Urban Biorefinery Demonstration: Production of Polyhydroxyalkanoates from a Municipal Solid Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Enrichment Fermentation
2.2. Scl-PHA Accumulation
2.3. Scl-PHA Extraction
2.4. Analytical Methods
2.4.1. Gas Chromatography Analysis of scl-PHA
2.4.2. Scl-PHA Determination in the Whole Cells
3. Results
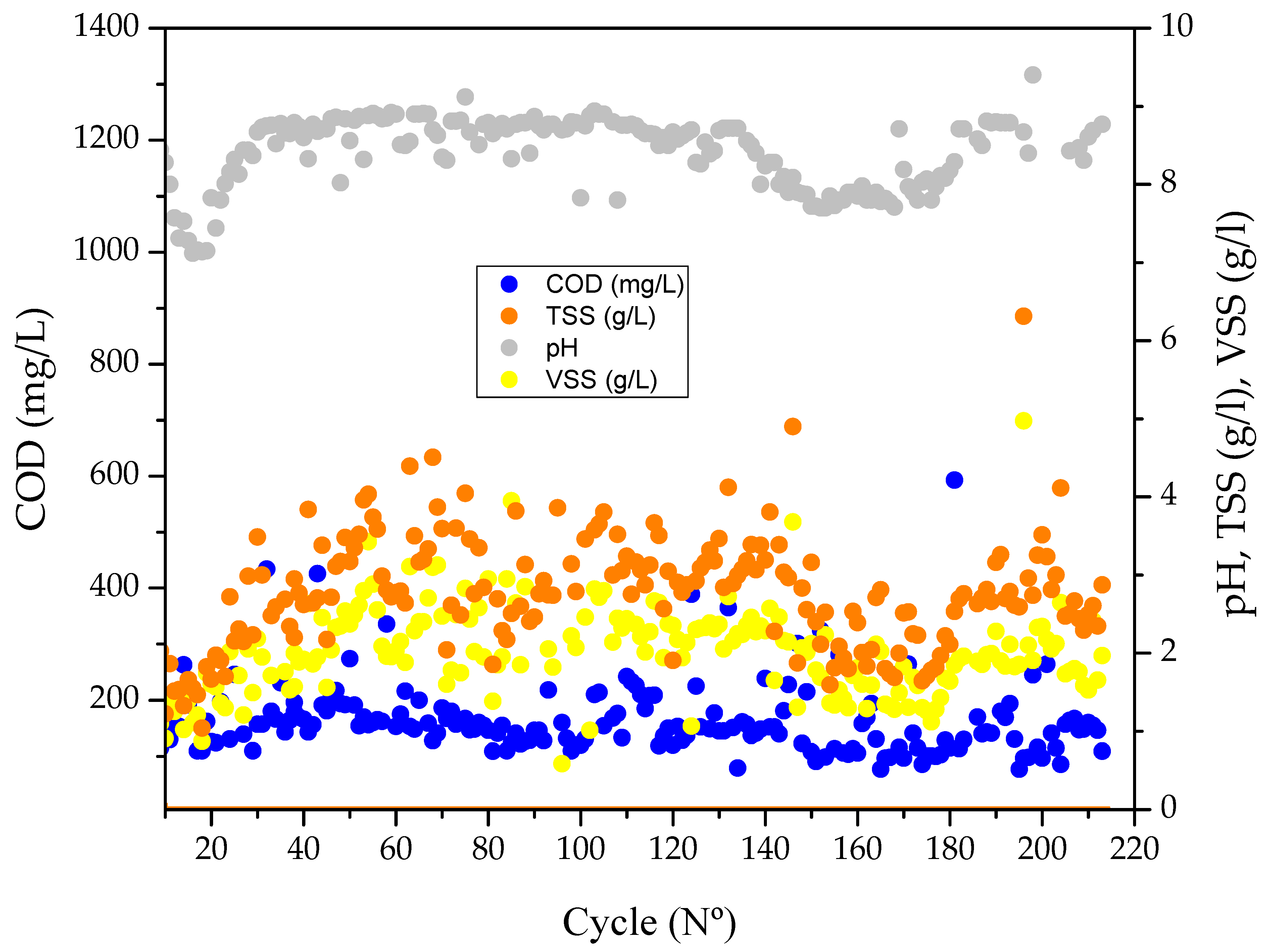
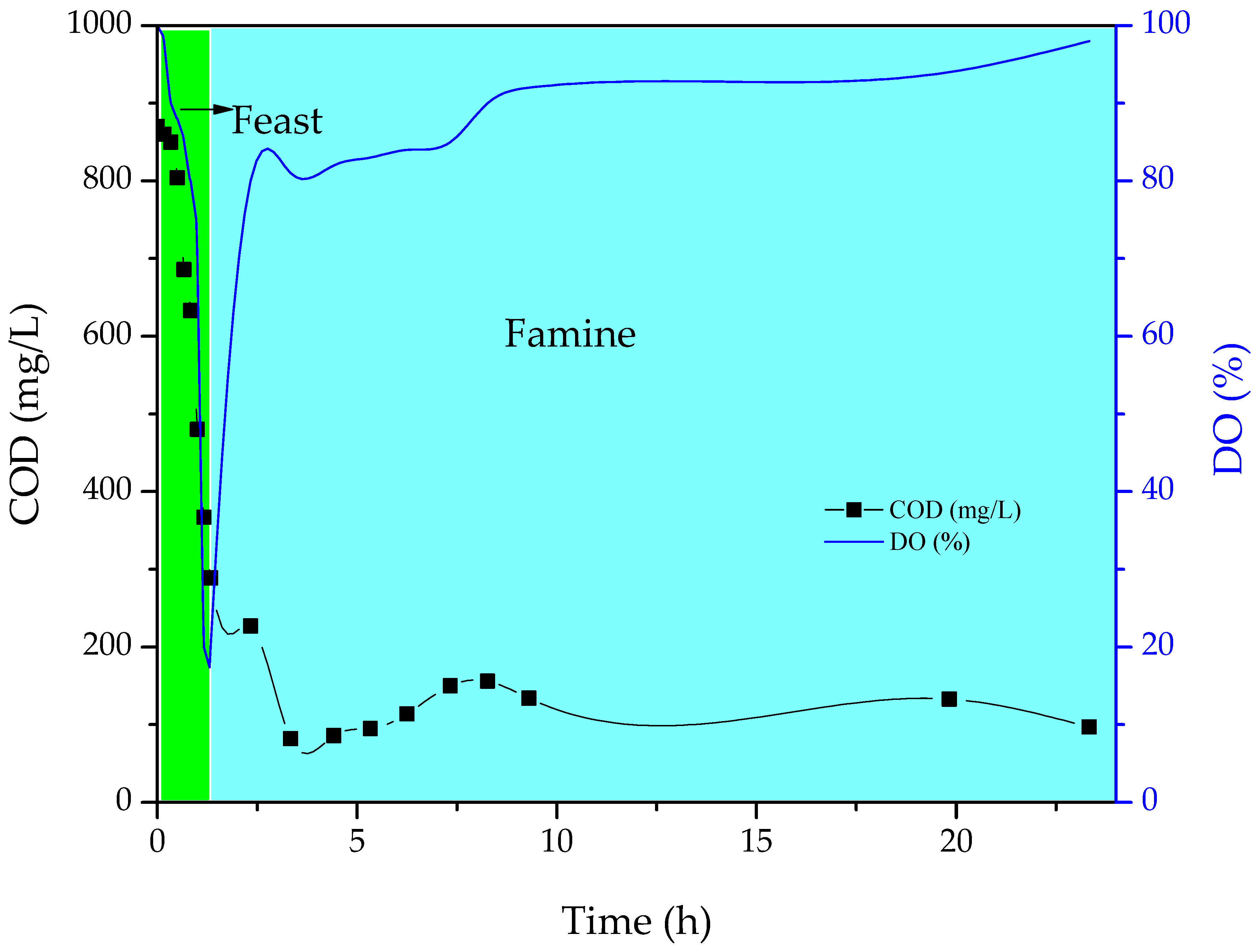
3.1. Enrichment of scl-PHA-Accumulating Microorganisms
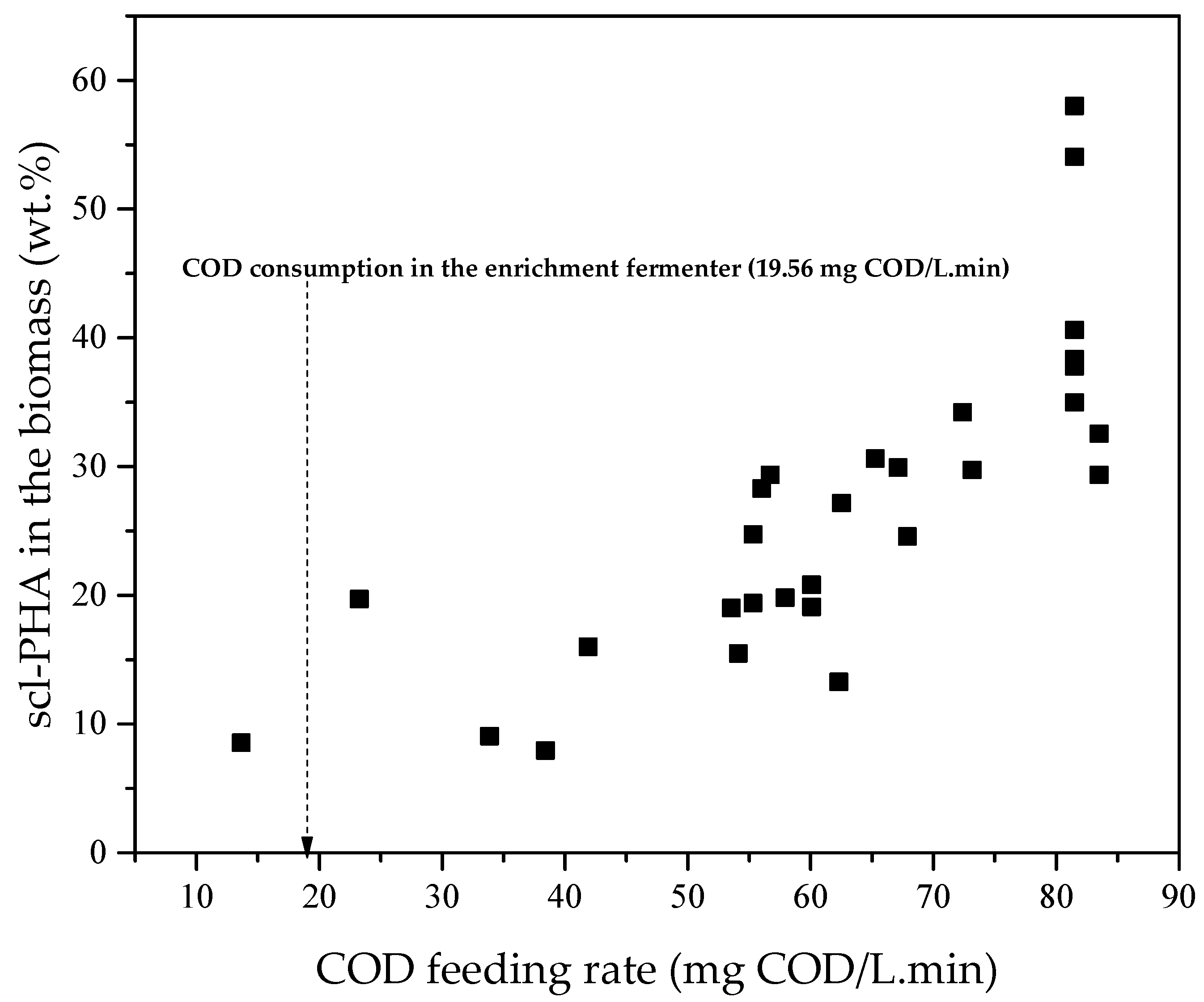
3.2. Accumulation of scl-PHA in Microorganisms
3.3. Extraction of scl-PHA from the Produced Biomass at Demo Plant
4. Discussion
4.1. Enrichment of scl-PHA-Accumulating Microorganisms
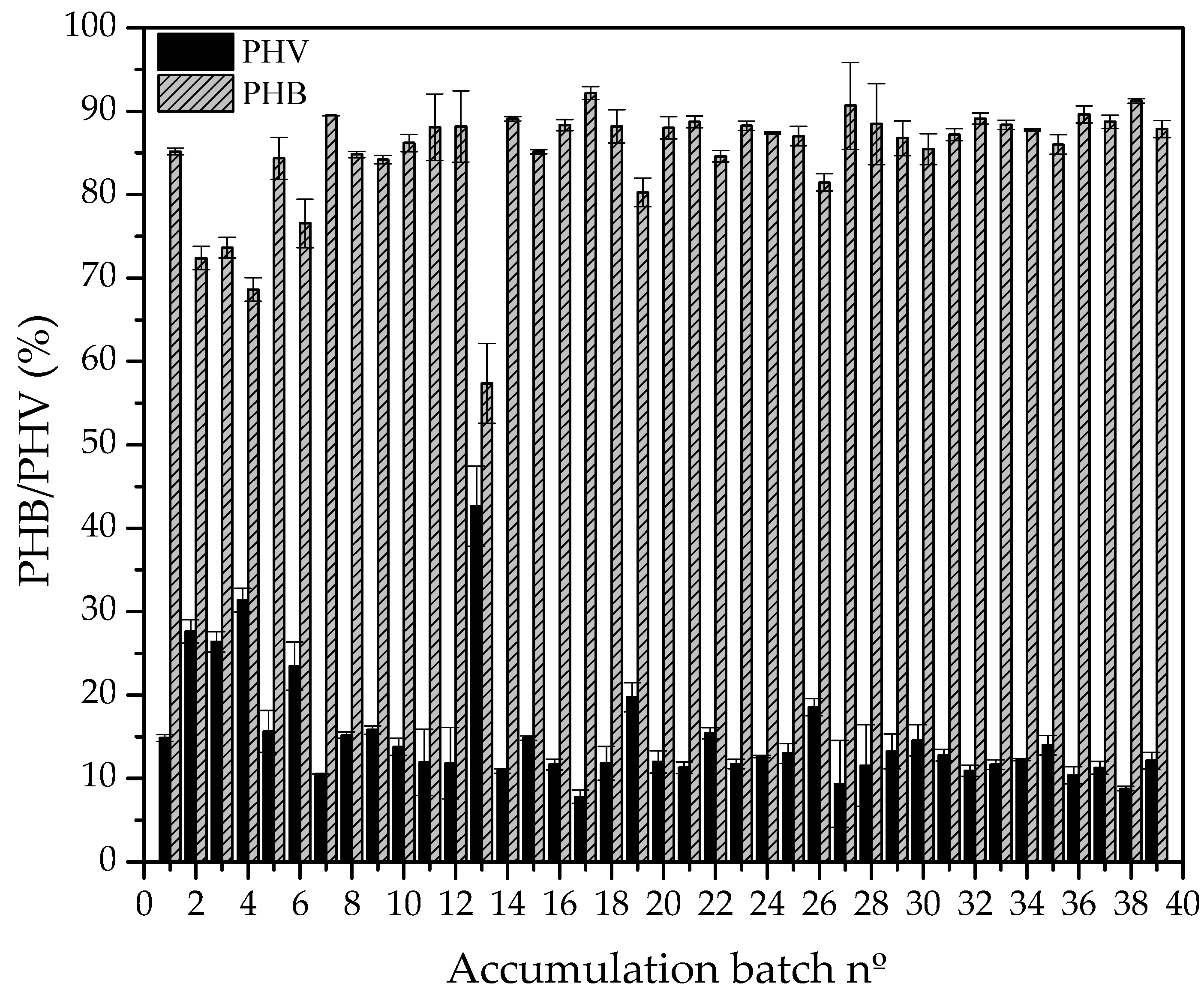
4.2. Accumulation of scl-PHA in Microorganisms
4.3. Extraction of scl-PHA from the Produced Biomass at Demo Plant
