Sustainable Solutions for Pollutants Removal with a Hybrid Multifunctional Adsorbent Based on Recycled Expanded Glass
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hybrid Adsorbent Synthesis
2.3. Modification of EGS with APTES and Goethite
2.4. Characterization of Hybrid Adsorbent
2.5. Batch Adsorption and Kinetics Studies
2.6. Fixed-Bed Column Breakthrough Studies
2.7. Desorption, Regeneration, Exhausted Adsorbent and Pollutants Stabilization, and Degradation Studies
2.7.1. Desorption and Regeneration Studies
2.7.2. Inorganic Pollutants’ Stabilization Studies
2.7.3. Photocatalytic Degradation of Fungicides
2.7.4. Stabilization of Exhausted EGS@APTES-GT Hybrid Adsorbent in UPe Resin Composites
2.7.5. Toxicity Characteristic Leaching Procedure (TCLP)
3. Results and Discussion
3.1. Characterization of the EGS@APTES-GT Hybrid Adsorbent
3.1.1. Particle Porosity, Amino-Group Content, and pHPZC
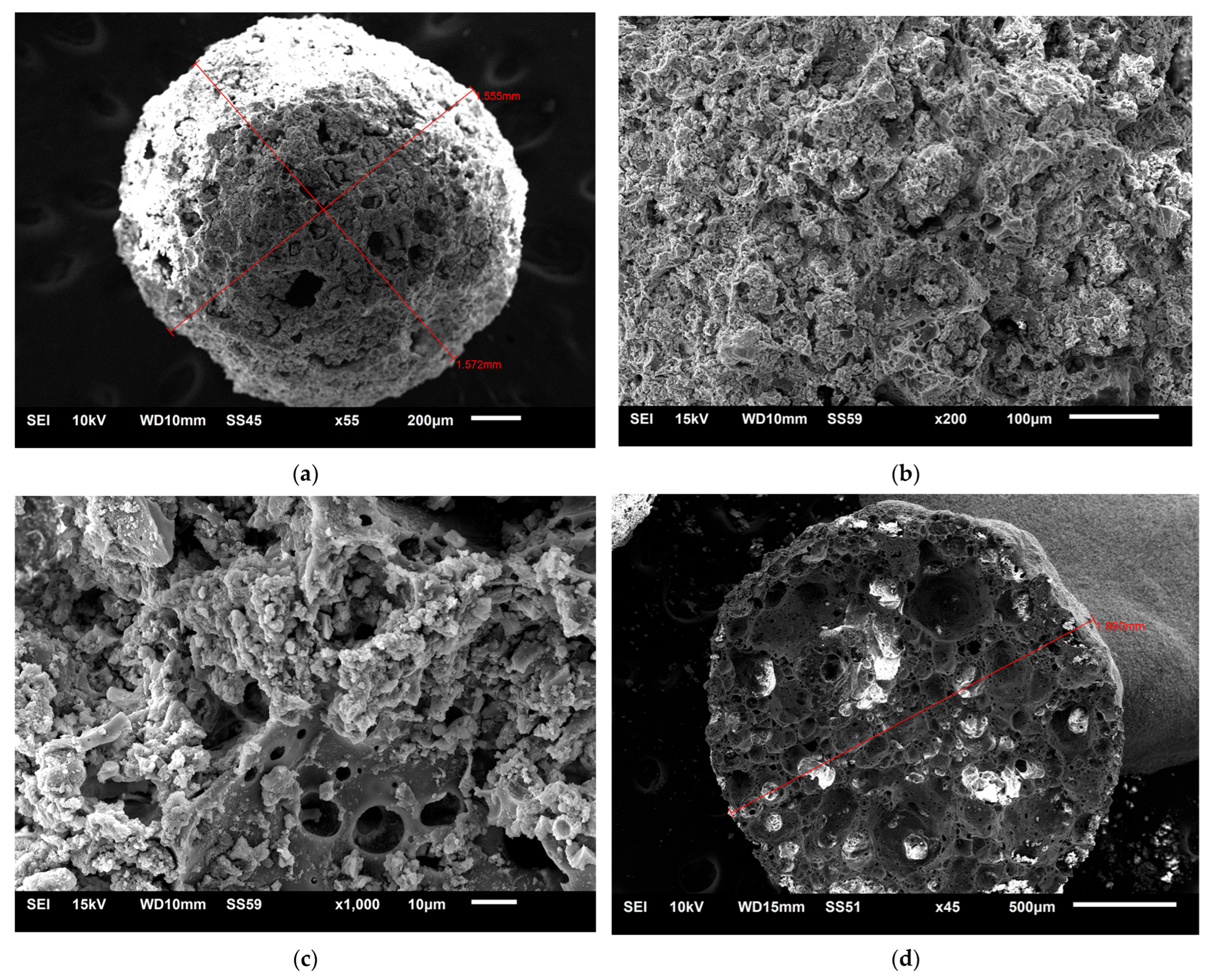
3.1.2. Morphological Characterization
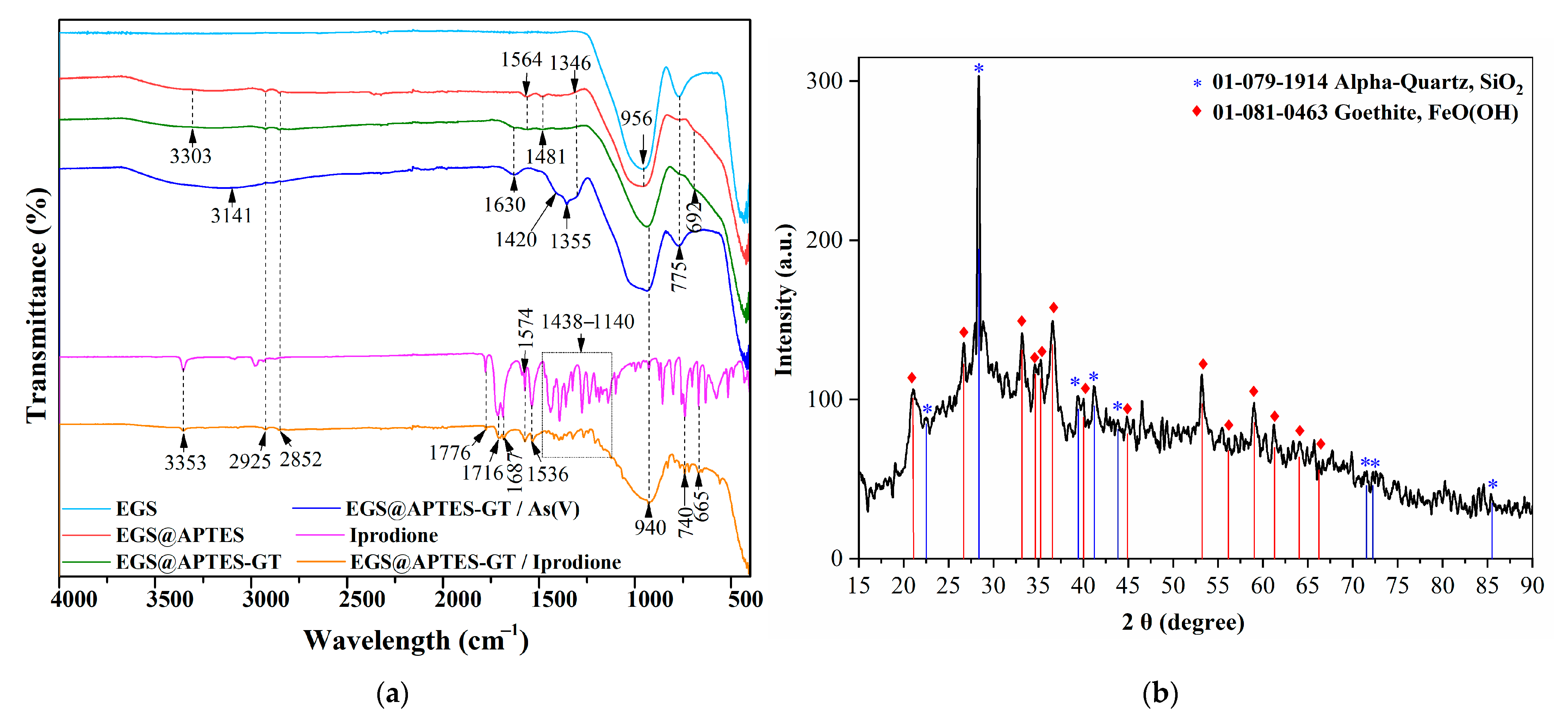
3.1.3. Structural Characterization (FTIR and XRD Analysis)
3.2. Batch Adsorption Performance of EGS@APTES-GT
3.2.1. Influence of Solution pH on Adsorbent Efficiency
3.2.2. Adsorbents Isotherms
3.2.3. Thermodynamic Study
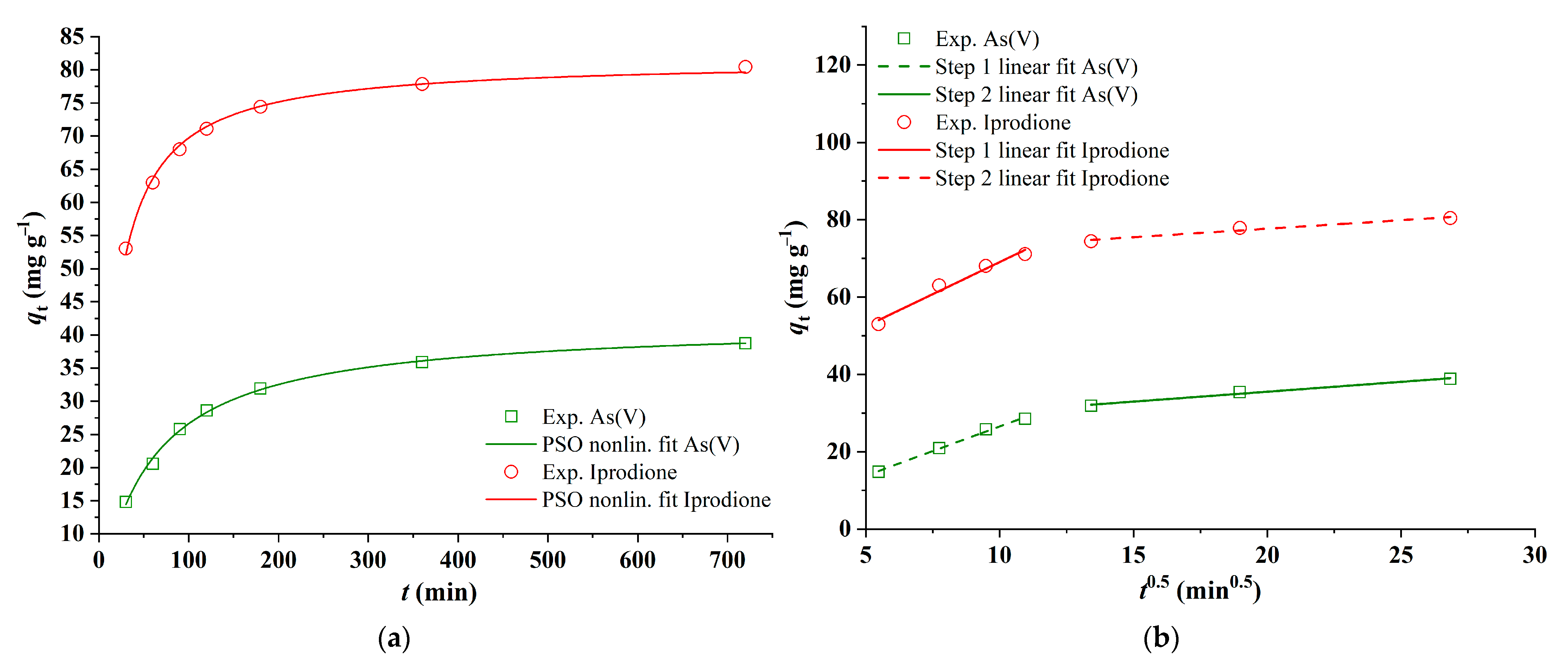
3.2.4. Adsorption Kinetics
3.3. Fixed-Bed Column Adsorption Performance of EGS@APTES-GT
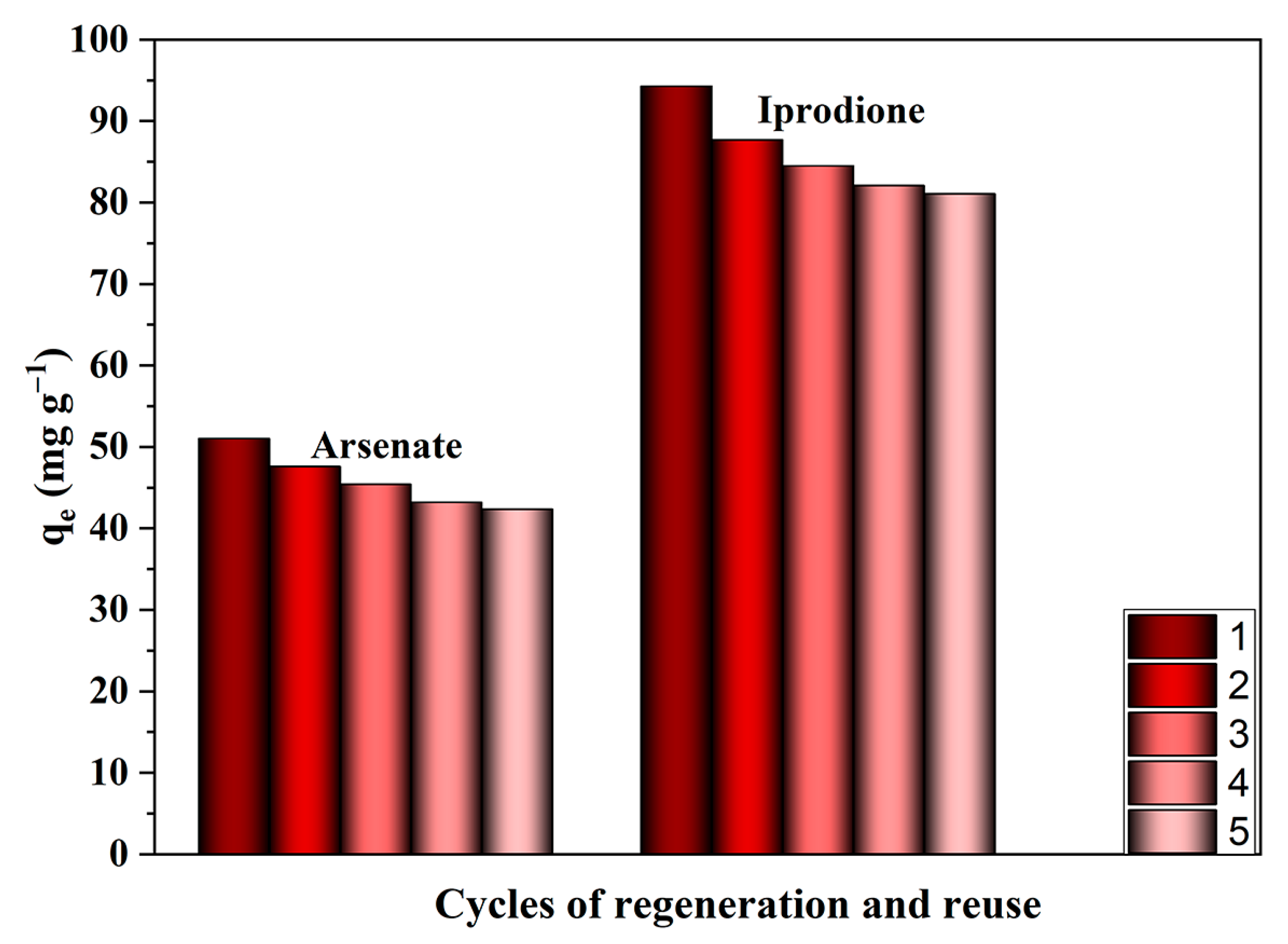
3.4. Desorption Studies
3.5. Developed Technologies for Exhausted Adsorbent and Desorption Solution Disposal
3.5.1. Arsenate Stabilization and Leaching Study
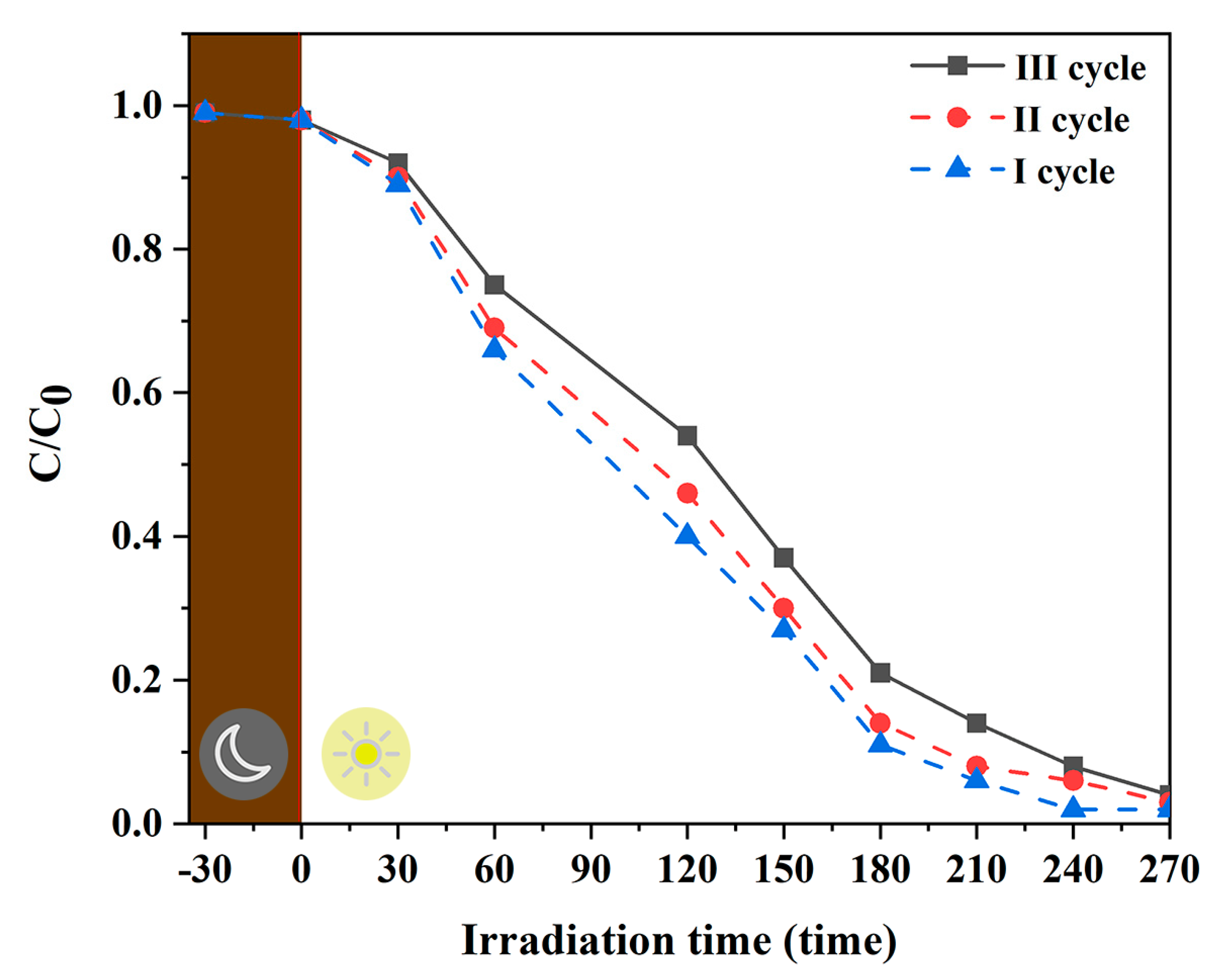
3.5.2. Photocatalytic Degradation of Iprodione
3.5.3. Stabilization of Exhausted EGS@APTES-GT Material in UPe-Based Composites
FTIR Analysis of UPe-Based Composites
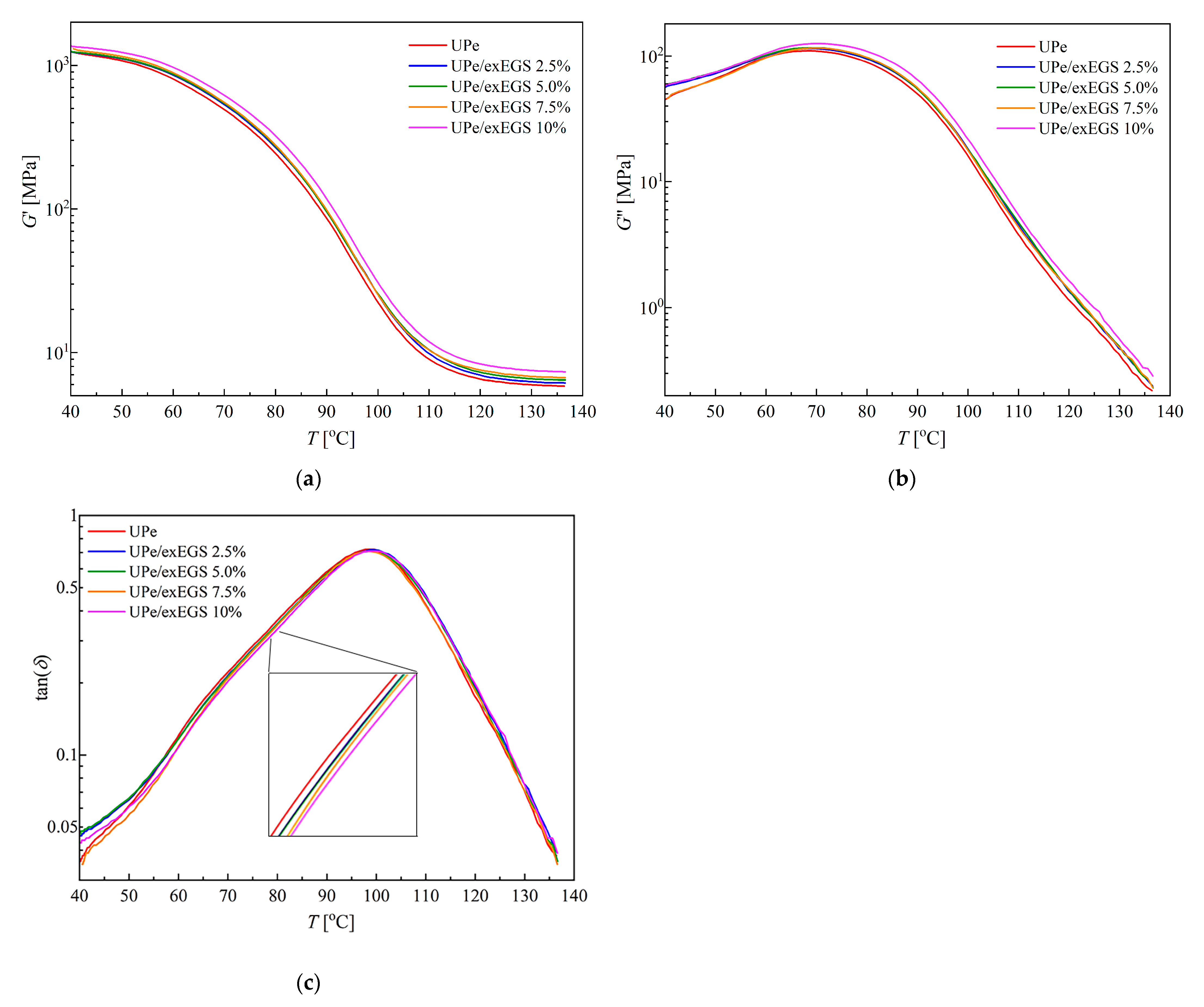
Dynamic–Mechanical Testing of Composite Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EGS | Expanded Glass Spheres |
| GT | Goethite |
| SEM | Scanning Electron Microscope |
| XRD | X-ray Diffraction |
| FTIR | Fourier-transform Infrared Spectroscopy |
| ICP-MS | Inductively Coupled Plasma Mass Spectrometer |
| UPe | Unsaturated Polyester Resin |
| APTES | (3-aminopropyl) triethoxysilane |
| Tg | Glass Transition Temperature |
| DMA | Dynamic–Mechanical Analysis |
References
- Abdelli, H.E.; Mokrani, L.; Kennouche, S.; de Aguiar, J.B. Utilization of Waste Glass in the Improvement of Concrete Performance: A Mini Review. Waste Manag. Res. J. Sustain. Circ. Econ. 2020, 38, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Meng, W.; Nassif, H.; Gou, H.; Bao, Y. New Perspectives on Recycling Waste Glass in Manufacturing Concrete for Sustainable Civil Infrastructure. Constr. Build. Mater. 2020, 257, 119579. [Google Scholar] [CrossRef]
- Chandra Paul, S.; Šavija, B.; Babafemi, A.J. A Comprehensive Review on Mechanical and Durability Properties of Cement-Based Materials Containing Waste Recycled Glass. J. Clean. Prod. 2018, 198, 891–906. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy Metal Water Pollution: A Fresh Look about Hazards, Novel and Conventional Remediation Methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Assessment of Heavy Metal Pollution from Anthropogenic Activities and Remediation Strategies: A Review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef]
