Sow and Piglet Behavior Characterization Using Visual Observation, Sensor Detection, and Video Recording
Abstract
1. Introduction
2. Materials and Methods
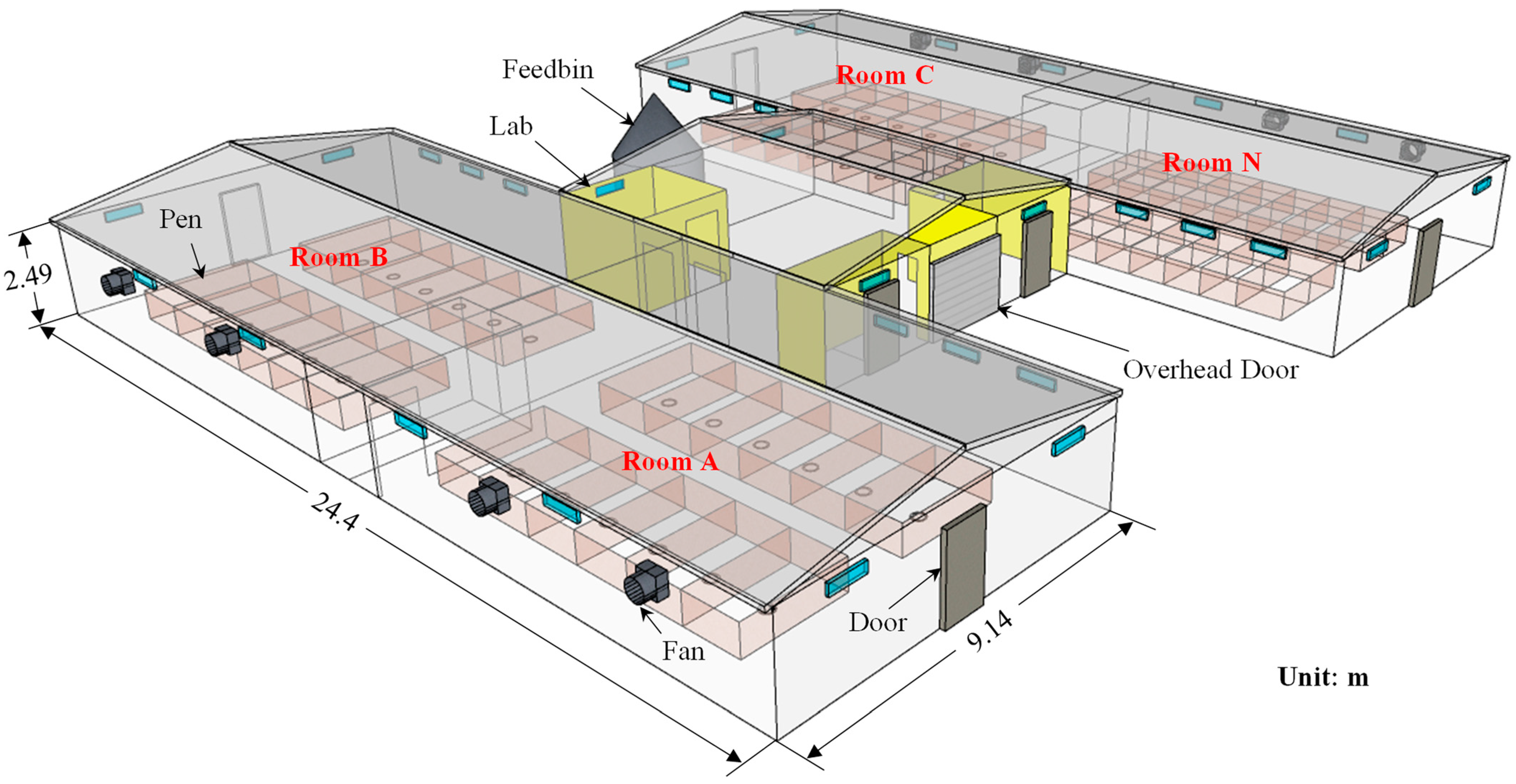
2.1. Pig Research Building
2.1.1. Layout and Ventilation of the Building
2.1.2. Environment Monitoring
2.2. Heat Stress and Floor Cooling Study
2.3. Pig Behavior Monitoring
2.3.1. Visual Behavior Observation
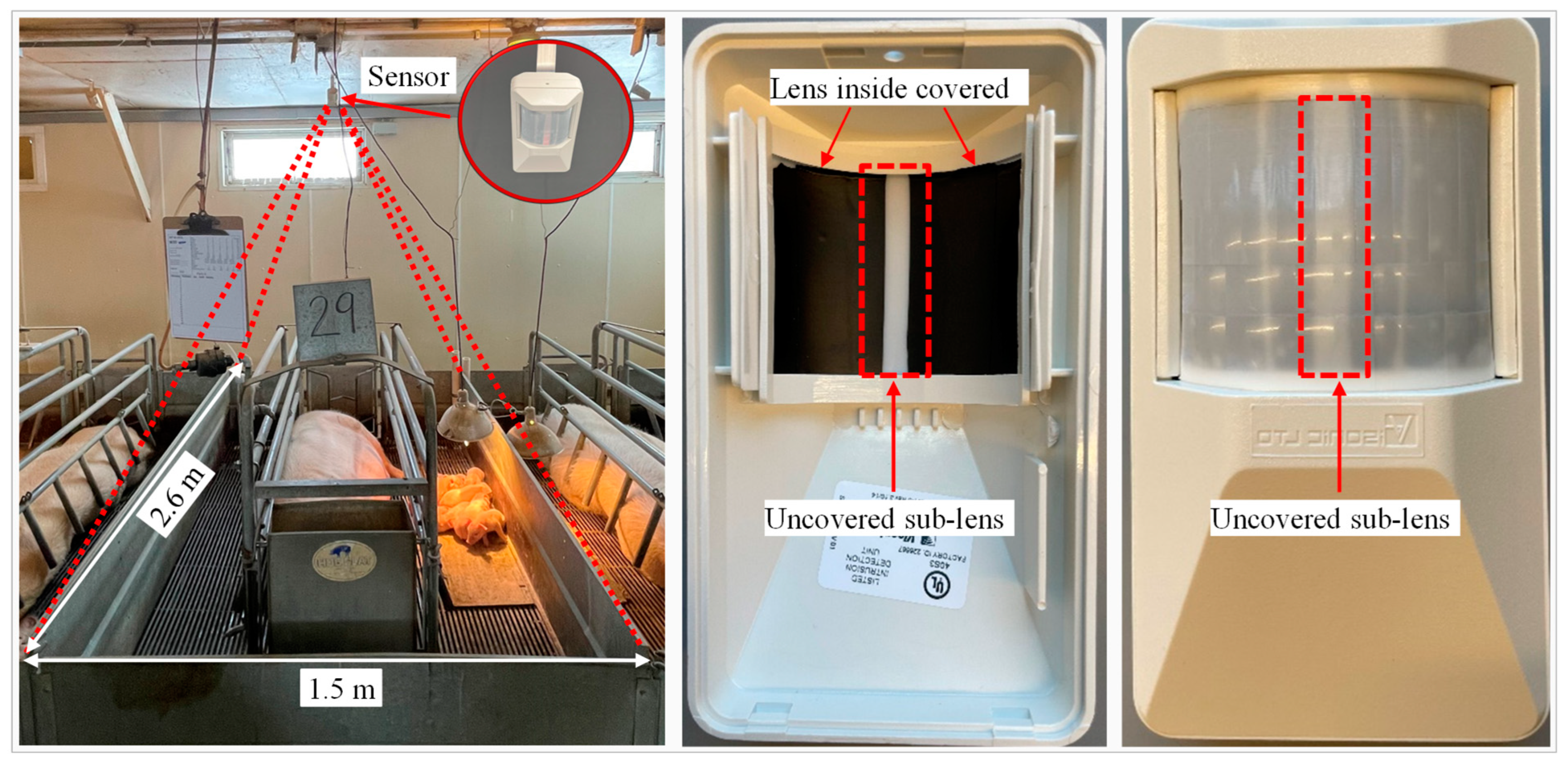
