Gagam-Palmultang Restores Immune Homeostasis and T Lymphocyte Activation in a Cyclophosphamide-Induced Immunosuppression Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Authentication of PMT
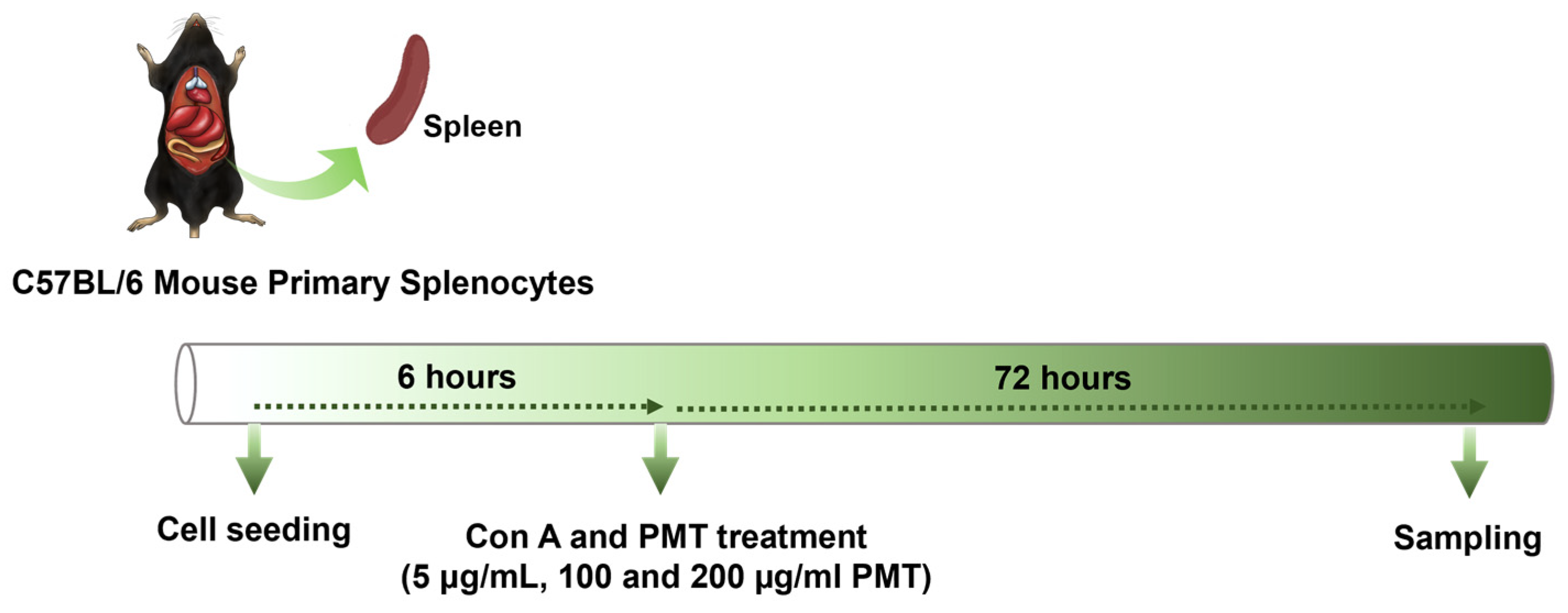
2.2. Mouse Primary Splenocyte Culture and Drug Treatment
2.3. ELISA
2.4. Real-Time qPCR
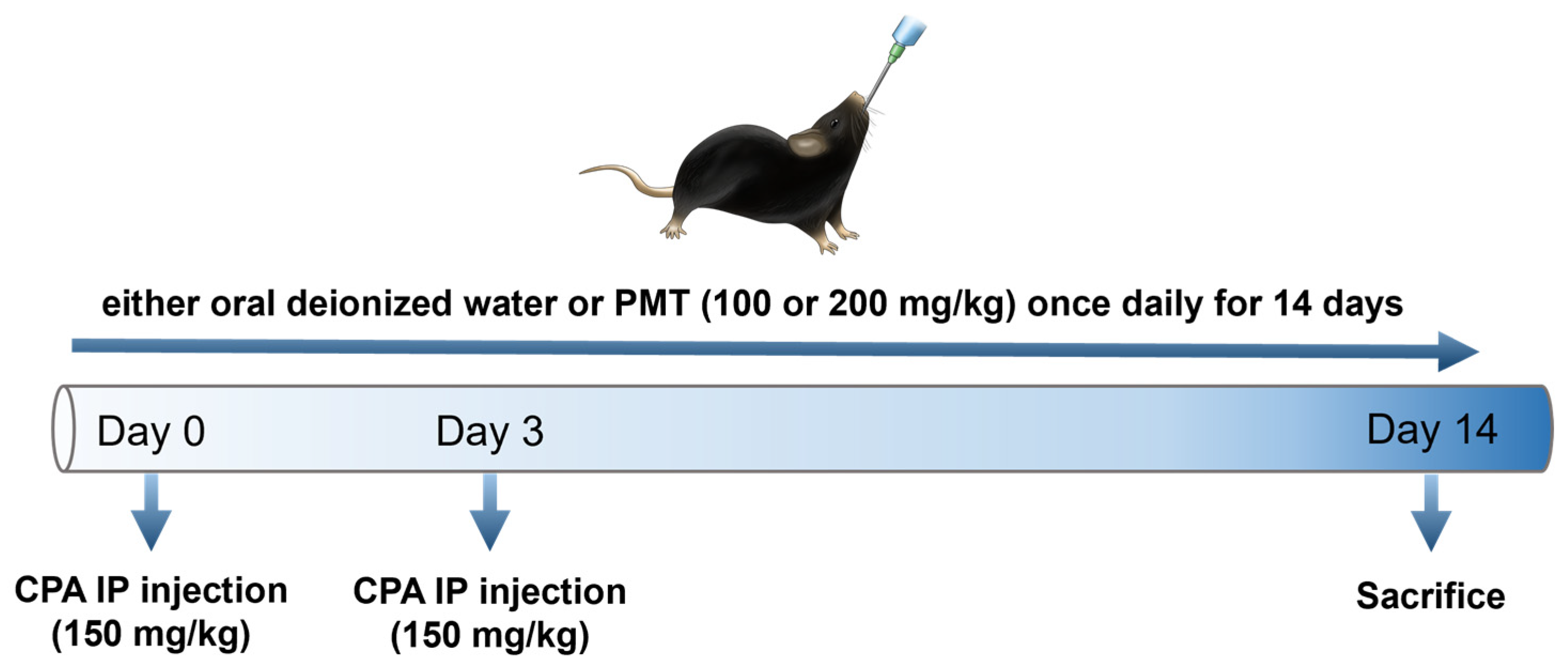
2.5. Animal Model and Treatment Protocol
2.6. Hematological Analysis
2.7. Weight Gain and Immune Organ Index Analysis
2.8. Hematoxylin and Eosin (H&E) Staining
2.9. Flow Cytometric Analysis
2.10. Immunohistochemistry
2.11. Statistical Analyses
3. Results
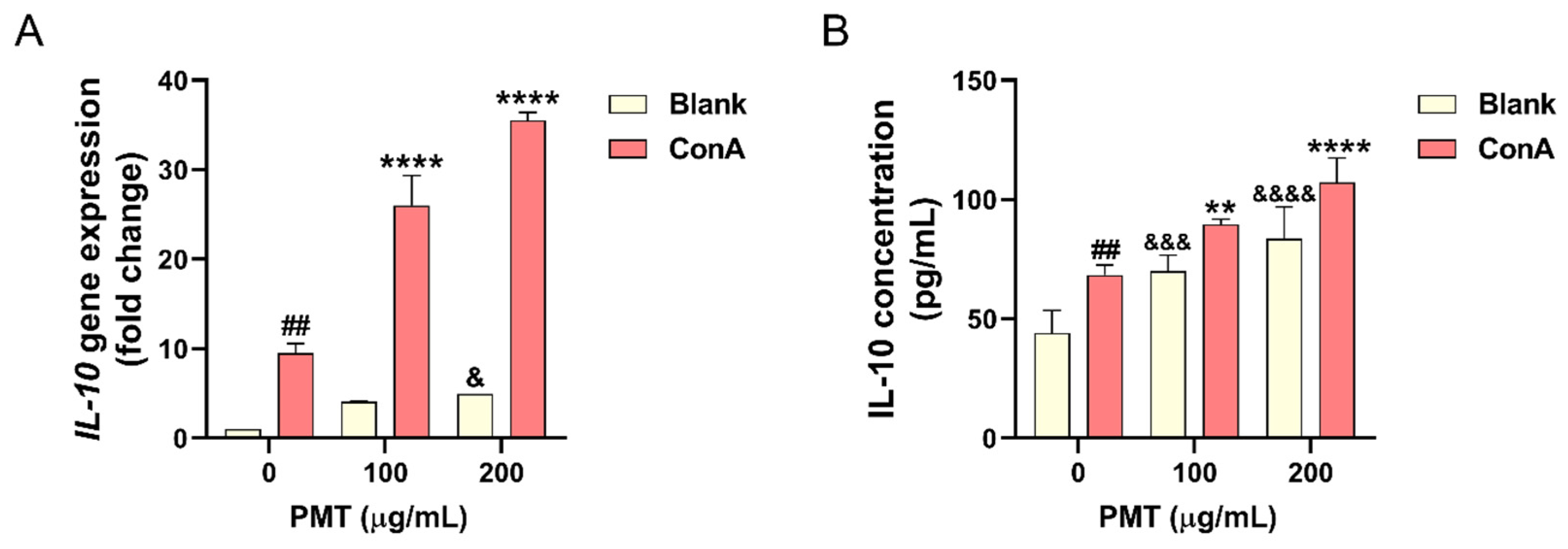
3.1. Effect of PMT on IL-10 Expression in the Con A-Treated Mouse Primary Splenocytes
3.2. Effect of PMT on Weight Gain and Hematological Parameters in the Immunosuppressed Mice
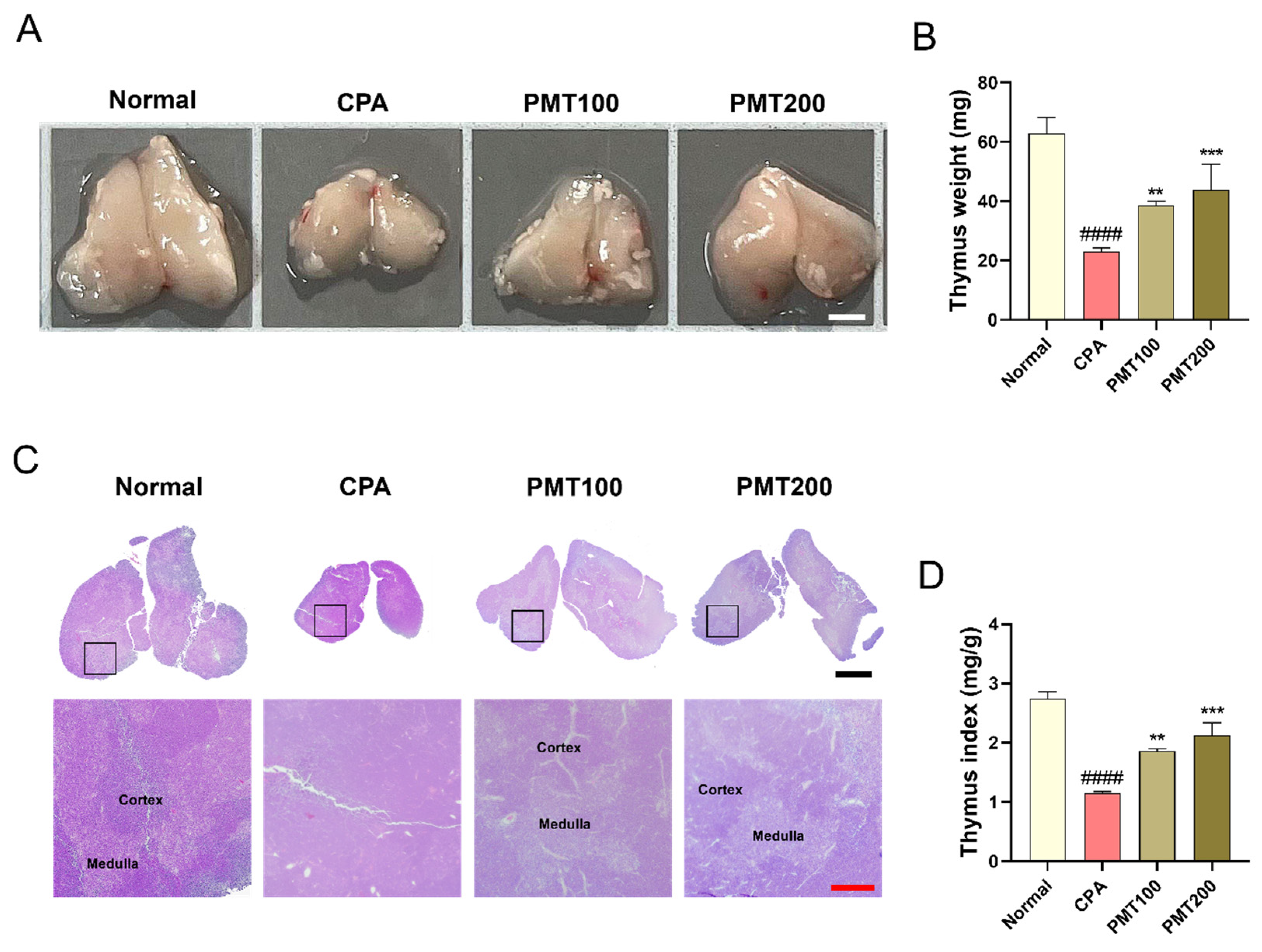
3.3. Effect of PMT on Thymus Morphology and Function in the Immunosuppressed Mice
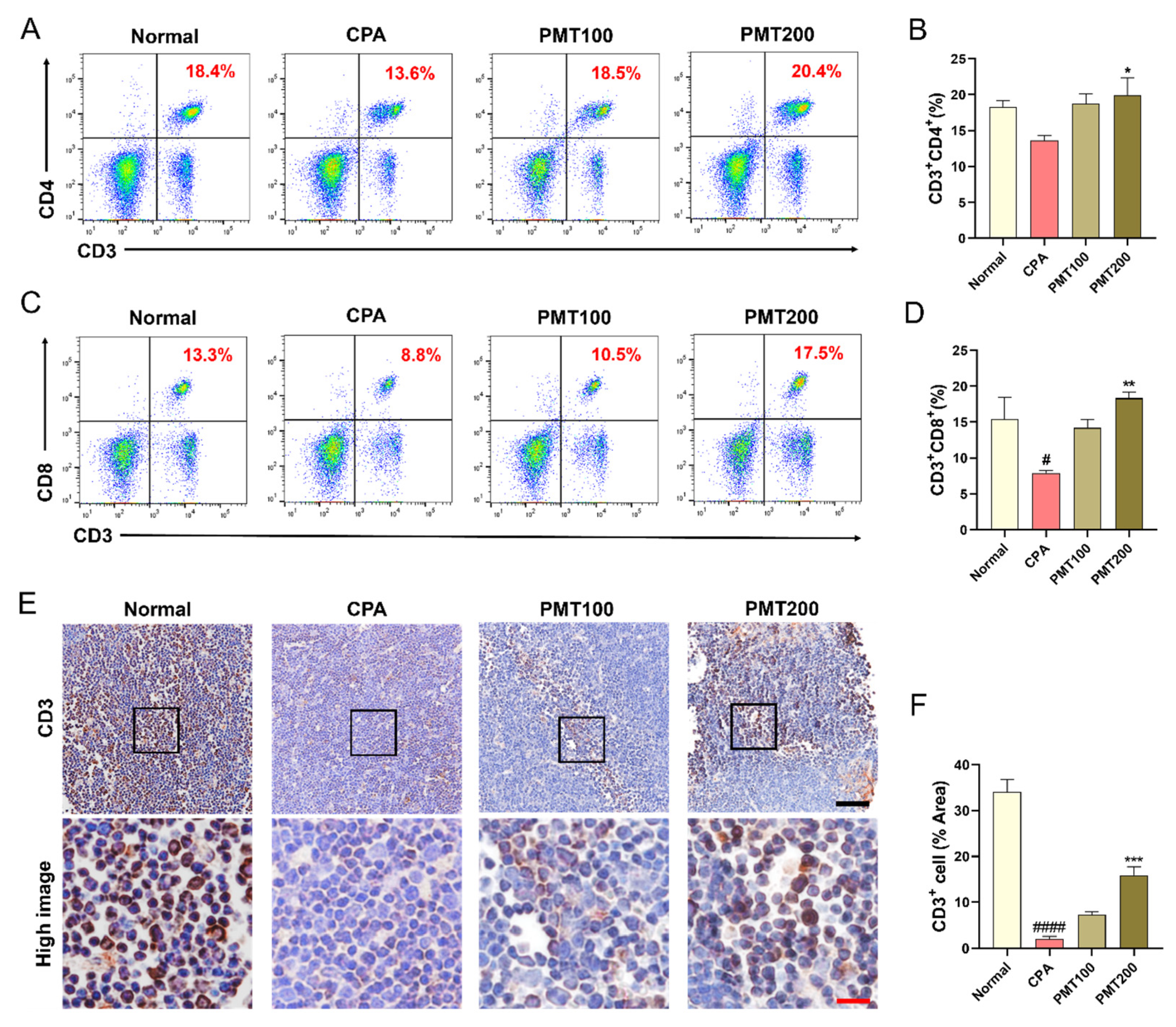
3.4. Effect of PMT on T Lymphocyte Populations in the Immunosuppressed Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BASO | Basophil |
| Con A | concanavalin A |
| CPA | cyclophosphamide |
| ELISA | enzyme-linked immunosorbent assay |
| EOS | eosinophil |
| H&E | hematoxylin and eosin |
| IACUC | Institutional Animal Care and Use Committee |
| IL | interleukin |
| LYM | lymphocyte |
| MONO | monocyte |
| NEU | neutrophil |
| PE | phycoerythrin |
| PMT | gagam-palmultang |
| qPCR | quantitative PCR |
| SEM | standard error of the mean |
| WBC | white blood cell |
References
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Moudgil, K.D.; Venkatesha, S.H. The Anti-Inflammatory and Immunomodulatory Activities of Natural Products to Control Autoimmune Inflammation. Int. J. Mol. Sci. 2022, 24, 95. [Google Scholar] [CrossRef] [PubMed]
- Reale, M.; Conti, L.; Velluto, D. Immune and Inflammatory-Mediated Disorders: From Bench to Bedside. J. Immunol. Res. 2018, 2018, 7197931. [Google Scholar] [CrossRef]
- Alhazmi, H.A.; Najmi, A.; Javed, S.A.; Sultana, S.; Al Bratty, M.; Makeen, H.A.; Meraya, A.M.; Ahsan, W.; Mohan, S.; Taha, M.M.E.; et al. Medicinal Plants and Isolated Molecules Demonstrating Immunomodulation Activity as Potential Alternative Therapies for Viral Diseases Including COVID-19. Front. Immunol. 2021, 12, 637553. [Google Scholar] [CrossRef]
