Study on the Influence of Clay Content on the Freeze–Thaw Characteristics and Mechanisms of Solidified Low-Liquid-Limit Clay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
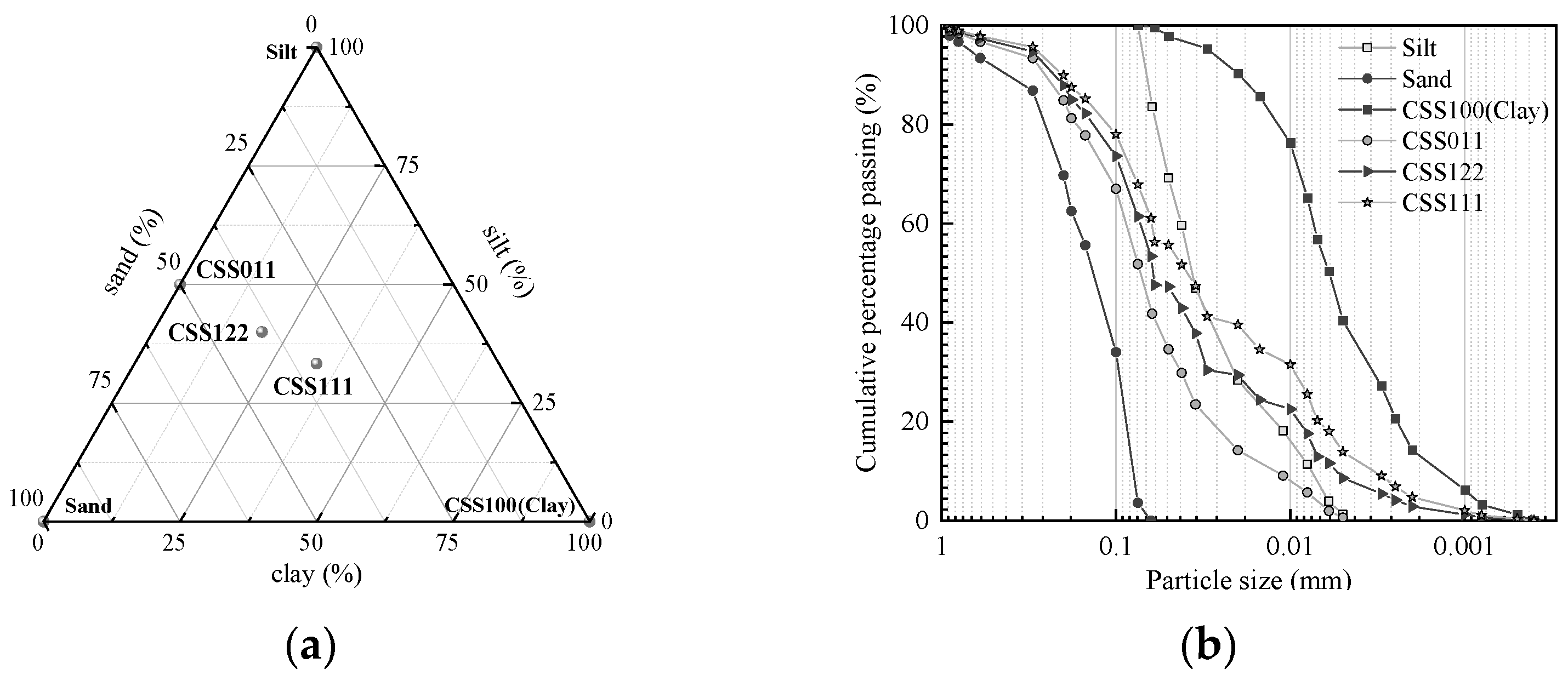
2.1.1. Test Soil
2.1.2. Binder Components and Ratios
2.2. Test Schemes and Test Methods
2.2.1. Schemes
2.2.2. Methods
- Sample preparation methods;
- 2.
- Unconfined compressive strength test
- 3.
