Accuracy of Measurement Tools for Ocular-Origin Anomalous Head Posture and the Cervical Range of Motion Kinematics in Children with an Anomalous Head Position
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design and Instrumentation
2.2.1. Digital Goniometer
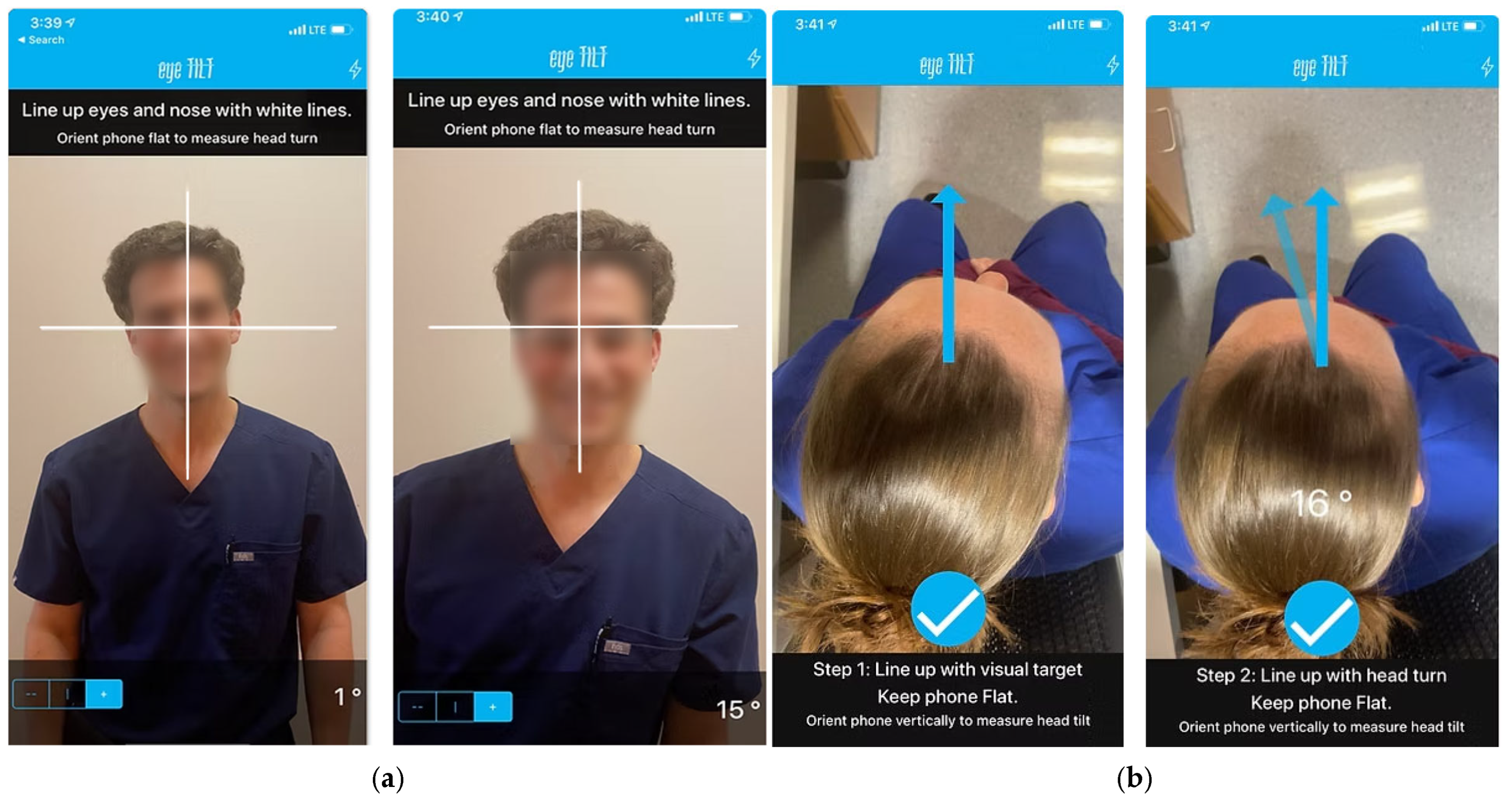
2.2.2. iOS Application
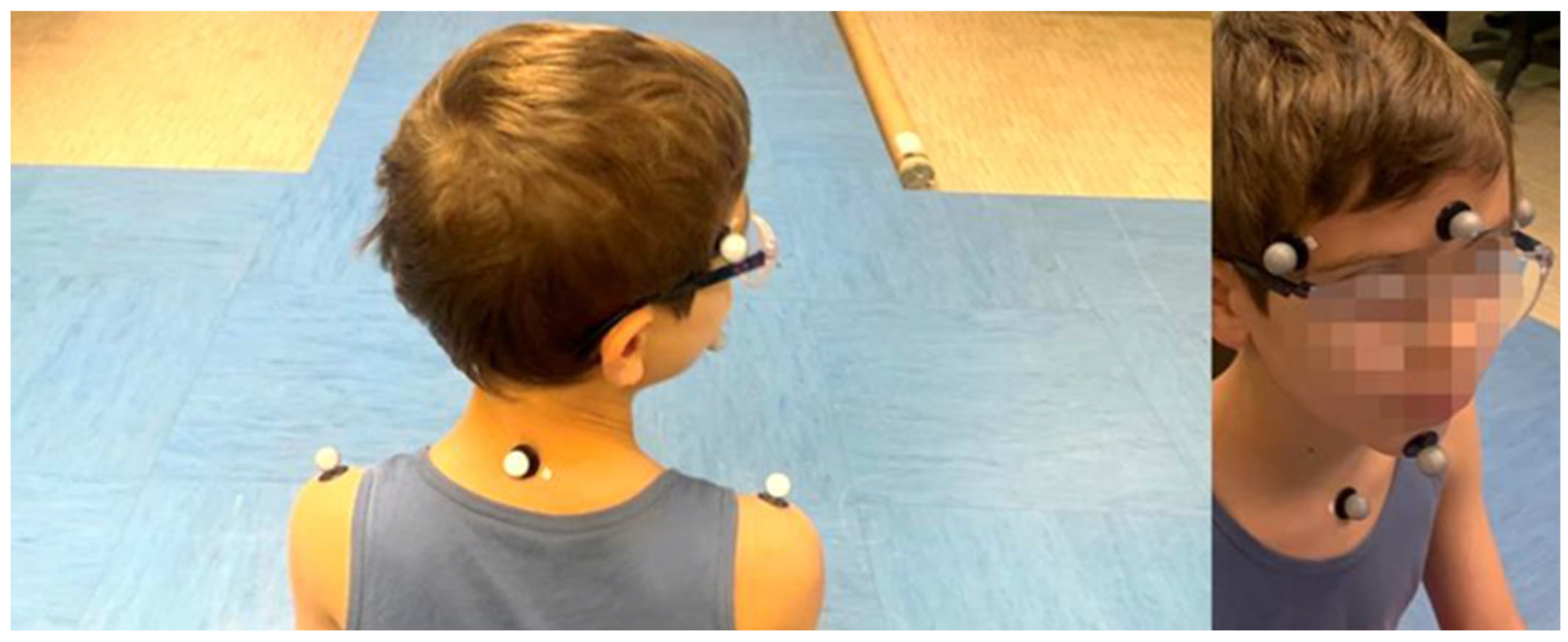
2.2.3. MoCap System
2.2.4. Experimental Procedures
- Head flexion/extension (Figure 3a): Starting from their natural neutral position, participants were instructed to perform controlled head flexion by gradually lowering their head forward and bringing the chin toward the chest. After returning to the neutral position, participants then executed a controlled head extension, tilting the head backward toward the scapulae and lifting the chin toward the ceiling.