- Isasi, J.; Arévalo, P.; Martin, E.; Martín-Hernández, F. Preparation and Study of Silica and APTES–Silica-Modified NiFe2O4 Nanocomposites for Removal of Cu2+ and Zn2+ Ions from Aqueous Solutions. J. Sol-Gel Sci. Technol. 2019, 91, 596–610. [Google Scholar] [CrossRef]
- Singh, N.B.; Nagpal, G.; Agrawal, S. Rachna Water Purification by Using Adsorbents: A Review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Liu, G.; Li, L.; Huang, X.; Zheng, S.; Xu, X.; Liu, Z.; Zhang, Y.; Wang, J.; Lin, H.; Xu, D. Adsorption and Removal of Organophosphorus Pesticides from Environmental Water and Soil Samples by Using Magnetic Multi-Walled Carbon Nanotubes @ Organic Framework ZIF-8. J. Mater. Sci. 2018, 53, 10772–10783. [Google Scholar] [CrossRef]
- Tang, Y.; Liang, S.; Wang, J.; Yu, S.; Wang, Y. Amino-Functionalized Core-Shell Magnetic Mesoporous Composite Microspheres for Pb(II) and Cd(II) Removal. J. Environ. Sci. 2013, 25, 830–837. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, V.; Pandita, S.; Singh, S.; Bhardwaj, R.; Varol, M.; Rodrigo-Comino, J. A Global Meta-Analysis of Toxic Metals in Continental Surface Water Bodies. J. Environ. Chem. Eng. 2023, 11, 109964. [Google Scholar] [CrossRef]
- Zolgharnein, J.; Shahmoradi, A.; Ghasemi, J. Pesticides Removal Using Conventional and Low-Cost Adsorbents: A Review. CLEAN Soil, Air, Water 2011, 39, 1105–1119. [Google Scholar] [CrossRef]
- Palma, P.; Köck-Schulmeyer, M.; Alvarenga, P.; Ledo, L.; Barbosa, I.R.; López de Alda, M.; Barceló, D. Risk Assessment of Pesticides Detected in Surface Water of the Alqueva Reservoir (Guadiana Basin, Southern of Portugal). Sci. Total Environ. 2014, 488–489, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Xi, C.; Wang, R.; Rao, P.; Zhang, W.; Yan, L.; Li, G.; Chai, F.; Cai, Y.; Luo, T.; Zhou, X. The Fabrication and Arsenic Removal Performance of Cellulose Nanocrystal-Containing Absorbents Based on the “Bridge Joint” Effect of Iron Ions. Carbohydr. Polym. 2020, 237, 116129. [Google Scholar] [CrossRef]
- Aminul Haque, M.; Chowdhury, R.A.; Islam, S.; Bhuiyan, M.S.; Ragib, A. Bin Sustainability Assessment of Arsenic-Iron Bearing Groundwater Treatment Soil Mixed Mortar in Developing Countries, Bangladesh. J. Environ. Manag. 2020, 261, 110257. [Google Scholar] [CrossRef]
- Ohta, M.; Okawa, H.; Kato, T.; Sugawara, K. Removal of Arsenite from Aqueous Solutions Using Ultrasonic Irradiation in the Presence of a Lead Electrode. Jpn. J. Appl. Phys. 2020, 59, SKKD01. [Google Scholar] [CrossRef]
- Gupta, V.K.; Carrott, P.J.M.; Ribeiro Carrott, M.M.L. Suhas Low-Cost Adsorbents: Growing Approach to Wastewater Treatment—A Review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 783–842. [Google Scholar] [CrossRef]
- Montazeri, B.; Koba-Ucun, O.; Arslan-Alaton, I.; Olmez-Hanci, T. Iprodione Removal by UV-Light-, Zero-Valent Iron- and Zero-Valent Aluminium-Activated Persulfate Oxidation Processes in Pure Water and Simulated Tertiary Treated Urban Wastewater. Water 2021, 13, 1679. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, X.; Khan, A.; Wang, P.; Liu, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Environmental Remediation and Application of Nanoscale Zero-Valent Iron and Its Composites for the Removal of Heavy Metal Ions: A Review. Environ. Sci. Technol. 2016, 50, 7290–7304. [Google Scholar] [CrossRef]