- Shi, J.; Weng, J.-H.; Mitchison, T.J. Immunomodulatory drug discovery from herbal medicines: Insights from organ-specific activity and xenobiotic defenses. eLife 2021, 10, e73673. [Google Scholar] [CrossRef]
- Gao, G.-Z.; Nan, Z. Korean-Chinese-English oriental medicine dictionary; Publication Department. Maeil Health Newspaper, 2001. [Google Scholar]
- Oh, Y.-C.; Jeong, Y.H.; Cho, W.-K.; Gu, M.-J.; Ma, J.Y. Inhibitory effects of palmultang on inflammatory mediator production related to suppression of NF-kappaB and MAPK pathways and induction of HO-1 expression in macrophages. Int. J. Mol. Sci. 2014, 15, 8443–8457. [Google Scholar] [CrossRef]
- Joo, J.-M.; Baek, S.-H.; Kim, E.-H.; Kim, D.-C. The effect of Palmultang (八物湯) on the ovarian functions and differential gene expression of caspase-3, MAPK and MPG in female mice. J. Korean Obstet. Gynecol. 2007, 20, 91–110. [Google Scholar]
- Shin, I.-S.; Lee, M.-Y.; Seo, C.-S.; Lim, H.-S.; Kim, J.-H.; Jeon, W.-Y.; Shin, H.-K.J. Palmul-tang, a traditional herbal formula, protects against ethanol-induced acute gastric injury in rats. J. Korean Med. 2011, 32, 74–84. [Google Scholar]
- Kim, Y.R.; Kwon, M.Y.; Pak, M.E.; Park, S.H.; Baek, J.U.; Choi, B.T. Beneficial Effects of Gagam-Palmultang on Scopolamine-Induced Memory Deficits in Mice. Evid.-Based Complement. Altern. Med. 2018, 2018, 3479083. [Google Scholar] [CrossRef]
- Rhim, E.-K.; Shin, S.-H.J. Protective effects of Palmul-tang on hypoxia-induced apoptosis in H9c2 cardiomyoblast cells. Soc. Korean Med. 2004, 25, 67–76. [Google Scholar]
- Choi, L.Y.; Kim, M.H.; Nam, Y.K.; Kim, J.H.; Cho, H.-Y.; Yang, W.M. Palmul-tang, a Korean medicine, promotes bone formation Via bmp-2 pathway in osteoporosis. Front. Pharmacol. 2021, 12, 643482. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.-W.; Myers, R.; Greenslade, A.; Mader, E.; Greiner, S.; Federspiel, M.J.; Dispenzieri, A.; Russell, S.J. Using clinically approved cyclophosphamide regimens to control the humoral immune response to oncolytic viruses. Gene Ther. 2013, 20, 255–261. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.Y.; Lee, J.; Yeo, C.; Jeon, W.-J.; Lee, Y.J.; Ha, I.-H. Immune-boosting effect of Yookgong-dan against cyclophosphamide-induced immunosuppression in mice. Heliyon 2024, 10, e24033. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of Interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Hsu, P.; Santner-Nanan, B.; Hu, M.; Skarratt, K.; Lee, C.H.; Stormon, M.; Wong, M.; Fuller, S.J.; Nanan, R. IL-10 Potentiates Differentiation of Human Induced Regulatory T Cells via STAT3 and Foxo1. J. Immunol. 2015, 195, 3665–3674. [Google Scholar] [CrossRef]