- Head lateral bending toward left/right (Figure 3b): From the neutral position, participants were asked to tilt their head laterally toward one shoulder, return to the neutral position, and then repeat the movement to the opposite side.
- Head rotation toward left/right (Figure 3c): Starting from their neutral position, participants performed a controlled head rotation to the right, moving the chin toward the shoulder. After returning to the neutral position, the same movement was repeated to the left.
- Returning to the neutral position, the same movement was repeated toward the left.
2.3. Data Analysis and Parameters
2.4. Statistical Analysis
3. Results
3.1. Comparison Between MoCap and Ophthalmologic Tools
3.2. Assessment of Head Movement Limitations in Individuals with AHP
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHP | Anomalous head posture; |
| ROM | Range of motion; |
| DG | Digital goniometer. |
References
- Akbari, M.R.; Khorrami-Nejad, M.; Kangari, H.; Akbarzadeh Baghban, A.; Ranjbar Pazouki, M. Ocular Abnormal Head Posture: A Literature Review. J. Curr. Ophthalmol. 2021, 33, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.R. Ocular torticollis. Trans. Am. Ophthalmol. Soc. 1999, 97, 697–769. [Google Scholar] [PubMed]
- Nucci, P.; Kushner, B.J.; Serafino, M.; Orzalesi, N. A multi-disciplinary study of the ocular, orthopedic, and neurologic causes of abnormal head postures in children. Am. J. Ophthalmol. 2005, 140, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, J.A. Abnormal head posture: An ophthalmological approach. Binocul. Vis. Strabismus Q. 2000, 15, 237–239. [Google Scholar]
- Campobasso, A.; Di Cosola, M.; Testa, N.F.; Lacarbonara, V.; Dioguardi, M.; Lo Muzio, L.; Lo Muzio, E.; Scarpa, M.; Covelli, M.; Crincoli, V.; et al. The influence of abnormal head posture on facial asymmetry. J. Biol. Regul. Homeost. Agents 2022, 36, 325–335. [Google Scholar]
- Yadegari, S. Approach to abnormal head posture. Strabismus 2024, 32, 287–293. [Google Scholar] [CrossRef]
- Davitt, B.V. Abnormal Head Postures: A Review. Am. Orthopt. J. 2001, 51, 137–143. [Google Scholar] [CrossRef]
- Amaral, D.M.; Cadilha, R.P.B.S.; Rocha, J.A.G.M.; Silva, A.I.G.; Parada, F. Congenital muscular torticollis: Where are we today? A retrospective analysis at a tertiary hospital. Porto Biomed. J. 2019, 4, e36. [Google Scholar] [CrossRef]
- Ballock, R.T.; Song, K.M. The prevalence of nonmuscular causes of torticollis in children. J. Pediatr. Orthop. 1996, 16, 500–504. [Google Scholar] [CrossRef]
- Kushner, B.J. Ocular causes of abnormal head postures. Ophthalmology 1979, 86, 2115–2125. [Google Scholar] [CrossRef]
- Rao, R.; Morton, G.V.; Kushner, B.J. Ocular torticollis and facial asymmetry. Binocul. Vis. Strabismus Q. 1999, 14, 27–32. [Google Scholar] [PubMed]
