Efficient Removal of Tetracycline Hydrochloride via Adsorption onto Modified Bentonite: Kinetics and Equilibrium Studies
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Modification of Bentonite
2.2. Characterization of Bentonite Materials
2.3. Preparation of TC Solutions
2.4. Adsorption Kinetics Studies
2.5. Adsorption Equilibrium Study
2.6. Influence of pH
2.7. Reuse of the Adsorbent
3. Results
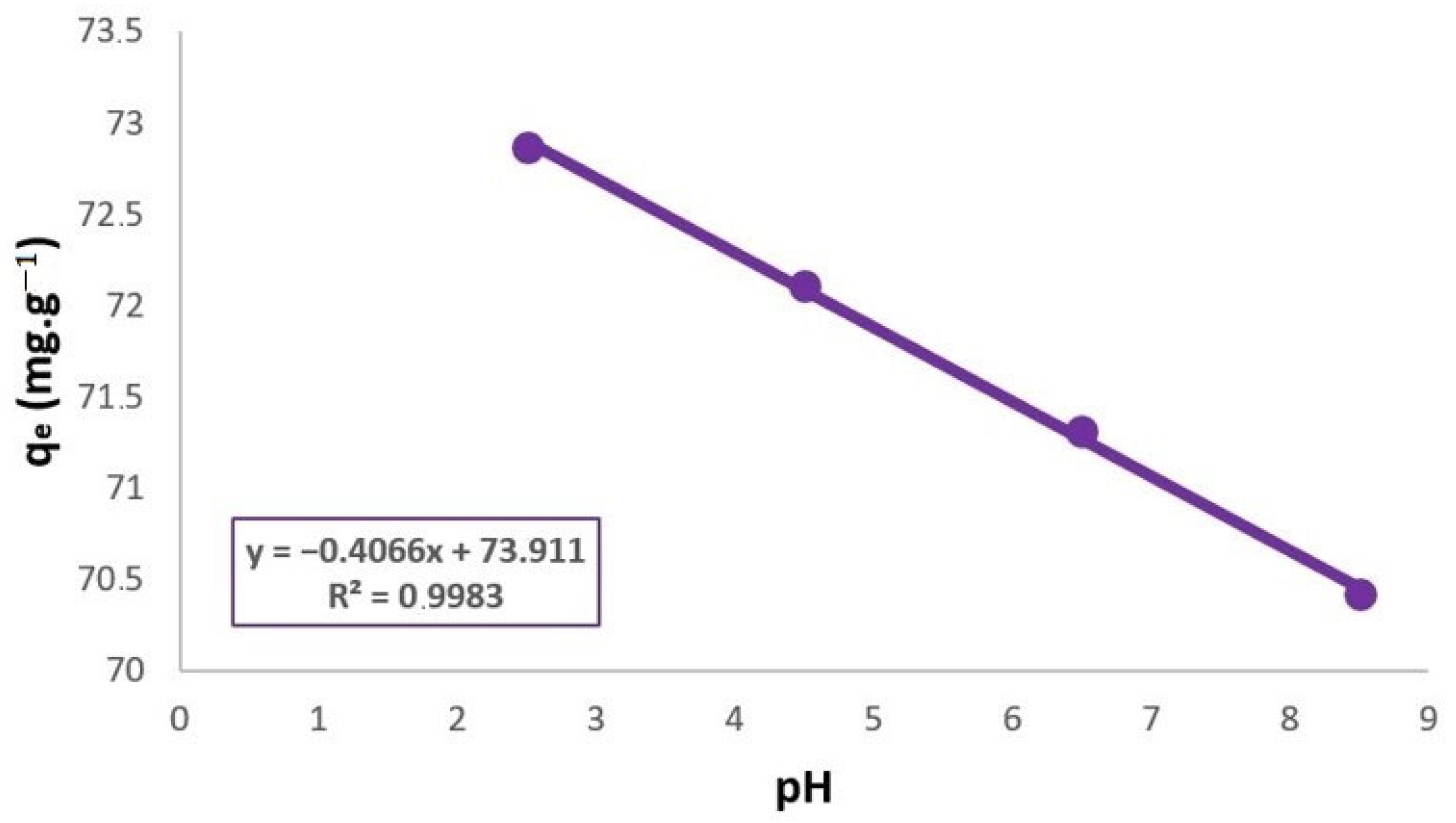
3.1. Influence of pH in TC Adsorption by Bentonite Materials
3.2. Compositional Analysis by X-Ray Fluorescence Spectroscopy (XRF)
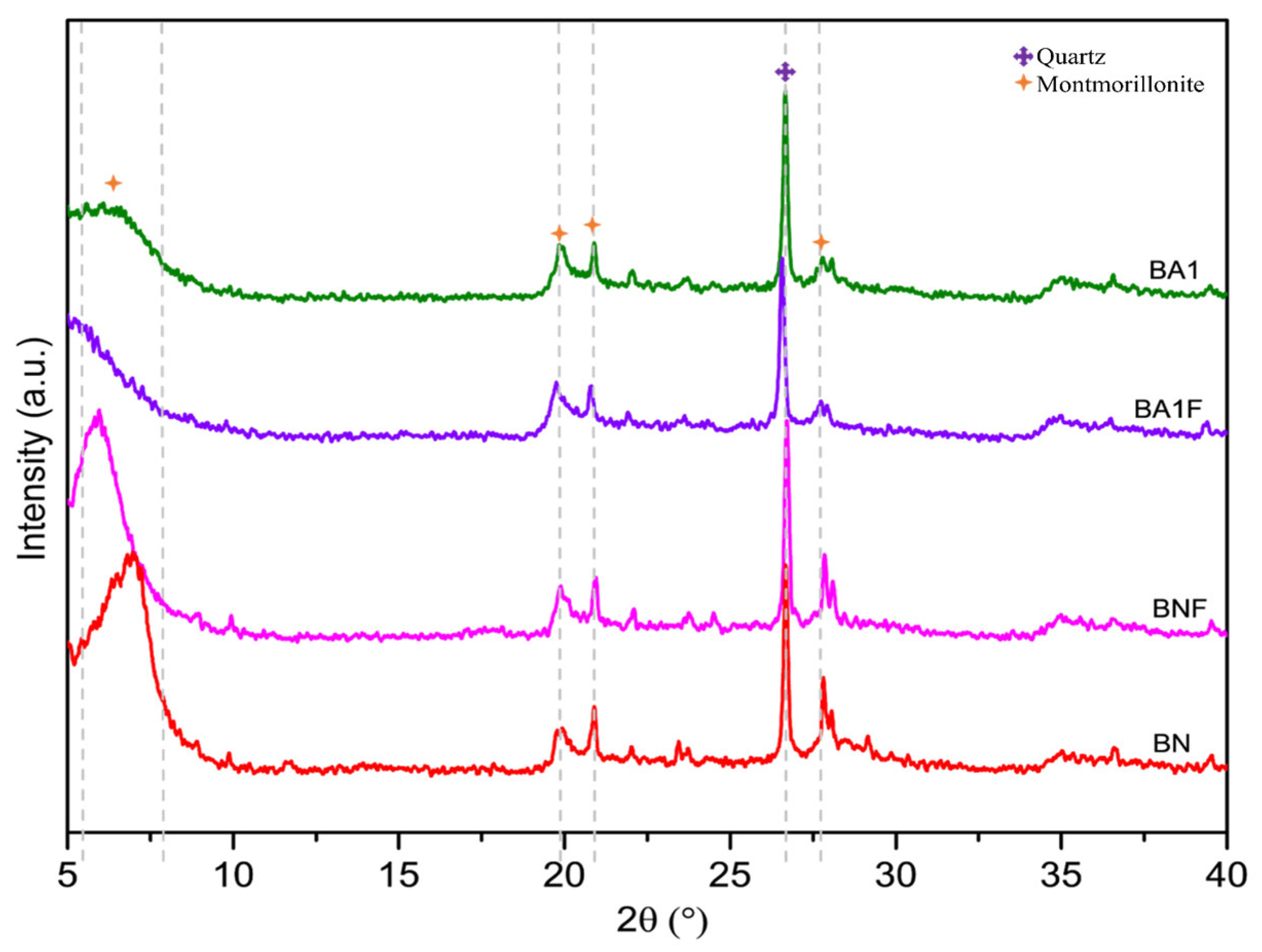
