1. Introduction
Central solar receiver system technology presents a promising solution for the advancement of concentrated solar power (CSP). These systems can reach higher operating temperatures, which improves efficiency and reduces costs compared to other CSP technologies [
1]. Currently, commercial central receiver power plants operate with receiver outlet temperatures below 565 °C, using a steam Rankine cycle as the power generation method [
2].
Supercritical carbon dioxide (S-CO
2) cycles have emerged as a promising alternative to steam Rankine cycles in solar tower power plants, primarily due to their higher efficiency. This efficiency arises from the relatively high density of S-CO
2, which decreases the specific work needed during compression. S-CO
2 cycles also reduce heat rejection to the environment, thanks to S-CO
2’s specific heat properties. The relatively high density leads to lower volumetric flow, allowing for more compact turbomachinery. Additionally, CO
2 is cost-effective, abundant, non-explosive, chemically stable, and less corrosive than other working fluids [
3].
The S-CO
2 Brayton cycle can operate with central solar receiver systems in direct or indirect mode. The direct method circulates CO
2 through both the power cycle and the solar receiver. The indirect method uses a different heat transfer fluid (HTF) and requires an intermediate heat exchanger to connect the two systems. This indirect method is preferred because it prevents the direct heating of S-CO
2 [
4]. Choosing the right HTF is crucial, as its maximum temperature determines the efficiency of the power cycle. Solar salt, a mix of 60% NaNO
3 and 40% KNO
3, is often used, reaching a maximum of 565 °C, which limits performance. Researchers are exploring chloride salts, a mix of MgCl
2 and KCl, because they can be obtained easily and economically through mining or desalination [
5,
6].
Several authors have compared different HTFs using S-CO
2 power cycles driven by central solar receiver systems. Wang et al. [
4] investigated six S-CO
2 Brayton cycles powered by a solar tower with thermal energy storage. The intercooling cycle had the highest efficiency, while the partial-cooling cycle yielded the greatest specific work. The straightforward recuperation, partial cooling, and precompression cycles presented the largest temperature difference. In the study referenced [
7], the authors examined three solar tower power cycles: Rankine recompression and partial cooling S-CO
2. They used three molten salts: NaNO
3–KNO
3, MgCl
2–KCl, and Li
2CO
3–Na
2CO
3–K
2CO
3. Conducted in Seville and Dubai, the study aimed to determine annual electricity production efficiency. The results showed that the partial cooling S-CO
2 cycle was the most efficient at both locations, with the best performance in Dubai using Li
