Abstract
Background: Strokes are a major public health concern, responsible for high mortality and long-term disability rates. Rehabilitation techniques aim to harness neuroplasticity—brain self-repair mechanisms that restore lost functions. Beyond traditional methods, therapies like Repetitive Transcranial Magnetic Stimulation (rTMS) and Extremely Low-Frequency Magnetic Fields (ELF-MFs) show promise in enhancing neuroplasticity. This pilot study explored the feasibility and safety of ELF-MFs in stroke rehabilitation. Methods: The study involved 44 patients randomized into three groups: magnetotherapy applied to the head (MT1), pelvis (MT2), or standard rehabilitation (control). Assessments included functional measures (FIM, Barthel Index, Tinetti Scale, SPPB, and Berg Balance Scale) and inflammatory markers (CRP, PCT). Results: All groups showed functional improvement, with CRP and PCT reductions highlighting potential benefits of ELF-MFs. No adverse effects or changes in blood or organ function were observed. Conclusions: ELF-MFs could be safely conducted in this group allowing for further research to confirm their efficacy in larger studies.
1. Introduction
Strokes are a significant public health issue, being one of the most common causes of death and often leading to permanent disability and impairment [1,2,3]. It is estimated that there are currently 9.53 million prevalent stroke cases in the European Union. By 2047, the number of prevalent stroke cases is expected to increase by more than a fourth, reaching over 12 million [3]. This increase is related to the aging of the population of the European Union, as age is one of the most significant risk factors for stroke [2,4].
A key aspect in counteracting the disabling effects of strokes is the initiation of early and comprehensive rehabilitation. Neurological rehabilitation of stroke patients is typically conducted based on activities such as kinesitherapy and therapy using specialized methods like NDT Bobath or PNF [5,6,7]. It also includes hand therapy, balance training, gait re-education, and occupational therapy (particularly self-care training and improving the ability to perform activities of daily living) [8]. All these elements should be introduced as early as the patient’s condition allows because, in the case of a stroke, we refer to the concept of a “therapeutic window”, denoting the period during which therapy has the highest likelihood of achieving the best outcome [7]. This is related to the intense course of neuroplasticity processes in the early period after a stroke. Neuroplasticity is a phenomenon that allows the recovery of functions lost by the brain. It is based on the restoration of connections between nerve cells that were lost due to damage (e.g., as a result of a stroke or a traumatic brain injury) [9,10]. This phenomenon involves a range of both intracellular and extracellular mechanisms that enable the brain’s self-repair and reorganization, allowing it to regain functions lost due to damage most effectively [11].
Promoting neuroplasticity in neurological rehabilitation is mainly achieved through appropriate exercises that engage or stimulate the patient’s motor system, allowing for the restoration of lost motor functions. However, in recent years, additional methods that may influence neuroplasticity and increase the effectiveness of rehabilitation have been explored [12,13]. Some of these methods involve using physical stimuli to enhance neuroplasticity processes.
In recent years, Repetitive Transcranial Magnetic Stimulation (rTMS) has gained significant popularity in the treatment of stroke patients [13,14]. Additionally, reports suggest that not only rTMS but also the application of Extremely Low-Frequency Magnetic Fields Therapy (ELF-MFs) may be useful in the treatment of neurological patients [12,13,15]. ELF-MFs could, therefore, be a therapy that, by supporting neuroplasticity, enhances the effectiveness of traditional physiotherapeutic methods in the rehabilitation of stroke patients.
The literature describes two areas for the application of this type of stimulation: the head and the pelvic region [13]. Some authors suggest that application to the head may induce epileptic seizures [12]; however, according to the available literature, no data support this [13,16]. Moreover, one study claims the safety of ELF-MF stimulation in the head area [16]. Therefore, an assessment of potential differences in the effectiveness of magnetic field application to the head versus the pelvic region in stroke patients is needed. However, due to uncertainties regarding the safety of this type of therapy, conducting such a study should be preceded by safety and feasibility studies. Thus, this pilot study aimed to assess the feasibility of the study, as well as the safety of the procedure.