- Nilesh, K.; Mukherji, S. Congenital muscular torticollis. Ann. Maxillofac. Surg. 2013, 3, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, S.L.; Coulter, C.; Sargent, B. Physical Therapy Management of Congenital Muscular Torticollis: A 2018 Evidence-Based Clinical Practice Guideline From the APTA Academy of Pediatric Physical Therapy. Pediatr. Phys. Ther. 2018, 30, 240–290. [Google Scholar] [CrossRef] [PubMed]
- Sudesh, P.; Bali, K.; Mootha, A.K.; Dhillon, M.S. Results of Bipolar Release in the Treatment of Congenital Muscular Torticolis in Patients Older Than 10 Years of Age. J. Child. Orthop. 2010, 4, 227–232. [Google Scholar] [CrossRef]
- Cheng, J.C.Y.; Au, A.W.Y. Infantile Torticollis: A Review of 624 Cases. J. Pediatr. Orthop. 1994, 14, 802–808. [Google Scholar] [CrossRef]
- Herman, M.J. Torticollis in infants and children: Common and unusual causes. Instr. Course Lect. 2006, 55, 647–653. [Google Scholar]
- Wang, P.; Lou, L.; Song, L. Design and Efficacy of Surgery for Horizontal Idiopathic Nystagmus with Abnormal Head Posture and Strabismus. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 2011, 31, 678–681. [Google Scholar] [CrossRef]
- Turan, K.E.; Şekeroğlu, H.T.; Koç, İ.; Vural, E.; Karakaya, J.; Şener, E.C.; Sanaç, A.Ş. Ocular Causes of Abnormal Head Position: Strabismus Clinic Data. Turk. J. Ophthalmol. 2017, 47, 211. [Google Scholar] [CrossRef]
- Reddy, A.C.; Portilla, A.; Donahue, S.P. Purely horizontal strabismus associated with head tilt. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2018, 22, 69–71. [Google Scholar] [CrossRef]
- Noval, S.; González-Manrique, M.; Valle, J.M.R.-D.; Rodríguez-Sánchez, J.M. Abnormal Head Position in Infantile Nystagmus Syndrome. ISRN Ophthalmol. 2011, 2011, 594848. [Google Scholar] [CrossRef][Green Version]
- Teodorescu, L. Anomalous head postures in strabismus and nystagmus diagnosis and management. Rom. J. Ophthalmol. 2015, 59, 137–140. [Google Scholar] [PubMed]
- Papageorgiou, E.; McLean, R.J.; Gottlob, I. Nystagmus in childhood. Pediatr. Neonatol. 2014, 55, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Zanin, E.; Gambarelli, N.; Denis, D. Distinctive Clinical Features of Bilateral Duane Retraction Syndrome. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2010, 14, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.R.; Manouchehri, V.; Mirmohammadsadeghi, A. Surgical treatment of Duane retraction syndrome. J. Curr. Ophthalmol. 2017, 29, 248–257. [Google Scholar] [CrossRef]
- Carreira, F.; Pereira, B.; Rodrigues, M.; Borges, M.; Silva, S.; Sá, C. Postural Changes in Adolescents with Refractive Errors. Revsalus—Rev. Científica Da Rede Académica Das Ciências Da Saúde Da Lusofonia. 2024. Available online: https://revsalus.com/index.php/RevSALUS/article/view/595/472 (accessed on 24 March 2025).