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khajuria, A.; Atienza, V.A.; Chavanich, S.; Henning, W.; Islam, I.; Kral, U.; Liu, M.; Liu, X.; Murthy, I.K.; Oyedotun, T.D.T.; et al. Accelerating circular economy solutions to achieve the 2030 agenda for sustainable development goals. Circ. Econ. 2022, 1, 100001. [Google Scholar] [CrossRef]
- Sengupta, D.; Ilankoon, I.M.S.K.; Dean Kang, K.; Nan Chong, M. Circular economy and household e-waste management in India: Integration of formal and informal sectors. Miner. Eng. 2022, 184, 107661. [Google Scholar] [CrossRef]
- Sakthivelmurugan, E.; Senthilkumar, G.; Karthick, K.N. Analysis of the impact of circular economy over linear economy in the paper processing industry. Mater. Today Proc. 2022, 66, 1446–1452. [Google Scholar] [CrossRef]
- Bongers, A.; Casas, P. The circular economy and the optimal recycling rate: A macroeconomic approach. Ecol. Econ. 2022, 199, 107504. [Google Scholar] [CrossRef]
- Noor, T.; Javid, A.; Hussain, A.; Bukhari, S.M.; Ali, W.; Akmal, M.; Hussain, S.M. Chapter 14—Types, sources and management of urban wastes. In Urban Ecology; Verma, P., Singh, P., Singh, R., Raghubanshi, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 239–263. [Google Scholar] [CrossRef]
- Pérez, V.; Pascual, A.; Rodrigo, A.; García Torreiro, M.; Latorre-Sánchez, M.; Coll Lozano, C.; David-Moreno, A.; Oliva-Dominguez, J.M.; Serna-Maza, A.; Herrero García, N.; et al. Chapter 2—Integrated innovative biorefinery for the transformation of municipal solid waste into biobased products. In Waste Biorefinery; Bhaskar, T., Pandey, A., Rene, E.R., Tsang, D.C.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 41–80. [Google Scholar] [CrossRef]
- URBIOFIN, Urban Biorefinery: From Waste to Bioproducts Through Biorefiney. Available online: https://circulareconomy.europa.eu/platform/en/good-practices/urbiofin-waste-bioproducts-through-biorefinery (accessed on 14 September 2023).