- Gao, Y.; Truong, Y.B.; Cacioli, P.; Butler, P.; Kyratzis, I.L. Bioremediation of Pesticide Contaminated Water Using an Organophosphate Degrading Enzyme Immobilized on Nonwoven Polyester Textiles. Enzyme Microb. Technol. 2014, 54, 38–44. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Robinson, B.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Farraji, H.; Vakili, M. Pesticides in Aquatic Environments and Their Removal by Adsorption Methods. Chemosphere 2020, 253, 126646. [Google Scholar] [CrossRef]
- Martinez–Vargas, S.; Martínez, A.I.; Hernández–Beteta, E.E.; Mijangos–Ricardez, O.F.; Vázquez–Hipólito, V.; Patiño-Carachure, C.; López–Luna, J. As(III) and As(V) Adsorption on Manganese Ferrite Nanoparticles. J. Mol. Struct. 2018, 1154, 524–534. [Google Scholar] [CrossRef]
- Owen, M.J.; Williams, D.E. Surface Modification by Fluoroalkyl-Functional Silanes. J. Adhes. Sci. Technol. 1991, 5, 307–320. [Google Scholar] [CrossRef]
- Castillo, X.; Pizarro, J.; Ortiz, C.; Cid, H.; Flores, M.; De Canck, E.; Van Der Voort, P. A Cheap Mesoporous Silica from Fly Ash as an Outstanding Adsorbent for Sulfate in Water. Microporous Mesoporous Mater. 2018, 272, 184–192. [Google Scholar] [CrossRef]
- Gérardin, C.; Reboul, J.; Bonne, M.; Lebeau, B. Ecodesign of Ordered Mesoporous Silica Materials. Chem. Soc. Rev. 2013, 42, 4217. [Google Scholar] [CrossRef]
- Acres, R.G.; Ellis, A.V.; Alvino, J.; Lenahan, C.E.; Khodakov, D.A.; Metha, G.F.; Andersson, G.G. Molecular Structure of 3-Aminopropyltriethoxysilane Layers Formed on Silanol-Terminated Silicon Surfaces. J. Phys. Chem. C 2012, 116, 6289–6297. [Google Scholar] [CrossRef]
- Arena, N.; Lee, J.; Clift, R. Life Cycle Assessment of Activated Carbon Production from Coconut Shells. J. Clean. Prod. 2016, 125, 68–77. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and Adsorption Capacities of Low-Cost Sorbents for Wastewater Treatment: A Review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Lata, S.; Samadder, S.R. Removal of Arsenic from Water Using Nano Adsorbents and Challenges: A Review. J. Environ. Manag. 2016, 166, 387–406. [Google Scholar] [CrossRef]
- Xu, W.; Yang, B.; Jia, F.; Chen, T.; Yang, L.; Song, S. Removal of As(V) from Aqueous Solution by Using Cement-Porous Hematite Composite Granules as Adsorbent. Results Phys. 2018, 11, 23–29. [Google Scholar] [CrossRef]
- Hu, X.; Ding, Z.; Zimmerman, A.R.; Wang, S.; Gao, B. Batch and Column Sorption of Arsenic onto Iron-Impregnated Biochar Synthesized through Hydrolysis. Water Res. 2015, 68, 206–216. [Google Scholar] [CrossRef]
- Fouda-Mbanga, B.G.; Onotu, O.; Tywabi-Ngeva, Z. Advantages of the Reuse of Spent Adsorbents and Potential Applications in Environmental Remediation: A Review. Green Anal. Chem. 2024, 11, 100156. [Google Scholar] [CrossRef]
- Baskar, A.V.; Bolan, N.; Hoang, S.A.; Sooriyakumar, P.; Kumar, M.; Singh, L.; Jasemizad, T.; Padhye, L.P.; Singh, G.; Vinu, A.; et al. Recovery, Regeneration and Sustainable Management of Spent Adsorbents from Wastewater Treatment Streams: A Review. Sci. Total Environ. 2022, 822, 153555. [Google Scholar] [CrossRef]
- Rial, J.B.; Ferreira, M.L. Potential Applications of Spent Adsorbents and Catalysts: Re-Valorization of Waste. Sci. Total Environ. 2022, 823, 153370. [Google Scholar] [CrossRef] [PubMed]
- Veličković, Z.; Vuković, G.D.; Marinković, A.D.; Moldovan, M.S.; Perić-Grujić, A.A.; Uskoković, P.S.; Ristić, M.D. Adsorption of Arsenate on Iron(III) Oxide Coated Ethylenediamine Functionalized Multiwall Carbon Nanotubes. Chem. Eng. J. 2012, 181–182, 174–181. [Google Scholar] [CrossRef]
