1. Introduction
Primary squamous cell carcinoma (SCC) of the temporal bone is an exceedingly rare malignancy, constituting only about 0.2% of all head and neck cancers. This anatomical site presents unique challenges for diagnosis and management due to its proximity to critical structures such as the cochlea, vestibular system, and the facial nerve. No universally adopted staging system for temporal bone tumors exists, complicating prognostication and treatment decisions. This study assesses the clinical utility of a previously proposed staging system (the Pittsburgh system) in patients with primary temporal bone SCC [
1]. Additionally, the study seeks to identify prognostic factors linked to both patient and tumor characteristics, evaluate clinical outcomes, and share the institutional experience with this rare and aggressive malignancy [
1].
In the literature currently available, some authors have proposed various staging systems, reviews, and case series of patients. The main issues in developing a unique classification and staging system are, in addition to the rarity of the tumor and the narrowness in the number of patients, the use of non-standardized surgical nomenclature in the case studies, which prevents a comprehensive analysis and comparison of outcomes, the wide variability in histological type of the neoplasm, which creates a disparity in classification criteria, and the difficulty in assessing the extent, subsite of origin, and spread vectors of the tumor in an anatomical site where several structures coexist in very narrow spaces, with complex anatomical relationships [
2].
Imaging techniques have revolutionized the diagnostic and therapeutic approach to this pathology. In the early decades of the 20th century, conventional radiography (X-ray) was the only available tool. Still, it offered limited visualization of the intricate structures of the temporalis, allowing only for the recognition of advanced bony changes. As technology has progressed, the introduction of methods such as ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), and, more recently, positron emission tomography (PET) has significantly expanded diagnostic capabilities, allowing for more detailed analysis of both bone components and surrounded soft tissues [
2].
Among these methods, multidetector CT stands out for its high resolution in evaluating bone structures and detecting post-treatment bone destruction or necrosis. At the same time, MRI offers an excellent definition of soft tissues, allowing tumor extension into adjacent tissues and neurovascular structures to be characterized. The addition of molecular imaging techniques, such as PET-CT, allows not only morphologic assessment but also functional and metabolic analysis of tumors, making it an indispensable tool for assessing recurrence and metastasis [
3].
Some studies have highlighted the superiority of CT in delineating bone erosion and structural details, while MRI excels in characterizing soft tissue involvement and identifying perineural or dural extension. Gillespie et al. demonstrated that while CT remains critical for staging advanced disease (T3/T4), MRI significantly improves diagnostic accuracy by detecting subtle soft tissue changes, particularly in early-stage tumors (T1/T2) [
4].
Despite these advances, staging the primary SCC of SUE (Superior External Ear) remains a challenge. The Pittsburgh staging system, proposed in the 1990s, is a widely accepted model for classifying the extent of disease and guiding treatment. Still, gaps in understanding therapeutic outcomes persist due to the limited number of patients and lack of prospective randomized trials [
5].
This retrospective study aims to analyze the use and accuracy of different imaging modalities in staging primary SCC of the SUE and evaluate their prognostic and therapeutic role in a group of patients treated at a referral hospital.
2. Materials and Methods
Between 2020 and 2023, a total of 2579 patients were reviewed in the Head and Neck Tumor Board at the Azienda Ospedaliera-Universitaria Policlinico Umberto I in Rome, of whom those with primary squamous cell carcinoma (SCC) of the temporal bone were selected. To be included in the study, patients had to (1) have a diagnosis of primary SCC originating from the cutaneous region of the temporal bone and (2) have undergone imaging of the temporal bone at our institution. Only patients with primary SCC of the temporal region without significant comorbidities were included in the study. A total of 15 patients who met these criteria were identified.
Patient information was obtained through a retrospective review of hospital and outpatient medical records, including discharge letters and follow-up examinations, with patient consent for study purposes. The factors assessed included demographic data, presenting symptoms, physical examination findings, radiological results, surgical treatment, histopathological findings, postoperative radiotherapy, chemotherapy, targeted therapy, and/or immunotherapy. Inclusion criteria were receiving a primary SCC (squamous cell carcinoma) diagnosis of the temporal skin region; patients must have undergone temporal imaging, and no other primary tumors.