2CO
3–Na
2CO
3–K
2CO
3. Regarding reference [
6], this study compares solar salts and a MgCl
2–KCl mixture as heat transfer fluids in a solar tower recompression S-CO
2 Brayton cycle. It evaluates thermal efficiency, specific work, and thermal storage capacity. The MgCl
2–KCl system shows the highest thermal efficiency and specific work due to its ability to operate at higher temperatures, but it has limitations from its low specific heat capacity and higher viscosity. Polimeni et al. [
1] compared two heat transfer fluids: liquid sodium and MgCl
2–KCl. They examined four S-CO
2 cycle configurations: a simple cycle, a recompression cycle, a partial cooling cycle, and a recompression main compressor intercooling cycle. They calculated the solar electricity efficiency in Seville, Spain, and Las Vegas, USA, under design and yearly conditions. The results showed that liquid sodium provided higher efficiency in both conditions. Qiu et al. [
2] optimized the regenerative and recompression cycle of S-CO
2 Brayton systems for a solar power application with a maximum operating temperature of 700 °C. The regenerative cycle achieved higher specific work and heat storage temperature difference, while the recompression cycle resulted in greater thermal efficiency. Agyekum et al. [
8] assessed a 100 MW solar tower CSP plant in Tibet, China, focusing on its technical and economic aspects. They compared two heat transfer fluids, solar salts and a mixture of 46.5% LiF, 11.5% NaF, and 42% KF, along with two power cycles, S-CO
2 and Rankine. Using the System Advisor Model (SAM) [
9], they calculated the net present value, payback period, and levelized cost of electricity. Their findings suggest that the S-CO
2 cycle with the specified mixture is the more economically favorable option.
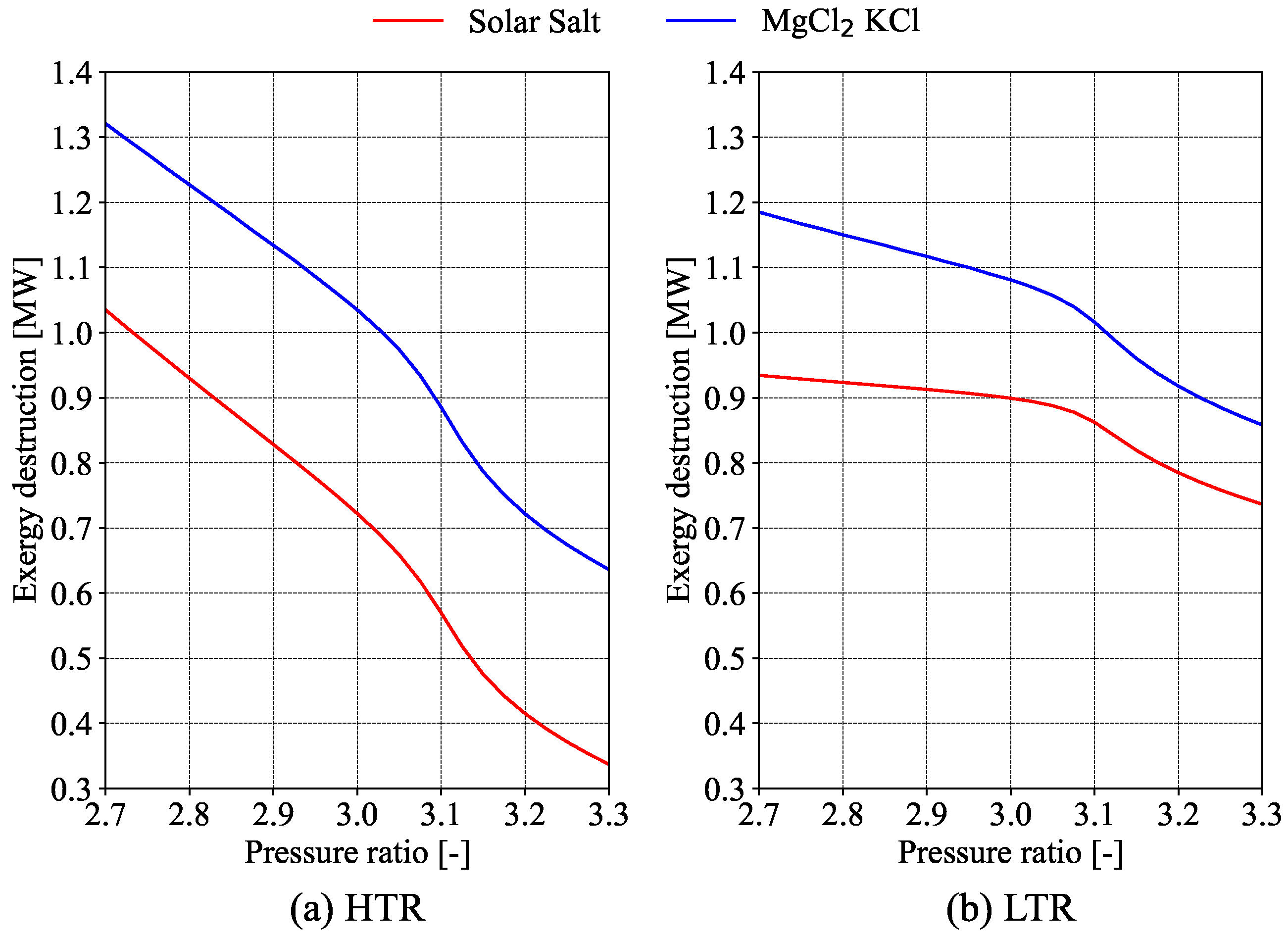
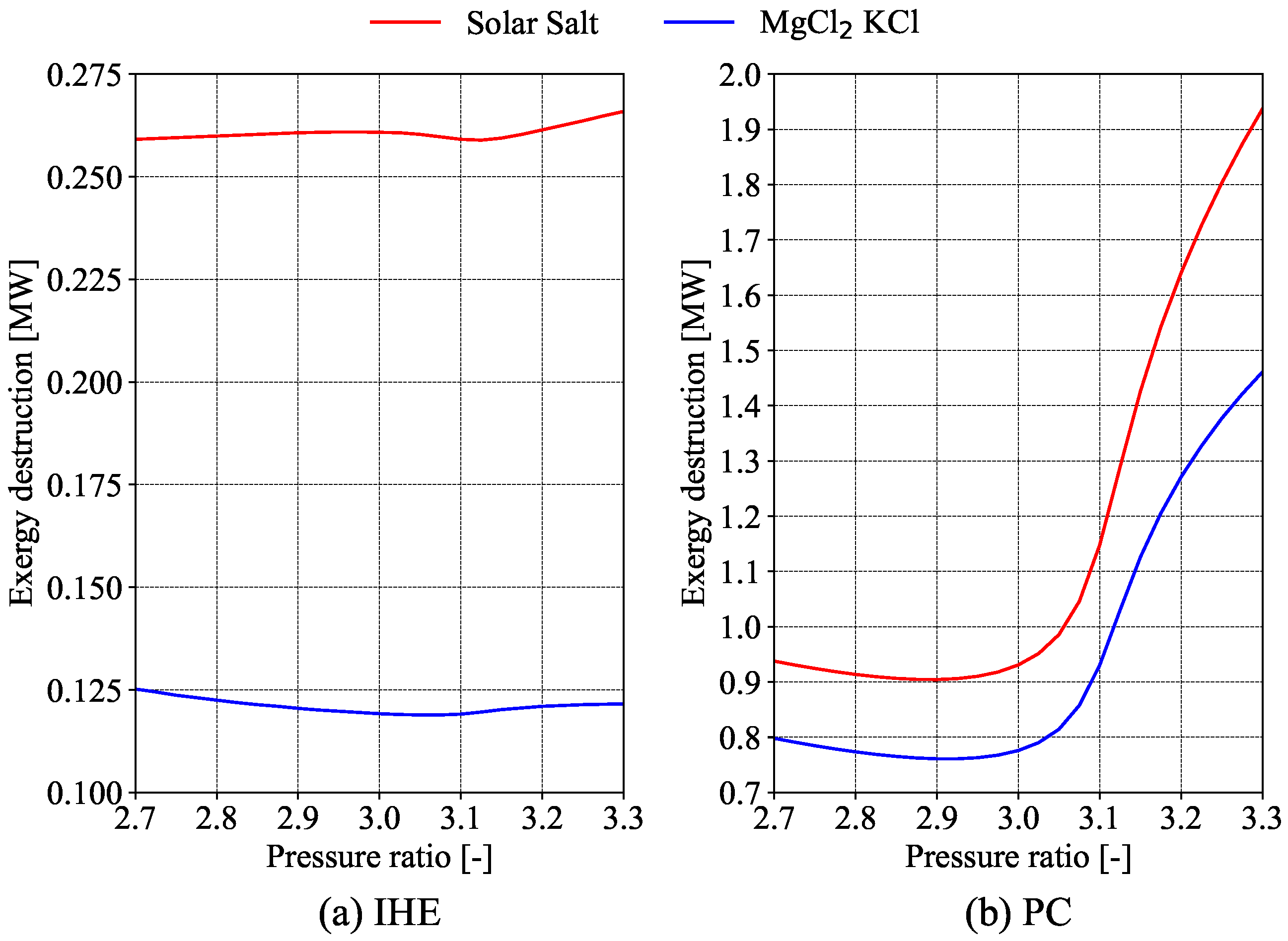
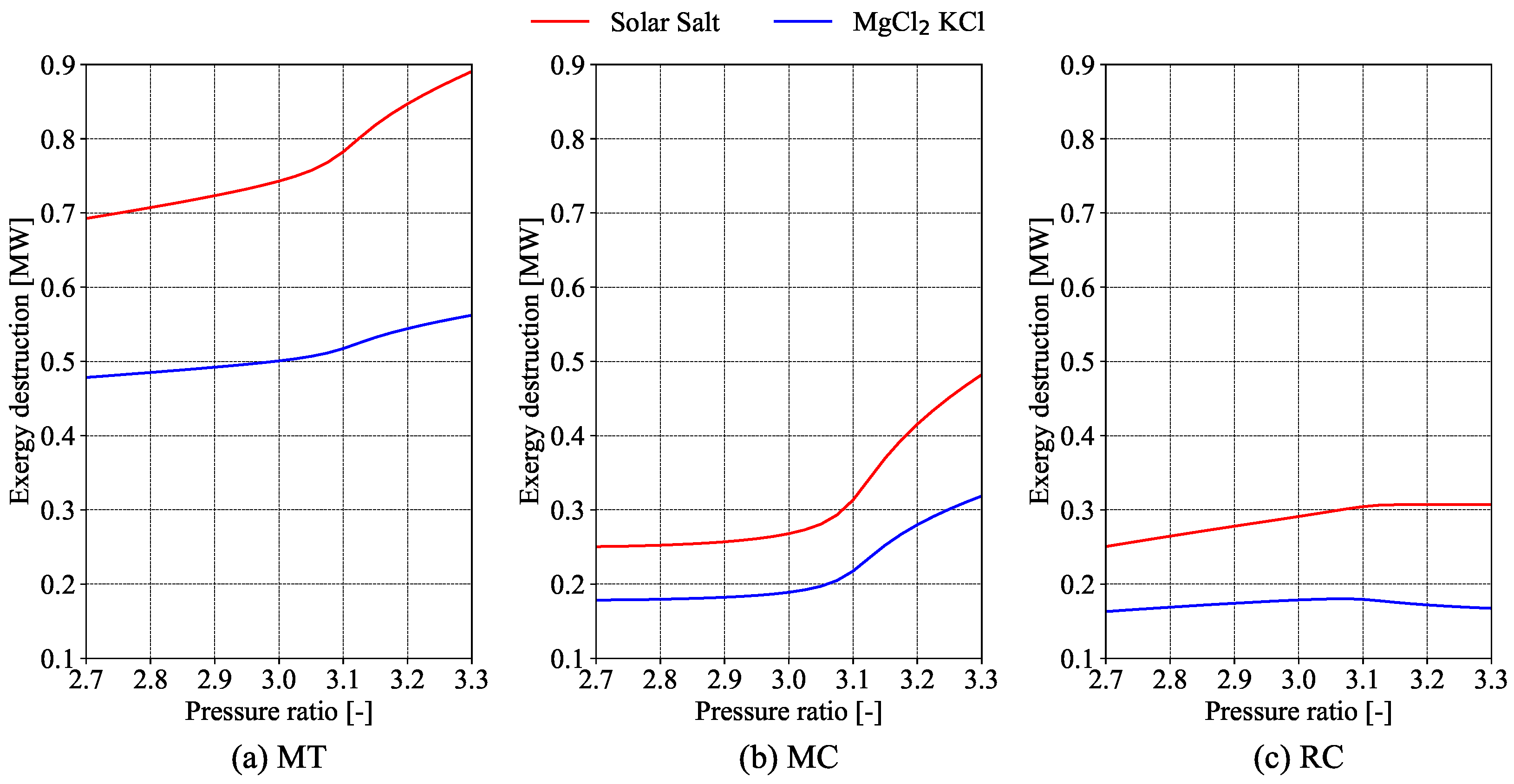
Evaluating the economics of S-CO
2 cycles is complicated due to a lack of economic data [
10]. One approach is to compare different equipment sizes using specific parameters, assuming that smaller equipment offers greater economic benefits. Salim et al. [
11] used this method in their exergy analysis of a recompression S-CO
2 cycle, including a preliminary cost analysis with size parameters (
) and the total heat transfer coefficient (
). Their study found that the turbine inlet temperature and split ratio are the most important for thermal efficiency. The precooler was highlighted as having the highest exergy destruction. Overall, as turbine and compressor inlet temperatures and pressures rise, total exergy destruction increases. While
improves with better thermal efficiency,
rises with higher inlet temperatures, showing a trade-off between cost and efficiency.