- Markovski, J.S.; Đokić, V.; Milosavljević, M.; Mitrić, M.; Perić-Grujić, A.A.; Onjia, A.E.; Marinković, A.D. Ultrasonic Assisted Arsenate Adsorption on Solvothermally Synthesized Calcite Modified by Goethite, α-MnO2 and Goethite/α-MnO2. Ultrason. Sonochem. 2014, 21, 790–801. [Google Scholar] [CrossRef]
- Hao, S.; Verlotta, A.; Aprea, P.; Pepe, F.; Caputo, D.; Zhu, W. Optimal Synthesis of Amino-Functionalized Mesoporous Silicas for the Adsorption of Heavy Metal Ions. Microporous Mesoporous Mater. 2016, 236, 250–259. [Google Scholar] [CrossRef]
- Đolić, M.; Karanac, M.; Radovanović, D.; Umićević, A.; Kapidžić, A.; Veličković, Z.; Marinković, A.; Kamberović, Ž. Closing the Loop: As(V) Adsorption onto Goethite Impregnated Coal-Combustion Fly Ash as Integral Building Materials. J. Clean. Prod. 2021, 303, 126924. [Google Scholar] [CrossRef]
- Pantić, K.; Bajić, Z.J.; Veličković, Z.S.; Nešić, J.Z.; Đolić, M.B.; Tomić, N.Z.; Marinković, A.D. Arsenic Removal by Copper-Impregnated Natural Mineral Tufa Part II: A Kinetics and Column Adsorption Study. Environ. Sci. Pollut. Res. 2019, 26, 24143–24161. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Nelson, J.H. Application of Gas Adsorption Kinetics I. A Theoretical Model for Respirator Cartridge Service Life. Am. Ind. Hyg. Assoc. J. 1984, 45, 509–516. [Google Scholar] [CrossRef]
- Bohart, G.S.; Adams, E.Q. Some Aspects of the Behavior of Charcoal with Respect to Chlorine. J. Franklin Inst. 1920, 189, 669. [Google Scholar] [CrossRef]
- Thomas, H.C. Heterogeneous Ion Exchange in a Flowing System. J. Am. Chem. Soc. 1944, 66, 1664–1666. [Google Scholar] [CrossRef]
- Perendija, J.; Velickovic, Z.; Drazevic, L.; Stojiljkovic, I.; Milcic, M.; Milosavljevic, M.; Marinkovic, A.; Pavlovic, V. Evaluation of Adsorption Performance and Quantum Chemical Modeling of Pesticides Removal Using Cell-MG Hybrid Adsorbent. Sci. Sinter. 2021, 53, 355–378. [Google Scholar] [CrossRef]
- Perendija, J.; Veličković, Z.S.; Cvijetić, I.; Rusmirović, J.D.; Ugrinović, V.; Marinković, A.D.; Onjia, A. Batch and Column Adsorption of Cations, Oxyanions and Dyes on a Magnetite Modified Cellulose-Based Membrane. Cellulose 2020, 27, 8215–8235. [Google Scholar] [CrossRef]
- Deshmukh, N.S.; Deosarkar, M.P. A Review on Ultrasound and Photocatalysis-Based Combined Treatment Processes for Pesticide Degradation. Mater. Today Proc. 2022, 57, 1575–1584. [Google Scholar] [CrossRef]
- Knežević, N.; Milanović, J.; Veličković, Z.; Milošević, M.; Vuksanović, M.M.; Onjia, A.; Marinković, A. A Closed Cycle of Sustainable Development: Effective Removal and Desorption of Lead and Dyes Using an Oxidized Cellulose Membrane. J. Ind. Eng. Chem. 2023, 126, 520–536. [Google Scholar] [CrossRef]
- Available online: https://www.epa.gov/hw-sw846/sw-846-test-method-1311-toxicity-characteristic-leaching-procedure (accessed on 16 January 2025).
- Athanasaki, G.; Sherrill, L.; Hristovski, K.D. The Pore Surface Diffusion Model as a Tool for Rapid Screening of Novel Nanomaterial-Enhanced Hybrid Ion-Exchange Media. Environ. Sci. Water Res. Technol. 2015, 1, 448–456. [Google Scholar] [CrossRef]
- Markovski, J.S.; Marković, D.D.; Đokić, V.R.; Mitrić, M.; Ristić, M.Đ.; Onjia, A.E.; Marinković, A.D. Arsenate Adsorption on Waste Eggshell Modified by Goethite, α-MnO2 and Goethite/α-MnO2. Chem. Eng. J. 2014, 237, 430–442. [Google Scholar] [CrossRef]