2. Materials and Methods
2.1. Study Design, Participants, Intervention, and Assesement
The study included patients from the Neuro-rehabilitation Ward of the Provincial Hospital in Poznan, Poland. The patients were admitted for rehabilitation following ischemic strokes. The study was conducted according to the Declaration of Helsinki, CONSORT guidelines [17] and the Guidelines for Designing and Evaluating Feasibility Pilot Studies [18]. The local Ethics Committee approved the study protocol (No. 978/22 and 849/22). To be eligible for the study, participants had to be 60 years of age or older and would have suffered an ischemic stroke within a maximum of two weeks prior to their admission. Patients with severe functional impairment, as indicated by a Functional Independence Measure (FIM) score of less than 37, a history of previous strokes, or other neurological diseases or conditions that could significantly impair functioning were excluded from the study. Additional exclusion criteria included contraindications to magnetotherapy, the inability to establish logical, verbal communication, and the lack of informed consent to participate. Participants were then randomly assigned to one of three groups. The first group, referred to as MT1, received magnetotherapy to the head in addition to standard neurological rehabilitation, with the intervention consisting of 10 sessions of low-frequency magnetotherapy (40 Hz, 5 mT) over 10 consecutive days, excluding weekends. The second group, MT2, received a similar intervention, but the magnetotherapy was applied to the pelvis rather than the head. The study employed a magnetotherapy protocol, including the dosage and application schedule, consistent with those used in studies conducted by other authors investigating the effects of this type of stimulation on post-stroke patients [19,20]. Although the coil is designed to maximize the effective delivery of the therapeutic dose to the patient, great care should be taken when positioning the patient during therapy. In particular, it is essential to ensure that the area intended for magnetotherapy exposure is precisely centered within the coil to achieve optimal treatment efficacy. Finally, the control group participants received only standard neurological rehabilitation without any additional magnetotherapy. The Magnetronic MF10 (EiE Elektronika i Elektromedycyna, Otwock, Poland) was used to deliver electromagnetic field stimulation. Regardless of group assignment, each study participant underwent neurological rehabilitation consisting of 120 min of exercise daily, six days a week. In addition to this, patients also participated in occupational therapy sessions, which were held six days a week for approximately 30 min each day. This consistent rehabilitation regimen was designed to support recovery and improve functional outcomes following a stroke. The study design is presented in Figure 1.
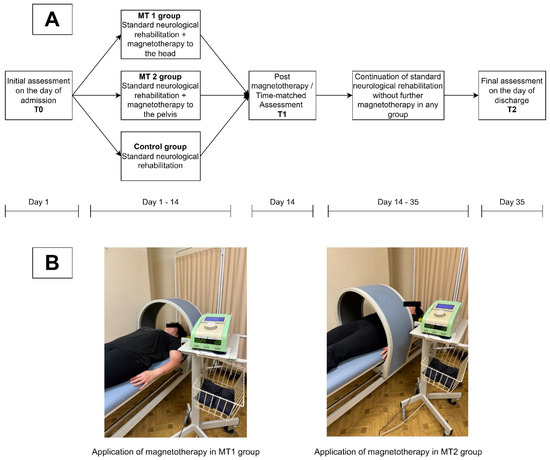
Figure 1.
Study design. (A) Schematic presentation of the experimental design, (B) presentation of differences in application of magnetotherapy between MT1 and MT2 group. Source: Authors’ own.
The study utilized several standardized assessment tools to evaluate various aspects of the participants’ functional recovery and mobility. The Functional Independence Measure (FIM) assessed the level of independence in performing daily activities such as self-care, mobility, and communication. Scores on the FIM range from 18 to 126, with higher scores reflecting greater independence [21]. The Barthel Index measured the ability to perform basic activities of daily living, including feeding, bathing, dressing, and mobility, with scores ranging from 0 to 20, where a higher score indicates greater independence [22,23]. The Tinetti Scale evaluated balance and gait to help determine the risk of falling, with scores ranging from 0 to 28, where lower scores signify a higher risk of falls [24]. The Berg Balance Scale assessed balance through various tasks, with scores ranging from 0 to 56, where lower scores indicate a higher risk of losing balance and falling [25]. Finally, the Short Physical Performance Battery (SPPB) assessed lower extremity function through tasks such as balance, walking speed, and chair stands, with scores ranging from 0 (worst performance) to 12 (best performance) [26].
