, M.M. Shedding Light on Cuprorivaite, the Egyptian Blue Pigment: Joining Neutrons and Photons for a Computational Spectroscopy Study. Cryst. Growth Des. 2023, 23, 4961–4969. [Google Scholar] [CrossRef]
- Anbalagan, G.; Prabakaran, A.R.; Gunasekaran, S. Spectroscopic characterization of Indian standard sand. J. Appl. Spectrosc. 2010, 77, 86–94. [Google Scholar] [CrossRef]
- Bishop, J.L.; Lane, M.D.; Dyar, M.D.; Brown, A.J. Reflectance and emission spectroscopy study of four groups of phyllosilicates: Smectites, kaolinite-serpentines, chlorites and micas. Clay Miner. 2008, 43, 35–54. [Google Scholar] [CrossRef]
- Silva, C.E.; Silva, L.P.; Edwards, H.G.M.; De Oliveira, L.F.C. Diffuse reflection FTIR spectral database of dyes and pigments. Anal. Bioanal. Chem. 2006, 386, 2183–2191. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Chervonnyi, A.D. Infrared Spectroscopy of Minerals and Related Compounds; Springer International Publishing: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Zilio, S.C.; Bagnato, V.S. Infrared Spectra of Natural Sodalite. J. Phys. Chem. 1984, 88, 1373–1376. [Google Scholar]
- Mofrad, A.M.; Peixoto, C.; Blumeyer, J.; Liu, J.; Hunt, H.K.; Hammond, K.D. Vibrational Spectroscopy of Sodalite: Theory and Experiments. J. Phys. Chem. C 2018, 122, 24765–24779. [Google Scholar] [CrossRef]
- Fernández-Carrasco, L.; Torrens-Martín, D.; Morales, L.M.; Martínez-Ramírez, S. Infrared spectroscopy in the analysis of building and construction materials. In Infrared Spectroscopy: Materials Science, Engineering and Technology; Theophanides, T., Ed.; InTech: Rijeka, Croatia, 2012; pp. 369–381. [Google Scholar]
- Friedrich, F.; Heissler, S.; Faubel, W.; Nüesch, R.; Weidler, P.G. Cu(II)-intercalated muscovite: An infrared spectroscopic study. Vib. Spectrosc. 2007, 43, 427–434. [Google Scholar] [CrossRef]
- Helwig, K. The Characterisation of Iron Earth Pigments Using Infrared Spectroscopy; Canadian Conservation Institute: Ottawa, ON, Canada, 1998. [Google Scholar]