2.3.2. Sensor Activity Detection
2.3.3. Video Monitoring
2.4. Data Processing and Analysis
2.4.1. Processing and Analysis of Observational and Environmental Data
2.4.2. Processing and Analysis of Sensor Data
2.4.3. Comparison of Video Recording and Sensor Detection
3. Results
3.1. Three Different Pig Behavior Monitoring Methods
3.2. Pig Behaviors from Direct Visual Observation
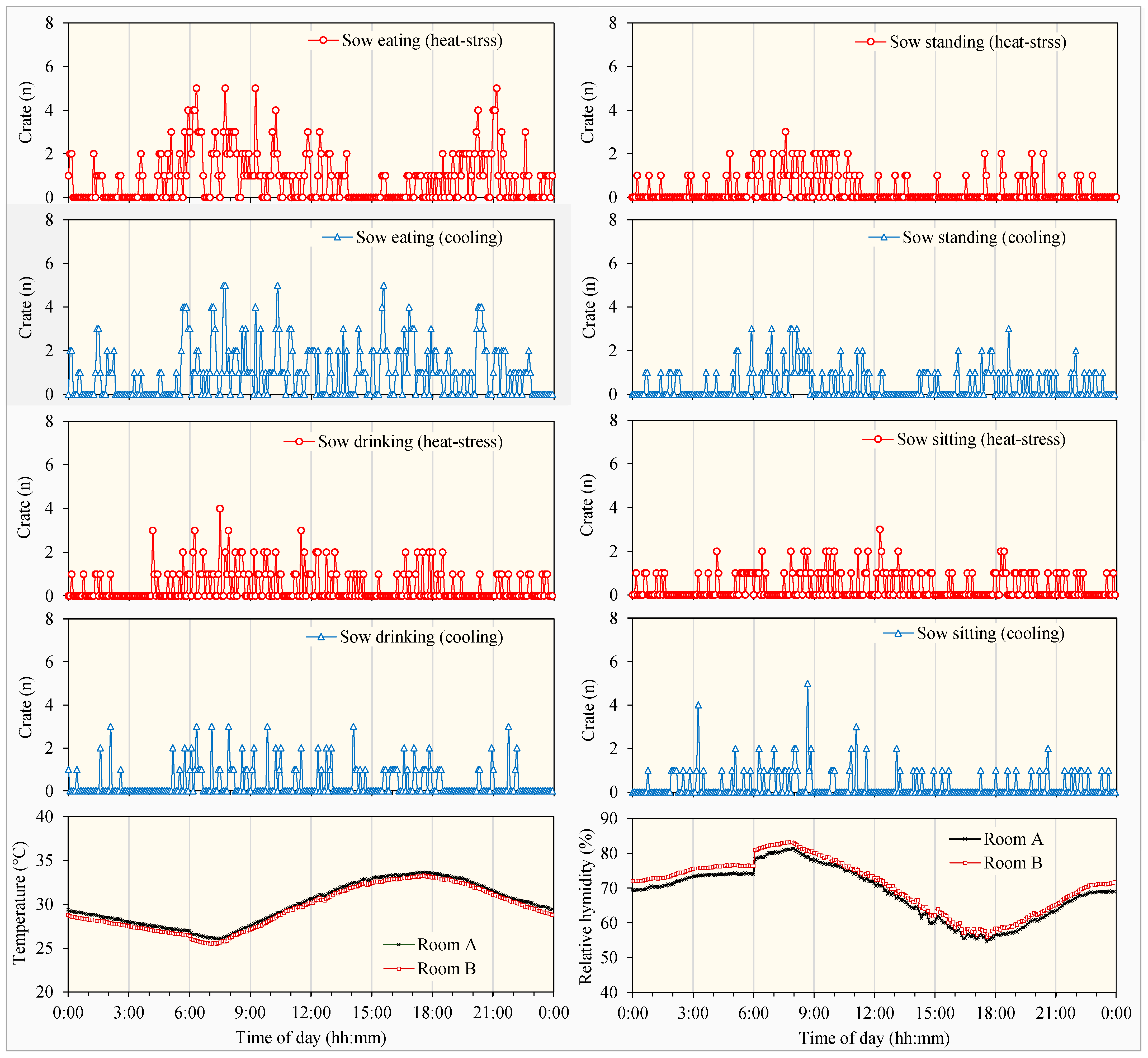
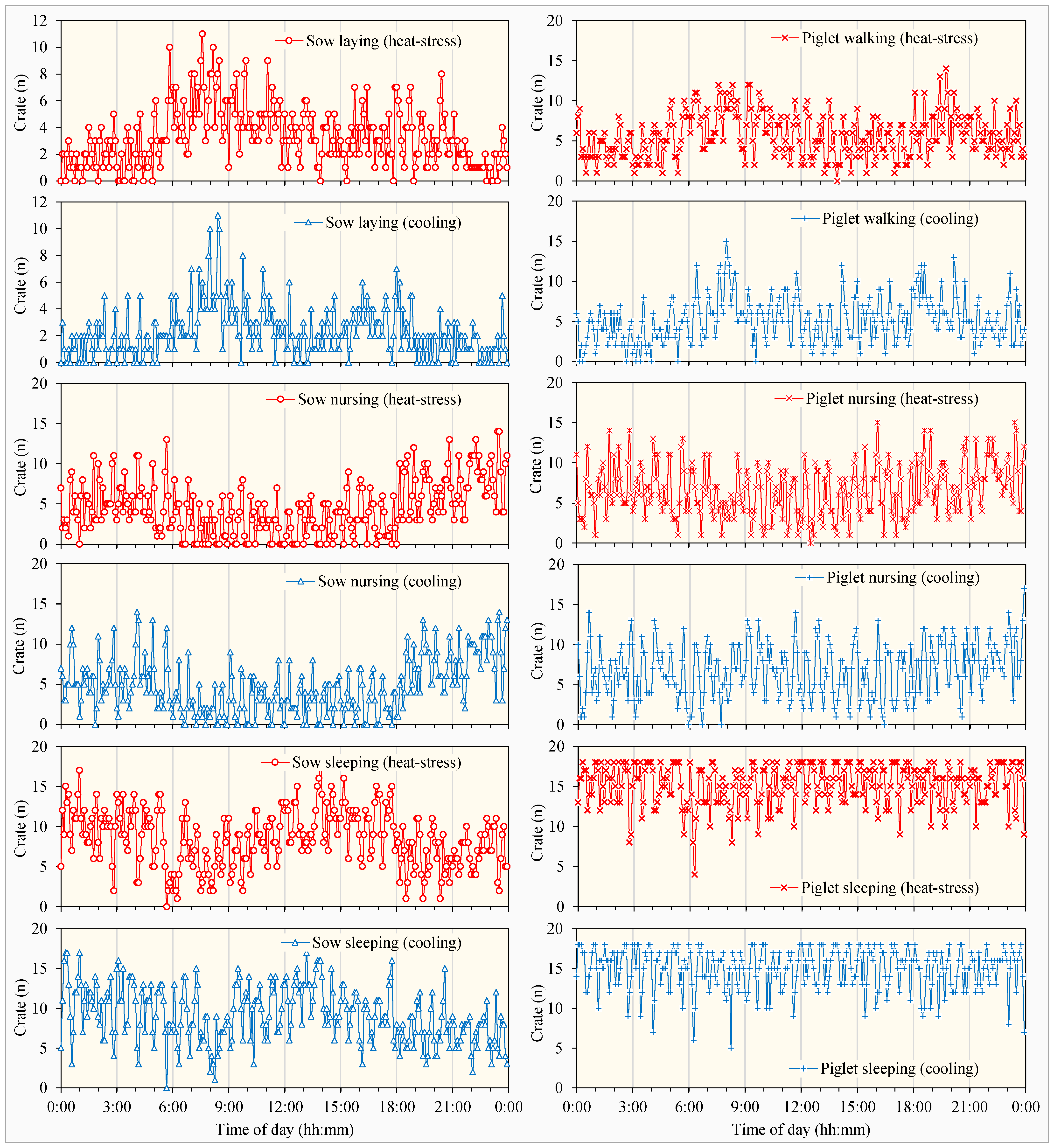