This work compares two types of molten salts used in S-CO
2 Brayton cycles with a central solar receiver system: traditional solar salts and MgCl
2–KCl. These two types of salts were selected to compare the current solar salts to one of the most promising alternatives for replacement due to their lower cost and higher operational temperature range. Energetic, exergetic and equipment sizing analyses are performed in order to determine the advantages and disadvantages of each type of molten salt. Among the several alternative S-CO
2 cycles [
12], the S-CO
2 recompression cycle has been selected because it achieves high efficiencies with a relatively simple configuration [
13]. In this cycle, the recuperator is divided into two heat exchangers: a high-temperature recuperator (HTR) and a low-temperature recuperator (LTR). An extra compressor, the re-compressor (RC), is included to minimize the differences in heat capacity between the two sides of the recuperator [
4].
The novelty of this work is the simultaneous analysis from the perspectives of energy, exergy, and equipment sizing for a solar tower-driven S-CO2 recompression system utilizing two types of molten salts: solar salts and MgCl2–KCl. This approach enhances our understanding of how the choice of molten salt affects the overall performance of the system. An exergy analysis will help us assess which system achieves higher performance and how each molten salt contributes to the irreversibilities present in each equipment of the system. Additionally, the equipment sizing analysis will provide a preliminary estimate of how selecting one molten salt over another impacts plant costs. As noted in the previously cited works, the influence of the heat transfer fluid (HTF) in an S-CO2 cycle is often studied primarily from an energy perspective, with efficiency, specific work, and the temperature differences of the HTF being the most commonly used parameters for comparison. Some studies extend this energy analysis by incorporating an economic analysis. This paper contributes to the existing literature by including an exergy analysis, which allows us to determine how the use of molten salts impacts the irreversibilities in various pieces of equipment within the S-CO2 cycle. Another significant contribution of this work is using volume as a key parameter to represent storage size. In the reviewed literature, the size of the storage system is typically related to the temperature difference of the HTF, where a larger temperature difference results in a smaller storage system. This study considers the density changes associated with temperature variations using volume as a parameter.
The paper is organized as follows:
Section 2 describes the central solar receiver system utilizing the recompression of S-CO
2 and details the thermophysical properties of both heat transfer fluids (HTFs).
Section 3 summarizes the simulation model developed for the various analyses. The validation of this model is presented in
Section 4. The results are presented in
Section 5, and finally,
Section 6 concludes with a summary of the findings and suggestions for future work.
2. System Description
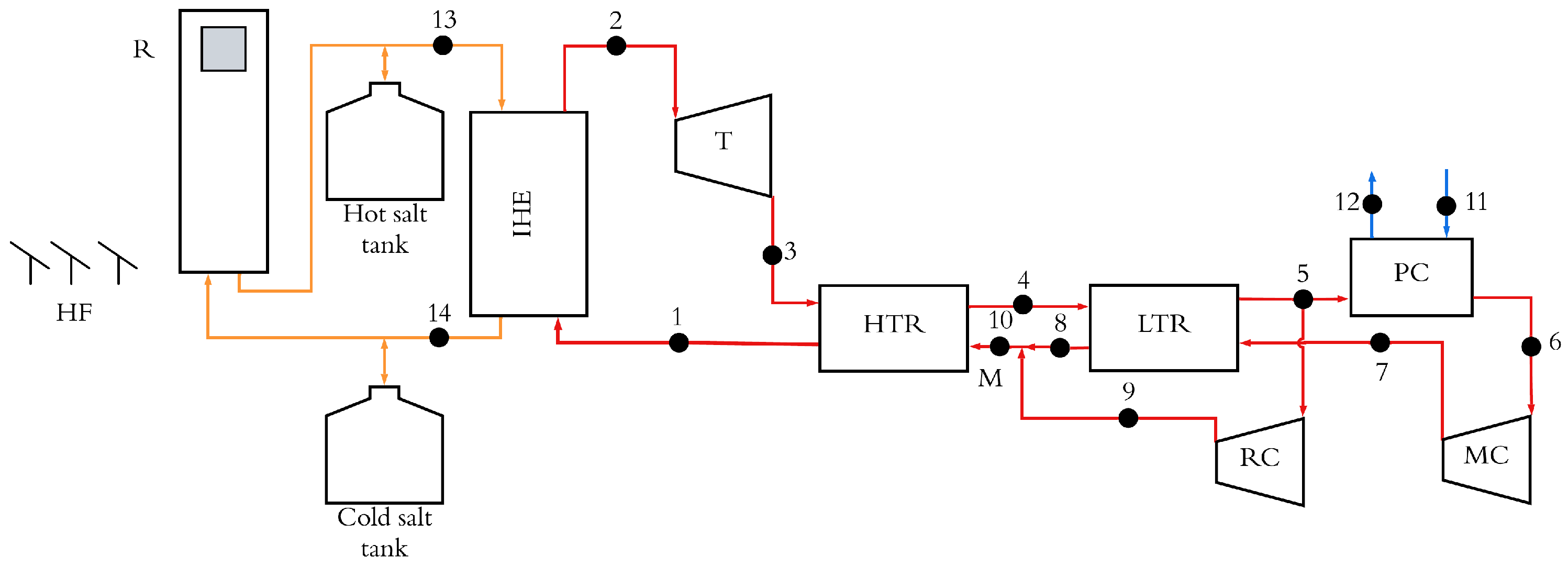
The layout of the solar tower integrated with the S-CO
2 recompression Brayton cycle is depicted in
Figure 1. The system consists of the heliostats field, solar receiver, thermal energy storage and the S-CO
2 recompression cycle. The solar radiation in the heliostat field (HF) is reflected and concentrated on the solar receiver (R), which is located at the top of the tower. This concentrated radiation heats the HTF, which flows through the solar receiver. The HTF is used to provide thermal power to the S-CO
2 power cycle. The thermal storage system is used to take advantage of times when there is a surplus of solar radiation. In conditions of elevated solar radiation, an excess of thermal energy is stored. Conversely, in conditions of reduced solar radiation, the thermal energy that has been stored is discharged. Both operation modes are oriented to maintain the desired electricity production of the cycle. The thermal storage system can consist of two tanks or a single thermocline tank. In this work, the two-tank system is adopted because this method is more reliable and, therefore, more used in commercial projects despite the fact that the method based on the thermocline tank presents lower costs [
14]. The intermediate heat exchanger connects the HTF circuit with the S-CO
2 cycle. This cycle consists of the following thermodynamic processes:
Process 1–2 and 13–14: Heat transfer in intermediate heat exchanger (IHE) between the molten salts (states 13–14) to the S-CO2 circulating through the power block (states 1–2).