- Havertape, S.A.; Cruz, O.A. Abnormal head posture associated with high hyperopia. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 1998, 2, 12–16. [Google Scholar] [CrossRef]
- Wu, P.; Ma, J.; Zhang, T.; Ma, D. Advances in the Genetics of Congenital Ptosis. Ophthalmic Res. 2022, 65, 131–139. [Google Scholar] [CrossRef]
- Kushner, B.J. The influence of head tilt on ocular torsion in patients with superior oblique muscle palsy. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2009, 13, 132–135. [Google Scholar] [CrossRef]
- Akbari, M.R.; Khorrami Nejad, M.; Askarizadeh, F.; Pour, F.F.; Ranjbar Pazooki, M.; Moeinitabar, M.R. Facial asymmetry in ocular torticollis. J. Curr. Ophthalmol. 2015, 27, 4–11. [Google Scholar] [CrossRef]
- Kraft, S.P.; O’Donoghue, E.P.; Roarty, J.D. Improvement of compensatory head postures after strabismus surgery. Ophthalmology 1992, 99, 1301–1308. [Google Scholar] [CrossRef]
- Kraft, S.P. Oblique Muscle Surgery for Head Tilt Caused by Congenital Motor Nystagmus. Am. Orthopt. J. 1996, 46, 143–149. [Google Scholar] [CrossRef]
- Burchell, V.-J.; Arblaster, G.; Buckley, D.; Wheat, J. Is a Depth Camera in Agreement with an Electromagnetic Tracking Device when Measuring Head Position? Br. Ir. Orthopt. J. 2021, 17, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Raya, R.; Garcia-Carmona, R.; Sanchez, C.; Urendes, E.; Ramirez, O.; Martin, A.; Otero, A. An Inexpensive and Easy to Use Cervical Range of Motion Measurement Solution Using Inertial Sensors. Sensors 2018, 18, 2582. [Google Scholar] [CrossRef] [PubMed]
- Kushner, B.J. The usefulness of the cervical range of motion device in the ocular motility examination. Arch. Ophthalmol. 2000, 118, 946–950. [Google Scholar] [PubMed]
- Tousignant, M.; Smeesters, C.; Breton, A.-M.; Breton, E.; Corriveau, H. Criterion validity study of the cervical range of motion (CROM) device for rotational range of motion on healthy adults. J. Orthop. Sports Phys. Ther. 2006, 36, 242–248. [Google Scholar] [CrossRef]
- Tousignant, M.; Duclos, E.; Laflèche, S.; Mayer, A.; Tousignant-Laflamme, Y.; Brosseau, L.; O’Sullivan, J.P. Validity study for the cervical range of motion device used for lateral flexion in patients with neck pain. Spine 2002, 27, 812–817. [Google Scholar] [CrossRef]
- Fletcher, J.P.; Bandy, W.D. Intrarater reliability of CROM measurement of cervical spine active range of motion in persons with and without neck pain. J. Orthop. Sports Phys. Ther. 2008, 38, 640–645. [Google Scholar] [CrossRef]