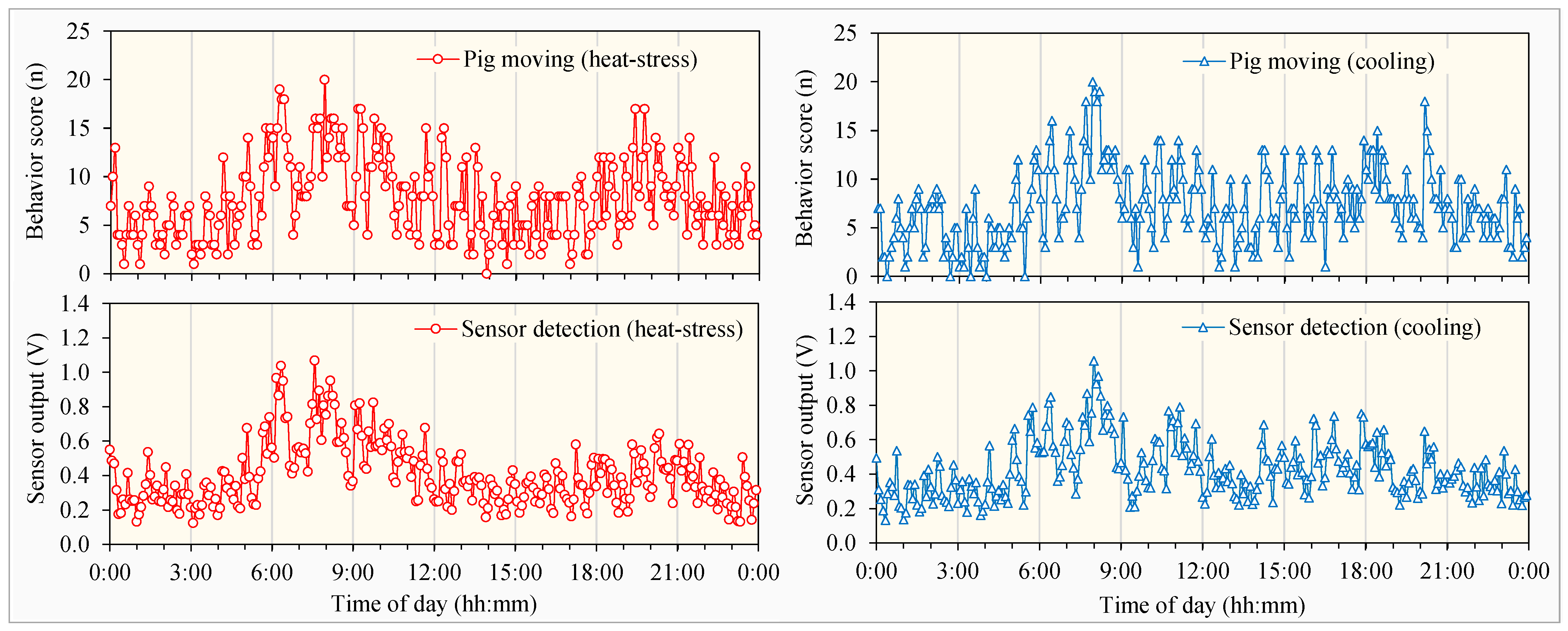
3.2.1. Circadian Rhythms in Pig Behaviors
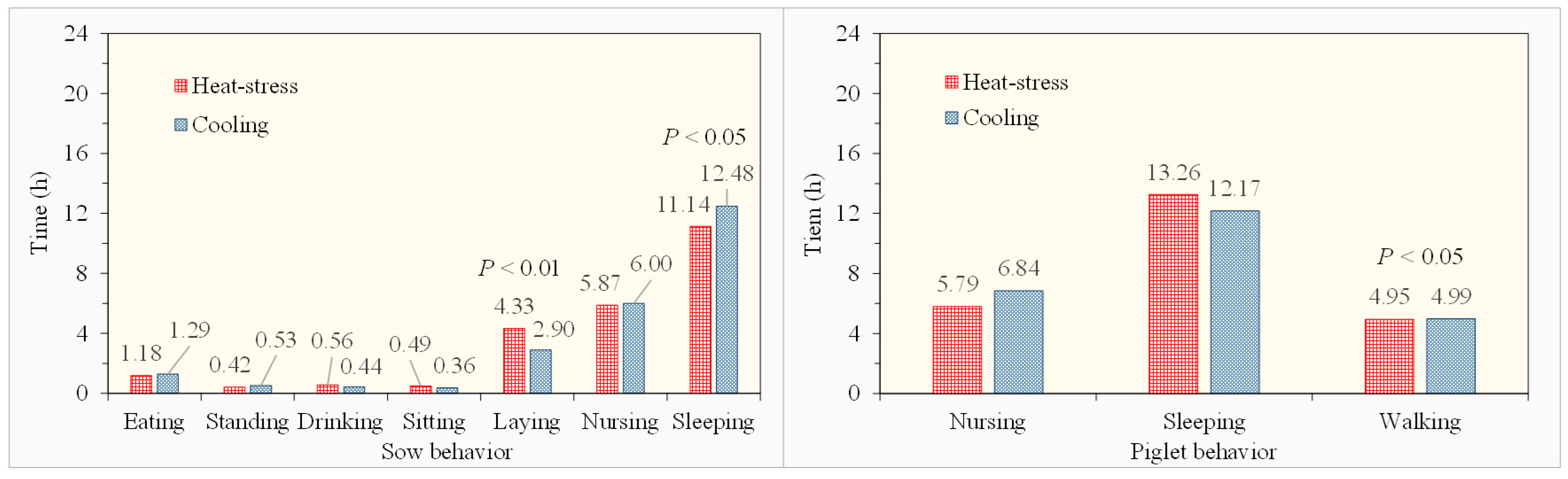
3.2.2. Behavioral Differences in Pigs Under Heat Stress and Cooling Treatments
3.3. Pig Behaviors as Detected by Sensors
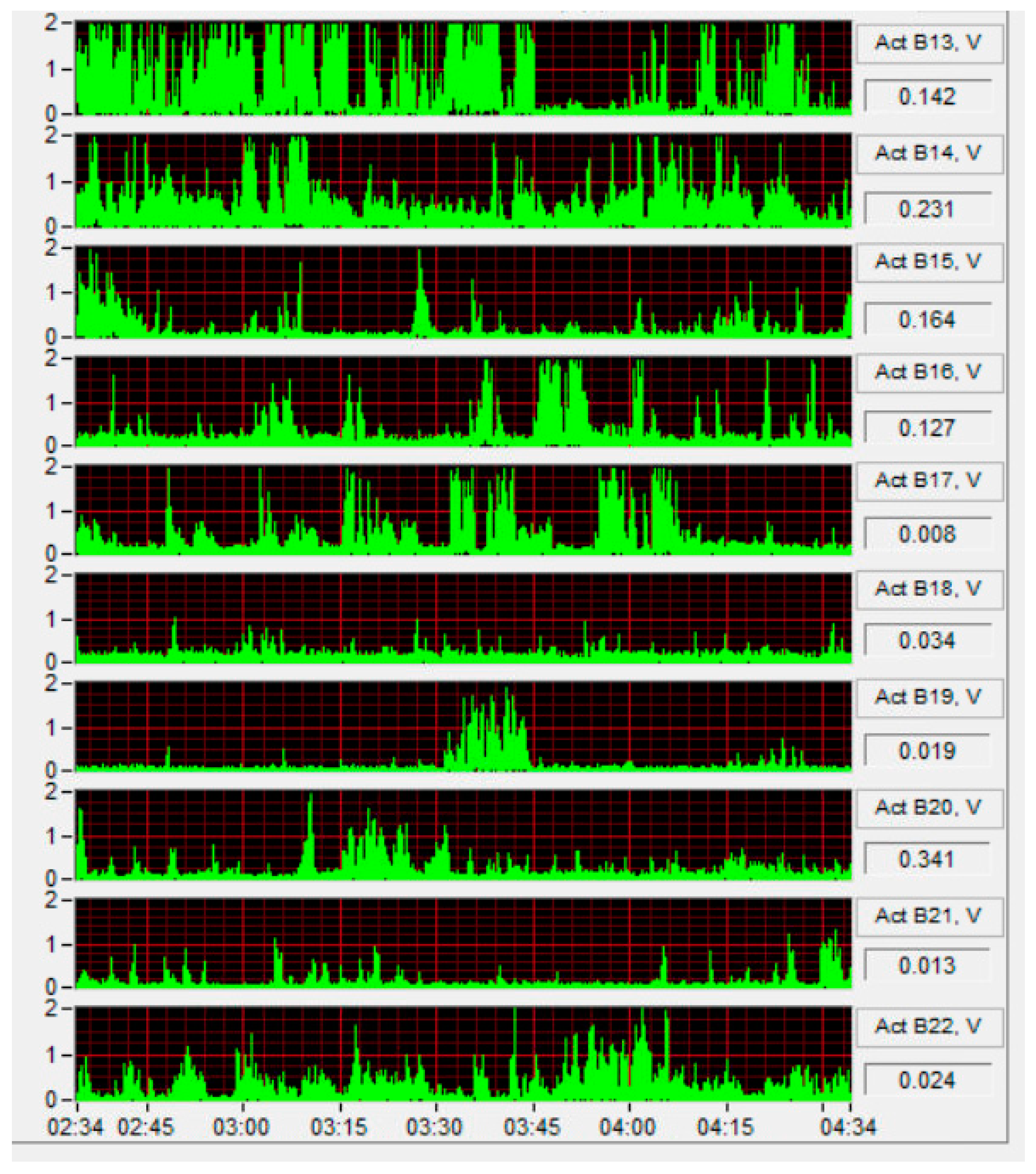
3.3.1. Sensor Detection Characteristics
3.3.2. Correlation Between Sensor Outputs and Pig Behaviors
3.4. Insights from the Video Monitoring of Pig Behaviors
3.4.1. Dynamic Behaviors of Sows and Piglets
3.4.2. Video Monitoring and Sensor Detection of Pig Behaviors
4. Conclusions
- (1)
- Sows and piglets showed more circadian rhythm variations in some behaviors than the others. Their active time distributions for different behaviors also varied between natural heat stress and floor cooling treatments.
- (2)
- The sensor outputs correlated positively with certain pig behaviors and negatively with others. The highest correlations were with the pig moving behaviors, showing correlation coefficients of 0.81 and 0.74 with heat stress and cooling treatments, respectively.
- (3)
- Video recording analysis confirmed that the sensor could quickly and sensitively detect changes in sow and piglet activities and even capture the movements of small body parts.
- (4)
- The pens near the room doors had a 21% higher sensor output and higher pig moving scores, demonstrating possible effects of the workers’ activities on the pigs’ behaviors.
- (5)
- Visual observation, sensor detection, and video recording methods for pig behavior monitoring each present their own set of advantages and limitations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. World Population Prospects 2022: Summary of Results; UN DESA/POP/2022/TR/NO. 3; United Nations, Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2022; ISBN 978-92-1-148373-4.