The study also included specific laboratory tests to monitor inflammatory and infection markers in the participants. C-reactive protein (CRP) concentrations were measured to assess the level of inflammation in the body. CRP is an important biomarker for detecting the presence and severity of infection or other inflammatory conditions, with results expressed in milligrams per liter (mg/L). Additionally, procalcitonin blood concentrations were assessed to monitor inflammatory processes and oxidative stress in stroke patients. Procalcitonin (PCT) results were expressed in nanograms per milliliter (ng/mL). As part of the safety assessment of the intervention, blood morphology and kidney and liver function tests were also performed. Blood was drawn via a venous puncture after overnight fasting. Venous blood samples were taken to measure hemoglobin (Hb), white blood cells (WBC), and red blood cells (RBC). Platelets were also obtained and processed by a centralized laboratory. Biochemical markers were measured using the Cobas 6000TM clinical chemistry analyzer (Roche; Mannheim, Germany). Hematological indices (complete blood count, hemoglobin) were analyzed in EDTA-blood with the XT-2000iTM (Sysmex Corporation; Kobe, Japan). We also measured alanine aminotransferase (ALT), aspartate aminotransferase (AST), sodium (NA), potassium (K), creatinine (CREA), and urea (UREA). These parameters were determined quantitatively in the serum using the Cobas 6000 analyzer. AST and ALT tests were performed using kinetic methods with absorbance measurements. High-sensitivity C-reactive protein (CRP) and procalcitonin (PCT) were determined using an immunoturbidimetric method enhanced with latex particles.
All these assessments were conducted on the day of admission, after the series of treatments (approximately on the 14th day of stay) and on the day of discharge (approximately on the 35th day of stay) by blinded assessors. Adverse events were recorded throughout the entire study period if any occurred.
2.2. Statistical Analysis
The study did not conduct advanced statistical analyses of the results regarding functional performance and inflammatory markers. This was due to the fact that the primary objective of this feasibility study was to evaluate feasibility and safety in the context of a planned full-scale study. Consequently, in line with the guidelines for conducting and assessing feasibility studies [18], advanced statistical analyses were not performed, as their utility is significantly limited in this context. Instead, descriptive statistics were provided, including changes between measurements at different study stages, with means and standard deviations calculated for quantitative variables and medians and ranges presented for ordinal variables [18].
3. Results
A total of 45 participants participated in the pilot study, with 15 participants in each of the MT1, MT2, and C groups. However, during the study, one participant from the control group developed pneumonia, resulting in 44 participants completing the study.
Functional improvements over time were assessed using several validated scales, including the FIM, Barthel, Tinetti, SPPB, and Berg scales. The results demonstrated a general trend of improvement across all these measures, reflecting enhanced functional abilities among the participants. These findings have been visualized in detail in Figure 2, which consolidates the results from all five scales into one figure. Each scale’s outcomes are represented in separate charts within the figure, labeled as follows: FIM outcomes in chart A, Barthel Index results in chart B, Tinetti scale data in chart C, SPPB scores in chart D, and Berg scale findings in chart E.
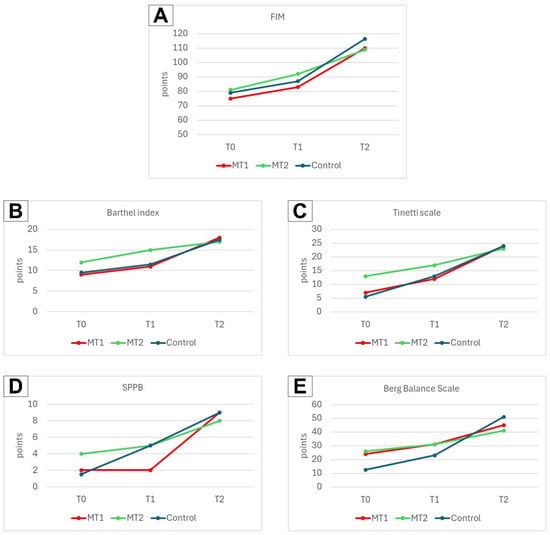
Figure 2.