Process 2–3: Non-isentropic expansion process in the turbine (T).
Process 3–4 and 1–10: Heat exchange in high-temperature recuperator (HTR).
Process 5–6 and 7–8: Heat exchange in low-temperature recuperator (LTR).
Process 6–7: Non-isentropic compression process in main compressor (MC).
Process 5–9: Non-isentropic compression process in recompression compressor (RC).
Process 9, 8 and 10: Adiabiatic mixed flow.
Process 5–6 and 11–12: Heat exchange process in precooler (PC) between the S-CO2 (states 5–6) and cooling water (states 11–12).
This paper examines two types of molten salts: solar salts and MgCl
2–KCl.
Table 1 summarizes the thermophysical properties of both molten salts. The thermophysical properties of the solar salts and MgCl
2–KCl were collected by Equation Engineering Solver (EES) [
15] from the reference materials [
16,
17], respectively. The minimum operating temperatures are set with a safety margin of 70 °C above the melting temperature [
6]. The specific heat for MgCl
2–KCl is 1.56 kJ/kg·K and is minimally influenced by temperature throughout the operating range. Generally, the specific heat of all chloride salts exhibits little dependence on temperature [
5]. Although the MgCl
2–KCl system presents higher operating temperatures, it has limitations due to its lower density and specific heat capacity, which should be taken into account.
3. Methodology
The energy and exergy analyses are carried out using Equation Engineering Solver (EES) [
15]. The thermophysical properties of CO
2, molten salts and water are calculated with the libraries included in EES. The following assumptions are adopted to develop both analysis:
The system operates in steady conditions.
The variations in kinetic and potential energies are neglected.
Pressure drops and heat losses are not considered in the design of heat exchangers and pipes.
A model based on effectiveness has been used for the recuperators. The effectiveness of each recuperator (Equations (
1) and (
2)) considers the side with the lower heat capacity [
2].
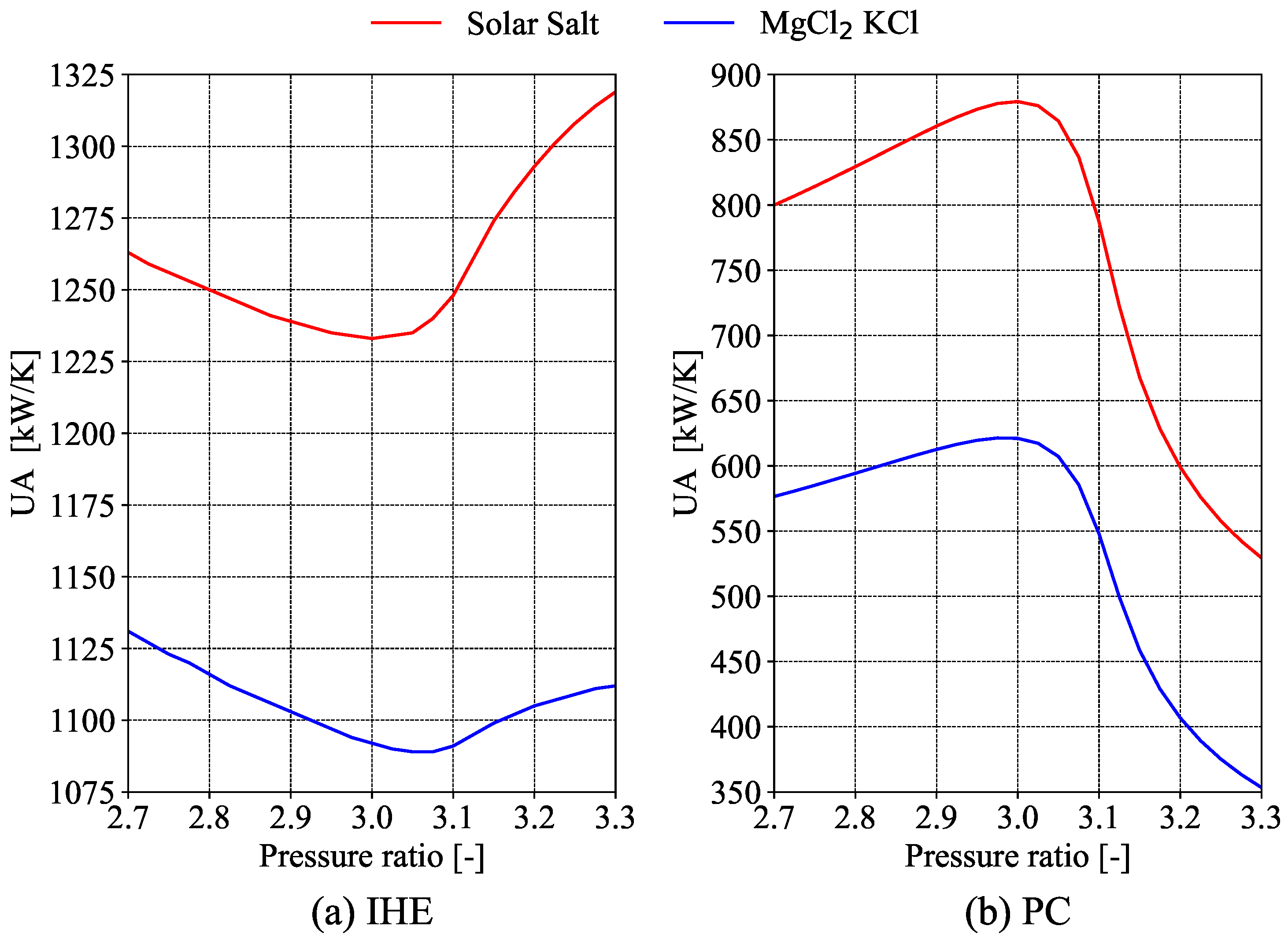
The power block absorbs the thermal power provided by the solar block in the inter-mediate heat exchanger, a counter-flow heat exchanger with a minimum temperature difference of 15 K between the molten salts and the S-CO
2 (Equation (
3)) [
18]. This value is different from the S-CO
2/S-CO
2 heat exchanger, where the minimum temperature difference is 5 K [
19]. The assumption of equal entropy generation at both terminals of this heat exchanger will be made (Equation (
4)) [
18].