- OECD-FAO. OECD-FAO Agricultural Outlook 2023–2032; OECD Publishing: Paris, France, 2023. [Google Scholar] [CrossRef]
- Ni, J.-Q.; Erasmus, M.A.; Croney, C.C.; Li, C.; Li, Y. A critical review of advancement in scientific research on food animal welfare-related air pollution. J. Hazard. Mater. 2021, 408, 124468. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, W.; Le, H.H.; Cottrell, J.J.; Green, M.P.; Leury, B.J.; Dunshea, F.R.; Bell, A.W. Review: What have we learned about the effects of heat stress on the pig industry? Animal 2022, 16, 13. [Google Scholar] [CrossRef]
- Johnson, J.S.; Stewart, K.R.; Safranski, T.J.; Ross, J.W.; Baumgard, L.H. In utero heat stress alters postnatal phenotypes in swine. Theriogenology 2020, 154, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.T.; Zhang, H.; He, Y.X.; Liu, T.H. A review in the automatic detection of pigs behavior with sensors. J. Sens. 2022, 2022, 17. [Google Scholar] [CrossRef]
- Liu, L.S.; Tai, M.; Yao, W.; Zhao, R.Q.; Shen, M.X. Effects of heat stress on posture transitions and reproductive performance of primiparous sows during late gestation. J. Therm. Biol. 2021, 96, 8. [Google Scholar] [CrossRef]
- Botermans, J.; Andersson, M. Growing-finishing pigs in an uninsulated house. 2. Pen function and thermal comfort. Swed. J. Agric. Res. 1995, 25, 83–92. [Google Scholar]
- Yang, Q.M.; Xiao, D.Q. A review of video-based pig behavior recognition. Appl. Anim. Behav. Sci. 2020, 233, 8. [Google Scholar] [CrossRef]
- Oczak, M.; Maschat, K.; Baumgartner, J. Dynamics of sows’ activity housed in farrowing pens with possibility of temporary crating might indicate the time when sows should be confined in a crate before the onset of farrowing. Animals 2020, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.Q.; Huang, H.S.; Zheng, B.; Li, S.M.; Gan, H.M.; Chen, C.X.; Yang, X.F.; Xue, Y.J. An automatic recognition framework for sow daily behaviours based on motion and image analyses. Biosyst. Eng. 2020, 192, 56–71. [Google Scholar] [CrossRef]
- Oliviero, C.; Pastell, M.; Heinonen, M.; Heikkonen, J.; Valros, A.; Ahokas, J.; Vainio, O.; Peltoniemi, O.A.T. Using movement sensors to detect the onset of farrowing. Biosyst. Eng. 2008, 100, 281–285. [Google Scholar] [CrossRef]
- Smulders, D.; Verbeke, G.; Mormède, P.; Geers, R. Validation of a behavioral observation tool to assess pig welfare. Physiol. Behav. 2006, 89, 438–447. [Google Scholar] [CrossRef]
- Pedersen, S.; Takai, H. Animal activity monitored by PID technique. In Proceedings of the AgEng '94 Conference on Agricultural Engineering, Milan, Italy, 29 August–1 September 1994. [Google Scholar]
- Pedersen, S.; Pedersen, C.B. Animal activity measured by infrared detectors. J. Agric. Eng. Res. 1995, 61, 239–246. [Google Scholar] [CrossRef]
- Ni, J.-Q.; Heber, A.J. An on-site computer system for comprehensive agricultural air quality research. Comput. Electron. Agr. 2010, 71, 38–49. [Google Scholar] [CrossRef]
- Ni, J.-Q.; Liu, S.; Radcliffe, J.S.; Vonderohe, C. Evaluation and characterization of passive infrared detectors to monitor pig activities in animal environmental research. Biosyst. Eng. 2017, 158, 86–94. [Google Scholar] [CrossRef]
- Besteiro, R.; Rodriguez, M.R.; Fernandez, M.D.; Ortega, J.A.; Velo, R. Agreement between passive infrared detector measurements and human observations of animal activity. Livest. Sci. 2018, 214, 219–224. [Google Scholar] [CrossRef]
- Von Jasmund, N.; Wellnitz, A.; Krommweh, M.S.; Büscher, W. Using passive infrared detectors to record group activity and activity in certain focus areas in fattening pigs. Animals 2020, 10, 792. [Google Scholar] [CrossRef]
- Besteiro, R.; Arango, T.; Ortega, J.; Fernandez, M.D.; Rodriguez, M.R. Long-term measurement of piglet activity using passive infrared detectors. Animals 2021, 11, 1607. [Google Scholar] [CrossRef]
- Drexl, V.; Siebler, D.; Dittrich, I.; Heins, R.; Diers, S.; Krieter, J. Use of a digital passive infrared motion detector in piglet rearing for the identification of animal activity. Smart Agric. Technol. 2023, 4, 100228. [Google Scholar] [CrossRef]
- Freson, L.; Godrie, S.; Bos, N.; Jourquin, J.; Geers, R. Validation of an infra-red sensor for oestrus detection of individually housed sows. Comput. Electron. Agr. 1998, 20, 21–29. [Google Scholar] [CrossRef]
- Kim, J.H.; Ni, J.-Q.; Stwalley, R.; Schinckel, A.P.; Marchant, J.N.; Johnson, J.S. A comprehensive microenvironment monitoring system for studying the productivity and welfare of sows and piglets. In Proceedings of the International Symposium on Animal Environment and Welfare, Chongqing, China, 20–23 October 2023; p. 8. [Google Scholar]