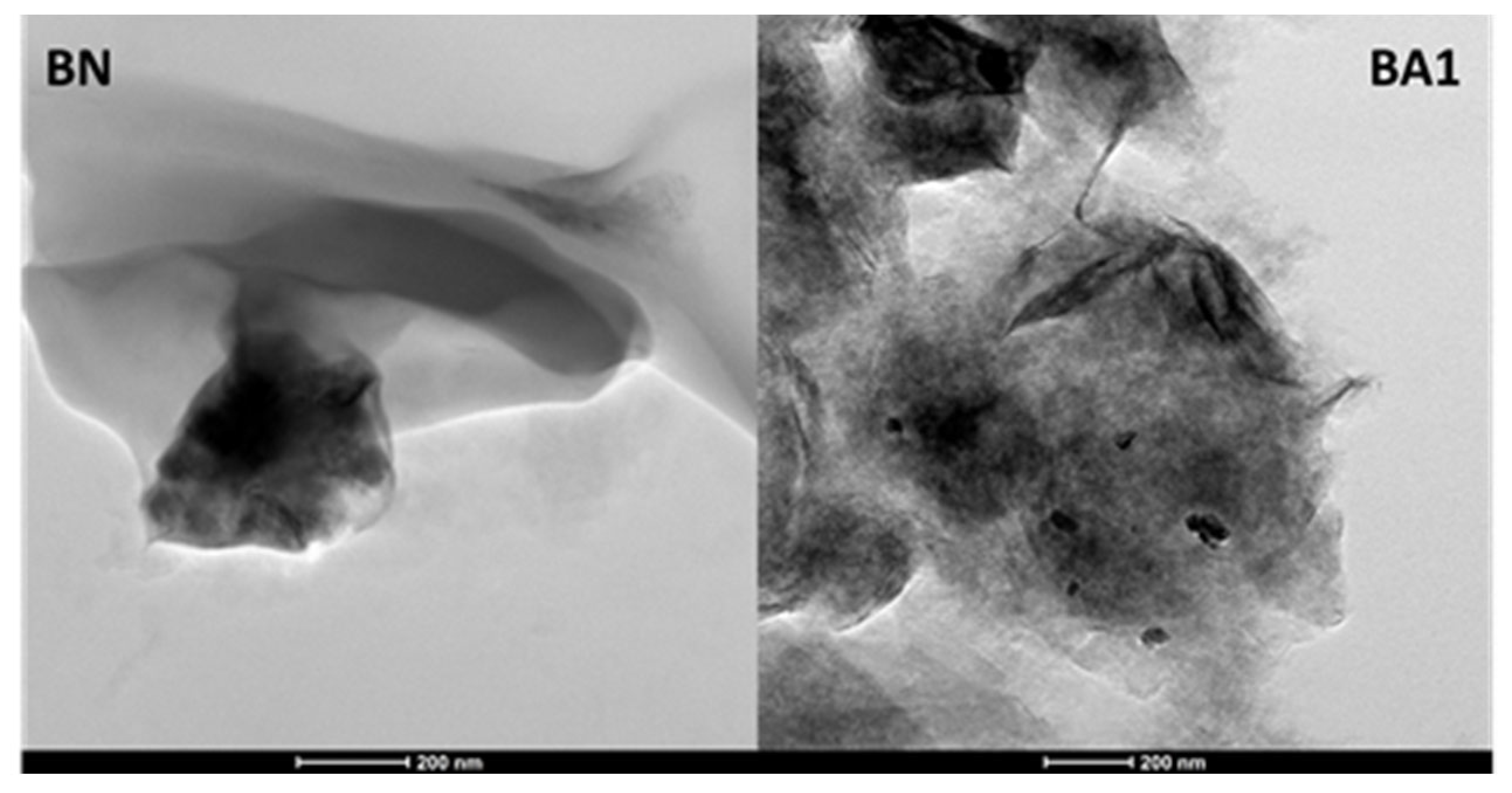
3.3. Structure and Morphology Assessment via XRD Patterns, SEM-EDS, and HRTEM Micrographs
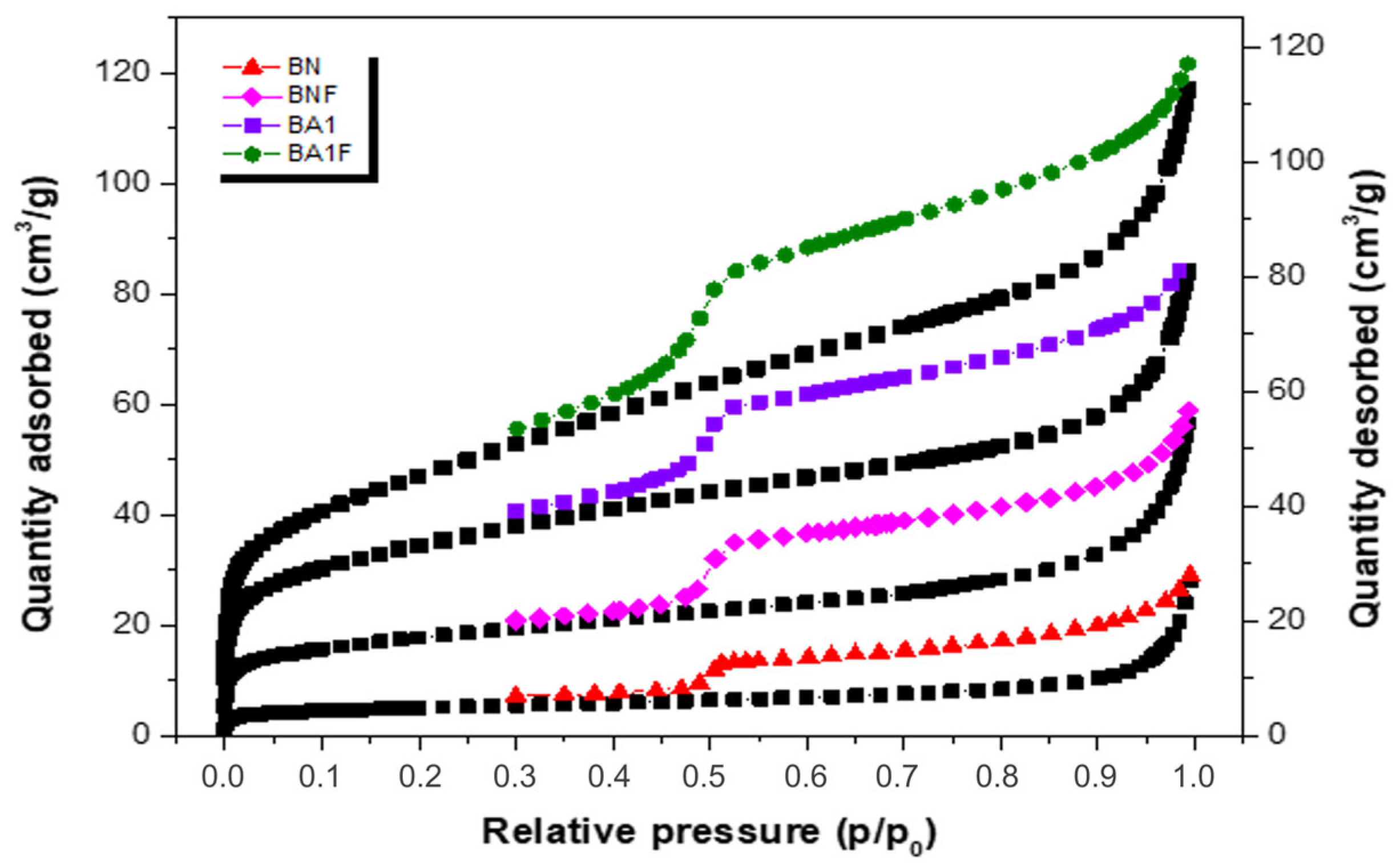
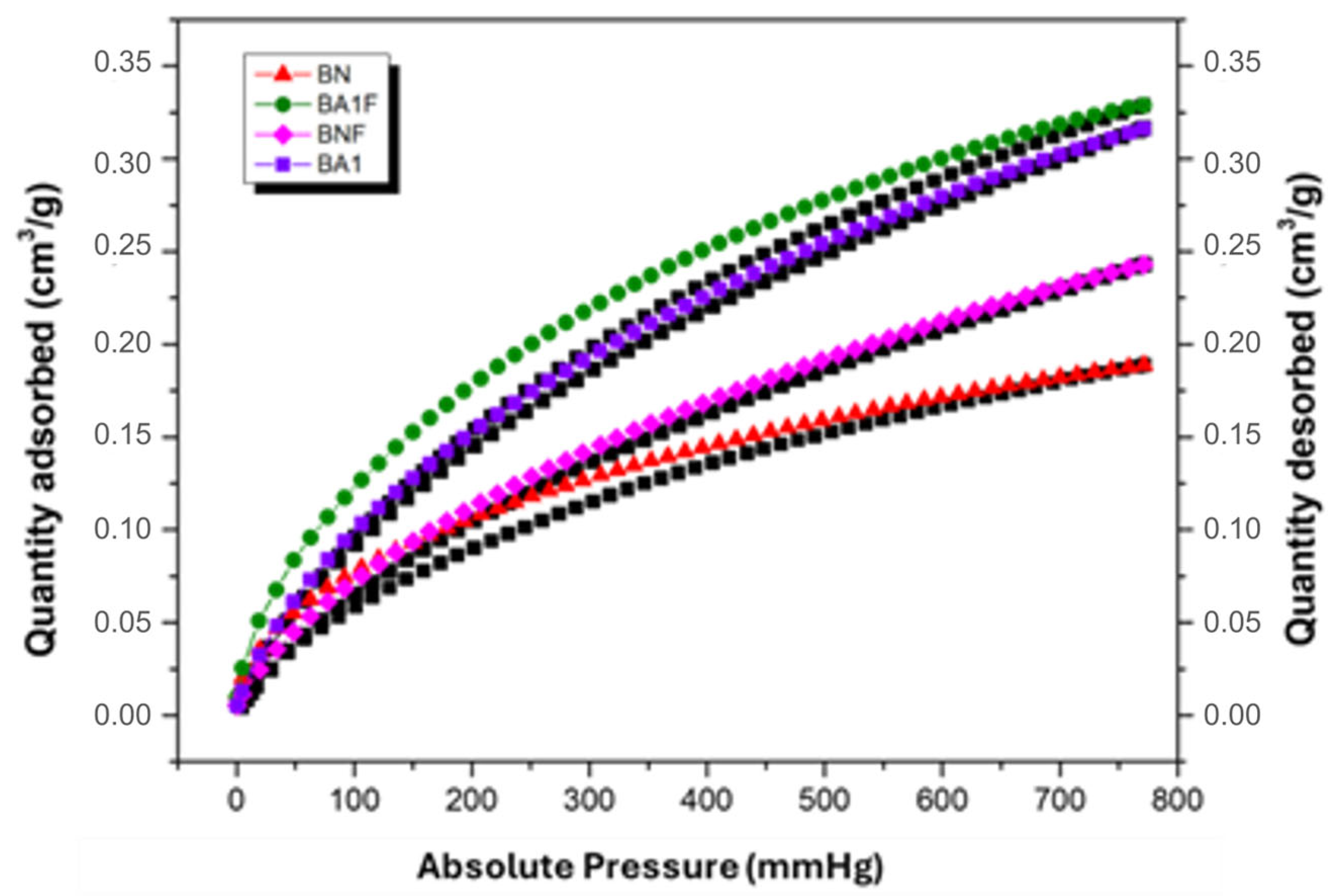
3.4. Textural Analysis by N2 Adsorption–Desorption Isotherms
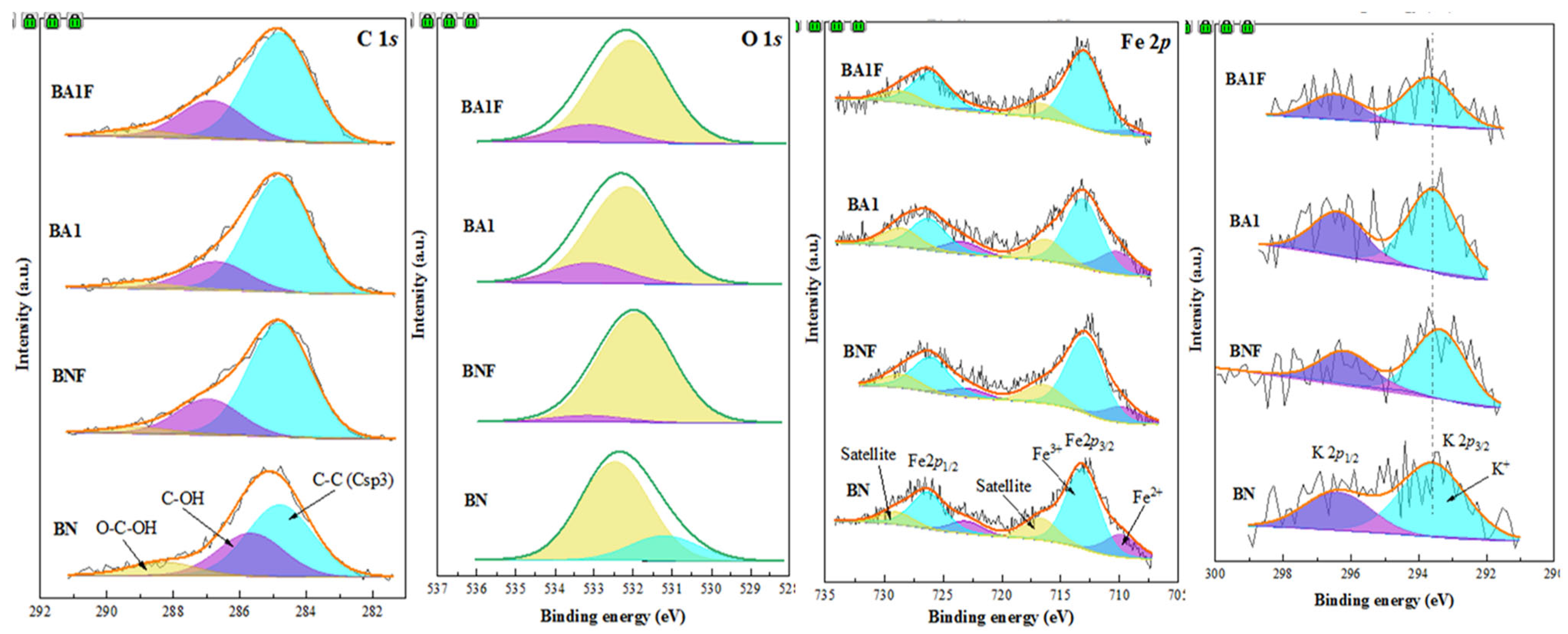
3.5. X-Ray Photoelectron Spectroscopy
3.6. CO2 Adsorption–Desorption Isotherms
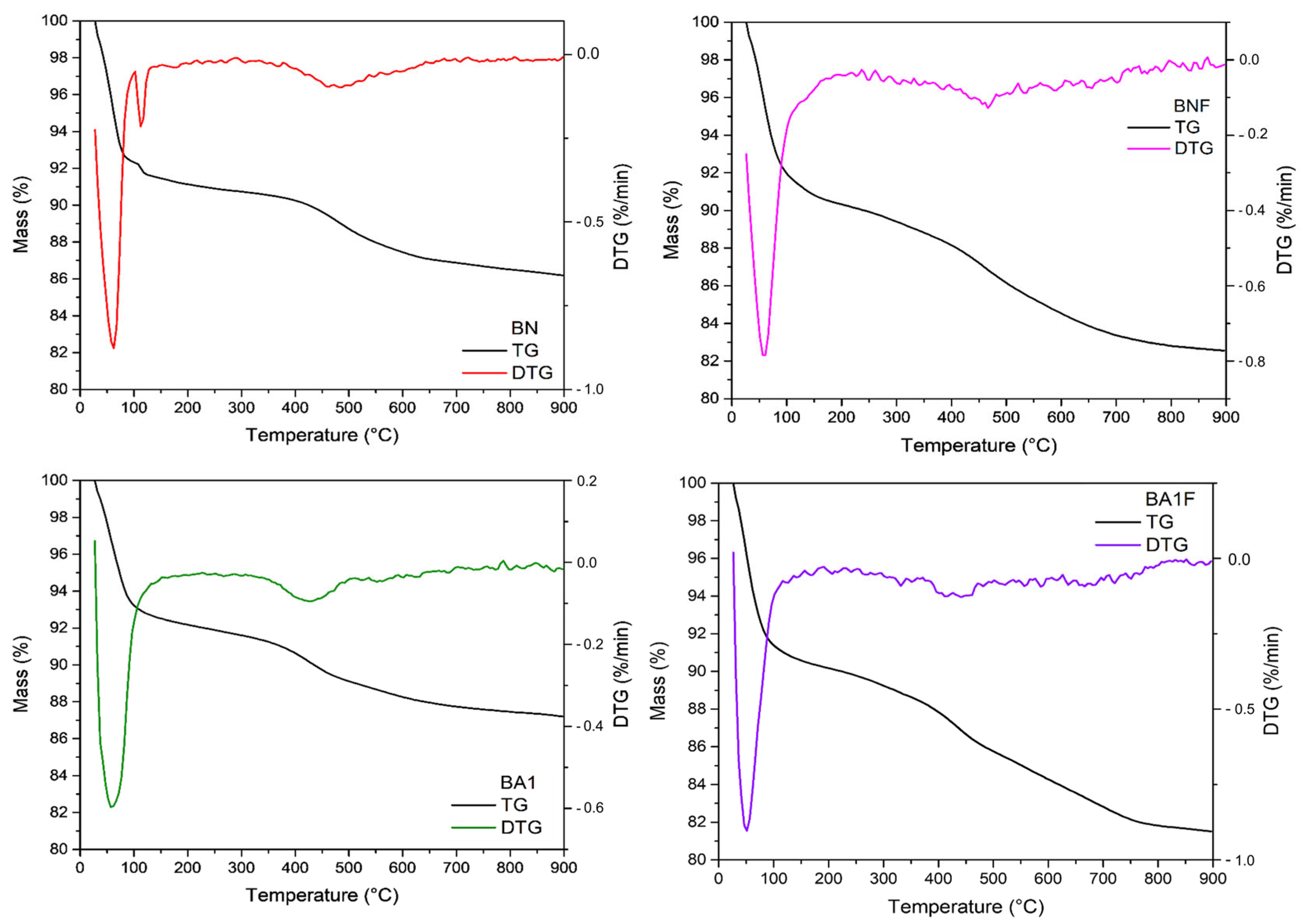
3.7. Thermogravimetric Analysis (TGA/DTG)
3.8. TC Adsorption Kinetics
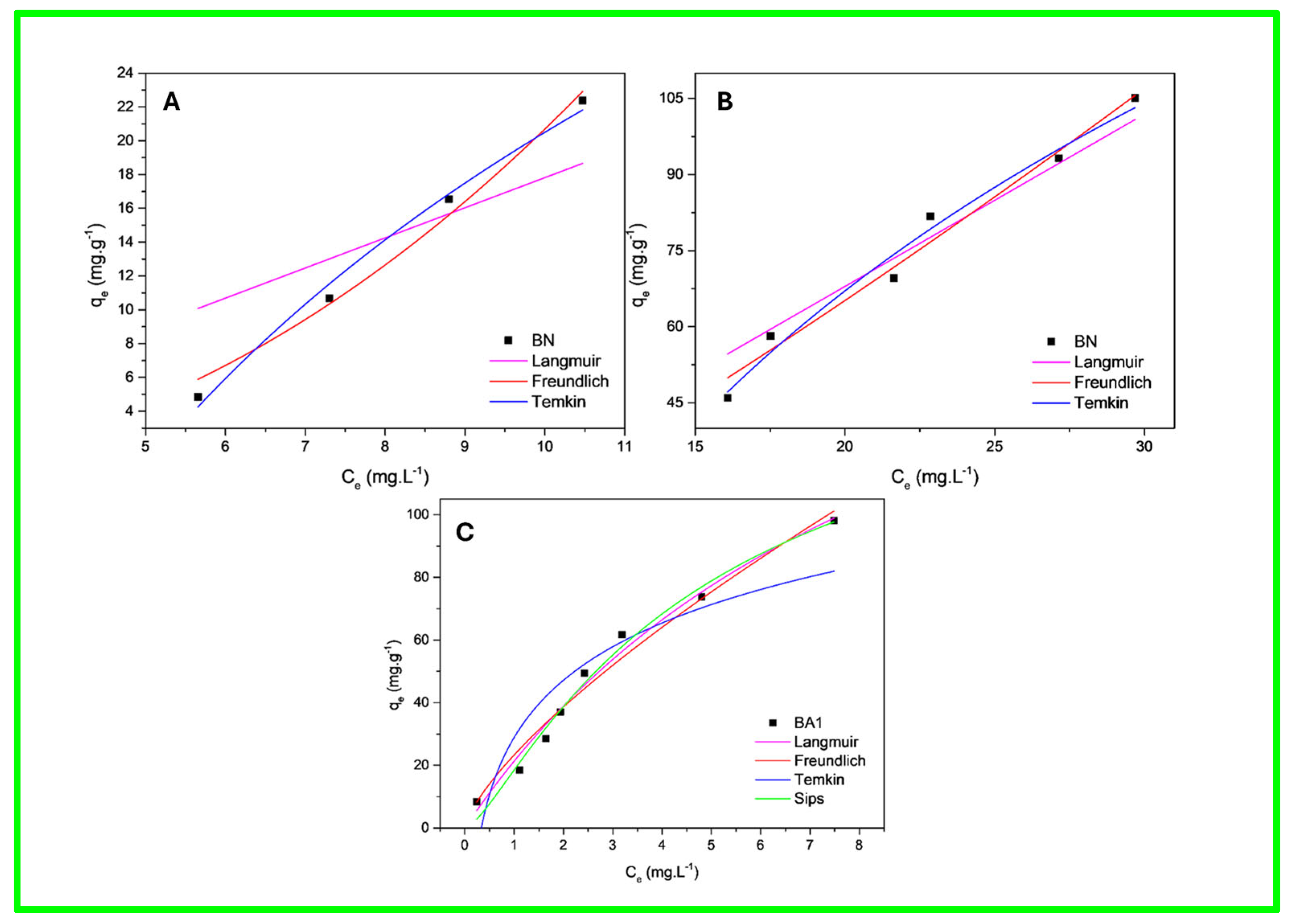
3.9. TC Adsorption Isotherms