Fifteen patients met these criteria and were included in the study.
Patients had no comorbidities or treatment of previous neoformations.
The study’s CT protocol for the temporal bone included high-resolution imaging with and without contrast in the axial plane, with a slice thickness of 1.25 mm. It reconstructed images in the coronal and sagittal planes. Iomeron, a non-ionic iodinated contrast medium with lower osmolality than ionic agents, was used to reduce the risk of side effects and adverse reactions. Both soft tissue and bone windows were used for interpretative purposes. Radiologists were blinded to pathologic findings, ensuring the objectivity of their assessments.
The MRI protocol for temporal bone imaging included axial T1-weighted (T1W) pre-contrast sequences (TE 10–20 ms; TR 400–600 ms), post-contrast T1W images in all three orthogonal planes, and an axial T2-weighted (T2W) sequence (TE 80–120 ms; TR 2000–3000 ms). Imaging was performed on a GE Discovery MR 3 Tesla scanner with a dedicated head and neck coil. Additionally, diffusion-weighted imaging (DWI) sequences (b = 0 and 1000 s/mm2) were used to assess the tumor’s characteristics, accurately evaluate its extension, and monitor treatment response. Fast spin echo (FSE) or turbo spin echo (TSE) sequences, particularly for T2W, helped reduce motion artifacts and shortened acquisition times by a factor of four. To enhance contrast and aid tumor mapping, fat-saturation (fat-sat) techniques were applied to both post-contrast T1W and T2W sequences. Gadolinium-based contrast agents (Gd) were used, specifically Gd-DOTA (Dotarem), a paramagnetic, non-specific gadolinium chelate, which accelerates the relaxation time (TR) in T1W sequences, increasing signal intensity and enhancing lesion characterization. A slice thickness of 3 mm with a gap of 0.5–1.0 mm between slices was used for all sequences.
The tumors in the 15 patients were staged using the Pittsburgh staging system for SCC of the external auditory canal (EAC), based on both preoperative CT findings and histopathological results (Table 2). Potential prognostic factors were compared for 13 patients with sufficient follow-up. Patients were considered to have no evidence of disease (NED) if they had free margins and no recurrence after at least 2 years of follow-up.
Statistical analysis was performed to assess the level of agreement between radiological and pathological staging. Cohen’s Kappa coefficient, commonly used to measure agreement between two qualitative classifications, was employed.
We used Cohen’s Kappa coefficient to assess the concordance between radiological and pathological stadia, as it is a particularly suitable statistical tool to measure the degree of agreement between two qualitative classifications, correcting for concordances that may occur by pure chance. This choice proved particularly relevant in our study, in which possible discrepancies between the two methodologies were analyzed, attributable to the following:
Limitations of imaging techniques:
Radiological images may underestimate or overestimate tumor extent due to the complexity of the anatomical structures involved and variability in the presentation of neoplasms.
Subjective interpretations: potential variations in radiologists’ assessments or in comparison with pathologic findings. The Kappa coefficient was employed to mitigate such discrepancies.
Random bias: In contrast to simple percent agreement, Cohen’s Kappa accounts for random agreement, providing a more robust measure of reliability.
Validation of diagnostic techniques:
The Kappa coefficient was used to quantify the effectiveness of radiology in predicting pathologic staging while highlighting any areas for improvement in diagnostic protocols or interpretive procedures.
The use of this method allowed for a more precise and valid assessment of concordance, providing our study with a solid foundation for statistical analysis and conclusions.
Additionally, statistical analysis was conducted to evaluate the association between various prognostic factors and clinical outcomes. Fisher’s exact test was used to identify factors significantly associated with an unfavorable clinical outcome, defined as death due to disease (DOD). A p-value < 0.05 was considered statistically significant. Analyses were performed using SPSS (Statistical Package for the Social Sciences) v29 software.