- Patra, A.K.; Kundu, S.K.; Bhaumik, A.; Kim, D. Morphology Evolution of Single-Crystalline Hematite Nanocrystals: Magnetically Recoverable Nanocatalysts for Enhanced Facet-Driven Photoredox Activity. Nanoscale 2016, 8, 365–377. [Google Scholar] [CrossRef]
- Hai, N.; Liu, X.; Li, Y.; Kong, F.; Zhang, Y.; Fang, S. Effects of Microplastics on the Adsorption and Bioavailability of Three Strobilurin Fungicides. ACS Omega 2020, 5, 30679–30686. [Google Scholar] [CrossRef]
- Weber, T.W.; Chakravorti, R.K. Pore and Solid Diffusion Models for Fixed-Bed Adsorbers. AIChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Karanac, M.; Đolić, M.; Veličković, Z.; Kapidžić, A.; Ivanovski, V.; Mitrić, M.; Marinković, A. Efficient Multistep Arsenate Removal onto Magnetite Modified Fly Ash. J. Environ. Manag. 2018, 224, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Gang, D.D.; Zhang, N.; Lin, L. Adsorptive Selenite Removal Using Iron-Coated GAC: Modeling Selenite Breakthrough with the Pore Surface Diffusion Model. J. Environ. Eng. 2013, 139, 213–219. [Google Scholar] [CrossRef]
- Toledo-Jaldin, H.P.; Sánchez-Mendieta, V.; Blanco-Flores, A.; López-Téllez, G.; Vilchis-Nestor, A.R.; Martín-Hernández, O. Low-Cost Sugarcane Bagasse and Peanut Shell Magnetic-Composites Applied in the Removal of Carbofuran and Iprodione Pesticides. Environ. Sci. Pollut. Res. 2020, 27, 7872–7885. [Google Scholar] [CrossRef]
- Abduarahman, M.A.; Vuksanović, M.M.; Knežević, N.; Banjanac, K.; Milošević, M.; Veličković, Z.; Marinković, A. MgAl-Layered Double Hydroxide-Coated Bio-Silica as an Adsorbent for Anionic Pollutants Removal: A Case Study of the Implementation of Sustainable Technologies. Int. J. Mol. Sci. 2024, 25, 11837. [Google Scholar] [CrossRef]
- Lin, S.; Yang, H.; Na, Z.; Lin, K. A Novel Biodegradable Arsenic Adsorbent by Immobilization of Iron Oxyhydroxide (FeOOH) on the Root Powder of Long-Root Eichhornia Crassipes. Chemosphere 2018, 192, 258–266. [Google Scholar] [CrossRef]
- Byambaa, E.; Seon, J.; Kim, T.-H.; Kim, S.D.; Ji, W.H.; Hwang, Y. Arsenic (V) Removal by an Adsorbent Material Derived from Acid Mine Drainage Sludge. Appl. Sci. 2020, 11, 47. [Google Scholar] [CrossRef]
- Shih, Y.-J.; Huang, R.-L.; Huang, Y.-H. Adsorptive Removal of Arsenic Using a Novel Akhtenskite Coated Waste Goethite. J. Clean. Prod. 2015, 87, 897–905. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1368. [Google Scholar] [CrossRef]
- Alkan, M.; Demirbas, O.; Dogan, M. Adsorption Kinetics and Thermodynamics of an Anionic Dye onto Sepiolite. Microporous Mesoporous Mater. 2007, 101, 388–396. [Google Scholar] [CrossRef]
- Taleb, K.; Markovski, J.; Veličković, Z.; Rusmirović, J.; Rančić, M.; Pavlović, V.; Marinković, A. Arsenic Removal by Magnetite-Loaded Amino Modified Nano/Microcellulose Adsorbents: Effect of Functionalization and Media Size. Arab. J. Chem. 2019, 12, 4675–4693. [Google Scholar] [CrossRef]
- Karanac, M.; Đolić, M.; Veljović, Đ.; Rajaković-Ognjanović, V.; Veličković, Z.; Pavićević, V.; Marinković, A. The Removal of Zn2+, Pb2+, and As(V) Ions by Lime Activated Fly Ash and Valorization of the Exhausted Adsorbent. Waste Manag. 2018, 78, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.epa.gov/dwreginfo/drinking-water-arsenic-rule-history (accessed on 16 January 2025).