- Mor, S.; Ravindra, K. Municipal solid waste landfills in lower- and middle-income countries: Environmental impacts, challenges and sustainable management practices. Process Saf. Environ. Prot. 2023, 174, 510–530. [Google Scholar] [CrossRef]
- Najar, I.N.; Sharma, P.; Das, R.; Tamang, S.; Mondal, K.; Thakur, N.; Gandhi, S.G.; Kumar, V. From waste management to circular economy: Leveraging thermophiles for sustainable growth and global resource optimization. J. Environ. Manag. 2024, 360, 121136. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Saravanan, A.; Varjani, S.; Ramamurthy, R. Bioconversion of municipal solid waste into bio-based products: A review on valorisation and sustainable approach for circular bioeconomy. Sci. Total Environ. 2020, 748, 141312. [Google Scholar] [CrossRef]
- Oliveira, M.; Cocozza, A.; Zucaro, A.; Santagata, R.; Ulgiati, S. Circular economy in the agro-industry: Integrated environmental assessment of dairy products. Renew. Sustain. Energy Rev. 2021, 148, 111314. [Google Scholar] [CrossRef]
- Muthuraj, R.; Valerio, O.; Mekonnen, T.H. Recent developments in short- and medium-chain- length Polyhydroxyalkanoates: Production, properties, and applications. Int. J. Biol. Macromol. 2021, 187, 422–440. [Google Scholar] [CrossRef]
- Taguchi, S.; Matsumoto, K.i. Evolution of polyhydroxyalkanoate synthesizing systems toward a sustainable plastic industry. Polym. J. 2020, 53, 67–79. [Google Scholar] [CrossRef]
- Zhuang, Q.; Wang, Q.; Liang, Q.; Qi, Q. Synthesis of polyhydroxyalkanoates from glucose that contain medium-chain-length monomers via the reversed fatty acid β-oxidation cycle in Escherichia coli. Metab. Eng. 2014, 24, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Mallick, N. Enhanced production of SCL-LCL-PHA co-polymer by sludge-isolated Pseudomonas aeruginosa MTCC 7925. Lett. Appl. Microbiol. 2008, 46, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.T.; Hosoe, Y.; Guex, M.; Tomoi, M.; Tomita, H.; Zinn, M.; Matsumoto, K.i. Directed Evolution of Sequence-Regulating Polyhydroxyalkanoate Synthase to Synthesize a Medium-Chain-Length–Short-Chain-Length (MCL–SCL) Block Copolymer. Biomacromolecules 2022, 23, 1221–1231. [Google Scholar] [CrossRef]
- Israni, N.; Shivakumar, S. Chapter 13—Polyhydroxybutyrate: Development and applications as a biodegradable biotextile. In Materials for Biomedical Engineering; Grumezescu, V., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 405–444. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Cammas-Marion, S.; Martínez-Barbosa, M.E. 15—Microbial polyesters: Synthesis and applications. In Fundamentals of Natural Fibres and Textiles; Mondal, M.I.H., Ed.; Woodhead Publishing: Cambridge, UK, 2021; pp. 515–555. [Google Scholar] [CrossRef]
- Chavan, S.; Yadav, B.; Tyagi, R.D.; Drogui, P. A review on production of polyhydroxyalkanoate (PHA) biopolyesters by thermophilic microbes using waste feedstocks. Bioresour. Technol. 2021, 341, 125900. [Google Scholar] [CrossRef]
- Allegue, L.D.; Ventura, M.; Melero, J.A.; Puyol, D. Unraveling PHA production from urban organic waste with purple phototrophic bacteria via organic overload. Renew. Sustain. Energy Rev. 2022, 166, 112687. [Google Scholar] [CrossRef]
- Rajesh Banu, J.; Ginni, G.; Kavitha, S.; Yukesh Kannah, R.; Kumar, V.; Adish Kumar, S.; Gunasekaran, M.; Tyagi, V.K.; Kumar, G. Polyhydroxyalkanoates synthesis using acidogenic fermentative effluents. Int. J. Biol. Macromol. 2021, 193, 2079–2092. [Google Scholar] [CrossRef] [PubMed]
- Corrado, I.; Varriale, S.; Pezzella, C. Microbial Processes for Upcycling Food Wastes into Sustainable Bioplastics. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Bellini, S.; Tommasi, T.; Fino, D. Poly(3-hydroxybutyrate) biosynthesis by Cupriavidus necator: A review on waste substrates utilization for a circular economy approach. Bioresour. Technol. Rep. 2022, 17, 100985. [Google Scholar] [CrossRef]
- Lorini, L.; Martinelli, A.; Pavan, P.; Majone, M.; Valentino, F. Downstream processing and characterization of polyhydroxyalkanoates (PHAs) produced by mixed microbial culture (MMC) and organic urban waste as substrate. Biomass Convers. Biorefinery 2021, 11, 693–703. [Google Scholar] [CrossRef]
- Crognale, S.; Tonanzi, B.; Valentino, F.; Majone, M.; Rossetti, S. Microbiome Dynamics and phaC Shyntase Genes Selected in a Pilot Plant Producing Polyhydroxyalkanoates Production from Organic Fraction of Urban Waste; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Francesco Valentino, M.R.; Silva, F.; Majone, M. Report on Optimization of PHA Production at Pilot Scale; European Comision: Brussels, Belgium, 2019. Available online: https://ec.europa.eu/research/participants/documents/downloadPublic?documentIds=080166e080165c080162e080107c080106&appId=PPGMS (accessed on 20 June 2022).