3. Results
The study group comprised 11 men and 4 women (
Table 1). Patient ages ranged from 53 to 94, with a mean age of 76. The most common presenting symptoms were otorrhea (12 patients [80%]), otalgia (9 [60%]), and hearing loss (6 [40%]). Less commonly, patients presented with facial paralysis (2 [13%]) or dizziness (1 [7%]).
Table 1.
Patient characteristics.
Table 1.
Patient characteristics.
| Variable | n (%) |
|---|
| Sex | |
| Male | 11 (73%) |
| Female | 4 (15%) |
| Symptoms | |
| Otorrhea | 12 (80%) |
| Otalgia | 9 (60%) |
| Hearing loss | 6 (40%) |
| The maximal extent of the disease | |
| EAC | 8 (53%) |
| Mastoid | 5 (33%) |
| Middle ear | 1 (7%) |
| Otic capsule | 2 (7%) |
Based on radiological interpretation, the maximal extent of the disease was found in the external auditory canal (EAC) in 8 (53%) of the 15 patients, in the mastoid in 5 (33%), in the middle ear in 1 (7%), and in the otic capsule in 1 (7%). Ten patients (66%) showed bone erosion in the EAC. Two patients (13%) had suspected cervical lymphadenopathy.
The temporal bone CT report was used to determine the preoperative stage according to the Pittsburgh staging system for the SCC of the EAC. The final stage was determined by intraoperative findings and the final pathological report (
Table 2).
The preoperative radiological staging and the final pathological staging correlated in 11 (73%) of the 15 cases. The 73% correlation between radiologic and pathologic staging is remarkable and suggests a moderate level of reliability of the imaging modalities used. However, the discrepancies observed, particularly in cases where radiologic staging underestimated or overestimated pathologic findings, warrant further analysis. Factors such as the resolution limits of imaging techniques, variability