- Chavoshan, S.; Khodadadi, M.; Nasseh, N. Photocatalytic Degradation of Penicillin G from Simulated Wastewater Using the UV/ZnO Process: Isotherm and Kinetic Study. J. Environ. Heal. Sci. Eng. 2020, 18, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Yusuff, A.S.; Taofeek Popoola, L.; Aderibigbe, E.I. Solar Photocatalytic Degradation of Organic Pollutants in Textile Industry Wastewater by ZnO/Pumice Composite Photocatalyst. J. Environ. Chem. Eng. 2020, 8, 103907. [Google Scholar] [CrossRef]
- Knežević, N.; Vuksanović, M.M.; Banjanac, K.; Pantić, K.; Veličković, Z.; Cvijetić, I.; Marinković, A.; Milošević, M. Cationic Waste Hemp Fibers-Based Membrane: Case Study of Anionic Pollutants Removal through Environmentally Friendly Processes. J. Environ. Manag. 2024, 371, 123174. [Google Scholar] [CrossRef]
- Salah Adeen Embirsh, H.; Vuksanović, M.M.; Mladenović, I.O.; Knežević, N.; Milošević, M.; Mijatov, S.; Jančić Heinemann, R.; Marinković, A. Unsaturated Polyester Resin Based Composites: A Case Study of Lignin Valorisation. Chemosphere 2024, 362, 142144. [Google Scholar] [CrossRef]
- Jing, N.; Jiang, X.; Wang, Q.; Tang, Y.; Zhang, P. Attenuated Total Reflectance/Fourier Transform Infrared (ATR/FTIR) Mapping Coupled with Principal Component Analysis for the Study of in Vitro Degradation of Porous Polylactide/Hydroxyapatite Composite Material. Anal. Methods 2014, 6, 5590–5595. [Google Scholar] [CrossRef]
- Franca, T.; Goncalves, D.; Cena, C. ATR-FTIR Spectroscopy Combined with Machine Learning for Classification of PVA/PVP Blends in Low Concentration. Vib. Spectrosc. 2022, 120, 103378. [Google Scholar] [CrossRef]
- Dinh, N.H.; Pham, H.H.; Kim, S.-H.; Choi, K.-K. Tensile Performance and Cracking Characteristics of Sustainable Textile-Reinforced Cementitious Composites Utilizing Expanded Glass Aggregate and Fly Ash Replacement. Constr. Build. Mater. 2024, 425, 136084. [Google Scholar] [CrossRef]
- Thomason, J.; Jenkins, P.; Yang, L. Glass Fibre Strength—A Review with Relation to Composite Recycling. Fibers 2016, 4, 18. [Google Scholar] [CrossRef]
- Aly, M.; Hashmi, M.S.J.; Olabi, A.G.; Messeiry, M.; Hussain, A.I. Effect of Nano Clay Particles on Mechanical, Thermal and Physical Behaviours of Waste-Glass Cement Mortars. Mater. Sci. Eng. A 2011, 528, 7991–7998. [Google Scholar] [CrossRef]
- Gorokhovsky, A.V.; Escalante-Garcia, J.I.; Gashnikova, G.Y.; Nikulina, L.P.; Artemenko, S.E. Composite Materials Based on Wastes of Flat Glass Processing. Waste Manag. 2005, 25, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Essabir, H.; Elkhaoulani, A.; Benmoussa, K.; Bouhfid, R.; Arrakhiz, F.Z.; Qaiss, A. Dynamic Mechanical Thermal Behavior Analysis of Doum Fibers Reinforced Polypropylene Composites. Mater. Des. 2013, 51, 780–788. [Google Scholar] [CrossRef]
- Essabir, H.; Hilali, E.; Elgharad, A.; El Minor, H.; Imad, A.; Elamraoui, A.; Al Gaoudi, O. Mechanical and Thermal Properties of Bio-Composites Based on Polypropylene Reinforced with Nut-Shells of Argan Particles. Mater. Des. 2013, 49, 442–448. [Google Scholar] [CrossRef]
- Rusmirović, J.; Galović, J.; Kluz, M.; Perković, S.; Brzić, S.; Bogosavljević, M.; Milojković, A.; Kovačević, T. Using Potential of Filament-Wound Carbon/Glass Polymeric Composites as Rocket Motor Thermal Insulation. Polym. Polym. Compos. 2021, 29, S1541–S1554. [Google Scholar] [CrossRef]
- Prabhu, P.; Karthikeyan, B.; Vannan, R.R.R.M.; Balaji, A. Mechanical, Thermal and Morphological Analysis of Hybrid Natural and Glass Fiber-Reinforced Hybrid Resin Nanocomposites. Biomass Convers. Biorefinery 2022, 14, 4941–4955. [Google Scholar] [CrossRef]
- Bassyouni, M.; Sherif, S.A.; Sadek, M.A.; Ashour, F.H. Synthesis and Characterization of Polyurethane—Treated Waste Milled Light Bulbs Composites. Compos. Part B Eng. 2012, 43, 1439–1444. [Google Scholar] [CrossRef]
- Achukwu, E.O.; Musa, H.; Daniel, D.; Yusuf, S.Y. Development of Waste Glass Particle-Reinforced Unsaturated Polyester Composites. Niger. J. Sci. Res. 2015, 14, 16–20. [Google Scholar]
- Rivera-Utrilla, J.; Bautista-Toledo, I.; Ferro-García, M.A.; Moreno-Castilla, C. Activated Carbon Surface Modifications by Adsorption of Bacteria and Their Effect on Aqueous Lead Adsorption. J. Chem. Technol. Biotechnol. 2001, 76, 1209–1215. [Google Scholar] [CrossRef]
- Sontheimer, H.; Crittenden, J.C.; Summers, R.S. Activated Carbon for Water Treatment, 2nd ed.; DVGW-Forschungsstelle, Engler-Bunte-Institut, Universitat Karlsruhe (TH): Karlsruhe, Germany, 1988. [Google Scholar]
- Obradović, N.; Rusmirović, J.; Filipović, S.; Kosanović, D.; Marinković, A.; Radić, D.; Pavlović, V. Porous Cordierite-Supported Polyethyleneimine Composites for Nickel(II) and Cadmium(II) Ions Removal. Desalin. Water Treat. 2020, 192, 283–296. [Google Scholar] [CrossRef]
- Vuković, G.D.; Marinković, A.D.; Škapin, S.D.; Ristić, M.T.; Aleksić, R.; Perić-Grujić, A.A.; Uskoković, P.S. Removal of Lead from Water by Amino Modified Multi-Walled Carbon Nanotubes. Chem. Eng. J. 2011, 173, 855–865. [Google Scholar] [CrossRef]
- Chiban, M.; Carja, G.; Lehutu, G.; Sinan, F. Equilibrium and Thermodynamic Studies for the Removal of As(V) Ions from Aqueous Solution Using Dried Plants as Adsorbents. Arab. J. Chem. 2016, 9, S988–S999. [Google Scholar] [CrossRef]
- Freundlich, H.M. Over the Adsorption in Solution. J. Phys. Chem. A 1906, 57, 385–470. [Google Scholar]
- Haghseresht, F.; Lu, G.Q. Adsorption Characteristics of Phenolic Compounds onto Coal-Reject-Derived Adsorbents. Energy and Fuels 1998, 12, 1100–1107. [Google Scholar] [CrossRef]
- Salih, R.; Veličković, Z.; Milošević, M.; Pavlović, V.P.; Cvijetić, I.; Sofrenić, I.V.; Gržetić, J.D.; Marinković, A. Lignin Based Microspheres for Effective Dyes Removal: Design, Synthesis and Adsorption Mechanism Supported with Theoretical Study. J. Environ. Manag. 2023, 326, 116838. [Google Scholar] [CrossRef]