- Mannina, G.; Presti, D.; Montiel-Jarillo, G.; Carrera, J.; Suárez-Ojeda, M.E. Recovery of polyhydroxyalkanoates (PHAs) from wastewater: A review. Bioresour. Technol. 2020, 297, 122478. [Google Scholar] [CrossRef]
- Oliveira, C.; Silva, C.; Carvalho, G.; Reis, M. Strategies for efficiently selecting PHA producing mixed microbial cultures using complex feedstocks: Feast and famine regime and uncoupled carbon and nitrogen availabilities. New Biotechnol. 2016, 37, 69–79. [Google Scholar] [CrossRef]
- Di Caprio, F.; Proietti Tocca, G.; Stoller, M.; Pagnanelli, F.; Altimari, P. Control of bacterial contamination in microalgae cultures integrated with wastewater treatment by applying feast and famine conditions. J. Environ. Chem. Eng. 2022, 10, 108262. [Google Scholar] [CrossRef]
- Jayakrishnan, U.; Deka, D.; Das, G. Waste as feedstock for polyhydroxyalkanoate production from activated sludge: Implications of aerobic dynamic feeding and acidogenic fermentation. J. Environ. Chem. Eng. 2021, 9, 105550. [Google Scholar] [CrossRef]
- Cui, Y.-W.; Zhang, H.-Y.; Lu, P.-F.; Peng, Y.-Z. Effects of carbon sources on the enrichment of halophilic polyhydroxyalkanoate-storing mixed microbial culture in an aerobic dynamic feeding process. Sci. Rep. 2016, 6, 30766. [Google Scholar] [CrossRef] [PubMed]
- Moretto, G.; Lorini, L.; Pavan, P.; Crognale, S.; Tonanzi, B.; Rossetti, S.; Majone, M.; Valentino, F. Biopolymers from Urban Organic Waste: Influence of the Solid Retention Time to Cycle Length Ratio in the Enrichment of a Mixed Microbial Culture (MMC). ACS Sustain. Chem. Eng. 2020, 8, 14531–14539. [Google Scholar] [CrossRef]
- Valentino, F.; Morgan-Sagastume, F.; Campanari, S.; Villano, M.; Werker, A.; Majone, M. Carbon recovery from wastewater through bioconversion into biodegradable polymers. New Biotechnol. 2017, 37, 9–23. [Google Scholar] [CrossRef]
- Majone, M.; Dircks, K.; Beim, J.J. Aerobic storage under dynamic conditions in activated sludge processes. The state of the art. Water Sci. Technol. 1999, 39, 61–73. [Google Scholar] [CrossRef]
- Sun, Y.; Angelotti, B.; Brooks, M.; Wang, Z.-W. Feast/famine ratio determined continuous flow aerobic granulation. Sci. Total Environ. 2021, 750, 141467. [Google Scholar] [CrossRef]
- Iorhemen, O.T.; Zaghloul, M.S.; Hamza, R.A.; Tay, J.H. Long-term aerobic granular sludge stability through anaerobic slow feeding, fixed feast-famine period ratio, and fixed SRT. J. Environ. Chem. Eng. 2020, 8, 103681. [Google Scholar] [CrossRef]
- Sousa, B.V.; Silva, F.; Reis, M.A.M.; Lourenço, N.D. Monitoring pilot-scale polyhydroxyalkanoate production from fruit pulp waste using near-infrared spectroscopy. Biochem. Eng. J. 2021, 176, 108210. [Google Scholar] [CrossRef]
- Albuquerque, M.; Martino, V.; Pollet, E.; Avérous, L.; Reis, M. Mixed culture polyhydroxyalkanoate (PHA) production from volatile fatty acid (VFA)-rich streams: Effect of substrate composition and feeding regime on PHA productivity, composition and properties. J. Biotechnol. 2010, 151, 66–76. [Google Scholar] [CrossRef]
- Chen, H.; Meng, H.; Nie, Z.; Zhang, M. Polyhydroxyalkanoate production from fermented volatile fatty acids: Effect of pH and feeding regimes. Bioresour. Technol. 2013, 128, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Mannina, G.; Presti, D.; Montiel-Jarillo, G.; Suárez-Ojeda, M.E. Bioplastic recovery from wastewater: A new protocol for polyhydroxyalkanoates (PHA) extraction from mixed microbial cultures. Bioresour. Technol. 2019, 282, 361–369. [Google Scholar] [CrossRef]
- Valentino, F.; Beccari, M.; Fraraccio, S.; Zanaroli, G.; Majone, M. Feed frequency in a Sequencing Batch Reactor strongly affects the production of polyhydroxyalkanoates (PHAs) from volatile fatty acids. New Biotechnol. 2014, 31, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Korkakaki, E.; Mulders, M.; Veeken, A.; Rozendal, R.; van Loosdrecht, M.C.M.; Kleerebezem, R. PHA production from the organic fraction of municipal solid waste (OFMSW): Overcoming the inhibitory matrix. Water Res. 2016, 96, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hebly, M.; Kleerebezem, R.; Muyzer, G.; van Loosdrecht, M.C.M. Metabolic modeling of mixed substrate uptake for polyhydroxyalkanoate (PHA) production. Water Res. 2011, 45, 1309–1321. [Google Scholar] [CrossRef]
- Choi, J.-i.; Lee, S.Y. Process analysis and economic evaluation for Poly(3-hydroxybutyrate) production by fermentation. Bioprocess Eng. 1997, 17, 335–342. [Google Scholar] [CrossRef]
- Burniol-Figols, A.; Varrone, C.; Le, S.B.; Daugaard, A.E.; Skiadas, I.V.; Gavala, H.N. Combined polyhydroxyalkanoates (PHA) and 1,3-propanediol production from crude glycerol: Selective conversion of volatile fatty acids into PHA by mixed microbial consortia. Water Res. 2018, 136, 180–191. [Google Scholar] [CrossRef]