- Kim, J.; Nam, K.W.; Jang, I.G.; Yang, H.K.; Kim, K.G.; Hwang, J.-M. Nintendo Wii remote controllers for head posture measurement: Accuracy, validity, and reliability of the infrared optical head tracker. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1388–1396. [Google Scholar] [CrossRef]
- Yamamoto, M.; Yagasaki, T.; Ichikawa, S.; Nakamura, T.; Konishi, Y. Accuracy and Correlation of the Kinect-Based Semi-Automatic Scoring Method for Measuring Anomalous Head Posture as Compared to the CROM® Device. Clin. Ophthalmol. 2022, 16, 4033–4040. [Google Scholar] [CrossRef]
- Oh, B.L.; Kim, J.; Kim, J.; Hwang, J.; Lee, J. Validity and Reliability of Head Posture Measurement Using Microsoft Kinect. Br. J. Ophthalmol. 2014, 98, 1560–1564. [Google Scholar] [CrossRef]
- Darby, J.; Sánchez, M.B.G.; Butler, P.B.; Loram, I.D. An Evaluation of 3D Head Pose Estimation Using the Microsoft Kinect V2. Gait Posture 2016, 48, 83–88. [Google Scholar] [CrossRef]
- Kim, S.J.; Jeong, S.H.; Yoon, T.L. The Effect of Visual Feedback of Head Angles with Using a Mobile Posture-Aware System on Craniocervical Angle and Neck and Shoulder Muscles Fatigue During Watching the Smartphone. J. Korean Phys. Ther. 2018, 30, 47–53. [Google Scholar] [CrossRef]
- Gallego-Izquierdo, T.; Arroba-Díaz, E.; García-Ascoz, G.; Val-Cano, M.D.A.; Pecos-Martín, D.; Cano-de-la-Cuerda, R. Psychometric Proprieties of a Mobile Application to Measure the Craniovertebral Angle a Validation and Reliability Study. Int. J. Environ. Res. Public Health 2020, 17, 6521. [Google Scholar] [CrossRef] [PubMed]
- Duc, C.; Salvia, P.; Lubansu, A.; Feipel, V.; Aminian, K. A wearable inertial system to assess the cervical spine mobility: Comparison with an optoelectronic-based motion capture evaluation. Med. Eng. Phys. 2014, 36, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.A.; McCarthy, C.J.; Chorti, A.; Cooke, M.W.; Gates, S. A Systematic Review of Reliability and Validity Studies of Methods for Measuring Active andPassive Cervical Range of Motion. J. Manip. Physiol. Ther. 2010, 33, 138–155. [Google Scholar] [CrossRef]
- Ferrario, V.F.; Sforza, C.; Serrao, G.; Grassi, G.; Mossi, E. Active range of motion of the head and cervical spine: A three-dimensional investigation in healthy young adults. J. Orthop. Res. 2002, 20, 122–129. [Google Scholar] [CrossRef]
- Palmieri, M.; Donno, L.; Cimolin, V.; Galli, M. Cervical Range of Motion Assessment through Inertial Technology: A Validity and Reliability Study. Sensors 2023, 23, 6013. [Google Scholar] [CrossRef]