- Moretto, L.M.; Orsega, E.F.; Mazzocchin, G.A. Spectroscopic methods for the analysis of celadonite and glauconite in Roman green wall paintings. J. Cult. Herit. 2011, 12, 384–391. [Google Scholar] [CrossRef]
- Fanost, A.; Gimat, A.; de Viguerie, L.; Martinetto, P.; Giot, A.C.; Clémancey, M.; Blondin, G.; Gaslain, F.; Glanville, H.; Walter, P.; et al. Revisiting the identification of commercial and historical green earth pigments. Colloids Surf. A Physicochem. Eng. Asp. 2020, 584, 124035. [Google Scholar] [CrossRef]
- Wang, F.; Qin, X.F.; Meng, Y.F.; Guo, Z.L.; Yang, L.X.; Ming, Y.F. Hydrothermal synthesis and characterization of α-Fe2O3 nanoparticles. Mater. Sci. Semicond. Process 2013, 16, 802–806. [Google Scholar] [CrossRef]
- Xiao, W.; Jones, A.M.; Collins, R.N.; Bligh, M.W.; David Waite, T. Use of fourier transform infrared spectroscopy to examine the Fe(II)-Catalyzed transformation of ferrihydrite. Talanta 2017, 175, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Salama, W.; El Aref, M.; Gaupp, R. Spectroscopic characterization of iron ores formed in different geological environments using FTIR, XPS, Mössbauer spectroscopy and thermoanalyses. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136, 1816–1826. [Google Scholar] [CrossRef] [PubMed]
- Wiesinger, R.; Pagnin, L.; Anghelone, M.; Moretto, L.M.; Orsega, E.F.; Schreiner, M. Pigment and Binder Concentrations in Modern Paint Samples Determined by IR and Raman Spectroscopy. Angew. Chem. Int. Ed. 2018, 57, 7401–7407. [Google Scholar] [CrossRef]
- Bai, Y.L.; Xu, H.B.; Zhang, Y.; Li, Z.H. Reductive conversion of hexavalent chromium in the preparation of ultra-fine chromia powder. J. Phys. Chem. Sol. 2006, 67, 2589–2595. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequenciues: Tables and Charts; John Wiley & Sons Ltd.: New York, NY, USA, 2004. [Google Scholar]
- Vahur, S.; Knuutinen, U.; Leito, I. ATR-FT-IR spectroscopy in the region of 500–230 cm−1 for identification of inorganic red pigments. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 73, 764–771. [Google Scholar] [CrossRef]