- Carlini, V.; Noonan, D.M.; Abdalalem, E.; Goletti, D.; Sansone, C.; Calabrone, L.; Albini, A. The multifaceted nature of IL-10: Regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front. Immunol. 2023, 14, 1161067. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Yao, J.; Zhou, G.; Xia, W.; Lyu, J.; Li, X.; Zhao, T.; Zhang, G.; Zhao, N.; Yang, J. Quantitative Proteomic Analysis Reveals That Arctigenin Alleviates Concanavalin A-Induced Hepatitis Through Suppressing Immune System and Regulating Autophagy. Front. Immunol. 2018, 9, 1881. [Google Scholar] [CrossRef] [PubMed]
- Tanno, D.; Yokoyama, R.; Kawamura, K.; Kitai, Y.; Yuan, X.; Ishii, K.; De Jesus, M.; Yamamoto, H.; Sato, K.; Miyasaka, T.; et al. Dectin-2-mediated signaling triggered by the cell wall polysaccharides of Cryptococcus neoformans. Microbiol. Immunol. 2019, 63, 500–512. [Google Scholar] [CrossRef]
- Gao, R.; Li, X.; Gao, H.; Zhao, K.; Liu, X.; Liu, J.; Wang, Q.; Zhu, Y.; Chen, H.; Xiang, S.; et al. Protein phosphatase 2A catalytic subunit β suppresses PMA/ionomycin-induced T-cell activation by negatively regulating PI3K/Akt signaling. FEBS J. 2022, 289, 4518–4535. [Google Scholar] [CrossRef]
- Ren, F.; Chen, X.; Hesketh, J.; Gan, F.; Huang, K. Selenium promotes T-cell response to TCR-stimulation and ConA, but not PHA in primary porcine splenocytes. PLoS ONE 2012, 7, e35375. [Google Scholar] [CrossRef]
- Shinohara, Y.; Tsukimoto, M. Adenine Nucleotides Attenuate Murine T Cell Activation Induced by Concanavalin A or T Cell Receptor Stimulation. Front. Pharmacol. 2017, 8, 986. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J.W.; Kim, Y.-K.; Ku, S.K.; Lee, H.-J. Immunoenhancement Effects of the Herbal Formula Hemomine on Cyclophosphamide-Induced Immunosuppression in Mice. Appl. Sci. 2022, 12, 4935. [Google Scholar] [CrossRef]
- Park, H.-R.; Kim, J.W.; Lee, J.-O.; Ahn, J.-D.; Yang, M.-C.; Bashir, K.M.I.; Choi, J.-S.; Ku, S.-K. Anti-Mutagenic and Immunomodulatory Effects of Astragali Radix Extract on a Cyclophosphamide-Induced Immunosuppressed Mouse Model. Appl. Sci. 2023, 13, 2959. [Google Scholar] [CrossRef]
- Huyan, X.-H.; Lin, Y.-P.; Gao, T.; Chen, R.-Y.; Fan, Y.-M. Immunosuppressive effect of cyclophosphamide on white blood cells and lymphocyte subpopulations from peripheral blood of Balb/c mice. Int. Immunopharmacol. 2011, 11, 1293–1297. [Google Scholar] [CrossRef]