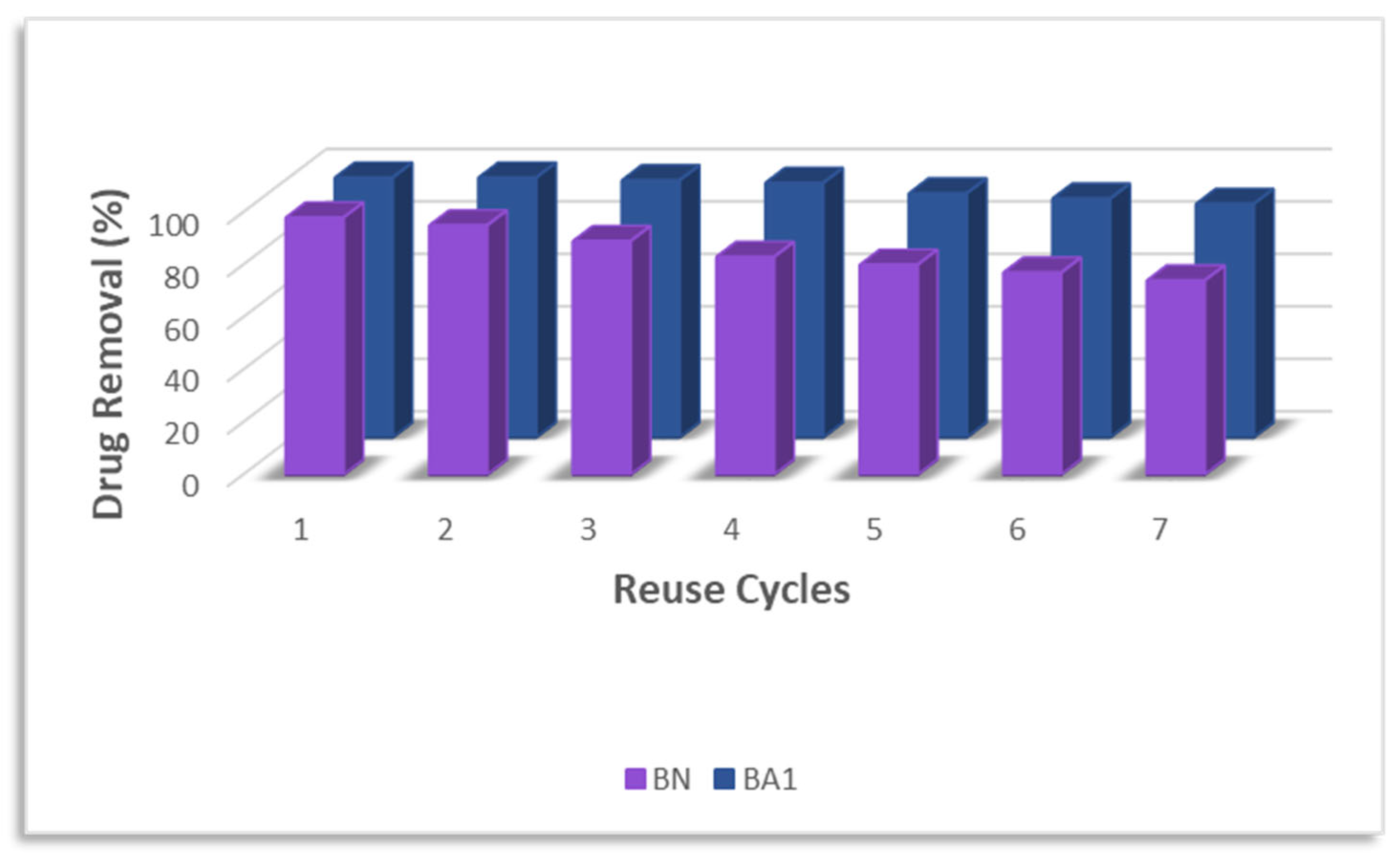
3.10. Adsorbent Recycling Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farto, C.D.; Athayde, G.B., Jr.; Sena, R.F.; Rosenhaim, R. Contaminantes de preocupação emergente no Brasil na década 2010–2019—Parte I: Ocorrência em diversos ambientes aquáticos. Rev. Gestão Água América Lat. 2021, 18, e6. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, X.; Feng, C.; Yang, S. Nano-clay montmorillonite removes tetracycline in water: Factors and adsorption mechanism in aquatic environments. iScience 2024, 27, 108952. [Google Scholar] [CrossRef] [PubMed]