in tumor presentation, radiologists’ experience, or ambiguous tumor features (e.g., small erosions or subtle soft tissue involvement) could contribute to these discrepancies. This finding could underscore the need for improving imaging protocols and staging accuracy with grids of observations. Radiological staging underestimated the stage in two cases and overestimated it in two others (
Table 3).
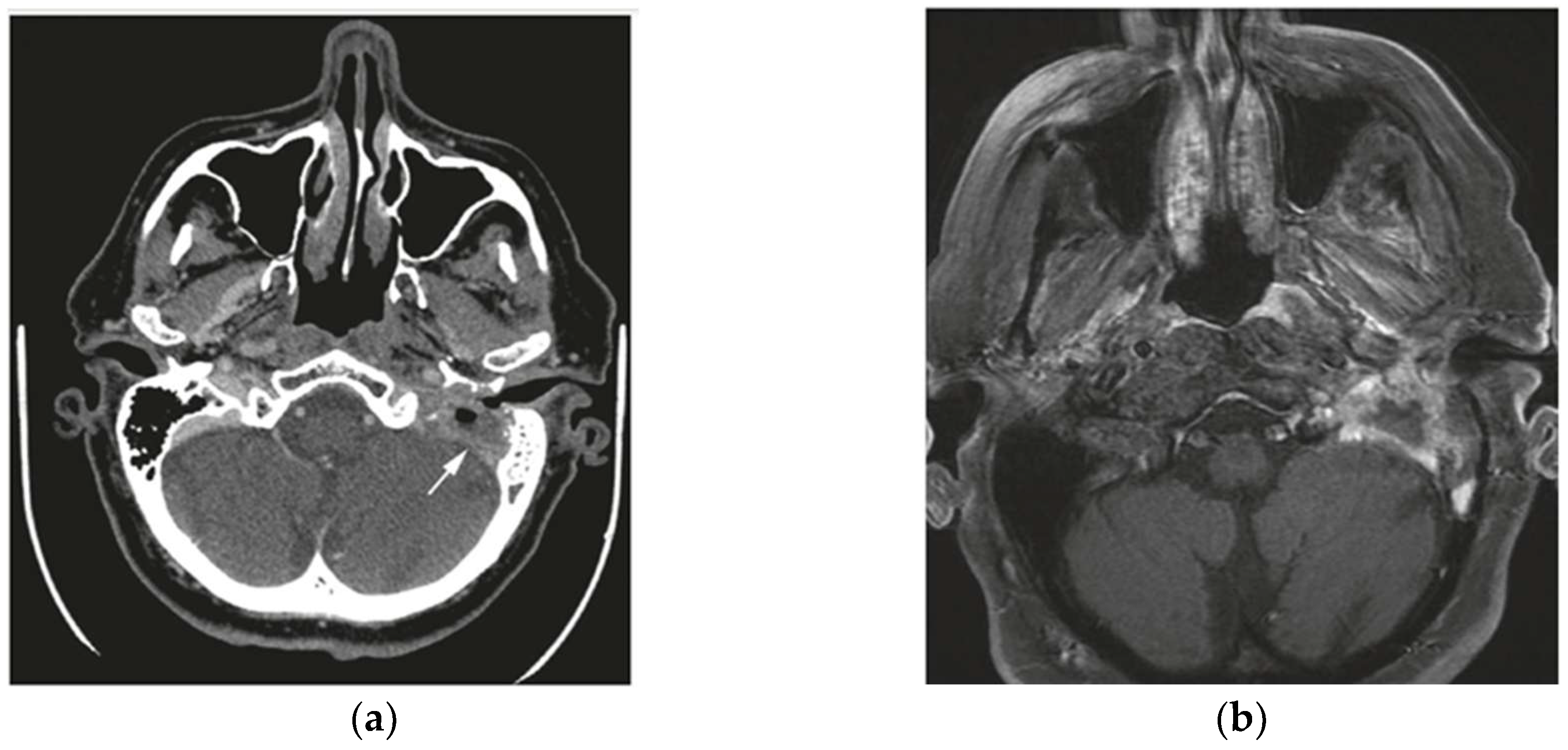
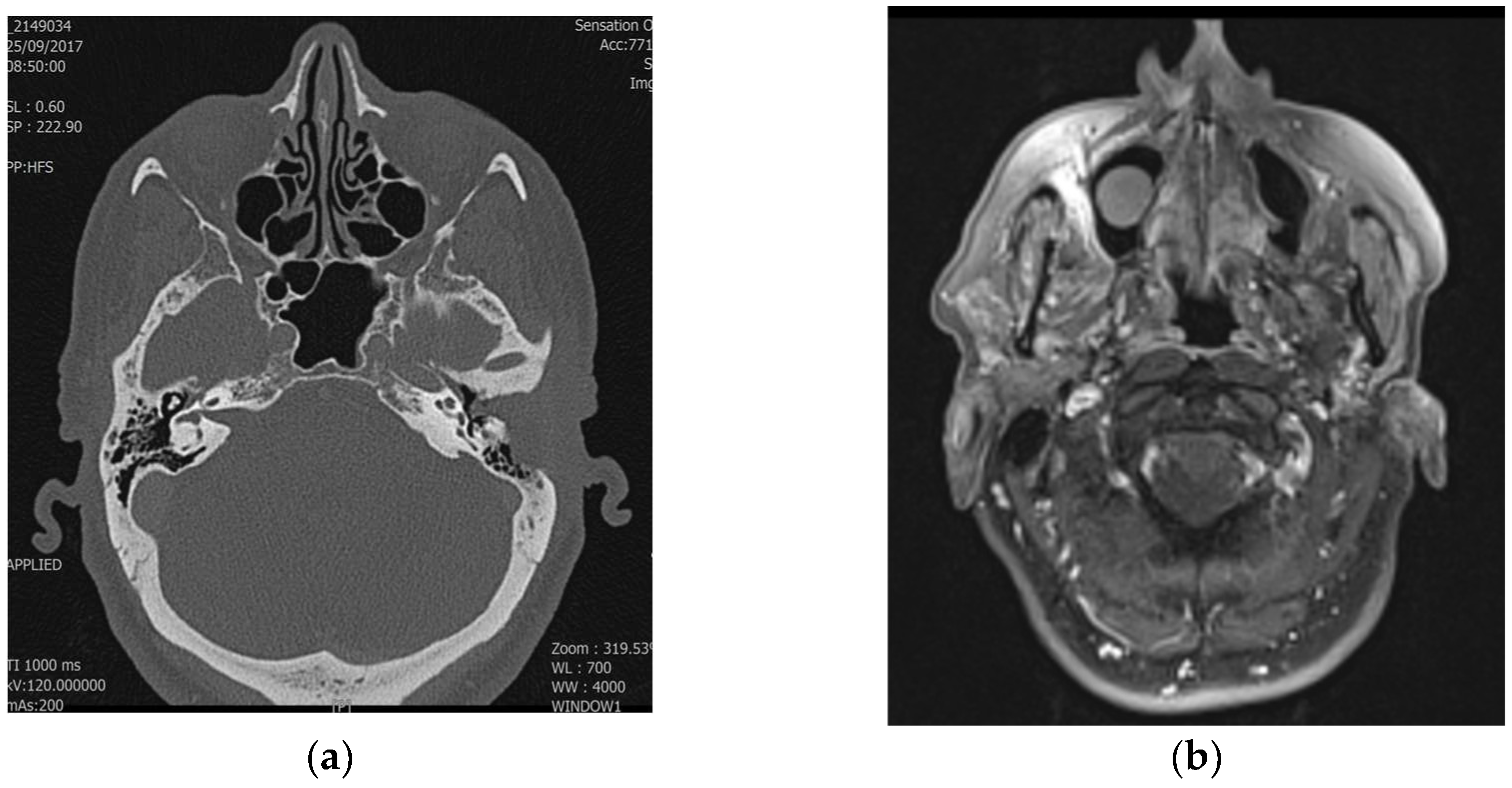
Table 3 highlights the discrepancies between radiological and pathological staging, illustrating cases where imaging underestimated or overestimated the tumor stage. These differences, seen in both T1–T2 and T3–T4 stages, emphasize the challenges in accurately determining tumor extent solely through imaging and underline the importance of multimodal evaluation to improve diagnostic precision (
Figure 1 and
Figure 2). Radiological staging correlated more frequently with pathological staging for T3 and T4 tumors compared to T1 and T2 tumors (86% vs. 63%). The Cohen’s Kappa value for evaluating the agreement between radiological and pathological staging was 0.66, indicating substantial agreement. This suggests a significant level of concordance between the two staging methods. Discrepancies may be attributed to the complexity of visualizing tumor extension through imaging or the possibility that some details were not easily identifiable with the imaging techniques. Therefore, a multimodal diagnosis, combining imaging and pathological evaluation, is emphasized to achieve accurate staging of temporal bone SCC.