- Kim, J.W.; Choi, J.-S.; Seol, D.J.; Choung, J.J.; Ku, S.K. Immunomodulatory Effects of Kuseonwangdogo-Based Mixed Herbal Formula Extracts on a Cyclophosphamide-Induced Immunosuppression Mouse Model. Evid.-Based Complement. Altern. Med. 2018, 2018, 6017412. [Google Scholar] [CrossRef] [PubMed]
- Refaie, M.M.; El-Hussieny, M.; Bayoumi, A.M.; Shehata, S.; Welson, N.N.; Abdelzaher, W.Y. Simvastatin cardioprotection in cyclophosphamide-induced toxicity via the modulation of inflammasome/caspase1/interleukin1β pathway. Hum. Exp. Toxicol. 2022, 41, 9603271221111440. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-K.; Kim, J.-Y.; Jang, S.-E.; Choi, M.-S.; Jang, H.-M.; Yoo, H.-H.; Kim, D.-H. Fermented Red Ginseng Alleviates Cyclophosphamide-Induced Immunosuppression and 2,4,6-Trinitrobenzenesulfonic Acid-Induced Colitis in Mice by Regulating Macrophage Activation and T Cell Differentiation. Am. J. Chin. Med. 2018, 46, 1879–1897. [Google Scholar] [CrossRef]
- Chen, L.-X.; Qi, Y.-L.; Qi, Z.; Gao, K.; Gong, R.-Z.; Shao, Z.-J.; Liu, S.-X.; Li, S.-S.; Sun, Y.-S. A Comparative Study on the Effects of Different Parts of Panax ginseng on the Immune Activity of Cyclophosphamide-Induced Immunosuppressed Mice. Molecules 2019, 24, 1096. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Liu, J.; Wang, Y.; Zhou, Q.; Wang, S.; Wang, X. Ginsenoside Rg3 improves cyclophosphamide-induced immunocompetence in Balb/c mice. Int. Immunopharmacol. 2019, 72, 98–111. [Google Scholar] [CrossRef]
- Jia, D.; Lu, W.; Wang, C.; Sun, S.; Cai, G.; Li, Y.; Wang, G.; Liu, Y.; Zhang, M.; Wang, D. Investigation on Immunomodulatory Activity of Calf Spleen Extractive Injection in Cyclophosphamide-induced Immunosuppressed Mice and Underlying Mechanisms. Scand. J. Immunol. 2016, 84, 20–27. [Google Scholar] [CrossRef]
- Monmai, C.; You, S.; Park, W.J. Immune-enhancing effects of anionic macromolecules extracted from Codium fragile on cyclophosphamide-treated mice. PLoS ONE 2019, 14, e0211570. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Dong, Z.; Sun, Y.; Li, S.; Zhao, Z. Protective Effect of Bergenin against Cyclophosphamide-Induced Immunosuppression by Immunomodulatory Effect and Antioxidation in Balb/c Mice. Molecules 2018, 23, 2668. [Google Scholar] [CrossRef] [PubMed]