- Microscopic examination
3. Clay Content on Freeze–Thaw Characteristics of Solidified Soils
3.1. Mass Loss Rate
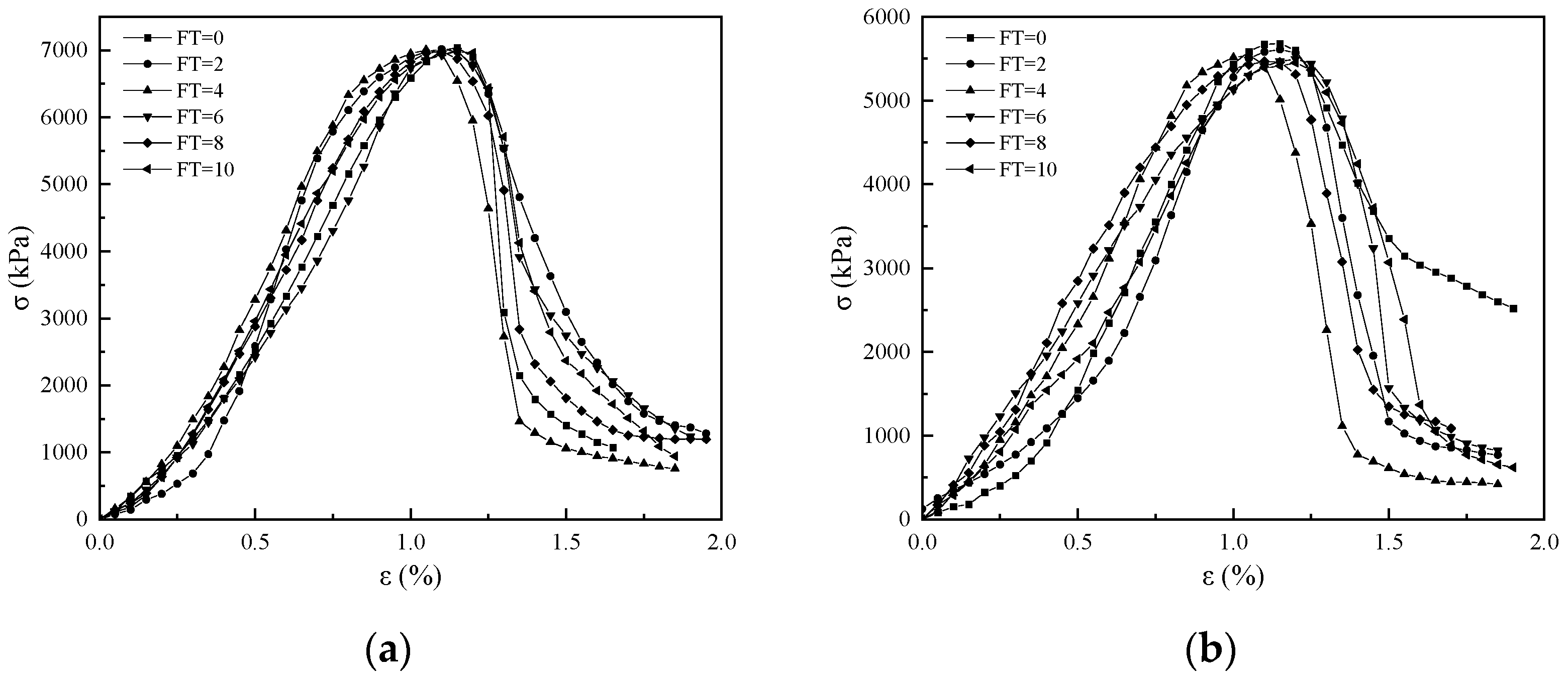
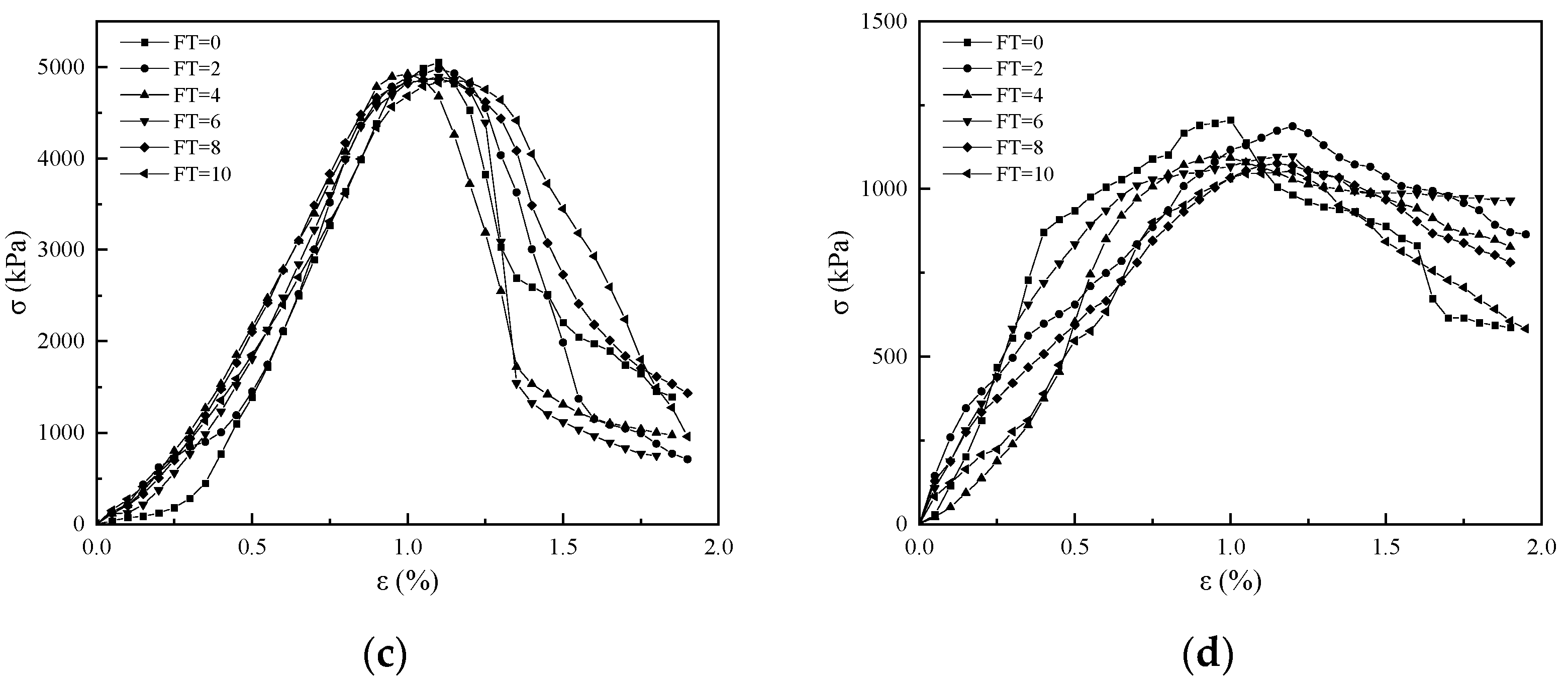
3.2. Stress–Strain Curves
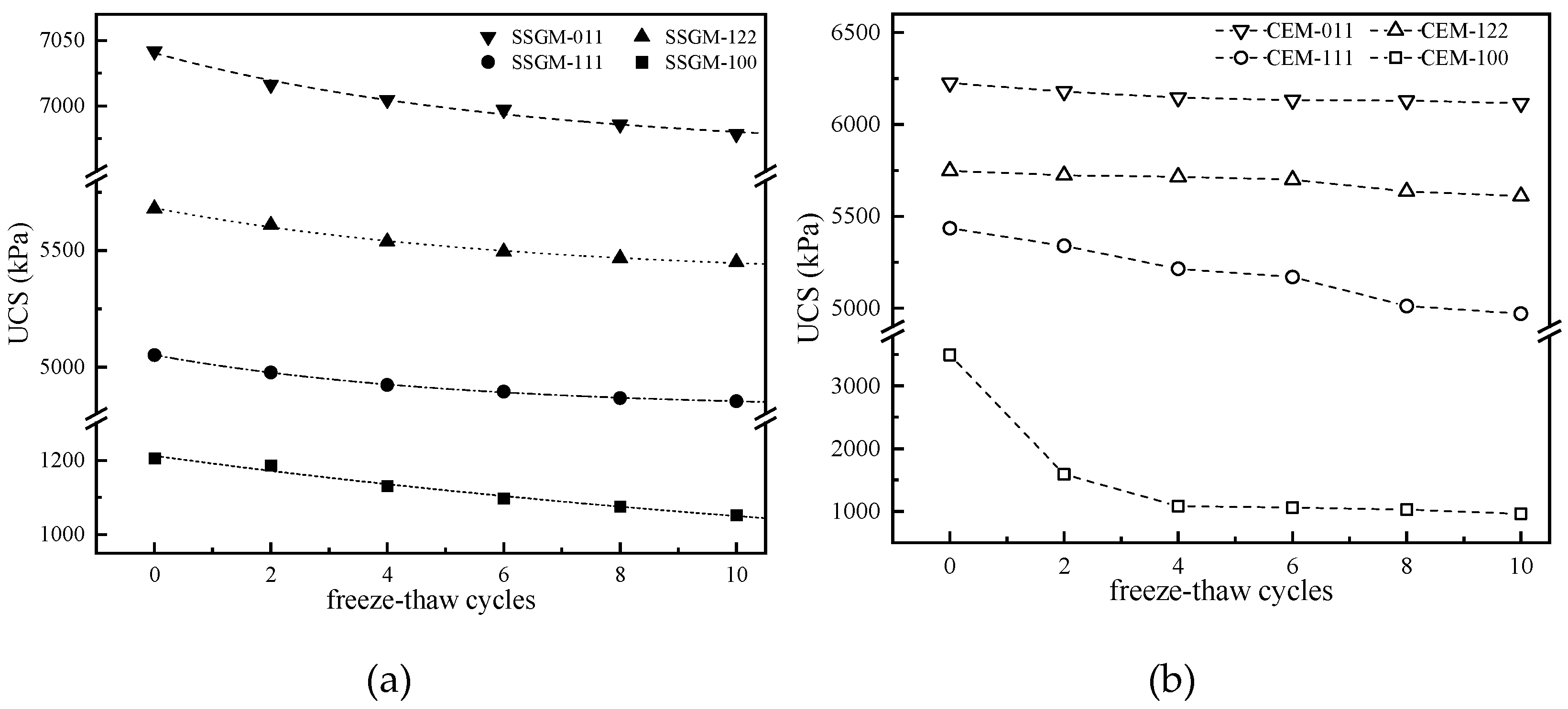
3.3. Strength Loss Rate
3.4. Variation in Deformation Modulus
3.5. Microscopic Properties
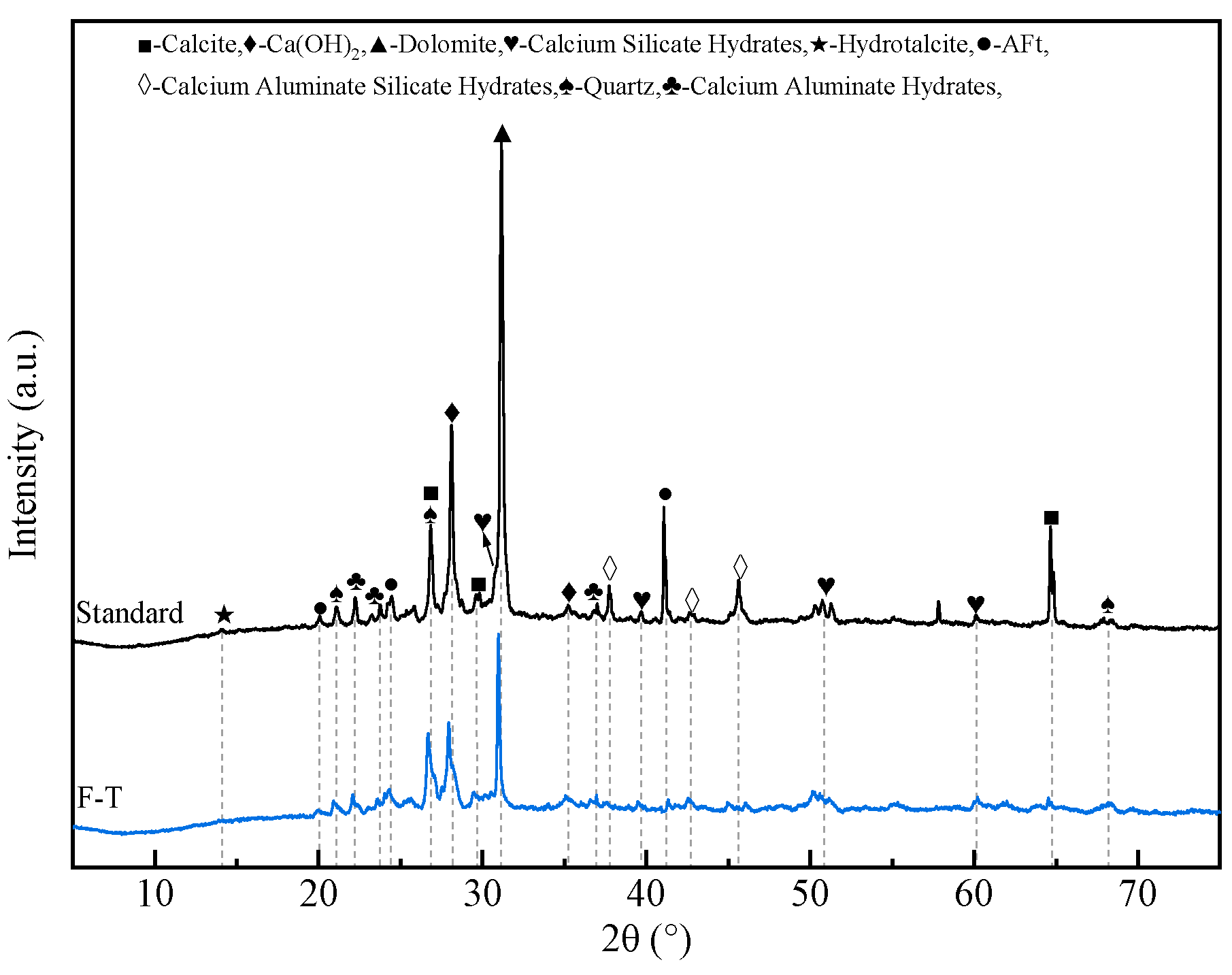
3.5.1. XRD Analysis
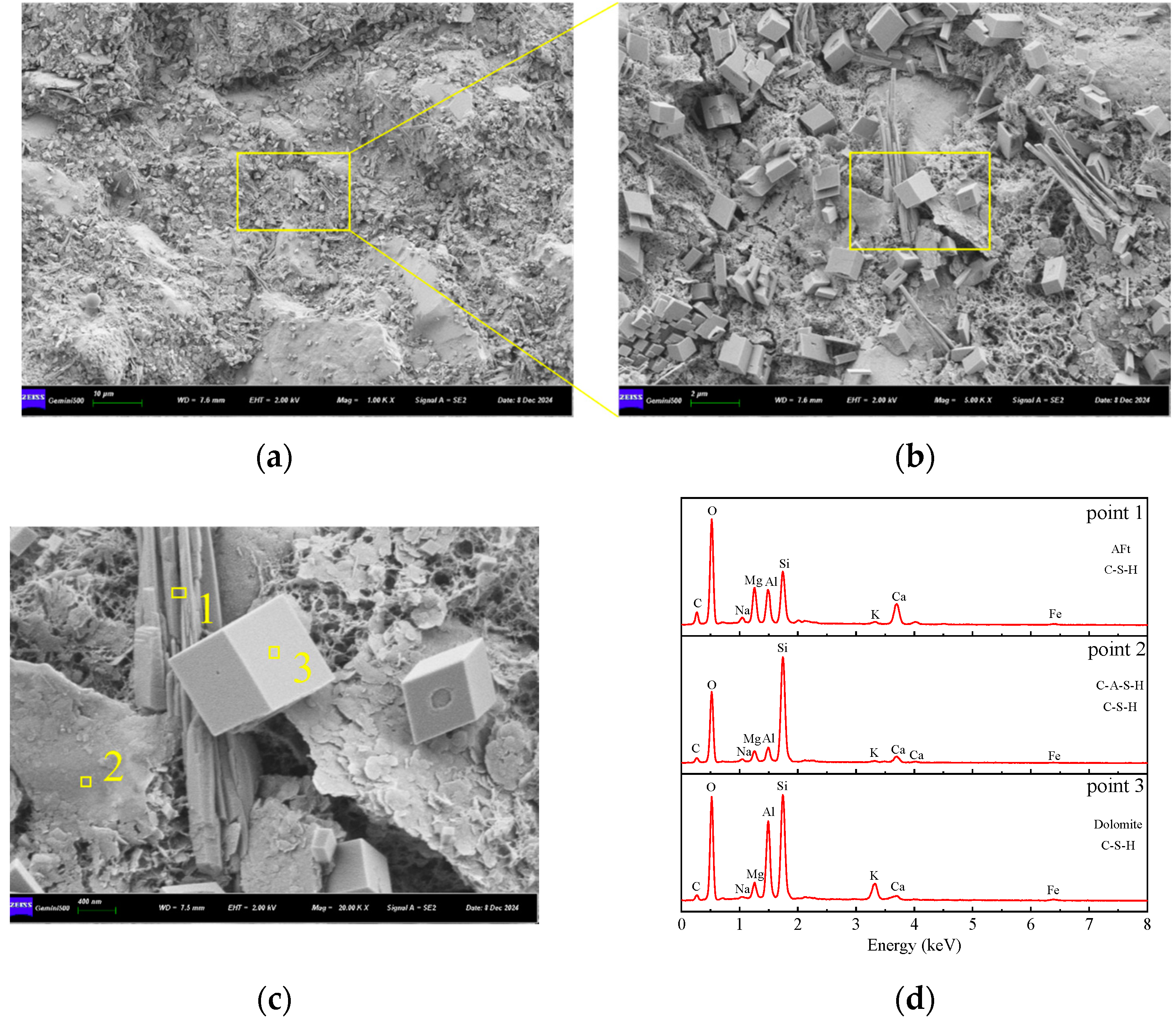
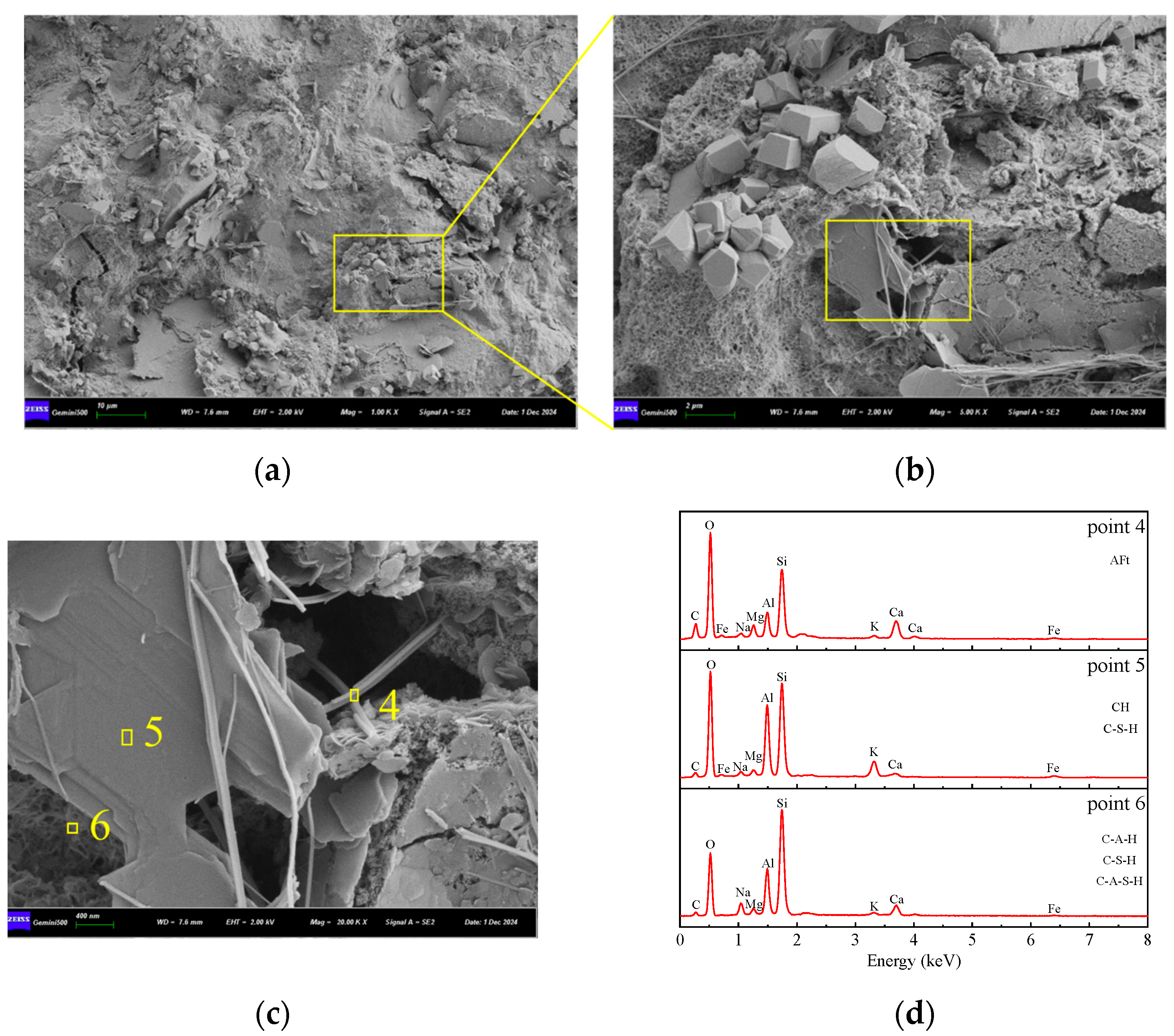
3.5.2. SEM-EDS Analysis
3.5.3. MIP Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luan, X.; Han, L. Variation Mechanism and Prediction of Soil–Water Characteristic Curve Parameters of Low-Liquid-Limit Silty Clay under Freeze–Thaw Cycles. Appl. Sci. 2022, 12, 10713. [Google Scholar] [CrossRef]
- Li, Z. The Analysis of The Impact of Liquid Limit on Mechanical Properties of Clayey Soil. IOP Conf. Ser. Earth Environ. Sci. 2020, 525, 012036. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, T.; Cai, G. Research on Technology and Engineering Application of Silt Subgrade Solidified by Lignin-based Industrial By-product. China J. Highw. Transp. 2018, 31, 1–11. [Google Scholar]
- Sun, R.; Fang, C.; Gao, F.; Ge, Z.; Zhang, H.; Lu, Q. Study on Pavement Performance and Solidified Mechanism of Solidified Soil Based on Solid Waste. China J. Highw. Transp. 2021, 34, 216–224. [Google Scholar]
- Chen, N. Study on the Solidification Mechanism and Road Performance of New Soil Curing Agent for Stabilizing and Low Liquid Limit Red Clay. Chongqing Jiaotong Univ. 2024. [CrossRef]
- Wang, Y.; Zhang, S.; Huang, X.; Li, J.; Liu, L.; Wang, S.; Cheng, X. Mechanical Property and Durability of Low Liquid Limit Silty Clay Solidified by Phosphogypsum⁃steel slag⁃ground granulated blast⁃furnace Slag. China Civ. Eng. J. 2023, 56 (Suppl. S1), 12–23. [Google Scholar]
- Chen, R.; Hao, R.; Li, D.; Bao, W.; Lai, H. Study on Road Performance and Freeze-thaw Resistance of Alkali Activated Material Stabilized Low-liquid-limit Silty Clay. J. Eng. Geol. 2022, 30, 327–337. [Google Scholar]