Three patients with T4 disease diagnosed by CT underwent preoperative contrast-enhanced temporal bone MRI. The MRI results did not alter the preoperative staging but provided additional information on tumor extension into soft tissues. MRI showed dural enhancement in two patients and parotid involvement in one patient, which was not visible on CT. Parotid involvement was suspected in one patient but confirmed in two (18%) of the eleven parotid specimens. Radical neck dissection was performed in six (40%) cases, with two (13%) showing lymphatic spread. In both cases of cervical metastasis, preoperative CT had suggested regional disease.
A multifactorial comparison of potential prognostic factors was performed on the 13 patients who either died due to disease or showed no evidence of disease after 2 years of follow-up. The remaining 2 patients were excluded from the analysis due to insufficient follow-up. The clinical outcome of patients was largely dependent on the extent of local disease, reflected in the severity of presenting symptoms, the stage of the primary tumor, and the extent of surgical resection. Statistical analysis using Fisher’s exact test revealed that certain prognostic factors were significantly associated with an unfavorable clinical outcome. In particular, p-values < 0.05 were obtained for the T3–T4 tumor stage (p = 0.004), subtotal resection (p = 0.02), facial nerve sacrifice (p = 0.03), positive surgical margin (p = 0.02), and postoperative radiotherapy (p = 0.02), suggesting that these factors are significantly associated with an increased likelihood of death due to disease (DOD). These findings imply that factors related to tumor aggressiveness (T3–T4) and the type of intervention performed (subtotal resection, facial nerve sacrifice, postoperative radiotherapy) play a crucial role in prognosis and should be considered when identifying patients at risk of negative clinical outcomes, thus guiding therapeutic decision-making.