The salt storage tank is well-insulated, and therefore, heat losses are considered negligible.
Table 2 summarizes the input parameters assumed for this study. The rated power is assumed to be 10 MW, which corresponds to the scale of a demonstration plant [
20]. The cooling water inlet and outlet temperatures for the precooler are 25 °C and 35 °C, following the recommendation of the reference [
21]. It is hypothesised that the compressor inlet temperature is 35 °C, a supposition that is in accordance with the recommendations set out in the references [
21,
22]. The efficiency of the power cycle significantly increases with higher recuperator effectiveness. It is asserted that an effectiveness level that exceeds 90% may not be economically viable, as indicated by reference [
22]. For this work, a conservative value of 86% is assumed. The assumed values for isentropic efficiency of the turbine (90%) and compressor (85%) are typical values that have been documented in the extant literature [
23]. The maximum cycle pressure, defined as the pressure at the outlet of the main compressor, is constant at 250 bar. This value signifies the upper limit of the mechanical strength of the materials commonly employed in S-CO
2 at operating temperatures [
1]. Throughout the study, this maximum cycle pressure remains constant. The minimum pressure, which is the pressure at the inlet of the main compressor, is determined based on the pressure ratio. The maximum temperature for each type of molten salt, which is the temperature at the outlet of the solar receiver, has been assumed to be the highest possible, as shown in