- Salvadó, N.; Butí, S.; Cotte, M.; Cinque, G.; Pradell, T. Shades of green in 15th century paintings: Combined microanalysis of the materials using synchrotron radiation XRD, FTIR and XRF. Appl. Phys. A 2013, 111, 47–57. [Google Scholar] [CrossRef]
- Prati, S.; Bonacini, I.; Sciutto, G.; Genty-Vincent, A.; Cotte, M.; Eveno, M.; Menu, M.; Mazzeo, R. ATR-FTIR microscopy in mapping mode for the study of verdigris and its secondary products. Appl. Phys. A 2016, 122, 10. [Google Scholar] [CrossRef]
- San Andrés, M.; De La Roja, J.M.; Baonza, V.G.; Sancho, N. Verdigris pigment: A mixture of compounds. Input from Raman spectroscopy. J. Raman Spectrosc. 2010, 41, 1468–1476. [Google Scholar] [CrossRef]
- Mokrzycki, W.; Tatol, M. Color difference Delta E-A survey Colour difference ∆E-A survey. MG&V 2011, 20, 383–411. [Google Scholar]
- Gomes, D.J.C.; De Souza, N.C.; Silva, J.R. Using a monocular optical microscope to assemble a wetting contact angle analyser. Measurement 2013, 46, 3623–3627. [Google Scholar] [CrossRef]
- Bico, J.; Thiele, U.; Quéré, D. Wetting of textured surfaces. Colloids Surf. A Physicochem. Eng. 2022, 206, 41–46. [Google Scholar] [CrossRef]
- Rubio, F.; Rubio, J.; Oteo, J.L. A FT-IR study of the hydrolysis of Tetraethylorthoselicate (TEOS). Spectrosc. Lett. 1998, 31, 199–219. [Google Scholar] [CrossRef]
- De Ferri, L.; Lottici, P.P.; Lorenzi, A.; Montenero, A.; Salvioli-Mariani, E. Study of silica nanoparticles-polysiloxane hydrophobic treatments for stone-based monument protection. J. Cult. Herit. 2011, 12, 356–363. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; Otero, J.; Alonso, P.; Mas i Barberà, X. Nanolime- and nanosilica-based consolidants applied on heated granite and limestone: Effectiveness and durability. Constr. Build. Mater. 2019, 201, 852–870. [Google Scholar] [CrossRef]
- Barberena-Fernández, A.M.; Carmona-Quiroga, P.M.; Blanco-Varela, M.T. Interaction of TEOS with cementitious materials: Chemical and physical effects. Cem. Concr. Compos. 2015, 55, 145–152. [Google Scholar] [CrossRef]