- Shirani, K.; Hassani, F.V.; Razavi-Azarkhiavi, K.; Heidari, S.; Zanjani, B.R.; Karimi, G. Phytotrapy of cyclophosphamide-induced immunosuppression. Environ. Toxicol. Pharmacol. 2015, 39, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, A.F.; Salazar, B.E.; Rodriguez, C.A.; Zapata, A.X.; Agudelo, M.; Vesga, O. Neutropenia induced in outbred mice by a simplified low-dose cyclophosphamide regimen: Characterization and applicability to diverse experimental models of infectious diseases. BMC Infect. Dis. 2006, 6, 55. [Google Scholar] [CrossRef]
- den Haan, J.M.; Arens, R.; van Zelm, M.C. The activation of the adaptive immune system: Cross-talk between antigen-presenting cells, T cells and B cells. Immunol. Lett. 2014, 162, 103–112. [Google Scholar] [CrossRef]
- Serbina, N.V.; Jia, T.; Hohl, T.M.; Pamer, E.G. Monocyte-mediated defense against microbial pathogens. Annu. Rev. Immunol. 2008, 26, 421–452. [Google Scholar] [CrossRef]
- Strandmark, J.; Rausch, S.; Hartmann, S. Eosinophils in Homeostasis and Their Contrasting Roles during Inflammation and Helminth Infections. Crit. Rev. Immunol. 2016, 36, 193–238. [Google Scholar] [CrossRef]
- Bronte, V.; Pittet, M.J. The spleen in local and systemic regulation of immunity. Immunity 2013, 39, 806–818. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, T.; Liao, L.; Fan, X.; Chang, L.; Hashimoto, K. Brain-spleen axis in health and diseases: A review and future perspective. Brain Res. Bull. 2022, 182, 130–140. [Google Scholar] [CrossRef]
- Kolb, J.-P.B.; Poupon, M.-F.M.; Lespinats, G.M.; Sabolovic, D.; Loisillier, F. Splenic modifications induced by cyclophosphamide in C3H/He, nude, and “B” mice. J. Immunol. 1977, 118, 1595–1599. [Google Scholar] [CrossRef]
- Krank, M.D.; MacQueen, G.M. Conditioned compensatory responses elicited by environmental signals for cyclophosphamide-induced suppression of antibody production in mice. Psychobiology 1988, 16, 229–235. [Google Scholar] [CrossRef]
- Feng, L.; Huang, Q.; Huang, Z.; Li, H.; Qi, X.; Wang, Y.; Liu, Z.; Liu, X.; Lu, L. Optimized Animal Model of Cyclophosphamide-induced Bone Marrow Suppression. Basic Clin. Pharmacol. Toxicol. 2016, 119, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Meng, Q.; Qiao, H.; Jiang, H.; Sun, X. Role of the spleen in cyclophosphamide-induced hematosuppression and extramedullary hematopoiesis in mice. Arch. Med. Res. 2009, 40, 249–255. [Google Scholar] [CrossRef]