- Freches, A.; Lemos, P.C. Microbial selection strategies for polyhydroxyalkanoates production from crude glycerol: Effect of OLR and cycle length. New Biotechnol. 2017, 39, 22–28. [Google Scholar] [CrossRef]
- Bengtsson, S.; Werker, A.; Christensson, M.; Welander, T. Production of polyhydroxyalkanoates by activated sludge treating a paper mill wastewater. Bioresour. Technol. 2008, 99, 509–516. [Google Scholar] [CrossRef]
- Jacquel, N.; Lo, C.-W.; Wei, Y.-H.; Wu, H.-S.; Wang, S.S. Isolation and purification of bacterial poly(3-hydroxyalkanoates). Biochem. Eng. J. 2008, 39, 15–27. [Google Scholar] [CrossRef]
- Koller, M.; Niebelschütz, H.; Braunegg, G. Strategies for recovery and purification of poly[(R)-3-hydroxyalkanoates] (PHA) biopolyesters from surrounding biomass. Eng. Life Sci. 2013, 13, 549–562. [Google Scholar] [CrossRef]
- Kosseva, M.R.; Rusbandi, E. Trends in the biomanufacture of polyhydroxyalkanoates with focus on downstream processing. Int. J. Biol. Macromol. 2018, 107, 762–778. [Google Scholar] [CrossRef] [PubMed]
- Samorì, C.; Basaglia, M.; Casella, S.; Favaro, L.; Galletti, P.; Giorgini, L.; Marchi, D.; Mazzocchetti, L.; Torri, C.; Tagliavini, E. Dimethyl carbonate and switchable anionic surfactants: Two effective tools for the extraction of polyhydroxyalkanoates from microbial biomass. Green Chem. 2015, 17, 1047–1056. [Google Scholar] [CrossRef]
- Mongili, B.; Abdel Azim, A.; Fraterrigo Garofalo, S.; Batuecas, E.; Re, A.; Bocchini, S.; Fino, D. Novel insights in dimethyl carbonate-based extraction of polyhydroxybutyrate (PHB). Biotechnol. Biofuels 2021, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- de Souza Reis, G.A.; Michels, M.H.A.; Fajardo, G.L.; Lamot, I.; de Best, J.H. Optimization of Green Extraction and Purification of PHA Produced by Mixed Microbial Cultures from Sludge. Water 2020, 12, 1185. [Google Scholar] [CrossRef]
- Samorì, C.; Abbondanzi, F.; Galletti, P.; Giorgini, L.; Mazzocchetti, L.; Torri, C.; Tagliavini, E. Extraction of polyhydroxyalkanoates from mixed microbial cultures: Impact on polymer quality and recovery. Bioresour. Technol. 2015, 189, 195–202. [Google Scholar] [CrossRef]
- Khosravi-Darani, K.; Vasheghani-Farahani, E.; Yamini, Y.; Bahramifar, N. Solubility of Poly(β-hydroxybutyrate) in Supercritical Carbon Dioxide. J. Chem. Eng. Data 2003, 48, 860–863. [Google Scholar] [CrossRef]
- Urbaser. (2020, Agosto 11). Urbaser Integra la Transformación de Residuos Orgánicos en Bioproductos Para su uso en otros Sectores. Urbaser. Available online: https://www.urbaser.com/2020/08/11/urbaser-integra-la-transformacion-de-residuos-organicos-en-bioproductos-para-su-uso-en-otros-sectores-2/ (accessed on 20 August 2023).
- Ospina, C.; Echeverri, S.; Rodriguez-Gonzalez, C.; Wist, J.; Combariza, M.; Sanabria, J. Enhancement of PHA Production by a Mixed Microbial Culture Using VFA Obtained from the Fermentation of Wastewater from Yeast Industry. Fermentation 2022, 8, 180. [Google Scholar] [CrossRef]
- Miu, D.M.; Eremia, M.C.; Moscovici, M. Polyhydroxyalkanoates (PHAs) as Biomaterials in Tissue Engineering: Production, Isolation, Characterization. Material 2022, 15, 1410. [Google Scholar] [CrossRef]
- Apha, A.; Barron, J. Standard Methods for the Examination of Water & Wastewater, 21st ed.; American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF): Washington, DC, USA, 2005. [Google Scholar]
- Huijberts, G.N.M.; van der Wal, H.; Wilkinson, C.; Eggink, G. Gas-chromatographic analysis of poly(3-hydroxyalkanoates) in bacteria. Biotechnol. Tech. 1994, 8, 187–192. [Google Scholar] [CrossRef]
- Wang, X.; Bengtsson, S.; Oehmen, A.; Carvalho, G.; Werker, A.; Reis, M.A.M. Application of dissolved oxygen (DO) level control for polyhydroxyalkanoate (PHA) accumulation with concurrent nitrification in surplus municipal activated sludge. New Biotechnol. 2019, 50, 37–43. [Google Scholar] [CrossRef]
- Pinto-Ibieta, F.; Serrano, A.; Cea, M.; Ciudad, G.; Fermoso, F.G. Beyond PHA: Stimulating intracellular accumulation of added-value compounds in mixed microbial cultures. Bioresour. Technol. 2021, 337, 125381. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.A.P.; Oehmen, A.; Reis, M.A.M. The impact of biomass withdrawal strategy on the biomass selection and polyhydroxyalkanoates accumulation of mixed microbial cultures. New Biotechnol. 2022, 66, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.K.; Chang, J.-S. Chapter 6—Bioprocessing for production and applications of bioplastics from algae. In Algae-Based Biomaterials for Sustainable Development; Ngo, H., Guo, W., Pandey, A., Chang, J.-S., Lee, D.-J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 105–132. [Google Scholar] [CrossRef]