- Paineau, E.; Bihannic, I.; Baravian, C.; Philippe, A.M.; Davidson, P.; Levitz, P.; Funari, S.S.; Rochas, C.; Michot, L.J. Aqueous suspensions of natural swelling clay minerals. 1. structure and electrostatic interactions. Langmuir 2011, 27, 5562–5573. [Google Scholar] [CrossRef]
- Jiménez-Desmond, D.; Pozo-Antonio, J.S.; Arizzi, A. Outdoor durability of nano-sized silica-based chromatic reintegrations. Influence of exposure conditions and pigment composition. Dyes Pig. 2025, 235, 112651. [Google Scholar] [CrossRef]
- Cockell, C.S.; Pybus, D.; Olsson-Francis, K.; Kelly, L.; Petley, D.; Rosser, N.; Howard, K.; Mosselmans, F. Molecular Characterization and Geological Microenvironment of a Microbial Community Inhabiting Weathered Receding Shale Cliffs. Environ. Microbiol. 2011, 61, 166–181. [Google Scholar] [CrossRef]
- Licchelli, M.; Malagodi, M.; Weththimuni, M.; Zanchi, C. Nanoparticles for conservation of bio-calcarenite stone. Appl. Phys. A 2014, 114, 673–683. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; Noya, D.; Montojo, C. Aesthetic Effects on Granite of Adding Nanoparticle TiO2 to Si-Based Consolidants (Ethyl Silicate or Nano-Sized Silica). Coatings 2020, 10, 215. [Google Scholar] [CrossRef]
- Borsoi, G.; Veiga, R.; Santos Silva, A. Effect of nanostructured lime-based and silica-based products on the consolidation of historical renders. In Proceedings of the 3rd Historic Mortars Conference, Glasgow, Scotland, 11–13 September 2013. [Google Scholar]
- López-Martínez, T.; Collado-Montero, F.J.; García-Bueno, A. Consolidation tests in archaeological wall painting: Comparing treatments depending on the painting technique. Conserv. Património 2022, 39, 33–44. [Google Scholar]
- Zhuravlev, L.T. The surface chemistry of amorphous silica. Zhuravlev model. Colloids Surf. A Physicochem. Eng. Asp. 2000, 173, 1–38. [Google Scholar] [CrossRef]
- Hofmann, C.; Hartl, A.; Ahn, K.; Druceikaite, K.; Henniges, U.; Potthast, A. Stabilization of Verdigris. J. Pap. Conserv. 2016, 17, 88–99. [Google Scholar] [CrossRef]
- Brostoff, L.B.; Connelly Ryan, C. Tracing the Alteration of Verdigris Pigment through Combined Raman Spectroscopy and X-ray Diffraction, Part I. Restaurator 2020, 4, 3–30. [Google Scholar] [CrossRef]