- Ogundare, W.; Teeple, K.; Fisher, E.; Davis, C.; Reis, L.G.; Jannasch, A.; Beckett, L.M.; Schinckel, A.; Minor, R.; Casey, T. Cooling lactating sows exposed to early summer heat wave alters circadian patterns of behavior and rhythms of respiration, rectal temperature, and saliva melatonin. PLoS ONE 2024, 19, e0310787. [Google Scholar] [CrossRef]
- Johnson, J.S.; Jansen, T.L.; Galvin, M.; Field, T.C.; Graham, J.R.; Stwalley, R.M.; Schinckel, A.P. Electronically controlled cooling pads can improve litter growth performance and indirect measures of milk production in heat-stressed lactating sows. J. Anim. Sci. 2022, 100, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, J.; Zhang, B.; Dai, S.; Shen, M. Visual detection on posture transformation characteristics of sows in late gestation based on Libra R-CNN. Biosyst. Eng. 2022, 223, 219–231. [Google Scholar] [CrossRef]
- Morello, G.M.; Lay, D.C.; Rodrigues, L.H.A.; Richert, B.T.; Marchant-Forde, J.N. Microenvironments in swine farrowing rooms: The thermal, lighting, and acoustic environments of sows and piglets. Scientia Agricola 2018, 75, 1–11. [Google Scholar] [CrossRef]

| Behavior | Sow * | Piglets ** |
|---|---|---|
| Eating | Head in the feeder and munching | NA |
| Standing | Erect on all four limbs (drinking) | NA |
| Drinking | Actively gulping water with the mouth through the water nozzle (sitting) | NA |
| Siting | Dog-sitting position with the hind limbs lateral to the floor (nursing) | NA |
| Lying | Flat on the floor, either on the side or the abdomen (nursing) | NA |
| Nursing | All-to-most piglets on the teats, actively suckling (sleeping) | ≥1 suckling |
| Sleeping | Lying with the eyes closed (lying or nursing) | ≥1 lying with the eyes closed |
| Walking | NA | ≥1 moving on all four limbs |
| Visual Observation | Sensor Detection | Video Recording | |
|---|---|---|---|
| Behavior distinction | Yes | No | Yes |
| Equipment requirement | No | Yes | Yes |
| Data collection method | Human observation | Sensor detection | Video shooting |
| Data collection mode | Discrete | Continuous | Continuous |
| Data collection frequency | Every 5 mins | 1 Hz | 20 frames per sec |
| Data recording | Notepad | Computer | Computer |
| Data type | Binary data | Analog data | Sequence of images |
| Response time | A couple of secs | ~1 s | <1 s |
| Researcher involvement | High | Low | Low |
| Invasiveness | Possible | No | No |
| Data storage requirement | Low | Medium | High |
| Data processing requirement | High | Low | High |
| Heat Stress | Cooling | |
|---|---|---|
| Individual behavior | ||
| Sow eating | 0.61 | 0.37 |
| Sow standing | 0.47 | 0.41 |
| Sow drinking | 0.38 | 0.27 |
| Sow sitting | 0.31 | 0.23 |
| Sow lying | 0.53 | 0.49 |
| Sow nursing | −0.10 | −0.14 |
| Sow sleeping | −0.61 | −0.50 |
| Piglet nursing | 0.05 | 0.12 |
| Piglet sleeping | −0.42 | −0.34 |
| Piglet walking | 0.69 | 0.64 |
| Behavior combination | ||
| Sow on-feet | 0.77 | 0.59 |
| Sow on-body | −0.77 | −0.56 |
| Piglet moving | 0.47 | 0.38 |
| Pig moving | 0.81 | 0.74 |
| Pig moving and lying | 0.75 | 0.70 |
| Sow on-feet and lying | 0.77 | 0.61 |
| Sow and piglet sleeping | −0.59 | −0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Ni, J.-Q.; Ogundare, W.; Schinckel, A.P.; Minor, R.C.; Johnson, J.S.; Casey, T.M. Sow and Piglet Behavior Characterization Using Visual Observation, Sensor Detection, and Video Recording. Appl. Sci. 2025, 15, 3018. https://doi.org/10.3390/app15063018
Kim JH, Ni J-Q, Ogundare W, Schinckel AP, Minor RC, Johnson JS, Casey TM. Sow and Piglet Behavior Characterization Using Visual Observation, Sensor Detection, and Video Recording. Applied Sciences. 2025; 15(6):3018. https://doi.org/10.3390/app15063018
Chicago/Turabian StyleKim, Jun Ho, Ji-Qin Ni, Wonders Ogundare, Allan P. Schinckel, Radiah C. Minor, Jay S. Johnson, and Theresa M. Casey. 2025. "Sow and Piglet Behavior Characterization Using Visual Observation, Sensor Detection, and Video Recording" Applied Sciences 15, no. 6: 3018. https://doi.org/10.3390/app15063018
APA StyleKim, J. H., Ni, J.-Q., Ogundare, W., Schinckel, A. P., Minor, R. C., Johnson, J. S., & Casey, T. M. (2025). Sow and Piglet Behavior Characterization Using Visual Observation, Sensor Detection, and Video Recording. Applied Sciences, 15(6), 3018. https://doi.org/10.3390/app15063018