- Leite, H. Debate Sobre Venda de Remédios em Supermercados e Pela Internet Coloca R$ 20 Bilhões em Disputa. 2022. Available online: https://cev.fgv.br/noticia/debate-sobre-venda-de-remedios-em-supermercados-e-pela-internet-coloca-r-20-bilhoes-em (accessed on 12 August 2024).
- Regitano, J.B. Comportamento e impacto ambiental de antibióticos usados na produção animal brasileira. Rev. Bras. Ciência Solo 2010, 34, 601–616. [Google Scholar] [CrossRef]
- Sun, Q.; Tang, M.; Hendriksen, P.V.; Chen, B. Biotemplated fabrication of a 3D hierarchical structure of magnetic ZnFe2O4/MgAl-LDH for efficient elimination of dye from water. J. Alloys Compd. 2020, 829, 154552. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, K.; Gao, B.; Zhang, G.; Liu, X.; Sentis, L. Adsorption of tetracycline on soil and sediment: Effects of pH and the presence of Cu(II). J. Hazard. Mater. 2011, 190, 856–862. [Google Scholar] [CrossRef]
- Zhao, Y.; Geng, J.; Wang, X.; Gu, X.; Gao, S. Tetracycline adsorption on kaolinite: pH, metal cations and humic acid effects. Ecotoxicology 2011, 20, 1141–1147. [Google Scholar] [CrossRef]
- Parolo, M.E.; Savini, M.C.; Vallés, J.M.; Baschini, M.T.; Avena, M.J. Tetracycline adsorption on montmorillonite: pH and ionic strength effects. Appl. Clay Sci. 2008, 40, 179–186. [Google Scholar] [CrossRef]
- Wu, H.; Xie, H.; He, G.; Guan, Y.; Zhang, Y. Effects of the pH and anions on the adsorption of tetracycline on iron-montmorillonite. Appl. Clay Sci. 2016, 119, 161–169. [Google Scholar] [CrossRef]
- Fernandez, F.A. Adsorción de Antibióticos Sobre Bentonitas Naturales y Modificadas. Neuquén. 2015. Available online: https://rdi.uncoma.edu.ar/bitstream/handle/uncomaid/15235/1%20Fernando%20Fernandez%202015.pdf?sequence=1&isAllowed=y (accessed on 1 February 2024).