- Damiani, D.; Gliozzo, E.; Memmi, I.T. The ‘Madonna and Child Enthroned with Saints’ of Ambrogio Lorenzetti in the St. Augustine Church (Siena, Italy): Raman microspectroscopy and SEM-EDS characterisation of the pigments. Archaeol. Anthropol. Sci. 2014, 6, 363–371. [Google Scholar] [CrossRef]
- Asensi Muñoz, R. Documentación y Análisis del Tríptico Ejecutado por Matarana en la Capilla del Colegio Corpus Christi. Ph.D. Thesis, Polytechnic University, Valencia, Spain, 2014. [Google Scholar]
- Bette, S.; Eggert, G.; Emmerling, S.; Etter, M.; Schleid, T.; Dinnebier, R.E. Crystal Structure, Polymorphism, and Anisotropic Thermal Expansion of α-Ca(CH3COO)2. Cryst. Growth. Des. 2020, 20, 5346–5355. [Google Scholar] [CrossRef]
- Toupance, T.; Kermarec, M.; Lambert, J.F.; Louis, C. Conditions of formation of copper phyllosilicates in silica-supported copper catalysts prepared by selective adsorption. J. Phys. Chem. B 2002, 106, 2277–2286. [Google Scholar] [CrossRef]
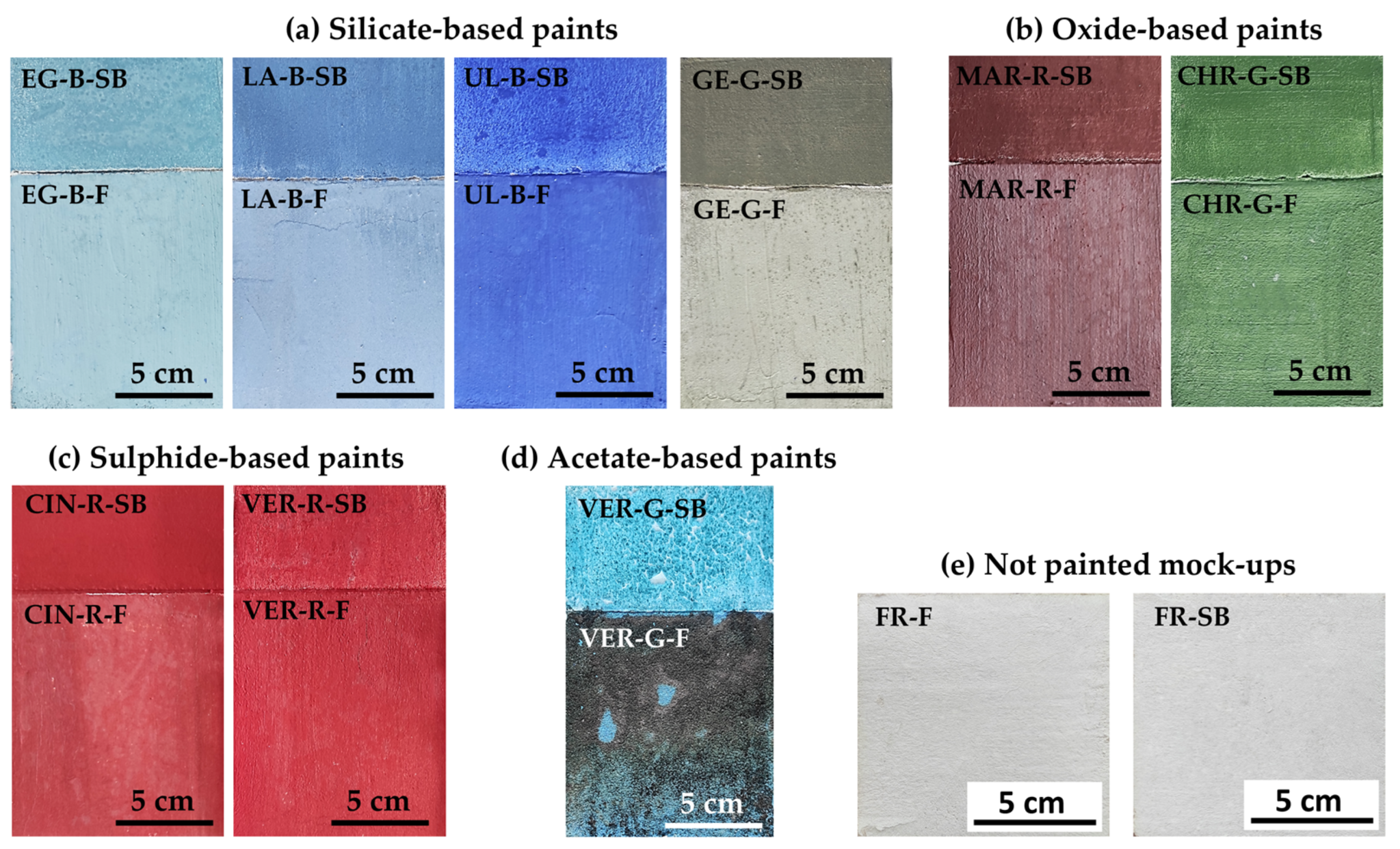

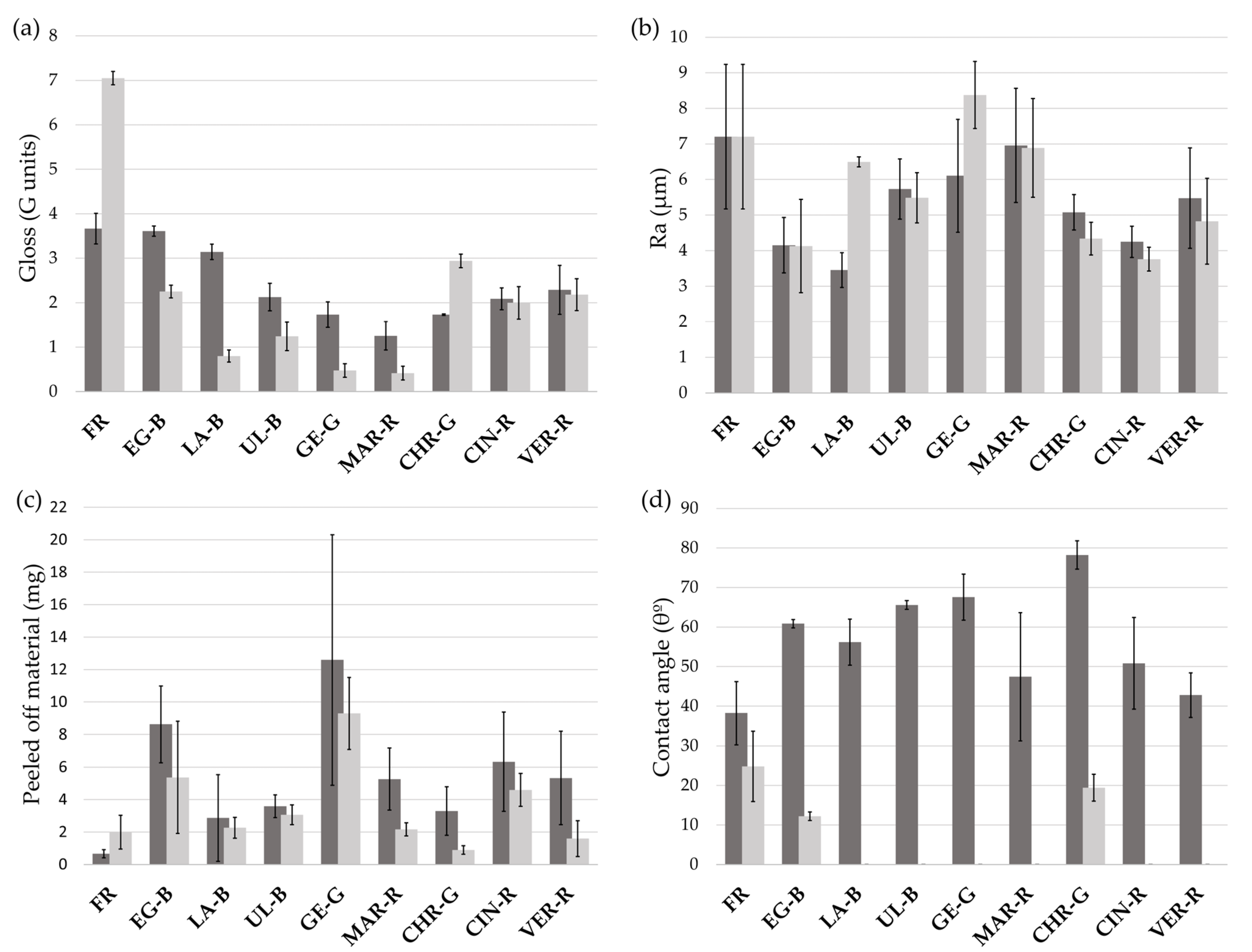
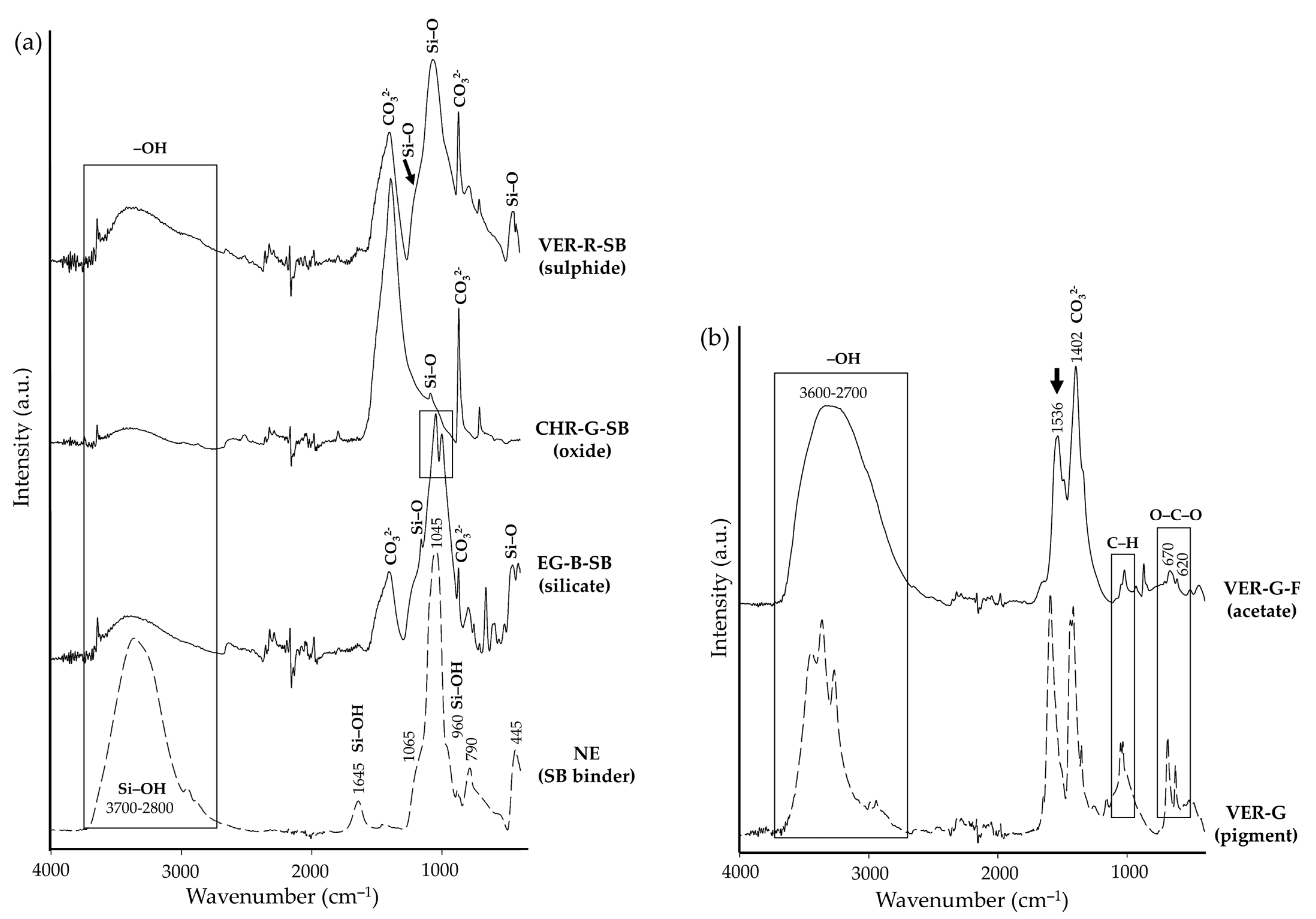
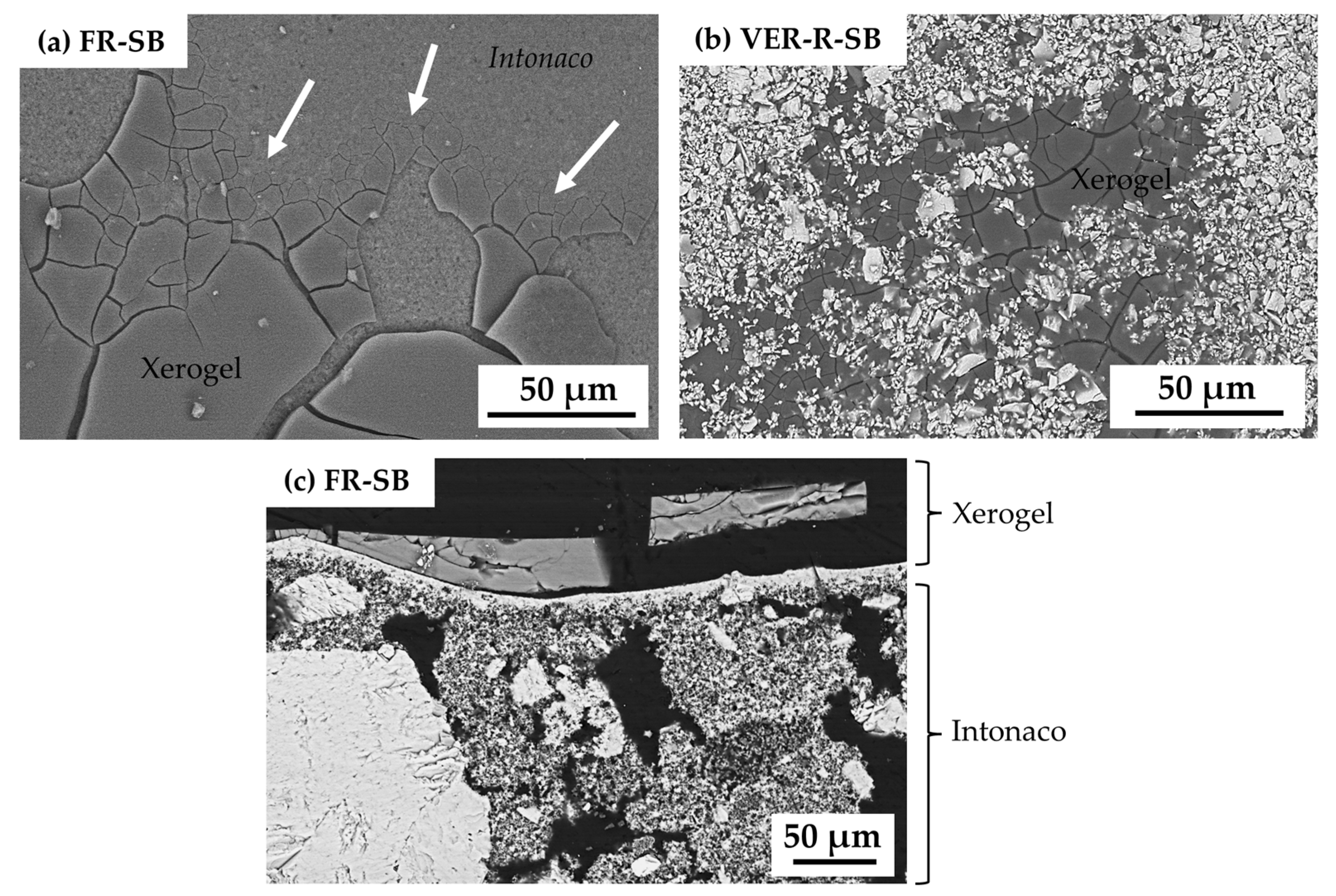
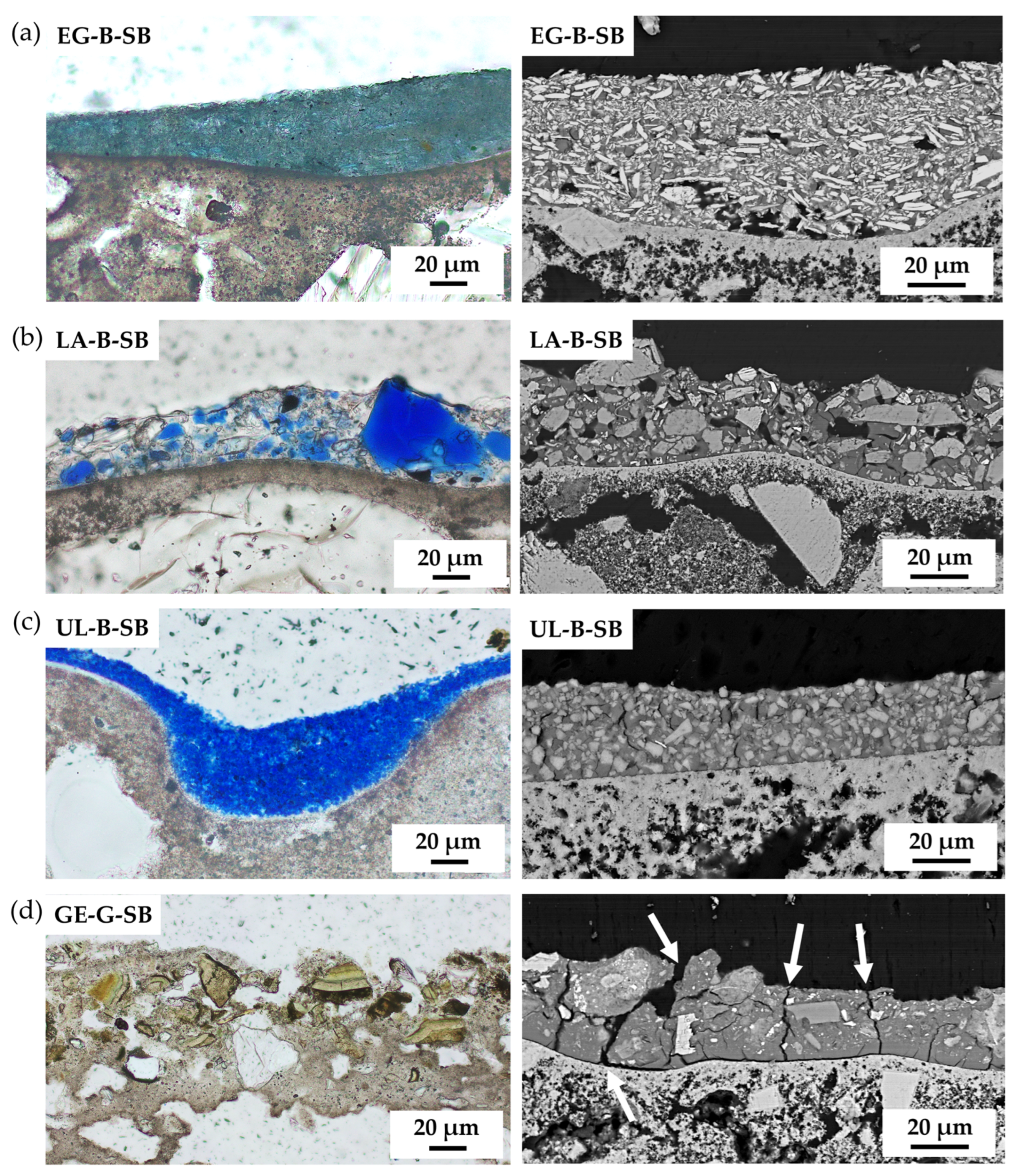
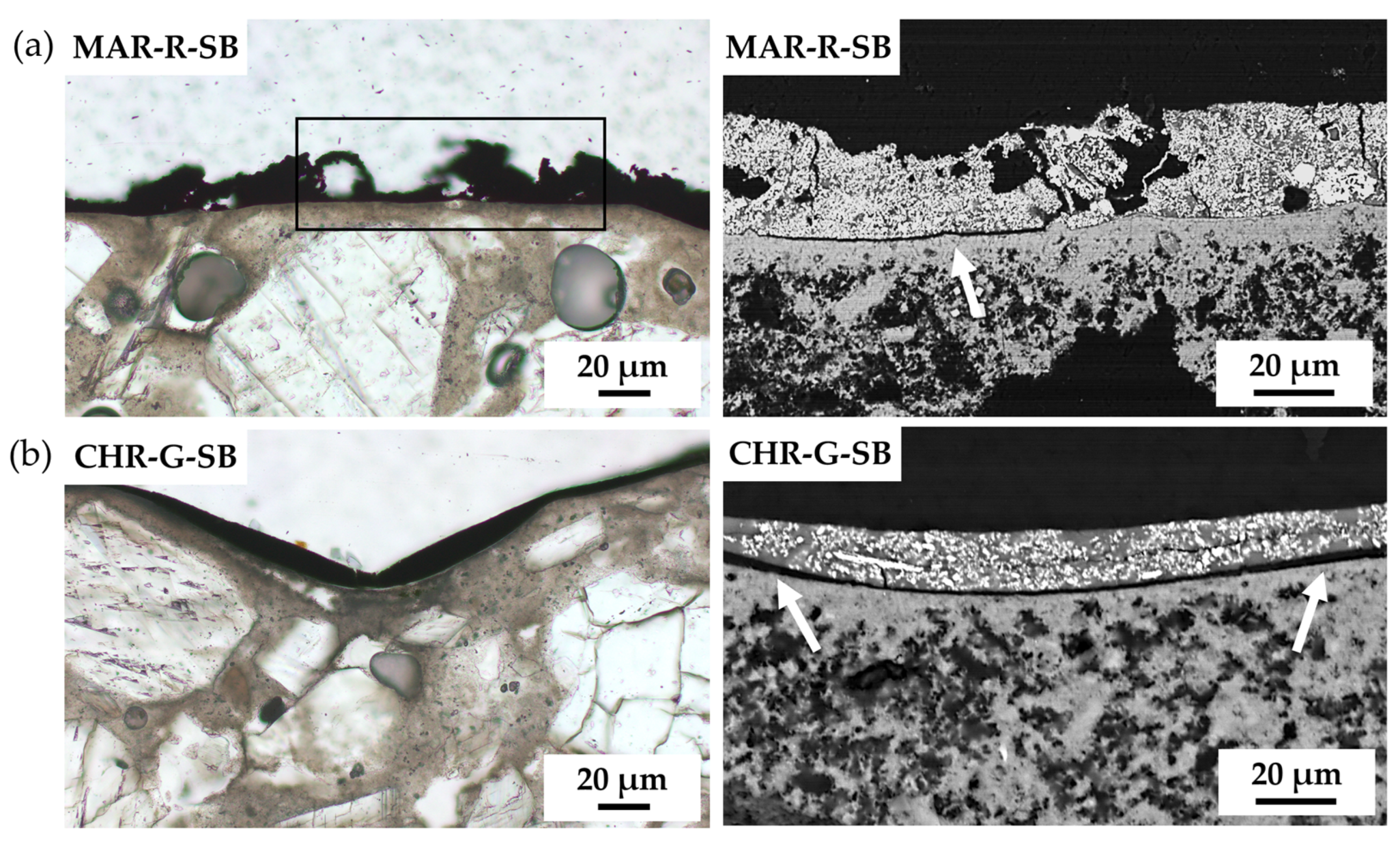

| Supplier’s Code | Authors Code | Supplier’s Particle Size | Authors Particle Size 1 | Supplier’s Composition | Authors Mineralogical Composition by XRD | Authors Chemical Composition by XRF |
|---|---|---|---|---|---|---|
| Calcitic lime paste | CLP | - | - | Calcium hydroxide, Ca(OH)2 | Portlandite, Ca(OH)2 Calcite, CaCO3 | - |
| Silica E1 JP | C-SI | 1–2 mm | R: 1.6–4 mm | Silica, SiO2 | Quartz, SiO2 Rutile, TiO2 Albite, NaAlSi3O8 | Si (97.39%) and Al (1.23%) <1%: Fe, Mg, Ca, Na, K, Ti, P, and S |
| Silica E1 A0052P JP | F-SI | 0.4–0.8 mm | R: 0.125–1.6 mm | Silica, SiO2 | Quartz, SiO2 Rutile, TiO2 Anorthoclase, (Na,K)AlSi3O Microcline, KAlSi3O8 | Si (93.03%), Al (3.23%), and K (2.41%) <1%: Fe, Mg, Ca, Na, Ti, P, and S |