- Ge, Y.; Yoon, S.-H.; Jang, H.; Jeong, J.-H.; Lee, Y.-M. Decursin promotes HIF-1α proteasomal degradation and immune responses in hypoxic tumour microenvironment. Phytomedicine 2020, 78, 153318. [Google Scholar] [CrossRef]
- Sestito, S.; Ibba, R.; Riu, F.; Carpi, S.; Carta, A.; Manera, C.; Habtemariam, S.; Yeskaliyeva, B.; Almarhoon, Z.M.; Sharifi-Rad, J.; et al. Anticancer potential of decursin, decursinol angelate, and decursinol from Angelica gigas Nakai: A comprehensive review and future therapeutic prospects. Food Sci. Nutr. 2024, 12, 6970–6989. [Google Scholar] [CrossRef]
- Xu, X.; Tao, N.; Sun, C.; Hoffman, R.D.; Shi, D.; Ying, Y.; Dong, S.; Gao, J. Ligustilide prevents thymic immune senescence by regulating Thymosin β15-dependent spatial distribution of thymic epithelial cells. Phytomedicine 2024, 123, 155216. [Google Scholar] [CrossRef]
- Yuan, C.; Sha, X.; Xiong, M.; Zhong, W.; Wei, Y.; Li, M.; Tao, S.; Mou, F.; Peng, F.; Zhang, C. Uncovering dynamic evolution in the plastid genome of seven Ligusticum species provides insights into species discrimination and phylogenetic implications. Sci. Rep. 2021, 11, 988. [Google Scholar] [CrossRef]
- Bhattamisra, S.K.; Koh, H.M.; Lim, S.Y.; Choudhury, H.; Pandey, M. Molecular and Biochemical Pathways of Catalpol in Alleviating Diabetes Mellitus and Its Complications. Biomolecules 2021, 11, 323. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Cho, S.Y.; Kang, S.S.; Lee, S.-H.; Baek, H.-Y.; Kim, Y.S. Orthogonal array design for optimizing extraction efficiency of active constituents from Jakyak-Gamcho Decoction, the complex formula of herbal medicines, Paeoniae Radix and Glycyrrhizae Radix. J. Ethnopharmacol. 2007, 113, 306–311. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, W. Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol. Ther. 2020, 207, 107452. [Google Scholar] [CrossRef]
- Zhang, R.-X.; Li, M.-X.; Jia, Z.-P. Rehmannia glutinosa: Review of botany, chemistry and pharmacology. J. Ethnopharmacol. 2008, 117, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-W.; Min, H.-Y. Ginseng, the ’Immunity Boost’: The Effects of Panax ginseng on Immune System. J. Ginseng Res. 2012, 36, 354–368. [Google Scholar] [CrossRef]