Change in scores of functional assessment scales observed in all groups between measurement points. (A) FIM outcomes, (B) Barthel Index outcomes, (C) Tinetti scale outcomes, (D) SPPB outcomes, and (E) Berg Balance scale outcomes.
Changes in CRP and PCT concentrations between measurement points are presented in Figure 3. In this case, an improvement was also observed across all groups, reflected in the reduction of these marker concentrations between the first and the final measurement.
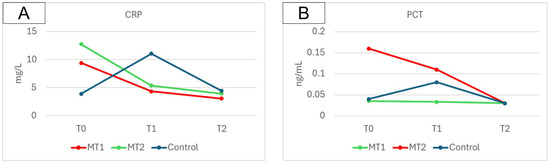
Figure 3.
Change in CRP (A) and PCT (B) concentrations observed in all groups between measurement points.
Based on the presented results, significant differences were observed in the parameters of the studied groups (MT1, MT2, and C) over time. The most notable changes for the CRP were observed in the T2–T0 difference, with a mean of −6.36 in the MT1 group, −8.82 in the MT2 group, and 0.54 in the C group.
The results of the measured parameters for each group at different time points are presented in Table 1. Individual data obtained from each participant in this study have been compiled into Table S1 and are provided in the Supplementary Materials section.

Table 1.
Scores of the studied variables at different measurement points for each group.
During the study period, none of the patients required the procedure to be stopped, nor did they report any discomfort related to the treatment, and no adverse outcomes have been reported. All patients willingly participated in the procedures. No adverse changes were observed in the blood morphological elements, kidney, or liver function among the participants during the study period (Table 2).

Table 2.
Blood analysis results.
4. Discussion
To the best of our knowledge, only one previous study in the literature has addressed the safety of using ELF-MFs on the head in stroke patients [16]. However, that study did not compare magnetotherapy on the head and the pelvic area, and it employed a different method of stimulation and parameters, with a gradual increase in dose. Our pilot study is the first to include a group receiving magnetotherapy on the head, a group receiving therapy on the pelvic area—both using a solenoid-shaped coil applicator—and a control group, which did not receive any magnetotherapy.
In our study, similar to the study by Capone et al., no adverse effects of therapy using ELF-MFs were observed. There were no adverse effects of magnetotherapy, regardless of the application area. During the study, none of the patients experienced epileptic seizures, nor did they report other symptoms such as discomfort, headaches, nausea, heart palpitations, breathing difficulties, or paresthesia. Except for one individual from the control group who developed pneumonia and had to be withdrawn from the study, the remaining participants in the treatment groups received all treatments using ELF-MFs and completed the study. This indicates a high acceptance rate for this type of procedure among this group of patients.
Our preliminary study results support the implementation of full-scale research in this area, which could significantly advance knowledge on adjunctive therapies in the rehabilitation of post-stroke patients. Identifying tools that enable the modulation of inflammatory processes may greatly improve outcomes for stroke patients. The modulation of inflammatory processes in stroke patients appears to play a crucial role in the recovery and rehabilitation process [20]. Inflammation significantly influences brain plasticity, tissue regeneration, and overall neurological recovery, which are critical factors in determining the effectiveness of post-stroke rehabilitation strategies. It is noted that elevated CRP is typically associated with poorer functional outcomes three months post-stroke [27]. By addressing and potentially mitigating these inflammatory responses, it may be possible to enhance the brain’s capacity to adapt and reorganize, thereby improving functional outcomes for patients. Given the central role of inflammation in the pathophysiology of strokes, exploring therapeutic approaches that target these processes could offer substantial benefits in optimizing recovery and maximizing the success of rehabilitation efforts [12,20].