| Bianco Carrara MK 000 | MRB-P | 0.0–0.7 mm | R: 0.0–0.5 mm | Not supplied | Calcite, CaCO3 Quartz, SiO2 Dolomite, CaMg(CO3)2 | Ca (55.55%) and Mg (1.08%) <1%: Si, Al, Fe, Na, K, P, and S |
| Ref. 100601 Egyptian blue | EG-B | <10 µm | R: 0.3–100 µm Mm: 20 µm | Cuprorivaite, CaCuSi4O10 | Cuprorivaite, CaCuSi4O10 Quartz, SiO2 | 1–49%: Si, Cu, and Ca <1%: Al |
| Ref. 10510 Lapis lazuli, medium quality from Afghanistan | LA-B | Not supplied | R: 0.3–100 µm Mm: 20 µm | Sodium calcium aluminium silicate, 3NaAlSiO4·NaS3 | Lazurite, Na3Ca(Al3Si3O12)S Sodalite, Na8Al6Si6O24Cl2 Calcite, CaCO3 Diopside, CaMgSi2O6 Pyrite, FeS2 Albite, (Na,Ca)(Si,Al)4O8 Muscovite, KAl2Si3AlO10(OH)2 Wollastonite, CaSiO3 | 1–49%: Si, Ca, Al, S, Fe, and Mg <1%: Cl and Ti |
| Ref. 45010 Ultramarine blue, dark, synthetic mineral pigment | UL-B | 2.50 µm | R: 0.7–35 µm Mm: 2.5 (25) µm | Sodium aluminium sulfo silicate, Na7Ai6Si6O24S3, and Kaolinite, Al2Si2O5(OH)4 | Lazurite, Na3Ca(Al3Si3O12)S Sodalite, Na8Al6Si6O24Cl2) Nepheline, Na,K(Al4Si4O16) Kaolinite, Al2Si2O5(OH)4 | 1–49%: Si, S, and Al <1%: Fe |
| Ref. 11010 Green Verona earth, extra fine celadonite | GE-G | 0–80 µm | R: 0.3–125 µm Mm: 35 (2.5) µm | Celadonite, K(Mg,Fe)Fe3+Si4O10(OH)2 | Glauconite, (K,Na)(Fe3+,Al,Mg)2(Si,Al)4O10(OH)2 Celadonite, K(Mg,Fe)Fe3+Si4O10(OH)2 Muscovite, KAl2(AlSi3O10)(OH)2 Calcite, CaCO3 Clinochlore, (Mg,Fe2+)5Al(Si3Al)O10(OH)8 Albite, NaAlSi3O8 Montmorillonite, (Na,Ca)0,3(Al,Mg)2Si4O10(OH)2 ·nH2O Kaolinite, Al2Si2O5(OH)4 | 1–49%: Si, Ca, Fe, Mg, Al, and Ti <1%: Mn |
| Ref. 48289 Iron oxide red, pure micronised, highly resistant to light | MAR-R | 0.97 µm | R: 0.15–40 µm Mm: 4 (15) µm | Synthetic iron (III) oxide αFe2O3 | Hematite, Fe2O3 | 1–49%: Fe and Mg <1%: Al, Mn, Cl, and Ca |
| Ref. 44200 Opaque chromium oxide green | CHR-G | 0.3 µm | R: 0.15–30 µm Mm: 1.8 (15) µm | Chrome (III) oxide, Cr2O3 | Eskolaite, Cr2O3 | 50–99%: Cr 1–49%: Mg and Al <1%: Ca and Mn |
| Ref. 10624 Cinnabar, very fine, chien t’ou | CIN-R | <20 µm | R: 0.15–35 µm Mm: 6 (0.35) µm | Cinnabar, HgS | Cinnabar, HgS | 50–99%: Hg 1–49%: S and Si <1%: Mo, Th, Rb, Nb, Sb, Ba, P, Fe, and Al |