Table 1. The maximum temperature of the molten salt determines the turbine inlet temperature on the power cycle by Equation (
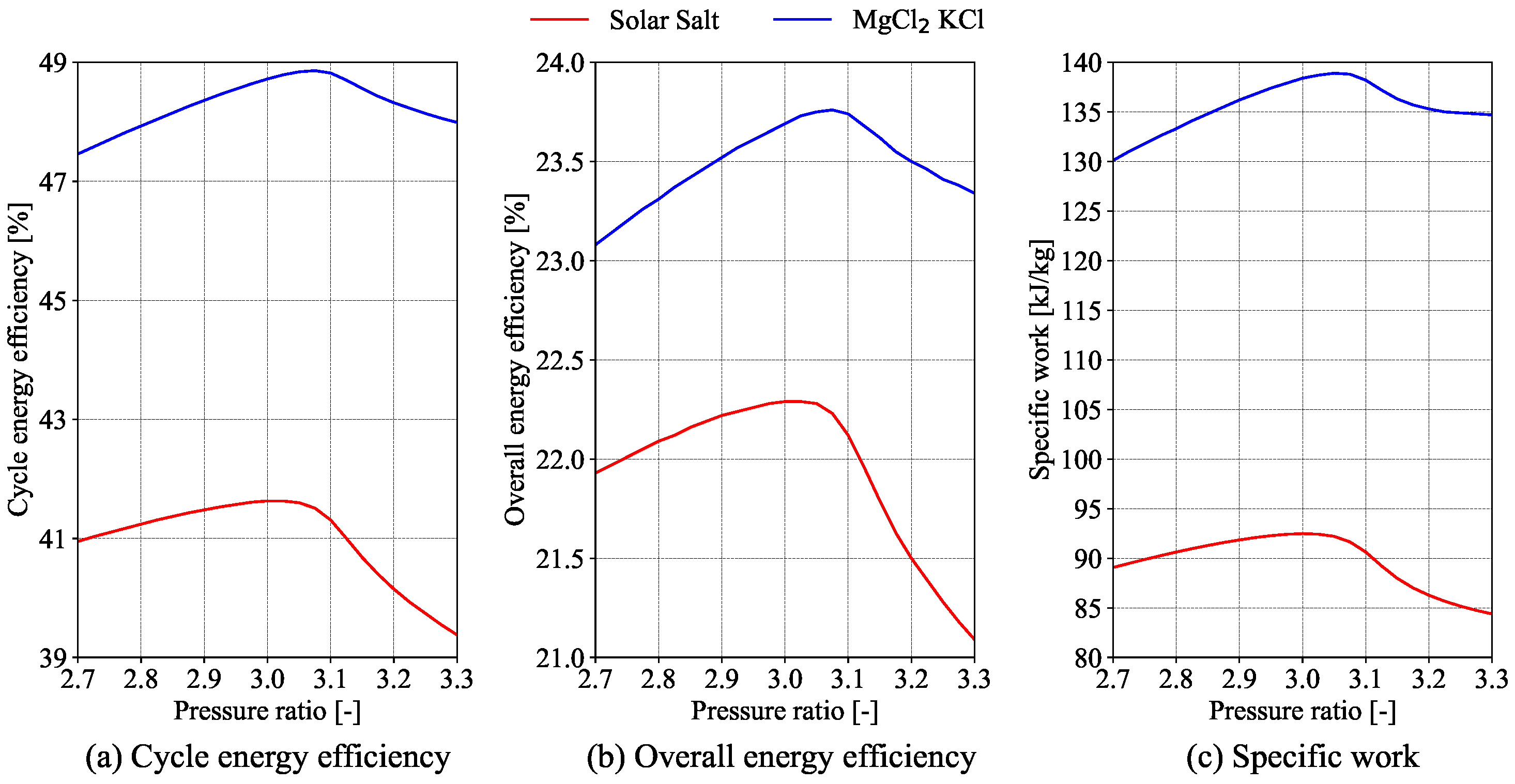
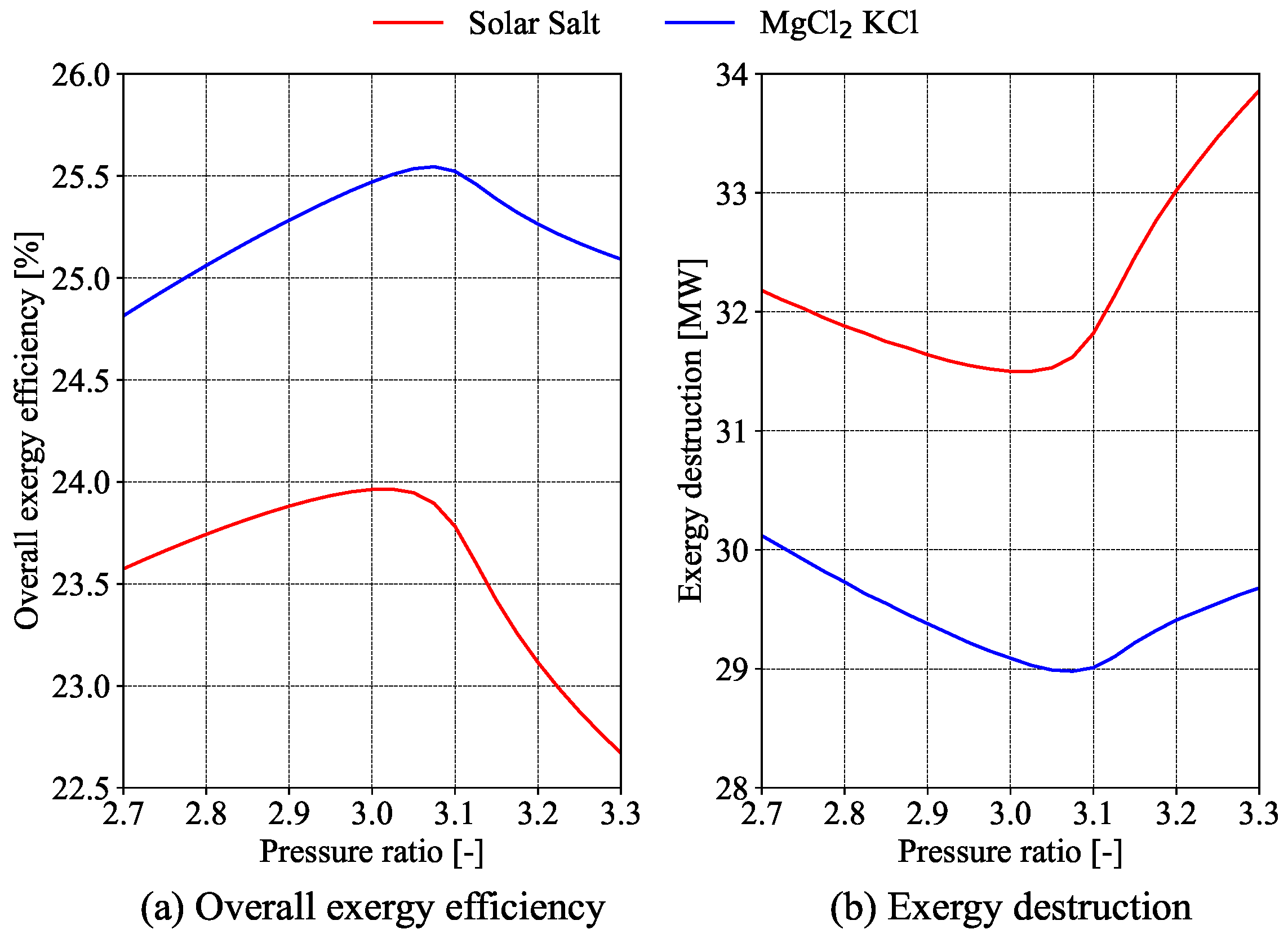
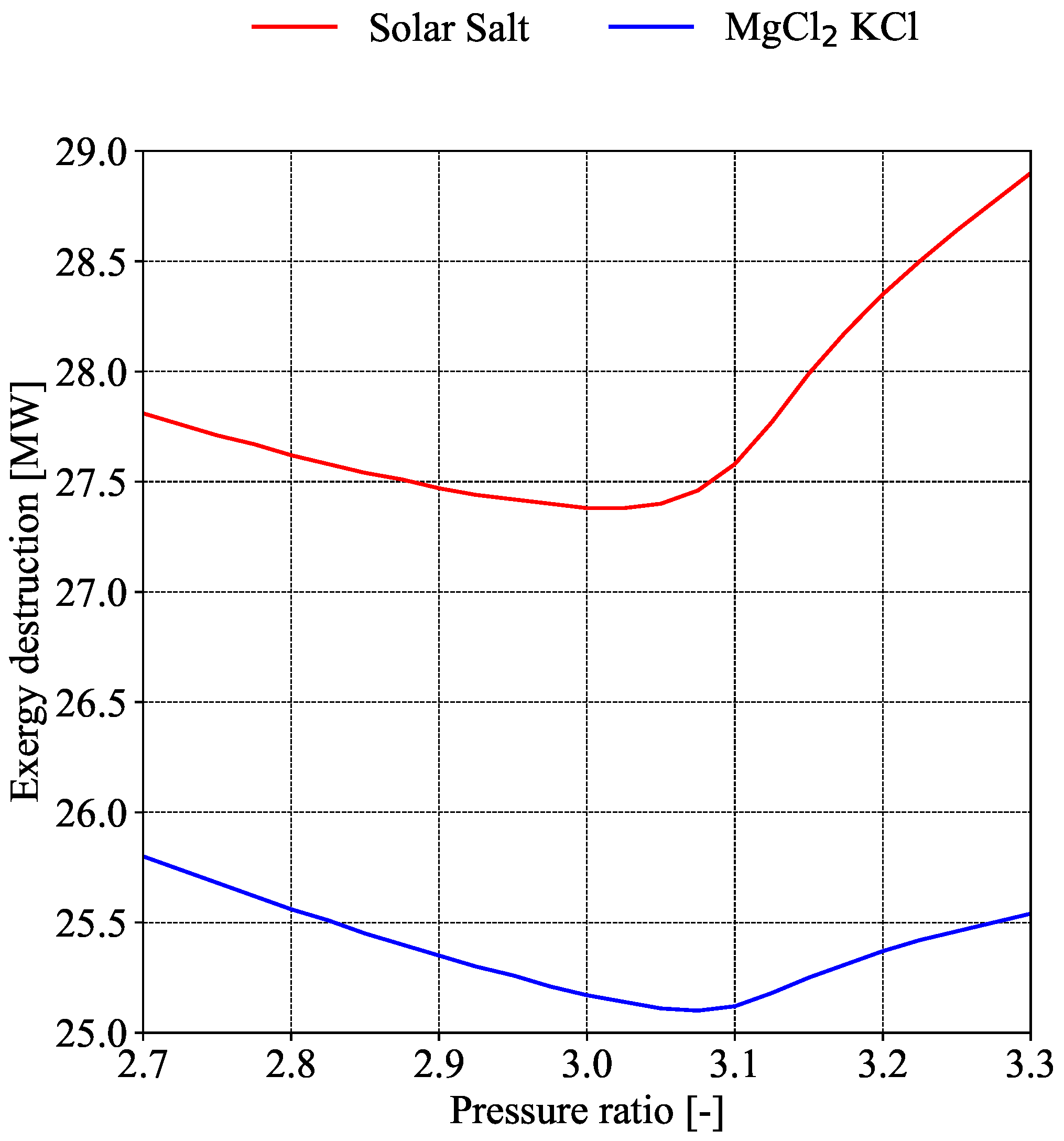
4). It is well established that the efficiency of the power cycle improves as the maximum turbine inlet temperature increases.
3.1. Energy Analysis
A mass balance and energy balance are conducted for each component. The equations obtained are summarized in
Table 3.
The parameter
indicated the split ratio. This parameter is defined as the mass flow rate circulating through the recompression compressor and the mass flow rate circulating through the turbine by Equation (
5).
The solar to thermal efficiency of the solar block
(heliostat field, central receiver and tower) is defined as the ratio between the power absorbed by the molten salts
in the receiver and the total power incident in the heliostat field
. The thermal power absorbed by the molten salts is determined by calculating the power absorbed by the solar receiver
and accounting for losses due to radiation
and convection
.
where
is the receiver absorptivity,
is the heliostat field efficiency,
C is the concentration ratio,
is the receiver emissivity,
is the Stefan–Boltzmann constant,
F is the view factor,
is the receiver surface temperature,
is the sky temperature,
is the receiver heat transfer coefficient and
is the direct normal irradiance [W/m
2]. The surface temperature of the receiver is calculated from the transfer coefficient from the receiver surface to HTF [
19]. The sky temperature
is calculated using the equation provided by [
24]. Equation (
6) is derived from the model proposed by reference [
19].