As the scientific literature on the use of ELF-MFs primarily focuses on examining the effects of magnetotherapy applied to the pelvic area [13], the findings of this study are particularly noteworthy. They suggest that magnetotherapy targeting the head is safe, opening the door to future research on its potential impact on post-stroke patients. Hypothetically, magnetotherapy applied to the head may be more effective than pelvic applications in stroke patients due to the closer proximity to the affected brain regions, potentially resulting in a stronger therapeutic effect. However, this hypothesis requires validation through further studies involving appropriately larger participant groups.
This study, despite its significant importance as the first of its kind, has certain limitations. The primary limitation is the exclusion of patients with severe functional impairment. This exclusion criterion was chosen because, in the planned full-scale study, we aim to obtain a relatively homogeneous group capable of performing functional assessment tests, which would not be possible if patients with severe disability, as measured by the FIM, were included. Another limitation is that the study was not designed to assess the effectiveness of the therapy, but rather to evaluate the feasibility of the study and the safety of the procedure. As a result, the data collected do not allow for conclusions that could be generalized about the therapy’s efficacy. Such conclusions can only be drawn from a full-scale study specifically designed to evaluate the therapeutic effects, which would be conducted in the future. The role of pilot studies is not to provide information on the effectiveness of the intervention being tested. Instead, they provide essential data for conducting future full-scale studies, allowing for the refinement of research procedures, identification of potential challenges, and assessment of safety. Their primary goal is to prevent unexpected difficulties and deficiencies in research methods from arising in the full-scale study [18,28]. Conducting pilot studies on appropriately smaller groups of participants does not allow for drawing reliable conclusions about the effectiveness of an intervention [18,28]. While this design is justified from the perspective of the planned full-scale study, it limits the generalizability of the safety evaluation of the procedure to patients who have been most severely affected by strokes in terms of functional disability.
5. Conclusions
In summary, the results of our pilot study indicate that conducting a full-scale investigation into the use of magnetotherapy for post-stroke patients is not only feasible but also safe. The study confirmed that magnetotherapy applied to the head is safe for patients with ischemic strokes and mild to moderate functional impairments. However, it is important to emphasize that basic safety precautions should always be followed during magnetotherapy treatment. The therapy should be promptly discontinued if there are any adverse symptoms or unexpected reactions. These findings provide a foundation for further research, enabling a study that compares the effectiveness of magnetotherapy applied to the pelvic area with that applied to the head in post-stroke patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15063182/s1: Table S1: Individual data regarding functional assessment and inflammatory markers obtained from each participant.
Author Contributions
Conceptualization, R.M., T.T. and K.H.; methodology, R.M., T.T. and K.H.; validation, R.M. and K.H.; formal analysis, R.M. and T.T.; investigation, R.M.; resources, K.H.; data curation, R.M. and T.T.; writing—original draft preparation, R.M., T.T. and K.H.; writing—review and editing, R.M., T.T. and K.H.; visualization, R.M. and T.T.; supervision, K.H.; project administration, R.M. and K.H.; funding acquisition, K.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Bioethics Committee at Poznan University of Medical Sciences (No. 978/22 (approval date: 8 December 2022) and 849/22 (approval date: 3 November 2022)).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| CREA | Creatinine |