- You, L.; Cha, S.; Kim, M.-Y.; Cho, J.Y. Ginsenosides are active ingredients in Panax ginseng with immunomodulatory properties from cellular to organismal levels. J. Ginseng Res. 2022, 46, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ye, Y.; Chen, H. Astragaloside IV inhibits cell migration and viability of hepatocellular carcinoma cells via suppressing long noncoding RNA ATB. Biomed. Pharmacother. 2018, 99, 134–141. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, C.; Gao, L.; Du, G.; Qin, X. Astragaloside IV derived from Astragalus membranaceus: A research review on the pharmacological effects. Adv. Pharmacol. 2020, 87, 89–112. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; Zhou, A.-G.; Zhang, L.; Chen, W.-J. Antioxidant status and immune activity of glycyrrhizin in allergic rhinitis mice. Int. J. Mol. Sci. 2011, 12, 905–916. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, X.; Wang, X.; Liang, F.; Tang, Y. Glycyrrhizin attenuates caspase-11-dependent immune responses and coagulopathy by targeting high mobility group box 1. Int. Immunopharmacol. 2022, 107, 108713. [Google Scholar] [CrossRef]
- Wei, C.; Wang, H.; Sun, X.; Bai, Z.; Wang, J.; Bai, G.; Yao, Q.; Xu, Y.; Zhang, L. Pharmacological profiles and therapeutic applications of pachymic acid (Review). Exp. Ther. Med. 2022, 24, 547. [Google Scholar] [CrossRef]
- Xu, H.; Van der Jeught, K.; Zhou, Z.; Zhang, L.; Yu, T.; Sun, Y.; Li, Y.; Wan, C.; So, K.M.; Liu, D.; et al. Atractylenolide I enhances responsiveness to immune checkpoint blockade therapy by activating tumor antigen presentation. J. Clin. Investig. 2021, 131, e146832. [Google Scholar] [CrossRef]
- Zhao, Z.-J.; Sun, Y.-L.; Ruan, X.-F. Bornyl acetate: A promising agent in phytomedicine for inflammation and immune modulation. Phytomedicine 2023, 114, 154781. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, Q.-L.; Hua, J.-W.; Cheng, W.-L.; Qin, L.-P. The traditional uses, phytochemistry, and pharmacology of Atractylodes macrocephala Koidz.: A review. J. Ethnopharmacol. 2018, 226, 143–167. [Google Scholar] [CrossRef]
- Liu, S.; Li, J.; Feng, L. Gallic acid regulates immune response in a mouse model of rheumatoid arthritis. Immun. Inflamm. Dis. 2023, 11, e782. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Han, L.; Liu, J.-M.; Zhang, J.; Wang, W.; Li, B.-G.; Dong, C.-X.; Bai, C.-C. Lycium Genus Polysaccharide: An Overview of its Extraction, Structures, Pharmacological Activities and Biological Applications. Separations 2022, 9, 197. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Li, X.; Zhang, L.; Ren, L. Flavonoids as Potential Antiviral Agents for Porcine Viruses. Pharmaceutics 2022, 14, 1793. [Google Scholar] [CrossRef] [PubMed]