- Arriaga, M.; Pinar, F.J.; Izarra, I.; Amo, J.D.; Vicente, J.; Fernández-Morales, F.J.; Mena, J. Valorization of Agri-Food Waste into PHA and Bioplastics: From Waste Selection to Transformation. Appl. Sci. 2025, 15, 1008. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Anwar, N.; Ma, Z.; Liu, G.; Zhang, R. Effect of Organic Loading Rate on Anaerobic Digestion of Food Waste under Mesophilic and Thermophilic Conditions. Energy Fuels 2017, 31, 2976–2984. [Google Scholar] [CrossRef]
- de Lucas, A.; Rodríguez, L.; Villaseñor, J.; Fernández, F.J. Biodegradation kinetics of stored wastewater substrates by a mixed microbial culture. Biochem. Eng. J. 2005, 26, 191–197. [Google Scholar] [CrossRef]
- Krzeminski, P.; Leverette, L.; Malamis, S.; Katsou, E. Membrane bioreactors—A review on recent developments in energy reduction, fouling control, novel configurations, LCA and market prospects. J. Membr. Sci. 2017, 527, 207–227. [Google Scholar] [CrossRef]
- Jwara, T.; Musonge, P.; Babatunde, F.; Mnguni, M. Biological Nutrient Removal Efficiencies for Hydraulically Overloaded Wastewater Works; WIT Press (Wessex Institute of Technology Press): Southampton, UK, 2019; pp. 223–231. [Google Scholar]
- Eriksson, E. Polyhydroxyalkanoate Production from Municipal Waste Streams. In Kth Skolan för Kemi, Bioteknologi Och Hälsa; KTH Royal Institute of Technology: Stockholm, Sweden, 2020. [Google Scholar]
- Lorini, L.; Di Re, F.; Majone, M.; Valentino, F. High rate selection of PHA accumulating mixed cultures in Sequencing Batch Reactors with uncoupled carbon and nitrogen feeding. New Biotechnol. 2020, 56, 140–148. [Google Scholar] [CrossRef]
- Valentino, F.; Moretto, G.; Lorini, L.; Bolzonella, D.; Pavan, P.; Majone, M. Pilot-Scale Polyhydroxyalkanoate Production from Combined Treatment of Organic Fraction of Municipal Solid Waste and Sewage Sludge. Ind. Eng. Chem. Res. 2019, 58, 12149–12158. [Google Scholar] [CrossRef]
- Singh, A.; Srivastava, V. Production of polyhydroxyalkanoates (PHA) by pseudomonas from waste products. Int. J. Health Sci. 2022, 6, 11450–11459. [Google Scholar] [CrossRef]
- Colombo, B.; Favini, F.; Scaglia, B.; Sciarria, T.P.; D’Imporzano, G.; Pognani, M.; Alekseeva, A.; Eisele, G.; Cosentino, C.; Adani, F. Enhanced polyhydroxyalkanoate (PHA) production from the organic fraction of municipal solid waste by using mixed microbial culture. Biotechnol. Biofuels 2017, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Nazir, H.; Batool, M.; Bolivar Osorio, F.J.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Inamuddin; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Pagliano, G.; Galletti, P.; Samorì, C.; Zaghini, A.; Torri, C. Recovery of Polyhydroxyalkanoates From Single and Mixed Microbial Cultures: A Review. Front. Bioeng. Biotechnol. 2021, 9, 624021. [Google Scholar] [CrossRef]




| Enrichment Fermenter | |
|---|---|
| Working volume (L) | 15,000 |
| HRT (d) | 5 |
| Cycle length (h) | 24 |
| Feeding period (min) | 30 |
| OLR (gCOD/L. d) | 1 |
| Aeration (m3/h) | 781 |
| Stirring (rpm) | 50 |
| pH | No control |
| Temperature | No control |
| Total COD (mg/L) | 62,165.00 ± 8922.16 |
|---|---|
| Soluble COD (mg/L) | 42,483.13 ± 8374.85 |
| N-NH4 (mg/L) | 1397.25 ± 161.12 |
| NT (mg/L) | 6450.00 ± 1990.81 |
| NO3− (mg/L) | 26.13 ± 14.99 |
| NO2− (mg/L) | 2.93 ± 1.48 |
| Ca (mg/L) | 2888.75 ± 620.89 |
| PT (mg/L) | 84.38 ± 30.53 |
| P-PO4 (mg/L) | 40.70 ± 19.25 |
| SST (g/L) | 2.06 ± 0.93 |
| SSV (g/L) | 1.55 ± 0.83 |
| Fe (mg/L) | 110.33 ± 60.37 |
| Mn (mg/L) | 15.31 ± 2.45 |
| Zn (mg/L) | 4.96 ± 2.87 |
| Cu (mg/L) | 5.00 ± 1.30 |
| Ca (mg/L) | 2888.75 ± 620.89 |
| Na (mg/L) | 1555.00 ± 77.78 |
| K (mg/L) | 3930.00 ± 49.49 |
| SO4 (mg/L) | 1778.00 ± 142.02 |
| Cycle | TSS (g/L) | VSS (g/L) | COD Consumption Rate in the Enrichment Fermenter (mg COD/L.Min) | g COD/g SSV·h |
|---|---|---|---|---|
| 29–34 | 2.89 ± 0.24 | 1.95 ± 0.27 | 10.11 | 0.31 |
| 35–62 | 3.05 ± 0.52 | 2.21 ± 0.45 | 10.41 | 0.28 |
| 63–81 | 3.30 ± 0.66 | 2.28 ± 0.48 | 11.98 | 0.32 |
| 82–132 | 2.99 ± 0.48 | 2.19 ± 0.66 | 13.91 | 0.38 |
| 133–214 | 2.91 ± 0.74 | 1.92 ± 0.55 | 19.56 | 0.61 |
| Nº | Cycle | COD Feeding Rate (mg COD/L.min) | Initial TSS (g/L) | Final TSS (g/L) | Scl-PHA in Final Biomass (wt.%) | Scl-PHA (g/L) | Scl-PHA Generated (kg)/100 kg COD Fed (%) | Final Soluble COD (mg/L) |
|---|---|---|---|---|---|---|---|---|
| 1 | 111 | 9.86 | 2.75 ± 0.26 | 4.61 ± 0.09 | 2.08 | 0.10 | 0.82 | <200 |
| 2 | 121 | 11.54 | 2.91 ± 0.08 | 5.19 ± 0.12 | 2.30 | 0.12 | 1.89 | |