| CRP | C-reactive Protein (High-sensitivity) |
| ELF-MFs | Extremely Low-Frequency Magnetic Fields Therapy |
| FIM | Functional Independence Measure |
| HCT | Hematocrit |
| HGB | Hemoglobin |
| K | Potassium |
| MCH | Mean Corpuscular Hemoglobin |
| MCHC | Mean Corpuscular Hemoglobin Concentration |
| MCV | Mean Corpuscular Volume |
| MPV | Mean Platelet Volume |
| MT1 | Group receiving magnetotherapy applied to the head |
| MT2 | Group receiving magnetotherapy applied to the pelvic area |
| NA | Sodium |
| PCT | Procalcitonin |
| PLT | Platelets |
| RBC | Red Blood Cells |
| RDW | Red Cell Distribution Width |
| rTMS | Repetitive Transcranial Magnetic Stimulation |
| SPPB | Short Physical Performance Battery |
| UREA | Urea |
| WBC | White Blood Cells |
References
- Coscia, M.; Wessel, M.J.; Chaudary, U.; Del Millán, J.R.; Micera, S.; Guggisberg, A.; Vuadens, P.; Donoghue, J.; Birbaumer, N.; Hummel, F.C. Neurotechnology-aided interventions for upper limb motor rehabilitation in severe chronic stroke. Brain 2019, 142, 2182–2197. [Google Scholar] [CrossRef] [PubMed]
- Wareńczak-Pawlicka, A.; Lisiński, P. Can We Target Close Therapeutic Goals in the Gait Re-Education Algorithm for Stroke Patients at the Beginning of the Rehabilitation Process? Sensors 2024, 24, 3416. [Google Scholar] [CrossRef] [PubMed]
- Wafa, H.A.; Wolfe, C.D.A.; Emmett, E.; Roth, G.A.; Johnson, C.O.; Wang, Y. Burden of Stroke in Europe: Thirty-Year Projections of Incidence, Prevalence, Deaths, and Disability-Adjusted Life Years. Stroke 2020, 51, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
- Yousufuddin, M.; Young, N. Aging and ischemic stroke. Aging 2019, 11, 2542–2544. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Chou, L.-W.; Hsieh, Y.-L. Proprioceptive Neuromuscular Facilitation-Based Physical Therapy on the Improvement of Balance and Gait in Patients with Chronic Stroke: A Systematic Review and Meta-Analysis. Life 2022, 12, 882. [Google Scholar] [CrossRef]
- Pathak, A.; Gyanpuri, V.; Dev, P.; Dhiman, N.R. The Bobath Concept (NDT) as rehabilitation in stroke patients: A systematic review. J. Fam. Med. Prim. Care 2021, 10, 3983–3990. [Google Scholar] [CrossRef]
- Błażejewska-Hyżorek, B.; Czernuszenko, A.; Członkowska, A.; Ferens, A.; Gąsecki, D.; Kaczorowski, R.; Karaszewski, B.; Karliński, M.; Kaźmierski, R.; Kłysz, B.; et al. Wytyczne postępowania w udarze mózgu. Pol. Przegl. Neurol. 2019, 15, 1–156. [Google Scholar] [CrossRef]
- García-Pérez, P.; Del Rodríguez-Martínez, M.C.; Lara, J.P.; La Cruz-Cosme, C.d. Early Occupational Therapy Intervention in the Hospital Discharge after Stroke. Int. J. Environ. Res. Public Health 2021, 18, 12877. [Google Scholar] [CrossRef]
- Dąbrowski, J.; Czajka, A.; Zielińska-Turek, J.; Jaroszyński, J.; Furtak-Niczyporuk, M.; Mela, A.; Poniatowski, Ł.A.; Drop, B.; Dorobek, M.; Barcikowska-Kotowicz, M.; et al. Brain Functional Reserve in the Context of Neuroplasticity after Stroke. Neural Plast. 2019, 2019, 9708905. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, C.; Wu, X.; Nie, D.; Yu, H. Neuroplasticity of Acupuncture for Stroke: An Evidence-Based Review of MRI. Neural Plast. 2021, 2021, 2662585. [Google Scholar] [CrossRef]
- Aderinto, N.; AbdulBasit, M.O.; Olatunji, G.; Adejumo, T. Exploring the transformative influence of neuroplasticity on stroke rehabilitation: A narrative review of current evidence. Ann. Med. Surg. 2023, 85, 4425–4432. [Google Scholar] [CrossRef] [PubMed]