- Akoglu, H. User’s guide to correlation coefficients. Turkish J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Kang, N.Y.; Isenberg, S.J. Kestenbaum Procedure with Posterior Fixation Suture for Anomalous Head Posture in Infantile Nystagmus. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 247, 981–987. [Google Scholar]
- Porro, G.; van der Linden, D.; van Nieuwenhuizen, O.; Wittebol-Post, D. Role of Visual Dysfunction in Postural Control in Children with Cerebral Palsy. Neural Plast. 2005, 12, 205–210. [Google Scholar] [CrossRef]
- Silverson, O.; Cascia, N.; Hettrich, C.M.; Heebner, N.R.; Uhl, T.L. Reliability and Validity of a Clinical Assessment Tool for Measuring Scapular Motion in All 3 Anatomical Planes. J. Athl. Train. 2020, 56, 586–593. [Google Scholar]
- Irving, F.; Russell, J.A.; Smith, T. Reliability of Knee Joint Position Sense Measurement: A Comparison Between Goniometry and Image Capture Methods. Eur. J. Physiother. 2016, 18, 95–102. [Google Scholar] [CrossRef][Green Version]
- Szczygieł, E.; Fudacz, N.; Golec, J.; Golec, E. The Impact of the Position of the Head on the Functioning of the Human Body: A Systematic Review. Int. J. Occup. Med. Environ. Health 2020, 33, 559–568. [Google Scholar] [PubMed]
- Farah; de Lima, M.; Santinello, M.; de Carvalho, L.E.M.R.; Uesugui, C.F.; Barcellos, R.B. Using a Smartphone as a Tool to Measure Compensatory and Anomalous Head Positions. Arq. Bras. Oftalmol. 2018, 81, 30–36. [Google Scholar] [PubMed]
- Keogh, J.W.L.; Cox, A.J.; Anderson, S.L.; Liew, B.X.W.; Olsen, A.; Schram, B.; Furness, J. Reliability and Validity of Clinically Accessible Smartphone Applications to Measure Joint Range of Motion: A Systematic Review. PLoS ONE 2019, 14, e0215806. [Google Scholar]
- Costa, A.C.R.V.d.; Lopes, M.C.B.; Nakanami, C.R. Influence of Head Posture on the Visual Acuity of Children with Nystagmus. Arq. Bras. Oftalmol. 2014, 77, 8–11. [Google Scholar]
- Qiu, X.; Wang, Z.; Pan, L.; Shen, T.; Deng, D.; Chen, Q.; Yan, J. Use of a Microelectromechanical Systems Sensor for Objective Measurements of Abnormal Head Posture in Congenital Superior Oblique Palsy Patients. Transl. Vis. Sci. Technol. 2024, 13, 30. [Google Scholar] [CrossRef]
- Narayanappa, D.; Rajani, H.S.; Anita, T.G.; Nagaraj, R. A Case of Ocular Torticollis. Oman Med. J. 2013, 28, e050. [Google Scholar]
- Nucci, P.; Curiel, B. Abnormal Head Posture due to Ocular Problems—A Review. Curr. Pediatr. Rev. 2009, 5, 105–111. [Google Scholar] [CrossRef]