Table 4 provides the value of the diferent parameters required to calculated the solar to thermal efficiency of the solar block using the Equation (
6). The input parameters assumed for the calculation of the solar to thermal efficiency of the solar block are summarized in
Table 4. These values are obtained from the reference [
19].
The thermal efficiency of the cycle power is defined as the ratio between the net power produced and the thermal power absorbed by the cycle (Equation (
7)).
The overall thermal efficiency of the systems is defined as the ratio of the net power and the incident power of the heliostat field.
The specific work is calculated as the ratio of net power to the mass flow rate of CO
2 circulating through the power cycle.
3.2. Exergy Analysis
The exergy analysis predicts the irreversibilities of a component quantifying its exergy destruction. After evaluating the system from the energy point of view, the exergy rate of each current is calculated. The exergy destruction for each component is calculated as the difference between the exergy rate of the fuel and the product. The exergy rate of product and fuel for each component are defined by applying the methodology explained in reference [
25]. The equations for each component are summarized in the
Table 5. The ambient temperature and pressure were established at 25 °C and 1 bar, respectively.
The solar block’s energy source is solar radiation. The exergy provided by this radiation can be expressed using Equation (
10), which is known as the Petela’s formula [
26].
where
is the total power incident in the heliostat field,
is the sun surface temperature (5700 K) and
is the ambient temperature.
The overall exergy efficiency of the system is expressed by Equation (
11)
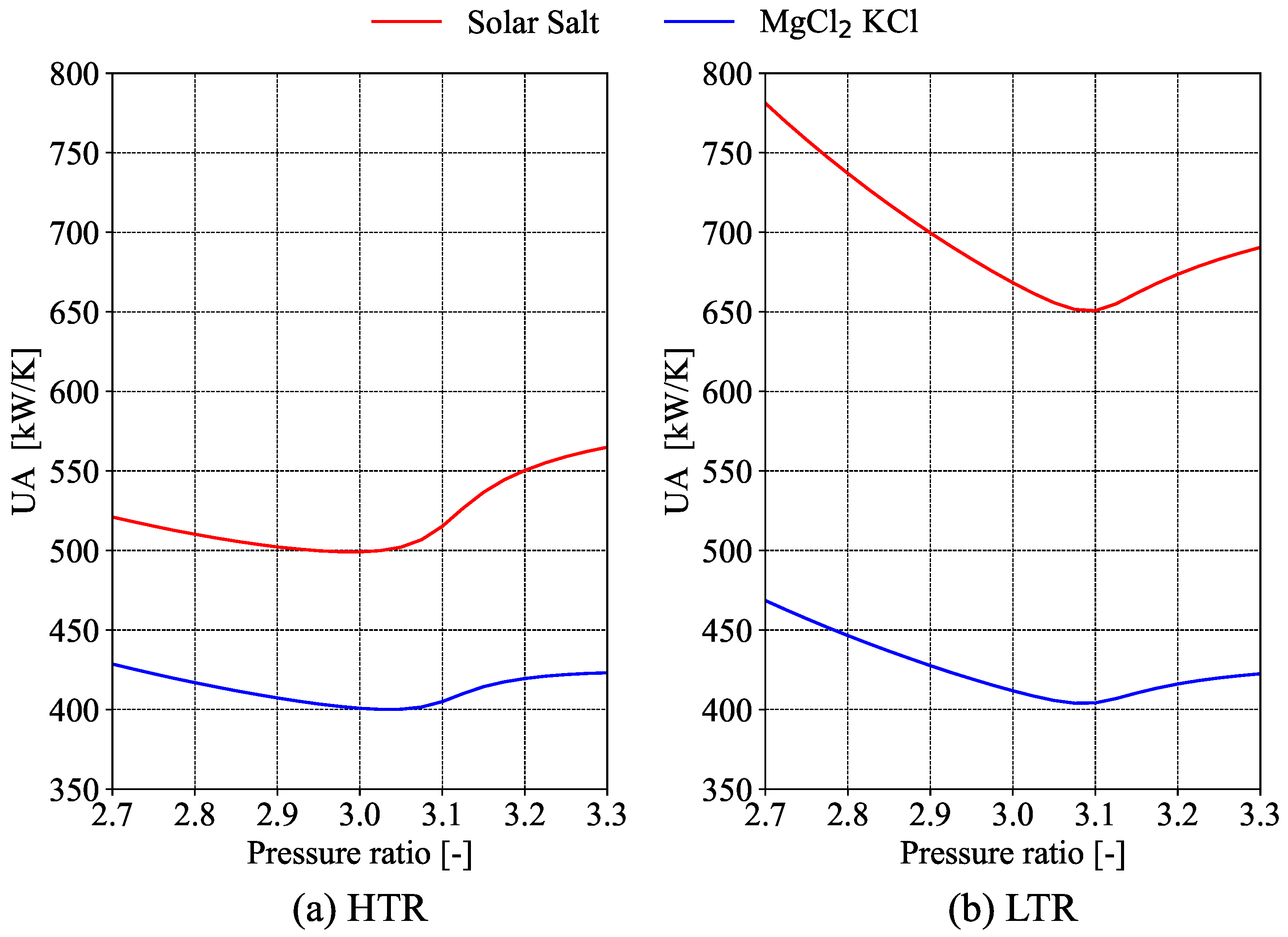
3.3. Sizing Analysis
The size of heat exchangers is determined by calculating the
coefficient, which is related to investment, operating, and maintenance costs [
11]. Due to the significant variation in the thermodynamic properties of S-CO
2, it is important to divide the heat exchanger into
N layers to calculate the
coefficient accurately. The
is calculated for the pre-cooler, low-temperature recuperator, high-temperature recuperator, and intermediate heat exchanger.