| Ref. 42000 Vermilion, synthetic mercury sulphide from China | VER-R | Not supplied | R: 0.15–40 µm Mm: 3 (0.35) µm | Mercuric sulphide, HgS <100% | Cinnabar, HgS | 50–99%: Hg 1–49%: S and Si <1%: Mo, Th, Rb, Nb, Fe, Al, and P |
| Ref. 44450 Verdigris, synthetic | VER-G | Not supplied | R: 0.3–550 µm Mm: 150 (4.5) µm | Copper (II)-acetate-1-hydrate, C4H6CuO4·H2O | Hoganite, Cu(CH3COO)2·H2O | 1–49%: Cu <1%: Al and Ca |
| Ref. 02002601 Nano Estel | NE | 10–20 nm | - | Colloidal aqueous dispersion of silica, SiO2 | - | - |
| ID | L* | a* | b* | C*ab | h* |
|---|---|---|---|---|---|
| FR-F | 94.33 ± 0.14 | 0.24 ± 0.05 | 2.54 ± 0.22 | 2.55 ± 0.22 | 84.51 ± 0.99 |
| FR-SB | 93.52 ± 0.42 | 0.29 ± 0.07 | 2.46 ± 0.32 | 2.48 ± 0.31 | 83.17 ± 1.82 |
| EG-B | 69.77 ± 4.31 | −10.81 ± 1.11 | −14.19 ± 1.24 | 17.85 ± 1.66 | 232.74 ± 0.44 |
| EG-B-F | 74.21 ± 1.08 | −9.52 ± 0.13 | −13.34 ± 0.84 | 16.40 ± 0.66 | 234.42 ± 1.88 |
| EG-B-SB | 66.39 ± 0.33 | −11.91 ± 0.13 | −15.17 ± 0.09 | 19.29 ± 0.03 | 231.87 ± 0.45 |
| LA-B | 56.53 ± 0.63 | −2.58 ± 0.03 | −23.81 ± 0.11 | 23.95 ± 0.12 | 263.82 ± 0.06 |
| LA-B-F | 70.98 ± 0.85 | −2.91 ± 0.08 | −13.92 ± 0.73 | 14.21 ± 0.72 | 258.19 ± 0.72 |
| LA-B-SB | 51.82 ± 2.37 | −2.91 ± 0.08 | −25.22 ± 0.78 | 25.39 ± 0.80 | 263.31 ± 0.80 |
| UL-B | 39.31 ± 1.05 | 20.04 ± 0.83 | −62.97 ± 0.86 | 66.08 ± 1.07 | 287.65 ± 0.46 |
| UL-B-F | 44.65 ± 0.65 | 14.97 ± 0.63 | −54.11 ± 0.58 | 56.15 ± 0.60 | 285.46 ± 0.63 |
| UL-B-SB | 39.61 ± 1.76 | 20.61 ± 1.73 | −61.68 ± 0.91 | 65.04 ± 1.43 | 288.45 ± 1.22 |
| GE-G | 56.45 ± 0.66 | −1.69 ± 0.03 | 11.65 ± 0.95 | 11.77 ± 0.95 | 98.27 ± 0.55 |
| GE-G-F | 72.52 ± 1.75 | −1.30 ± 0.07 | 8.77 ± 0.52 | 8.87 ± 0.52 | 98.43 ± 0.25 |
| GE-G-SB | 54.07 ± 1.86 | −1.51 ± 0.22 | 13.87 ± 1.43 | 13.95 ± 1.39 | 96.33 ± 1.52 |
| MAR-R | 34.58 ± 1.22 | 18.97 ± 1.80 | 11.00 ± 0.66 | 21.93 ± 1.89 | 30.16 ± 0.90 |
| MAR-R-F | 42.81 ± 1.04 | 13.08 ± 1.21 | 4.75 ± 1.11 | 13.93 ± 1.51 | 19.71 ± 2.70 |
| MAR-R-SB | 33.54 ± 0.04 | 17.45 ± 0.20 | 10.44 ± 0.09 | 20.33 ± 0.21 | 30.88 ± 0.16 |