- Ibrahim, H.S.; Ammar, N.S.; Abdel Ghafar, H.H.; Farahat, M. Adsorption of Cd(II), Cu(II) and Pb(II) Using Recycled Waste Glass: Equilibrium and Kinetic Studies. Desalin. Water Treat. 2012, 48, 320–328. [Google Scholar] [CrossRef]
- Jaiswal, A.; Banerjee, S.; Mani, R.; Chattopadhyaya, M.C. Synthesis, Characterization and Application of Goethite Mineral as an Adsorbent. J. Environ. Chem. Eng. 2013, 1, 281–289. [Google Scholar] [CrossRef]
- Ruj, D.B.; Chakrabortty, D.S.; Nayak, D.J.; Chatterjee, R. Treatment of Arsenic Sludge Generated from Groundwater Treatment Plant: A Review towards a Sustainable Solution. S. Afr. J. Chem. Eng. 2021, 37, 214–226. [Google Scholar] [CrossRef]
- Clancy, T.M.; Hayes, K.F.; Raskin, L. Arsenic Waste Management: A Critical Review of Testing and Disposal of Arsenic-Bearing Solid Wastes Generated during Arsenic Removal from Drinking Water. Environ. Sci. Technol. 2013, 47, 10799–10812. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, B.; Ning, P.; Qu, G.; Li, J.; Wang, X.; Xie, R. Stabilization of Arsenic in Waste Slag Using FeCl2 or FeCl3 Stabilizer. RSC Adv. 2017, 7, 54956–54963. [Google Scholar] [CrossRef]
- Majzlan, J.; Drahota, P.; Filippi, M.; Grevel, K.-D.; Kahl, W.-A.; Plášil, J.; Boerio-Goates, J.; Woodfield, B.F. Thermodynamic Properties of Scorodite and Parascorodite (FeAsO4·2H2O), Kaňkite (FeAsO4·3.5H2O), and FeAsO4. Hydrometallurgy 2012, 117–118, 47–56. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Sun, L.; Hu, D.; Zhang, Z.; Deng, X. Oxidative Degradation of Methylene Blue via PDS-Based Advanced Oxidation Process Using Natural Pyrite. Int. J. Environ. Res. Public Health 2019, 16, 4773. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Lichtfouse, E. Advantages and Disadvantages of Techniques Used for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Saeed, K.; Khan, I.; Gul, T.; Sadiq, M. Efficient Photodegradation of Methyl Violet Dye Using TiO2/Pt and TiO2/Pd Photocatalysts. Appl. Water Sci. 2017, 7, 3841–3848. [Google Scholar] [CrossRef]
- Naghash-Hamed, S.; Arsalani, N.; Mousavi, S.B. The Catalytic Reduction of Nitroanilines Using Synthesized CuFe2O4 Nanoparticles in an Aqueous Medium. ChemistryOpen 2022, 11. [Google Scholar] [CrossRef]
- Yang, C.; Li, Q.; Tang, L.; Xin, K.; Bai, A.; Yu, Y. Synthesis, Photocatalytic Activity, and Photogenerated Hydroxyl Radicals of Monodisperse Colloidal ZnO Nanospheres. Appl. Surf. Sci. 2015, 357, 1928–1938. [Google Scholar] [CrossRef]
- Saeed, K.; Khan, I.; Park, S.-Y. TiO2/Amidoxime-Modified Polyacrylonitrile Nanofibers and Its Application for the Photodegradation of Methyl Blue in Aqueous Medium. Desalin. Water Treat. 2015, 54, 3146–3151. [Google Scholar] [CrossRef]
- Balu, S.; Uma, K.; Pan, G.-T.; Yang, T.C.-K.; Ramaraj, S.K. Degradation of Methylene Blue Dye in the Presence of Visible Light Using SiO2@α-Fe2O3 Nanocomposites Deposited on SnS2 Flowers. Materials 2018, 11, 1030. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-Assisted Photocatalytic Degradation of Azo Dyes in Aqueous Solution: Kinetic and Mechanistic Investigations. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Li, Y.; Wu, M.; Yang, D.; Zeng, H.; Zhang, T.; Shen, J.; Zhang, B.; Li, Q. Novel High Efficiency Layered Oxide Photocatalyst Li2SnO3 for Rhodamine B and Tetracycline Degradation. Catalysts 2019, 9, 712. [Google Scholar] [CrossRef]
- Available online: https://www.epa.gov/sites/default/files/2013-08/documents/risk_rep.pdf (accessed on 16 January 2025).
- Mahedi, M.; Cetin, B.; Dayioglu, A.Y. Leaching Behavior of Aluminum, Copper, Iron and Zinc from Cement Activated Fly Ash and Slag Stabilized Soils. Waste Manag. 2019, 95, 334–355. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.B.; Vanapalli, K.R.; Barnwal, V.K.; Dubey, B.; Bhattacharya, J. Evaluation of Heavy Metal Leaching under Simulated Disposal Conditions and Formulation of Strategies for Handling Solar Panel Waste. Sci. Total Environ. 2021, 780, 146645. [Google Scholar] [CrossRef] [PubMed]
- Rinnan, Å.; Van Den Berg, F.; Engelsen, S.B. Review of the Most Common Pre-Processing Techniques for near-Infrared Spectra. TrAC Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Davallo, M.; Pasdar, H.; Mohseni, M. Mechanical Properties of Unsaturated Polyester Resin. Int. J. ChemTech Res. 2010, 2, 2113–2117. [Google Scholar]
- Embirsh, H.S.A.; Stajčić, I.; Gržetić, J.; Mladenović, I.O.; Anđelković, B.; Marinković, A.; Vuksanović, M.M. Synthesis, Characterization and Application of Biobased Unsaturated Polyester Resin Reinforced with Unmodified/Modified Biosilica Nanoparticles. Polymers 2023, 15, 3756. [Google Scholar] [CrossRef]
- Sudirman; Anggaravidya, M.; Budianto, E.; Gunawan, I. Synthesis and Characterization of Polyester-Based Nanocomposite. Procedia Chem. 2012, 4, 107–113. [CrossRef]
- Halim, Z.A.A.; Yajid, M.A.M.; Nurhadi, F.A.; Ahmad, N.; Hamdan, H. Effect of Silica Aerogel—Aluminium Trihydroxide Hybrid Filler on the Physio-Mechanical and Thermal Decomposition Behaviour of Unsaturated Polyester Resin Composite. Polym. Degrad. Stab. 2020, 182, 109377. [Google Scholar] [CrossRef]






| Models and Parameters | Temperature (K) | |||
|---|---|---|---|---|
| 298 | 308 | 318 | ||
| As(V) adsorption | ||||
| Langmuir model | qmax, (mg g−1) | 51.01 ± 5.26 | 53.58 ± 5.59 | 56.11 ± 5.92 |
| KL, (dm3 mg−1) | 1.097 ± 0.21 | 1.121 ± 0.22 | 1.155 ± 0.22 | |
| RL | 0.159 ± 0.01 | 0.156 ± 0.02 | 0.152 ± 0.02 | |
| R2 | 0.980 | 0.980 | 0.981 | |
| Freundlich model | KF, (mg g−1) (dm3 mg−1)1/n | 25.17 ± 0.13 | 26.92 ± 0.14 | 28.89 ± 0.16 |
| 1/n | 0.593 ± 0.02 | 0.602 ± 0.02 | 0.610 ± 0.02 | |
| R2 | 0.999 | 0.999 | 0.999 | |
| Temkin model | AT, (dm3 g−1) | 23.01 ± 6.90 | 24.13 ± 7.30 | 25.47 ± 7.76 |
| B, (mg g−1) | 7.855 ± 1.17 | 8.116 ± 1.23 | 8.377 ± 1.29 | |
| R2 | 0.900 | 0.896 | 0.894 | |
| Iprodione adsorption | ||||
| Langmuir model | qmax, (mg g−1) | 94.28 ± 7.04 | 103.7 ± 8.39 | 114.0 ± 9.70 |