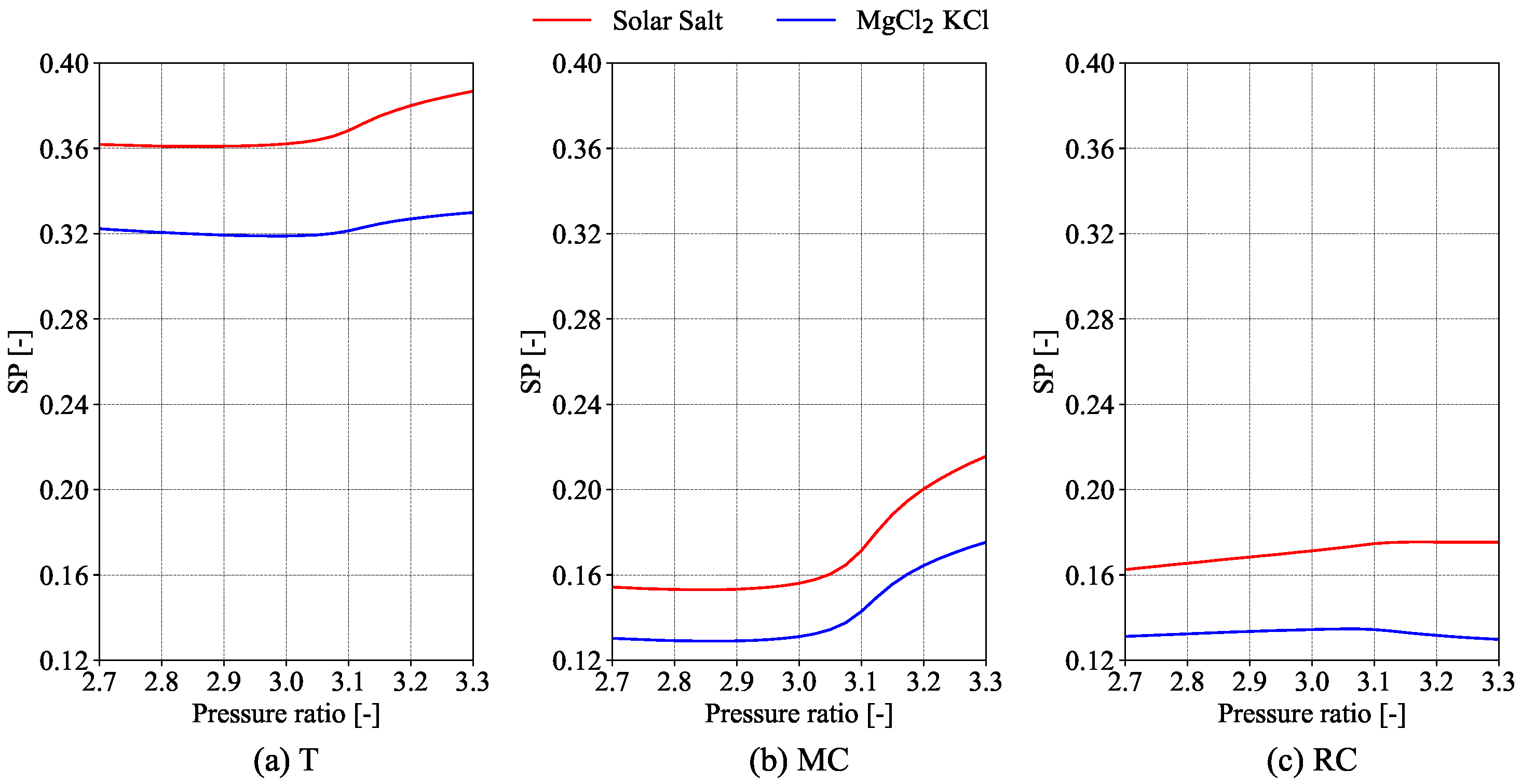
The size parameter (
) is used to estimate the dimensions and associated costs of the turbo components [
27]. The equations pertaining to the size parameter for the turbine, main compressor and recompression compressors, respectively, are represented by Equations (
12)–(
14).
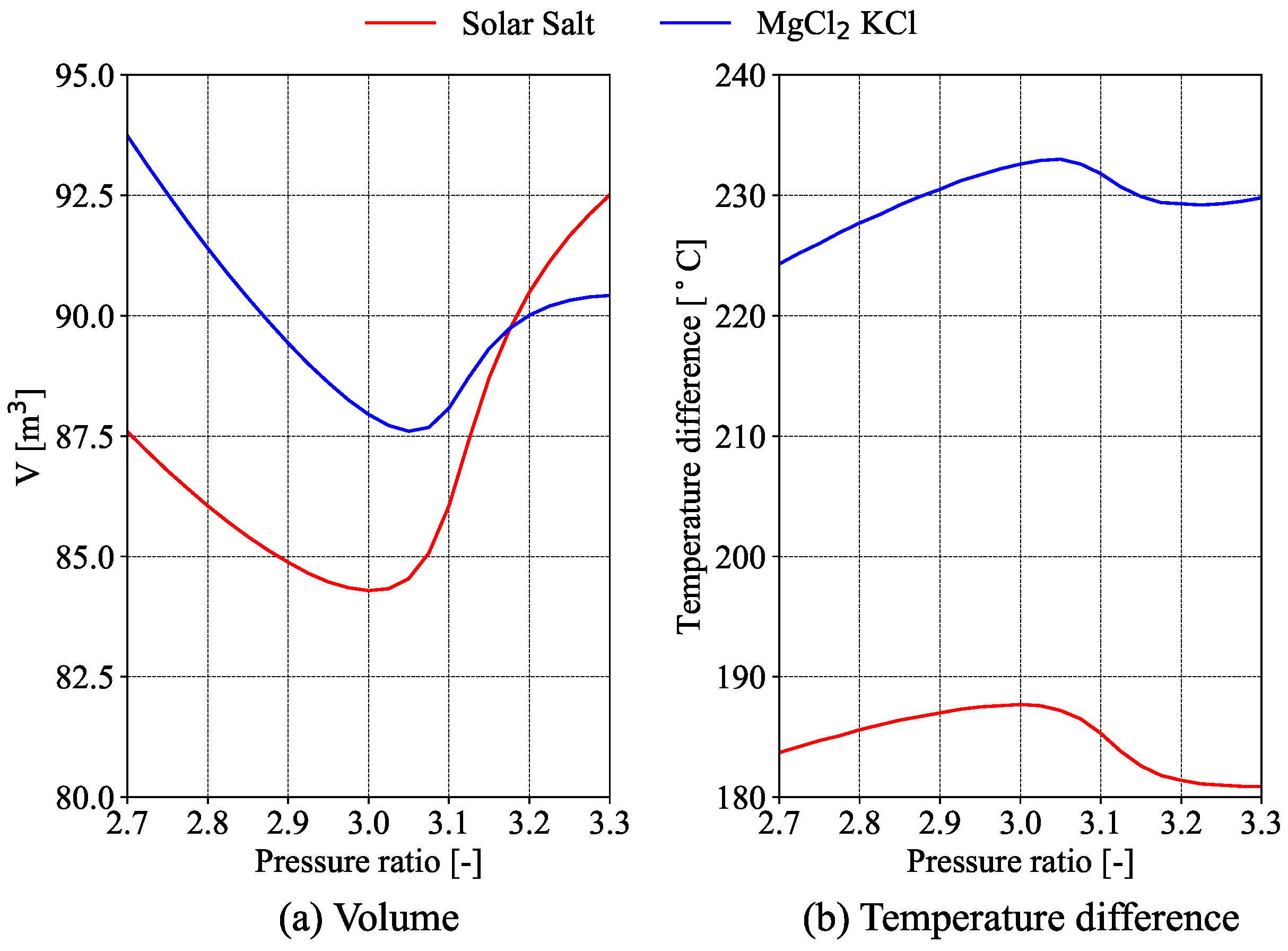
Storage tanks are the main component of the thermal energy storage. The volume of the storage system in the literature [
3] is often associated with the temperature difference of the heat transfer fluid (HTF) between the inlet and outlet of the solar receiver. In this study, the volume required to store the thermal energy needed by the power block for one hour is used as a representative parameter for the size of the storage system. This approach will also account for the different densities and the specific heat capacities of both types of molten salts. The volume of the storage tanks required for the cycle to operate at full load for one hour is expressed by Equation (
15).
where
is the density of the molten salts at the temperature of the cold tank and
is the density of the molten salts at the temperature of the hot tank. Since it is assumed that the tank’s heat losses are negligible, both temperatures coincide with the inlet and outlet temperatures of the solar receiver.