4. Discussion
In the present study, T1–T2 tumors showed 100% 5-year survival, while T3 and T4 tumors had survival rates of 25% and 0%, respectively. Zhang et al. found a 5-year survival of 100% for T1–T2 tumors, 69% for T3 tumors, and 20% for T4 tumors, while Arriaga et al. reported a 2-year survival of 100% for T1-T2 tumors, 50% for T3 tumors, and 15% for T4 tumors [
6,
7]. These results demonstrate the reliability of the Pittsburgh system in predicting survival based on the extent of the disease. Arriaga et al. demonstrated that preoperative CT has 98% accuracy in predicting surgical findings. However, it does not always detect spread into soft tissue through structures such as Santorini’s incisura and Huschke’s foramen. MRI suggested as an adjunct to CT, is useful in determining extension into soft tissues and improving overall assessment. The Pittsburgh system was compared with AJCC, focusing on head and neck skin tumors. While the AJCC shows significant differences only in disease-specific survival (DSS) in T3–T4 tumors, the Pittsburgh system discriminates DSS, disease-free survival (DFS), and overall survival (OS). However, the AJCC system does not consider the anatomical specificities of the EAC, limiting its applicability. Injuries extending toward the parotid gland and temporomandibular joint (TMJ) have a better prognosis than those involving the otic capsule or mastoid. Mazzoni et al. suggested dividing the T4 category into T4a: lesions in the peri-auricular soft tissues and parotid space. T4b: lesions involving the temporal and skull base [
8,
9].
A retrospective cohort analysis on patients who were treated for their primary EAC SCC pointed out that certain limitations in the predictive performance of the Pittsburgh classification system may be due to cancers classified as T4: more specifically, the Pittsburgh classification and modified PSS do not discriminate extension and invasion vectors in the different directions anterior, posterior, medial, lateral, superior, and inferior, thus creating a bias in the survival analysis and best treatment choice [
10]. Evidence suggests that tumors spreading anteriorly, in parotid space and preauricular region, are related to a notably higher DFS compared to other T4-classified tumors with other spreading vectors [
11]. A subclassification that considers the spreading direction of the T4-staged tumor was proposed by Lavieille et al. and subdivided such neoplasm’s feature into extracranial extension (T4a), intrapetrous bone and extradural extension (T4b), and meningeal or intradural extension/involvement (T4c) [
12].
Mao et al. observed that the Pittsburgh system, while effective, has limitations when applied to temporal tumors as a whole. Combining high-resolution CT and MRI allows for improved staging, especially for T1–T2 tumors, where subtle bone changes may be misinterpreted. A subdivision of T2 is proposed for T2a: soft tissue involvement of less than 0.5 mm. T2b: extensive involvement without bone erosion, potentially mediated by Santorini’s incisura or Huschke’s foramen. This integrated approach improves the diagnostic accuracy and effectiveness of the Pittsburgh system in prognosis prediction and treatment planning.
5. Conclusions
While the limited sample size is a constraint of this study, the results align with those from other studies concerning the utility of imaging modalities and the reliability of the Pittsburgh staging system. The system demonstrated a good correlation between radiological and pathological stages and clinical outcomes, highlighting its relevance in clinical practice. The combination of CT and MRI provides a comprehensive assessment of tumor extent, crucial for accurate staging and optimal treatment planning. These findings underscore the importance of multimodal imaging in managing temporal bone SCC and suggest that the Pittsburgh system offers a viable approach to staging. However, further validation in larger cohorts is warranted.