- Maia, P.P.; Rath, S.; Reyes, F.G.R. Antimicrobianos em alimentos de origem vegetal: Uma revisão. Segurança Aliment. Nutr. 2009, 16, 49–64. [Google Scholar] [CrossRef]
- Salgado-Campos, V.M.J.; Bertolino, L.C.; Silva, F.J.; Mendes, J.C.; Neumann, R. Mineralogy and chemistry of a new halloysite deposit from the Rio de Janeiro pegmatite province, south-eastern Brazil. Clay Miner. 2021, 56, 1–15. [Google Scholar] [CrossRef]
- Sadjadi, S.; Malmir, M.; Heravi, M.M. A novel magnetic heterogeneous catalyst based on decoration of halloysite with ionic liquid-containing dendrimer. Appl. Clay Sci. 2019, 168, 184–195. [Google Scholar] [CrossRef]
- Zhirong, L.; Azhar Uddin, M.; Zhanxue, S. FT-IR and XRD analysis of natural Na-bentonite and Cu(II)-loaded Na-bentonite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Usman, F.; Muhammad, A. Preparation and characterization of bentonite clay catalysts for transesterification of waste cooking oil into biodiesel. FUDMA J. Sci. 2024, 8, 204–211. [Google Scholar] [CrossRef]
- Nicolás, M.F.; Chávez, M.M.C.; Vlasova, M.; Puig, T.P. Low-temperature sintering of ceramic bricks from clay, waste glass and sand. Boletín Soc. Española Cerámica Vidr. 2024, 63, 377–388. [Google Scholar] [CrossRef]
- Barrera, D.; Villarroel, J.; Torres, D.; Sapag, K. Síntesis y Caracterización de Extruidos Cerámicos de Bentonita para la Remoción de pb (ii) en Solución Acuosa. IBEROMET XIX CONAMET/SAM. 2 al 5 de Noviembre de 2010, Viña del Mar, Chile. Available online: https://www.researchgate.net/publication/267800608_SINTESIS_Y_CARACTERIZACION_DE_EXTRUIDOS_CERAMICOS_DE_BENTONITA_PARA_LA_REMOCION_DE_Pb_II_EN_SOLUCION_ACUOSA (accessed on 21 October 2024).
- Pokharel, B.; Siddiqua, S. Effect of calcium bentonite clay and fly ash on the stabilization of organic soil from Alberta, Canada. Eng. Geol. 2021, 293, 106291. [Google Scholar] [CrossRef]
- Liu, W.T.; Tsai, S.C.; Tsai, T.L.; Lee, C.P.; Lee, C.H. Characteristic study for the uranium and cesium sorption on bentonite by using XPS and XANES. J. Radioanal. Nucl. Chem. 2017, 314, 2237–2241. [Google Scholar] [CrossRef]
- Bharali, P. Acid Treatment on Bentonite Clay for the Removal of Fast Green FCF Dye from Aqueous Solution. Environ. Qual. Manag. 2024, 34, e22274. [Google Scholar] [CrossRef]
- Rianna, M.; Harahap, R.A.L.; Ananda, N.; Situmorang, P.C.; Sembiring, T.; Sianturi, H.A.; Amiruddin, E.; Hussain, M.K.; Setiadi, E.A.; Tetuko, A.P. Experimental investigation on ceramic materials of wood flour and bentonite. J. Phys. Conf. Ser. 2024, 2733, 012016. [Google Scholar] [CrossRef]
- Kgabi, D.P.; Ambushe, A.A. Characterization of South African Bentonite and Kaolin Clays. Sustainability 2023, 15, 12679. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, S.; Meng, X.; Shi, L.; Liu, N. Acid Treatment on Bentonite Catalysts for Alkylation of Diphenylamine. Catal. Lett. 2024, 154, 4928–4940. [Google Scholar] [CrossRef]
- Huang, C.; Ma, Q.; Zhou, M.; Wang, J.; Feng, Z. Adsorption Effect of Oxalic Acid-Chitosan-Bentonite Composite on Cr6+ in Aqueous Solution. Water Air Soil Pollut. 2023, 234, 540. [Google Scholar] [CrossRef]
- Ortiz-Ramos, U.; Leyva-Ramos, R.; Mendoza-Mendoza, E.; Aragón-Piña, A. Removal of tetracycline from aqueous solutions by adsorption on raw Ca-bentonite. Effect of operating conditions and adsorption mechanism. Chem. Eng. J. 2022, 432, 134428. [Google Scholar] [CrossRef]
- Sudan, S.; Kaushal, J.; Khajuria, A.; Goyal, H.; Mantri, A. Bentonite clay-modified coconut biochar for effective removal of fluoride: Kinetic, isotherm studies. Adsorption 2024, 30, 389–401. [Google Scholar] [CrossRef]
- Sales, R.V. Avaliação da Dessulfurização de Diesel Utilizando Adsorventes Mesoporosos Modificados Pós-Situ Com Íons Metálicos. 2015. 117f. Tese (Doutorado em Química)—Programa de Pós-Graduação em Química; Universidade Federal do Rio Grande do Norte: Natal, Brazil, 2015. [Google Scholar]
- Khalaf, S.M.; Al-Mahmoud, S.M. Adsorption of Tetracycline Antibiotic from Aqueous Solutions Using Natural Iraqi Bentonite. Egypt. J. Chem. 2021, 64, 5511–5519. [Google Scholar] [CrossRef]
- Dolatabadi, M.; Mehrabpour, M.; Esfandyari, M.; Ahmadzadeh, S. Adsorption of tetracycline antibiotic onto modified zeolite: Experimental investigation and modeling. MethodsX 2020, 7, 100885. [Google Scholar] [CrossRef]
- Elodoma, M.A. Studies on the isotherm, kinetic, and thermos-sorption aspects of the Cr (VI) uptake onto commercial bentonite. Jouf Univ. Sci. Eng. J. JUSEJ 2023, 10, 8–9. [Google Scholar]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H. Of the adsorption of gases. section ii. kinetics and energetics of gas adsorption. introductory paper to section ii. Trans. Faraday Soc. 1932, 28, 195–201. [Google Scholar] [CrossRef]
- Aguareles, M.; Barrabés, E.; Myers, T.; Valverde, A. Mathematical analysis of a Sips-based model for column adsorption. Phys. D Nonlinear Phenom. 2023, 448, 133690. [Google Scholar] [CrossRef]
- Hai, L.; Wang, J. Experimental study on the heat treatment reaction process of bentonite. Sci. Rep. 2024, 14, 16649. [Google Scholar] [CrossRef]