- Cichoń, N.; Czarny, P.; Bijak, M.; Miller, E.; Śliwiński, T.; Szemraj, J.; Saluk-Bijak, J. Benign Effect of Extremely Low-Frequency Electromagnetic Field on Brain Plasticity Assessed by Nitric Oxide Metabolism during Poststroke Rehabilitation. Oxid. Med. Cell. Longev. 2017, 2017, 2181942. [Google Scholar] [CrossRef] [PubMed]
- Marchewka, R.; Trzmiel, T.; Hojan, K. The Effect of Extremely Low-Frequency Magnetic Field on Stroke Patients: A Systematic Review. Brain Sci. 2024, 14, 430. [Google Scholar] [CrossRef]
- Fan, H.; Song, Y.; Cen, X.; Yu, P.; Bíró, I.; Gu, Y. The Effect of Repetitive Transcranial Magnetic Stimulation on Lower-Limb Motor Ability in Stroke Patients: A Systematic Review. Front. Hum. Neurosci. 2021, 15, 620573. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Capone, F.; Apollonio, F.; Borea, P.A.; Cadossi, R.; Fassina, L.; Grassi, C.; Liberti, M.; Paffi, A.; Parazzini, M.; et al. A consensus panel review of central nervous system effects of the exposure to low-intensity extremely low-frequency magnetic fields. Brain Stimul. 2013, 6, 469–476. [Google Scholar] [CrossRef]
- Capone, F.; Liberti, M.; Apollonio, F.; Camera, F.; Setti, S.; Cadossi, R.; Quattrocchi, C.C.; Di Lazzaro, V. An open-label, one-arm, dose-escalation study to evaluate safety and tolerability of extremely low frequency magnetic fields in acute ischemic stroke. Sci. Rep. 2017, 7, 12145. [Google Scholar] [CrossRef]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016, 2, 64. [Google Scholar] [CrossRef]
- Teresi, J.A.; Yu, X.; Stewart, A.L.; Hays, R.D. Guidelines for Designing and Evaluating Feasibility Pilot Studies. Med. Care 2022, 60, 95–103. [Google Scholar] [CrossRef]
- Cichon, N.; Synowiec, E.; Miller, E.; Sliwinski, T.; Ceremuga, M.; Saluk-Bijak, J.; Bijak, M. Effect of Rehabilitation with Extremely Low Frequency Electromagnetic Field on Molecular Mechanism of Apoptosis in Post-Stroke Patients. Brain Sci. 2020, 10, 266. [Google Scholar] [CrossRef]
- Cichon, N.; Saluk-Bijak, J.; Miller, E.; Sliwinski, T.; Synowiec, E.; Wigner, P.; Bijak, M. Evaluation of the effects of extremely low frequency electromagnetic field on the levels of some inflammatory cytokines in post-stroke patients. J. Rehabil. Med. 2019, 51, 854–860. [Google Scholar] [CrossRef]
- Linacre, J.M.; Heinemann, A.W.; Wright, B.D.; Granger, C.V.; Hamilton, B.B. The structure and stability of the functional independence measure. Arch. Phys. Med. Rehabil. 1994, 75, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Collin, C.; Wade, D.T.; Davies, S.; Horne, V. The Barthel ADL Index: A reliability study. Int. Disabil. Stud. 1988, 10, 61–63. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, H.; Weatherall, M.; Taylor, W.; McPherson, K. Factors influencing rate of Barthel Index change in hospital following stroke. Clin. Rehabil. 2001, 15, 422–427. [Google Scholar] [CrossRef]
- Raîche, M.; Hébert, R.; Prince, F.; Corriveau, H. Screening older adults at risk of falling with the Tinetti balance scale. Lancet 2000, 356, 1001–1002. [Google Scholar] [CrossRef]
- Steffen, T.M.; Hacker, T.A.; Mollinger, L. Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Phys. Ther. 2002, 82, 128–137. [Google Scholar] [CrossRef]
- Western, M.J.; Malkowski, O.S. Associations of the Short Physical Performance Battery (SPPB) with Adverse Health Outcomes in Older Adults: A 14-Year Follow-Up from the English Longitudinal Study of Ageing (ELSA). Int. J. Environ. Res. Public Health 2022, 19, 16319. [Google Scholar] [CrossRef]
- Bian, J.; Guo, S.; Huang, T.; Li, X.; Zhao, S.; Chu, Z.; Li, Z. CRP as a potential predictor of outcome in acute ischemic stroke. Biomed. Rep. 2023, 18, 17. [Google Scholar] [CrossRef]
- Lowe, N.K. What Is a Pilot Study? J. Obstet. Gynecol. Neonatal Nurs. 2019, 48, 117–118. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).