- Pázmándi, K.; Szöllősi, A.G.; Fekete, T. The “root” causes behind the anti-inflammatory actions of ginger compounds in immune cells. Front. Immunol. 2024, 15, 1400956. [Google Scholar] [CrossRef]

| Scientific Name of the Herb | Source | Part | Amount (%) |
|---|---|---|---|
| Angelica gigas Nakai | Republic of Korea | Root | 8.98 |
| Ligusticum officinale (Makino) Kitag | Republic of Korea | Rhizome | 5.99 |
| Paeonia japonica (Makino) Miyabe & H.Takeda | Republic of Korea | Root | 5.99 |
| Rehmannia glutinosa (Gaertn.) DC. | Republic of Korea | Root | 11.98 |
| Panax ginseng C.A.Mey. | Republic of Korea | Root | 5.99 |
| Poria cocos | Republic of Korea | Dried sclerotia | 5.99 |
| Astragalus mongholicus Bunge. | Republic of Korea | Root | 11.98 |
| Glycyrrhiza uralensis Fisch. | Republic of Korea | Radices | 5.99 |
| Amomum villosum Lour. | Vietnam | Dry ripe fruit | 4.19 |
| Atractylodes macrocephala Koidz. | Republic of Korea | Rhizome | 5.99 |
| Dimocarpus longan Lour. | Thailand | Aril | 11.98 |
| Lycium chinense Mill. | Republic of Korea | Fruit | 5.99 |
| Crataegus pinnatifida Bunge | Republic of Korea | Fruit | 5.99 |
| Zingiber officinale Roscoe | Republic of Korea | Dry rhizome | 2.99 |
| Total amount | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.Y.; Choi, B.R.; Park, D.R.; Yoon, J.E.; Shin, J.Y.; Lee, Y.J.; Ha, I.-H. Gagam-Palmultang Restores Immune Homeostasis and T Lymphocyte Activation in a Cyclophosphamide-Induced Immunosuppression Mouse Model. Appl. Sci. 2025, 15, 3235. https://doi.org/10.3390/app15063235
Hong JY, Choi BR, Park DR, Yoon JE, Shin JY, Lee YJ, Ha I-H. Gagam-Palmultang Restores Immune Homeostasis and T Lymphocyte Activation in a Cyclophosphamide-Induced Immunosuppression Mouse Model. Applied Sciences. 2025; 15(6):3235. https://doi.org/10.3390/app15063235
Chicago/Turabian StyleHong, Jin Young, Bo Ram Choi, Doo Ri Park, Jee Eun Yoon, Ji Yun Shin, Yoon Jae Lee, and In-Hyuk Ha. 2025. "Gagam-Palmultang Restores Immune Homeostasis and T Lymphocyte Activation in a Cyclophosphamide-Induced Immunosuppression Mouse Model" Applied Sciences 15, no. 6: 3235. https://doi.org/10.3390/app15063235
APA StyleHong, J. Y., Choi, B. R., Park, D. R., Yoon, J. E., Shin, J. Y., Lee, Y. J., & Ha, I.-H. (2025). Gagam-Palmultang Restores Immune Homeostasis and T Lymphocyte Activation in a Cyclophosphamide-Induced Immunosuppression Mouse Model. Applied Sciences, 15(6), 3235. https://doi.org/10.3390/app15063235