| 3 | 119 | 12.07 | 3.05 ± 0.10 | 2.61 ± 0.50 | 2.00 | 0.05 | 0.68 | |
| 4 | 117 | 13.59 | 3.67 ± 0.59 | 5.01 ± 0.17 | 1.72 | 0.09 | 0.97 | |
| 5 | 117 | 13.80 | 3.51 ± 0.47 | 3.69 ± 0.57 | 2.48 | 0.09 | 0.88 | |
| 6 | 127 | 17.05 | 3.16 ± 0.10 | 6.92 ± 0.87 | 2.61 | 0.18 | 2.72 | |
| 7 | 132 | 20.28 | 4.12± 0.90 | 14.72 ± 0.15 | 6.71 | 0.99 | 12.16 | |
| 8 | 128 | 20.61 | 3.32 ± 0.18 | 6.43 ± 0.21 | 7.65 | 0.49 | 6.35 | |
| 9 | 129 | 24.44 | 3.16 ± 0.05 | 6.11 ± 0.11 | 12.44 | 0.76 | 6.11 | 237 |
| 10 | 131 | 24.44 | 2.84 ± 0.28 | 7.44 ± 0.27 | 16.06 | 1.19 | 10.48 | 299 |
| Nº | Cycle | Real COD Feeding Rate Applied in the Accumulation Reaction (mg COD/L.min) | TSS Initial (g/L) | TSS Final (g/L) | Scl-PHA in Biomass (wt.%) | Scl-PHA (g/L) | Scl-PHA Generated (kg)/100 kg COD Fed (%) | Final Soluble COD (mg/L) |
|---|---|---|---|---|---|---|---|---|
| 11 | 136 | 13.63 | 3.18 ± 0.05 | 7.74 ± 0.11 | 8.55 | 0.66 | 5.23 | 198 |
| 12 | 138 | 23.27 | 3.39 ± 0.15 | 7.04 ± 0.47 | 19.71 | 1.39 | 11.61 | 375 |
| 13 | 133 | 33.85 | 2.99 ± 0.08 | 8.32 ± 0.81 | 9.06 | 0.75 | 4.97 | 222 |
| 14 | 141 | 38.41 | 3.91 ± 1.09 | 9.92 ± 0.30 | 7.94 | 0.79 | 4.83 | 478 |
| 15 | 140 | 41.90 | 3.81 ± 0.08 | 10.17 ± 1.12 | 16.00 | 1.63 | 8.22 | 200 |
| 16 | 209 | 53.55 | 2.29 ± 0.04 | 4.52 ± 0.27 | 19.00 | 0.86 | 6.06 | 405 |
| 17 | 214 | 54.11 | 2.79 ± 0.79 | 4.26 ± 1.48 | 15.49 | 0.65 | 4.83 | 489 |
| 18 | 208 | 55.32 | 2.44 ± 0.07 | 4.09 ± 0.21 | 24.73 | 1.01 | 6.39 | 510 |
| 19 | 214 | 55.32 | 2.79 ± 0.79 | 4.35 ± 0.30 | 19.39 | 0.84 | 6.46 | 456 |
| 20 | 206 | 56.03 | 2.50 ± 0.15 | 4.18 ± 0.13 | 28.30 | 1.18 | 8.25 | 501 |
| 21 | 207 | 56.71 | 2.67 ± 0.14 | 5.40 ± 0.40 | 29.35 | 1.58 | 10.48 | 375 |
| 22 | 213 | 57.92 | 2.87 ± 0.67 | 4.40 ± 0.16 | 19.82 | 0.87 | 5.02 | 406 |
| 23 | 208 | 60.07 | 2.44 ± 0.07 | 4.35 ± 0.36 | 20.80 | 0.90 | 5.81 | 375 |
| 24 | 209 | 60.07 | 2.29 ± 0.04 | 3.89 ± 0.23 | 19.09 | 0.74 | 4.71 | 401 |
| 25 | 205 | 62.52 | 2.47 ± 0.05 | 5.90 ± 0.26 | 27.15 | 1.60 | 10.23 | 346 |
| 26 | 211 | 62.31 | 2.61 ± 0.25 | 2.98 ± 0.08 | 13.28 | 0.40 | 2.40 | 479 |
| 27 | 134 | 65.26 | 3.00 ± 0.27 | 11.97 ± 0.97 | 30.62 | 3.67 | 15.67 | 198 |
| 28 | 201 | 67.13 | 3.23 ± 0.37 | 6.79 ± 0.01 | 29.92 | 2.03 | 9.55 | 245 |
| 29 | 198 | 67.88 | 2.74 ± 0.12 | 3.58 ± 0.46 | 24.57 | 0.88 | 3.99 | 605 |
| 30 | 200 | 72.38 | 3.51 ± 0.51 | 3.57 ± 0.06 | 34.22 | 1.22 | 6.90 | 547 |
| 31 | 197 | 73.16 | 2.96 ± 0.07 | 3.98 ± 0.43 | 29.73 | 1.18 | 6.43 | 705 |
| 32 | 181 | 81.50 | 2.22 ± 0.04 | 1.99 ± 0.01 | 54.05 | 1.08 | 3.63 | 458 |
| 33 | 185 | 81.50 | 2.54 ± 0.12 | 1.78 ± 0.16 | 37.77 | 0.67 | 2.91 | 427 |
| 34 | 187 | 81.50 | 2.71 ± 0.01 | 2.00 ± 0.03 | 34.98 | 0.70 | 3.25 | 515 |
| 35 | 190 | 81.50 | 3.16 ± 0.20 | 4.94 ± 0.15 | 58.00 | 2.87 | 11.68 | 470 |
| 36 | 194 | 81.50 | 2.61 ± 0.13 | 9.5 ± 0.30 | 38.34 | 3.64 | 22.32 | 200 |
| 37 | 196 | 81.50 | 3.00 ± 0.34 | 4.53 ± 0.70 | 40.61 | 1.84 | 8.66 | 347 |
| 38 | 203 | 83.50 | 3.00 ± 0.27 | 5.63 ± 0.09 | 32.54 | 1.83 | 7.65 | 750 |
| 39 | 204 | 83.50 | 4.11 ± 1.30 | 4.64 ± 0.14 | 29.35 | 1.36 | 5.69 | 874 |
| Data Before Solvent Extraction | |
|---|---|
| Wet biomass treated (after centrifugation) (kg) | 543 |
| Scl-PHA content (wt.%) | 22.3 |
| Humidity (%) | 80 |
| Scl-PHA content (kg) | 24.21 |
| Data after solvent extraction | |
| Remaining scl-PHA in the biomass after the first extraction with DMC (wt.%) | 7.56 |
| Remaining scl-PHA in the biomass after the second extraction with DMC (wt.%) | 4.17 |
| Remaining scl-PHA in the biomass after the third extraction with DMC (wt.%) | 1.6 |
| Total extracted scl-PHA (kg) | 22.4 |
| Extraction yield (%) (respect to the wet biomass treated) | 92.78 |
| Purity (%) | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izarra, I.; Álvarez, I.; Pinar, F.J.; Mena, J. Urban Biorefinery Demonstration: Production of Polyhydroxyalkanoates from a Municipal Solid Waste. Appl. Sci. 2025, 15, 3272. https://doi.org/10.3390/app15063272
Izarra I, Álvarez I, Pinar FJ, Mena J. Urban Biorefinery Demonstration: Production of Polyhydroxyalkanoates from a Municipal Solid Waste. Applied Sciences. 2025; 15(6):3272. https://doi.org/10.3390/app15063272
Chicago/Turabian StyleIzarra, Irene, Irene Álvarez, F. Javier Pinar, and Javier Mena. 2025. "Urban Biorefinery Demonstration: Production of Polyhydroxyalkanoates from a Municipal Solid Waste" Applied Sciences 15, no. 6: 3272. https://doi.org/10.3390/app15063272
APA StyleIzarra, I., Álvarez, I., Pinar, F. J., & Mena, J. (2025). Urban Biorefinery Demonstration: Production of Polyhydroxyalkanoates from a Municipal Solid Waste. Applied Sciences, 15(6), 3272. https://doi.org/10.3390/app15063272