- Subbarayalu, A.V.; Ameer, M.A. Relationships among head posture, pain intensity, disability and deep cervical flexor muscle performance in subjects with postural neck pain. J. Taibah Univ. Med. Sci. 2017, 12, 541–547. [Google Scholar] [CrossRef]

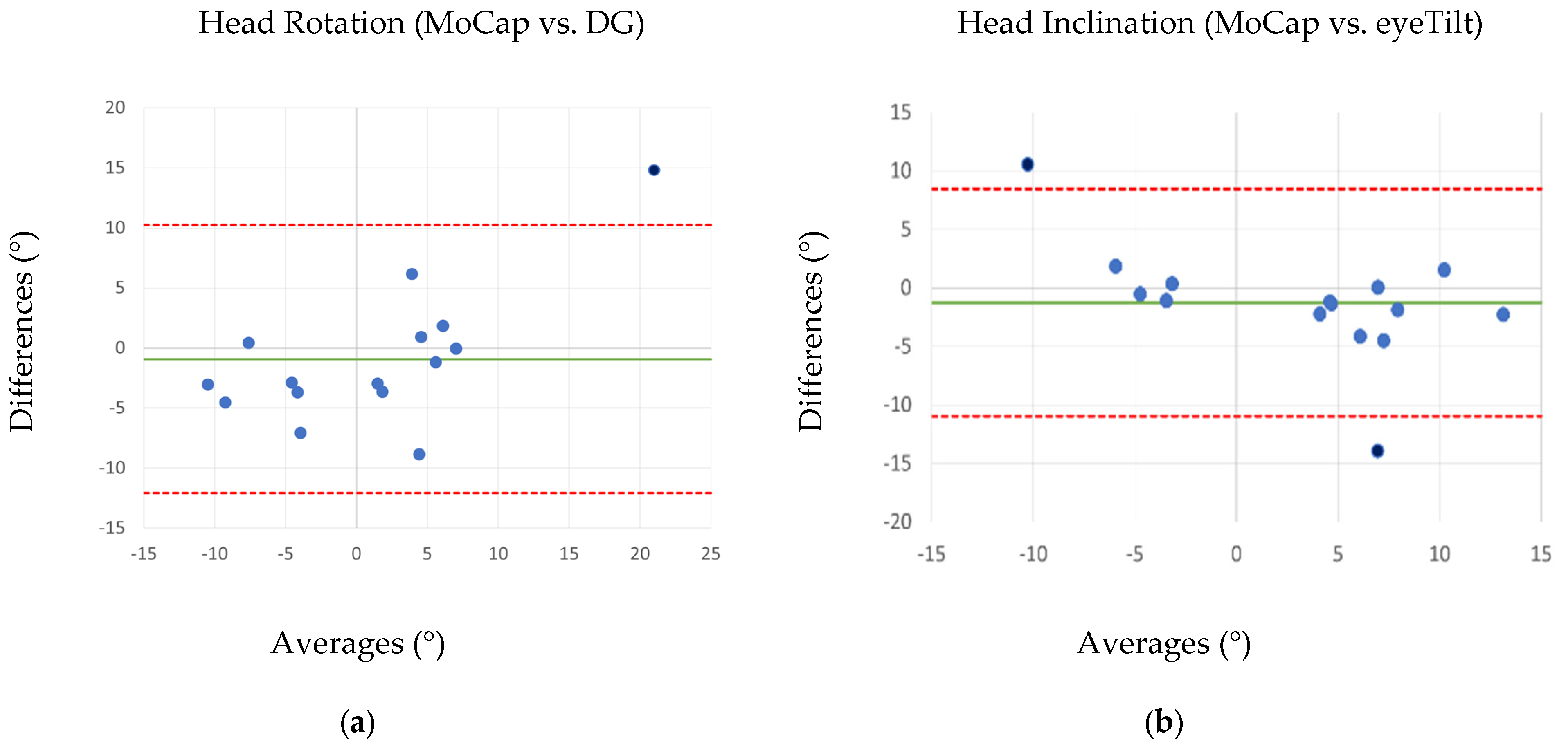
| Ophthalmological Tool | MoCap | p-Value (t-Test) | PCC | Accuracy (%) | ICC | RMSE (°) | |
|---|---|---|---|---|---|---|---|
| Head Rotation (°) | 0.60 ± 10.20 | 1.53 ± 6.48 | 0.538 | 0.86 * | 7.24 ± 4.08 | 0.79 | 3.43 |
| Head Inclination (°) | 2.33 ± 5.75 | 3.58 ± 8.40 | 0.346 | 0.82 * | 7.83 ± 3.78 | 0.77 | 5.00 |
| Task | ROM (AHP Group) | ROM (CG) |
|---|---|---|
| Right Rotation (°) | 67.7 ± 11.4 | 61.3 ± 10.1 |
| Left Rotation (°) | 61.7 ± 12.3 | 67.48 ± 9.0 |
| Flexion (°) | 50.49 ± 10.7 * | 42.2 ± 12.8 |
| Extension (°) | 43.0 ± 18.1 | 33.6 ± 9.4 |
| Right Lateral Bending (°) | 38.5 ± 10.9 | 38.7 ± 9.1 |
| Left Lateral Bending (°) | 36.4 ± 9.6 | 35.5 ± 8.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerfoglio, S.; Bonsignore, F.; Bernardelli, G.; Donno, L.; Aili, F.; Galli, M.; Nucci, F.; Villani, E.; Nucci, P.; Cimolin, V. Accuracy of Measurement Tools for Ocular-Origin Anomalous Head Posture and the Cervical Range of Motion Kinematics in Children with an Anomalous Head Position. Appl. Sci. 2025, 15, 3642. https://doi.org/10.3390/app15073642
Cerfoglio S, Bonsignore F, Bernardelli G, Donno L, Aili F, Galli M, Nucci F, Villani E, Nucci P, Cimolin V. Accuracy of Measurement Tools for Ocular-Origin Anomalous Head Posture and the Cervical Range of Motion Kinematics in Children with an Anomalous Head Position. Applied Sciences. 2025; 15(7):3642. https://doi.org/10.3390/app15073642
Chicago/Turabian StyleCerfoglio, Serena, Francesco Bonsignore, Giuseppina Bernardelli, Lucia Donno, Fabiana Aili, Manuela Galli, Francesca Nucci, Edoardo Villani, Paolo Nucci, and Veronica Cimolin. 2025. "Accuracy of Measurement Tools for Ocular-Origin Anomalous Head Posture and the Cervical Range of Motion Kinematics in Children with an Anomalous Head Position" Applied Sciences 15, no. 7: 3642. https://doi.org/10.3390/app15073642
APA StyleCerfoglio, S., Bonsignore, F., Bernardelli, G., Donno, L., Aili, F., Galli, M., Nucci, F., Villani, E., Nucci, P., & Cimolin, V. (2025). Accuracy of Measurement Tools for Ocular-Origin Anomalous Head Posture and the Cervical Range of Motion Kinematics in Children with an Anomalous Head Position. Applied Sciences, 15(7), 3642. https://doi.org/10.3390/app15073642