- Sarode, U.K.; Vaidya, P.D. Catalytic desorption of CO2-loaded solutions of diethylethanolamine using bentonite catalyst. Can. J. Chem. Eng. 2023, 102, 1262–1271. [Google Scholar] [CrossRef]
- Elbastamy, E.E.; Ibrahim, L.A.; Ghandour, A.; Zelenakova, M.; Vranayova, Z.; Abu-Hashim, M. Efficiency of natural clay mineral adsorbent filtration systems in wastewater treatment for potential irrigation purposes. Sustainability 2021, 13, 5738. [Google Scholar] [CrossRef]
- Nunes FIlho, F.G.; Silva Filho, E.C.; Osajima, J.A.; Alves, A.P.M.; Fonseca, M.G. Adsorption of tetracycline using chitosan–alginate–bentonite composites. Appl. Clay Sci. 2023, 239, 106952. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.; Su, Y.; Liu, Z.; Du, C. Indium oxide/Halloysite composite as highly efficient adsorbent for tetracycline Removal: Key roles of hydroxyl groups and interfacial interaction. Appl. Surf. Sci. 2021, 566, 150708. [Google Scholar] [CrossRef]
- Erdem, S.; Öztekin, M.; Sağ Açıkel, Y. Investigation of tetracycline removal from aqueous solutions using halloysite/chitosan nanocomposites and halloysite nanotubes/alginate hydrogel beads. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100576. [Google Scholar] [CrossRef]
- Silva, A.J.F. Adsorção dos Íons de Cobre Usando pó da Palha da Carnaúba e Bentonita: Estudo Cinético, Termodinâmico e de Equilíbrio. 2019 162f. Dissertação (Mestrado em Química)—Programa de Pós-Graduação em Engenharia Química; Universidade Federal do Rio Grande do Norte: Natal, Brazil, 2019. [Google Scholar]
- de Carvalho, R.S.; Arguelho, M.L.P.M.; Faccioli, G.G.; de Oliveira, R.A.; Passos, E.S.; Silva, A.V.; dos Santos, B.F.S. Utilização do biocarvão de bagaço de laranja na remoção de tetraciclina em água residuária. Matéria 2021, 26, e12980. [Google Scholar] [CrossRef]
- Tang, R.; Zhang, H.; Lu, C.; Liu, K.; Yu, S.; Tong, Z. Adsorption of Ciprofloxacin and Tetracycline by Organically Modified Magnetic Bentonite. E3S Web Conf. 2022, 350, 03006. [Google Scholar] [CrossRef]



| Element | BN—Composition (a%) | BA1—Composition (a%) |
|---|---|---|
| Si | 52.35 | 62.51 |
| Fe | 18.57 | 13.13 |
| Al | 15.18 | 15.83 |
| K | 3.42 | 3.28 |
| Ca | 2.73 | 1.23 |
| Na | 0.87 | 0.00 |
| Mg | 2.06 | 1.93 |
| Others | 4.65 | 2.06 |
| Samples | SBET (m2·g−1) | Vpa (cm3·g−1) | Pore Diameter (Å) |
|---|---|---|---|
| BN | 63 | 0.06 | 37.43 |
| BNF | 17.8 | 0.02 | 43.78 |
| BA1 | 165 | 0.15 | 35.29 |
| BA1F | 121 | 0.09 | 32.67 |
| Kinetic Parameters | BN | BA1 |
|---|---|---|
| qe,exp (mg·g−1) | 3.84 | 4.08 |
| Pseudo-first order | ||
| k1 (min−1) | −0.0008 | −0.0026 |
| qe,cal (mg·g−1) | 0.271 | 0.483 |
| R2 | 0.9799 | 0.848 |
| Pseudo-second order | ||
| k2 (g·mg−1·min−1) | 0.0071 | 0.1232 |
| qe,cal (mg·g−1) | 3.87 | 4.04 |
| R2 | 0.9998 | 0.9998 |
| Elovich | ||
| α (mg·g−1·min−1) | 7.30 × 10−5 | 1.30 × 10−2 |
| β (g·mg−1) | 0.309 | 0.906 |
| R2 | 0.9681 | 0.9679 |
| Clay Mineral | Isotherm | Kinetics | Time (min) | Removal (%) | Author |
|---|---|---|---|---|---|
| BN | Freundlich | Pseudo-second order | 30 | 97 | This study |
| BA1 | Freundlich | Pseudo-second order | 30 | 99 | This Study |
| Nano montmorillonite | Langmuir | Pseudo-first order | 60 | 90 | [4] |
| 20% In2O3/Halloysite | Langmuir | Pseudo-second order | 60 | 98 | [35] |
| 25% In2O3/Halloysite | Freundlich | Pseudo-second order | 1440 | 88.3 | [36] |
| Carbon–Cu composite | Langmuir | Pseudo-second order | 120 | 75 | [37] |
| Bentonite | Langmuir | Pseudo-second order | 1440 | - | [9] |
| Orange biocarbon | Langmuir | Pseudo-second order | 16 | 57.69 | [38] |
| Magnetic bentonite/CMC | Langmuir | Pseudo-second order | 120 | 96 | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.; Freitas, A.; Silva, M.; Camara, A.; Moura, H.; Ballesteros-Plata, D.; Rodríguez-Castellón, E.; de Carvalho, L. Efficient Removal of Tetracycline Hydrochloride via Adsorption onto Modified Bentonite: Kinetics and Equilibrium Studies. Appl. Sci. 2025, 15, 3372. https://doi.org/10.3390/app15063372
Pereira A, Freitas A, Silva M, Camara A, Moura H, Ballesteros-Plata D, Rodríguez-Castellón E, de Carvalho L. Efficient Removal of Tetracycline Hydrochloride via Adsorption onto Modified Bentonite: Kinetics and Equilibrium Studies. Applied Sciences. 2025; 15(6):3372. https://doi.org/10.3390/app15063372
Chicago/Turabian StylePereira, Aisha, Adriano Freitas, Mariana Silva, Anne Camara, Heloise Moura, Daniel Ballesteros-Plata, Enrique Rodríguez-Castellón, and Luciene de Carvalho. 2025. "Efficient Removal of Tetracycline Hydrochloride via Adsorption onto Modified Bentonite: Kinetics and Equilibrium Studies" Applied Sciences 15, no. 6: 3372. https://doi.org/10.3390/app15063372
APA StylePereira, A., Freitas, A., Silva, M., Camara, A., Moura, H., Ballesteros-Plata, D., Rodríguez-Castellón, E., & de Carvalho, L. (2025). Efficient Removal of Tetracycline Hydrochloride via Adsorption onto Modified Bentonite: Kinetics and Equilibrium Studies. Applied Sciences, 15(6), 3372. https://doi.org/10.3390/app15063372