| CHR-G | 51.41 ± 0.90 | −15.74 ± 0.37 | 17.11 ± 1.36 | 23.26 ± 1.25 | 132.68 ± 1.59 |
| CHR-G-F | 49.57 ± 0.25 | −14.10 ± 0.20 | 14.77 ± 0.41 | 20.42 ± 0.43 | 133.68 ± 0.39 |
| CHR-G-SB | 50.32 ± 1.01 | −15.58 ± 0.80 | 17.57 ± 0.94 | 23.48 ± 1.22 | 131.55 ± 0.54 |
| CIN-R | 48.73 ± 1.07 | 36.96 ± 3.37 | 22.69 ± 2.46 | 43.38 ± 4.05 | 31.52 ± 1.33 |
| CIN-R-F | 52.03 ± 0.49 | 35.53 ± 0.55 | 21.01 ± 0.96 | 41.28 ± 0.96 | 30.58 ± 0.78 |
| CIN-R-SB | 45.73 ± 1.59 | 37.02 ± 0.61 | 23.62 ± 0.44 | 43.91 ± 0.75 | 32.54 ± 0.16 |
| VER-R | 48.10 ± 0.63 | 41.74 ± 2.16 | 24.72 ± 2.56 | 48.52 ± 3.09 | 30.58 ± 1.56 |
| VER-R-F | 49.17 ± 0.62 | 40.99 ± 0.89 | 24.17 ± 0.94 | 47.59 ± 1.23 | 30.51 ± 1.23 |
| VER-R-SB | 47.67 ± 0.16 | 43.49 ± 0.89 | 26.59 ± 1.77 | 50.97 ± 2.46 | 31.42 ± 2.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Desmond, D.; Pozo-Antonio, J.S.; Arizzi, A.; López-Martínez, T. Physico-Chemical Compatibility of an Aqueous Colloidal Dispersion of Silica Nano-Particles as Binder for Chromatic Reintegration in Wall Paintings. Appl. Sci. 2025, 15, 3690. https://doi.org/10.3390/app15073690
Jiménez-Desmond D, Pozo-Antonio JS, Arizzi A, López-Martínez T. Physico-Chemical Compatibility of an Aqueous Colloidal Dispersion of Silica Nano-Particles as Binder for Chromatic Reintegration in Wall Paintings. Applied Sciences. 2025; 15(7):3690. https://doi.org/10.3390/app15073690
Chicago/Turabian StyleJiménez-Desmond, Daniel, José Santiago Pozo-Antonio, Anna Arizzi, and Teresa López-Martínez. 2025. "Physico-Chemical Compatibility of an Aqueous Colloidal Dispersion of Silica Nano-Particles as Binder for Chromatic Reintegration in Wall Paintings" Applied Sciences 15, no. 7: 3690. https://doi.org/10.3390/app15073690
APA StyleJiménez-Desmond, D., Pozo-Antonio, J. S., Arizzi, A., & López-Martínez, T. (2025). Physico-Chemical Compatibility of an Aqueous Colloidal Dispersion of Silica Nano-Particles as Binder for Chromatic Reintegration in Wall Paintings. Applied Sciences, 15(7), 3690. https://doi.org/10.3390/app15073690