| KL, (dm3 mg−1) | 2.087 ± 0.43 | 2.012 ± 0.43 | 1.963 ± 0.43 | |
| RL | 0.047 ± 0.03 | 0.048 ± 0.03 | 0.049 ± 0.03 | |
| R2 | 0.957 | 0.955 | 0.956 | |
| Freundlich model | KF, (mg g−1) (dm3 mg−1)1/n | 54.19 ± 1.26 | 59.23 ± 1.15 | 65.09 ± 1.09 |
| 1/n | 0.367 ± 0.13 | 0.386 ± 0.10 | 0.407 ± 0.08 | |
| R2 | 0.988 | 0.992 | 0.994 | |
| Temkin model | AT, (dm3 g−1) | 40.75 ± 10.6 | 42.98 ± 13.2 | 43.44 ± 14.1 |
| B, (mg g−1) | 16.04 ± 1.43 | 17.13 ± 1.85 | 18.56 ± 2.20 | |
| R2 | 0.962 | 0.945 | 0.935 | |
| Adsorbent | Pollutant | Conditions | qe (mg g−1) | Isotherm Models | Ref. |
|---|---|---|---|---|---|
| FeOOH immobilized on the biodegradable root powder (waste biomass) | As(V) | Ci = 10 ppm, T = 30 °C, pH = 9.0, m/V = 1 g L−1 | 9.21 | Langmuir | [56] |
| Goethite-impregnated fly ash (FAG) | As(V) | Ci = 5.0 ppm, T= 45 °C, pH = 6.0 ± 0.1, m/V = 0.2–2.0 g L−1 | 31.7 | Langmuir | [37] |
| Magnetite-impregnated fly ash (FAM) | As(V) | Ci = 5.0 ppm, T = 45 °C, pH = 6.0, m/V = 0.2–2.0 g L−1 | 19.1 | Langmuir | [51] |
| As(V) | Ci = 20.0 ppm, T = 45 °C, pH = 6.0 ± 0.1, m/V = not given |
| Freundlich | [56] |
| As(V) | Ci = 50 ppm, T = ./. pH = 6.6, m/V = 0.01–10 g L−1 |
| Langmuir Freundlich | [57] |
| As(V) | Ci = 10 ppm, T = room, pH = 3.5, m/V = 1 g L−1 |
| Langmuir Freundlich | [58] |
| Cell-MG hybrid membrane | Azoxystrobin | Ci = 6.1 ppm, T = 25 °C, pH = 6.0, m/V = 0.11–1.11 g L−1 | 35.3 | Langmuir | [42] |
| Iprodione | Ci = 5.1 ppm, T = 25 °C, pH = 6.0, m/V = 0.11–1.11 g L−1 | 30.2 | |||
| Iprodione | Ci = 30 ppm, T = 25 °C, m/V = 2.0 g L−1 |
| Langmuir Freundlich Sips | [53] |
| Expanded Glass Spheres modified with APTES and goethite (EGS@APTES-GT) | As(V) | Ci = 4.83 ppm, T = 25 °C, pH = 7.0 ± 0.1, m/V = 0.1–1.0 g L−1 | 51.0 | Freundlich Langmuir Temkin | This work |
| Iprodione | Ci = 9.81 ppm, T = 25 °C, pH = 7.0 ± 0.1, m/V = 0.1–1.0 g L−1 | 94.3 | Freundlich Langmuir Temkin | This work |
| Adsorbate | ΔGΘ (kJ mol−1) | ΔHΘ (kJ mol−1) | ΔSΘ (J mol−1 K−1) | R2 | ||
|---|---|---|---|---|---|---|
| 298 K | 308 K | 318 K | ||||
| Arsenate | −40.99 | −43.15 | −45.16 | 21.3 | 208.9 | 0.996 |
| Iprodione | −43.53 | −45.03 | −46.54 | 1.34 | 150.5 | 0.991 |
| Model | Parameter | As(V) | Iprodione |
|---|---|---|---|
| Pseudo-second-order | k2 (g mg−1 min−1) | 4.18 × 10−4 ± 2.1 × 10−5 | 7.25 × 10−4 ± 3.1 × 10−5 |
| qe (mg g−1) | 41.83 ± 0.47 | 81.52 ± 0.57 | |
| R2 | 0.997 | 0.996 | |
| Elovich | α (mg g−1 h−1) | 2.13 ± 0.51 | 244.9 ± 235.1 |
| β (mg g−1) | 0.130 ± 0.01 | 0.119 ± 0.02 | |
| R2 | 0.966 | 0.921 | |
| Weber–Morris Step 1 (Film/Intra-particle diffusion) Step 2 (Equilibrium) | kid1 (mg g−1 min−0.5) | 2.54 ± 0.14 | 3.318 ± 0.38 |
| C1 (mg g−1) | 1.10 ± 1.18 | 35.87 ± 3.26 | |
| R12 | 0.994 | 0.975 | |
| kid2 (mg g−1 min−0.5) | 0.511 ± 0.05 | 0.439 ± 0.08 | |
| C2 (mg g−1) | 25.30 ± 1.05 | 68.90 ± 1.69 | |
| R22 | 0.990 | 0.966 |
| Column Models/Parameters | Flow Rate (cm3 min−1) | |||
|---|---|---|---|---|
| 0.5 | 1.0 | 1.5 | ||
| As(V) | ||||
| B–A | KBA (dm3 mg−1 min−1) | 0.082 ± 0.002 | 0.155 ± 0.003 | 0.224 ± 0.007 |
| N0 (mg dm−3) | 41.62 ± 0.236 | 36.86 ± 0.248 | 32.24 ± 0.313 | |
| R2 | 0.998 | 0.998 | 0.996 | |
| Y–N | KYN (min−1) | 0.788 ± 0.017 | 0.748 ± 0.017 | 0.755 ± 0.022 |
| θ (min) | 5.558 ± 0.031 | 4.923 ± 0.033 | 4.305 ± 0.042 | |
| R2 | 0.998 | 0.998 | 0.996 | |
| Thomas | KTH (dm3 mg−1 min−1) | 0.163 ± 0.004 | 0.155 ± 0.003 | 0.156 ± 0.004 |
| q0 (mg g−1) | 41.62 ± 0.236 | 36.86 ± 0.248 | 32.24 ± 0.312 | |
| R2 | 0.998 | 0.998 | 0.996 | |
| Iprodione | ||||
| B–A | KBA (dm3 mg−1 min−1) | 0.060 ± 0.001 | 0.123 ± 0.001 | 0.201 ± 0.002 |
| N0 (mg dm−3) | 89.81 ± 0.293 | 80.06 ± 0.172 | 70.51 ± 0.148 | |
| R2 | 0.998 | 0.999 | 0.999 | |
| Y–N | KYN (min−1) | 0.594 ± 0.012 | 0.603 ± 0.007 | 0.657 ± 0.007 |
| θ (min) | 11.80 ± 0.038 | 10.52 ± 0.023 | 9.266 ± 0.020 | |
| R2 | 0.998 | 0.999 | 0.999 | |
| Thomas | KTH (dm3 mg−1 min−1) | 0.121 ± 0.002 | 0.123 ± 0.001 | 0.134 ± 0.001 |
| q0 (mg g−1) | 89.73 ± 0.292 | 80.00 ± 0.172 | 70.45 ± 0.148 | |
| R2 | 0.998 | 0.999 | 0.999 | |
| Sample | Mechanical Properties | DMA Properties | |||
|---|---|---|---|---|---|
| Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) | Tg (°C) | Tan δ Peak | |
| UPe | 22.48 | 0.79 | 612.4 | 97.70 | 0.710 |
| UPe/exEGS 2.5 wt.% | 23.15 | 0.83 | 668.1 | 98.62 | 0.711 |
| UPe/exEGS 5.0 wt.% | 25.94 | 0.97 | 712.4 | 98.63 | 0.712 |
| UPe/exEGS 7.5 wt.% | 28.31 | 1.11 | 796.9 | 98.65 | 0.724 |
| UPe/exEGS 10 wt.% | 27.83 | 1.04 | 765.0 | 98.64 | 0.721 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almazoug, A.A.; Mijatov, S.; Vuksanović, M.M.; Milosavljević, M.; Mohammed, A.J.M.; Milošević, M.D.; Marinković, A.; Bartula, M. Sustainable Solutions for Pollutants Removal with a Hybrid Multifunctional Adsorbent Based on Recycled Expanded Glass. Appl. Sci. 2025, 15, 3093. https://doi.org/10.3390/app15063093
Almazoug AA, Mijatov S, Vuksanović MM, Milosavljević M, Mohammed AJM, Milošević MD, Marinković A, Bartula M. Sustainable Solutions for Pollutants Removal with a Hybrid Multifunctional Adsorbent Based on Recycled Expanded Glass. Applied Sciences. 2025; 15(6):3093. https://doi.org/10.3390/app15063093
Chicago/Turabian StyleAlmazoug, Ali Abdussalam, Slavko Mijatov, Marija M. Vuksanović, Milutin Milosavljević, Asifa Jasim Mohammed Mohammed, Milena D. Milošević, Aleksandar Marinković, and Mirjana Bartula. 2025. "Sustainable Solutions for Pollutants Removal with a Hybrid Multifunctional Adsorbent Based on Recycled Expanded Glass" Applied Sciences 15, no. 6: 3093. https://doi.org/10.3390/app15063093
APA StyleAlmazoug, A. A., Mijatov, S., Vuksanović, M. M., Milosavljević, M., Mohammed, A. J. M., Milošević, M. D., Marinković, A., & Bartula, M. (2025). Sustainable Solutions for Pollutants Removal with a Hybrid Multifunctional Adsorbent Based on Recycled Expanded Glass. Applied Sciences, 15(6), 3093. https://doi.org/10.3390/app15063093