- Chang, R.; Yang, J.; Wu, Y.; Lu, R. Strength and Leaching Characteristics of CGF Solidification/stabilization Composite Heavy Metal Contaminated Soil. China Environ. Sci. 2024, 44, 2580–2594. [Google Scholar]
- Wu, Y. Soil Binder: Materials, Mechanism and Applications, 1st ed.; Chemical Industry: Beijing, China, 2022. [Google Scholar]
- Gong, Q.; Ouyang, Y.; Kou, D. Comprehensive Treatment and Utilization of Salt Sludge in Chlor Alkali Production. China Chlor-Alkali 2023, 12, 52–55. [Google Scholar]
- Zhang, J.; Zhang, B.; Zhang, G. Study on the Characteristics of White Mud and Salt Mud and the Properties of Prepared Engineering Soi; Soda Industry: Mersin, Turkey, 2025; pp. 22–27. [Google Scholar]
- Yan, X.; Zhang, X.; Wu, X. Research Progress on Comprehensive Utilization of Brine Sludge Resources. Environ. Prot. Sci. 2023, 49, 85–92. [Google Scholar]
- Chen, H.; Guo, W.; Zhang, Z.; Li, J.; Zhao, Q.; Qiu, Y.; Guo, R. Synthesis of a Novel One-Part Alkali-Activated Material from Solid Wastes Salt Sludge and Soda Residue. Constr. Build. Mater. 2024, 450, 138734. [Google Scholar] [CrossRef]
- Nan, X.; Yang, L.; Tang, W. Gray correlation analysis of the influence of particle size distribution of steel slag on hydraulic activity of steel slag cement. J. Lanzhou Univ. Technol. 2021, 47, 138–143. [Google Scholar]
- Su, Z.; Liu, Z.; Wang, H.; Xu, S.; Wang, D.; Han, F. Reaction Kinetics, Microstructure and Phase Evolution of Alkali-Activated Si-Mn Slag during Early Age. Constr. Build. Mater. 2022, 333, 127437. [Google Scholar] [CrossRef]
- Liu, K.; Duan, J.; Sun, J. Effect of different alkaline environment on the hydration and microstructure of converter steel slag. J. Chin. Electron Microsc. Soc. 2021, 40, 687–694. [Google Scholar]
- Rui, Y. Soundness, Activity and Mechanisms of Carbon Fixing Steel Slag Powder in Portland Cement-Based Materials. Southeast Univ. 2023. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, P.; Liu, S. Microwave Pre-Curing of Portland Cement-Steel Slag Powder Composite for Its Hydration Properties. Constr. Build. Mater. 2018, 189, 1093–1104. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Wang, D.; Yan, P.; Luo, L.; Zhang, H.; Zhang, H.; Gu, X. Hydration Superposition Effect and Mechanism of Steel Slag Powder and Granulated Blast Furnace Slag Powder. Constr. Build. Mater. 2023, 366, 130101. [Google Scholar] [CrossRef]
- Yön, M.Ş.; Karataş, M. Evaluation of the Mechanical Properties and Durability of Self-Compacting Alkali-Activated Mortar Made from Boron Waste and Granulated Blast Furnace Slag. J. Build. Eng. 2022, 61, 105263. [Google Scholar] [CrossRef]
- Wu, P.; Wang, Q.; Liu, Q. Preparation and hydration characteristics of soda residue-slag based cementitious materials. J. China Univ. Min. Technol. 2022, 51, 802–811. [Google Scholar]
- Dai, X.; Aydin, S.; Yardimci, M.Y.; Lesage, K.; De Schutter, G. Early Age Reaction, Rheological Properties and Pore Solution Chemistry of NaOH-Activated Slag Mixtures. Cem. Concr. Compos. 2022, 133, 104715. [Google Scholar] [CrossRef]
- Nan, X.; Yang, X.; Zhang, Y.; Tang, W.; Zhang, F. Synergistic Hydration Mechanism of Steel Slag-Slag Based Cementitious Material. J. Build. Mater. 2024, 27, 366–374. [Google Scholar]
- German, A.; Winnefeld, F.; Lura, P.; Rentsch, D.; Lothenbach, B. Hydrous Carbonate-Containing Brucite (HCB) in MgO/Hydromagnesite Blends. Cem. Concr. Res. 2023, 173, 107304. [Google Scholar] [CrossRef]
- Montserrat-Torres, P.; Winnefeld, F.; Lothenbach, B. Impact of Nesquehonite on Hydration and Strength of MgO-Based Cements. Cem. Concr. Res. 2025, 189, 107772. [Google Scholar] [CrossRef]
- Jansen, D.; German, A.; Ectors, D.; Winnefeld, F. Stacking Faults of the Hydrous Carbonate-Containing Brucite (HCB) Phase in Hydrated Magnesium Carbonate Cements. Cem. Concr. Res. 2024, 175, 107371. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, J.; Wu, Y.; Lu, R.; Li, Y.; Zhang, L.; Guo, J. Study on the Properties of All-Solid Waste Fluidized Filling Materials Applied to Mine Void Area Filling Engineering. Materials 2024, 17, 5154. [Google Scholar] [CrossRef]
- GB/T50145; Standard for Engineering Classification of Soil. Ministry of Housing and Urban-Rural Development of the People’s Republic of China: Beijing, China, 2007.
- ASTM D560-03; Standard Test Methods for Freezing and Thawing Compacted Soil-Cement Mixtures. American Society for Testing Materials, USA, 2012. Available online: https://webstore.ansi.org/standards/astm/astmd56003 (accessed on 4 March 2025).
- Zhang, Y.; Lu, Y.; Liu, S. Effects of cyclic freezing and thawing on volume changes and mechanical properties of clay-gravel mixtures. Chin. J. Rock Mech. Eng. 2021, 40 (Suppl. S2), 3323–3333. [Google Scholar]
- Li, J.; Tan, S.; Yang, C.; Chen, H.; Lin, Y. Analysis of Damage Characteristics for Skarn Subjected to Freeze-Thaw Cycles Based on Fractal Theory. Fractal Fract. 2023, 7, 354. [Google Scholar] [CrossRef]

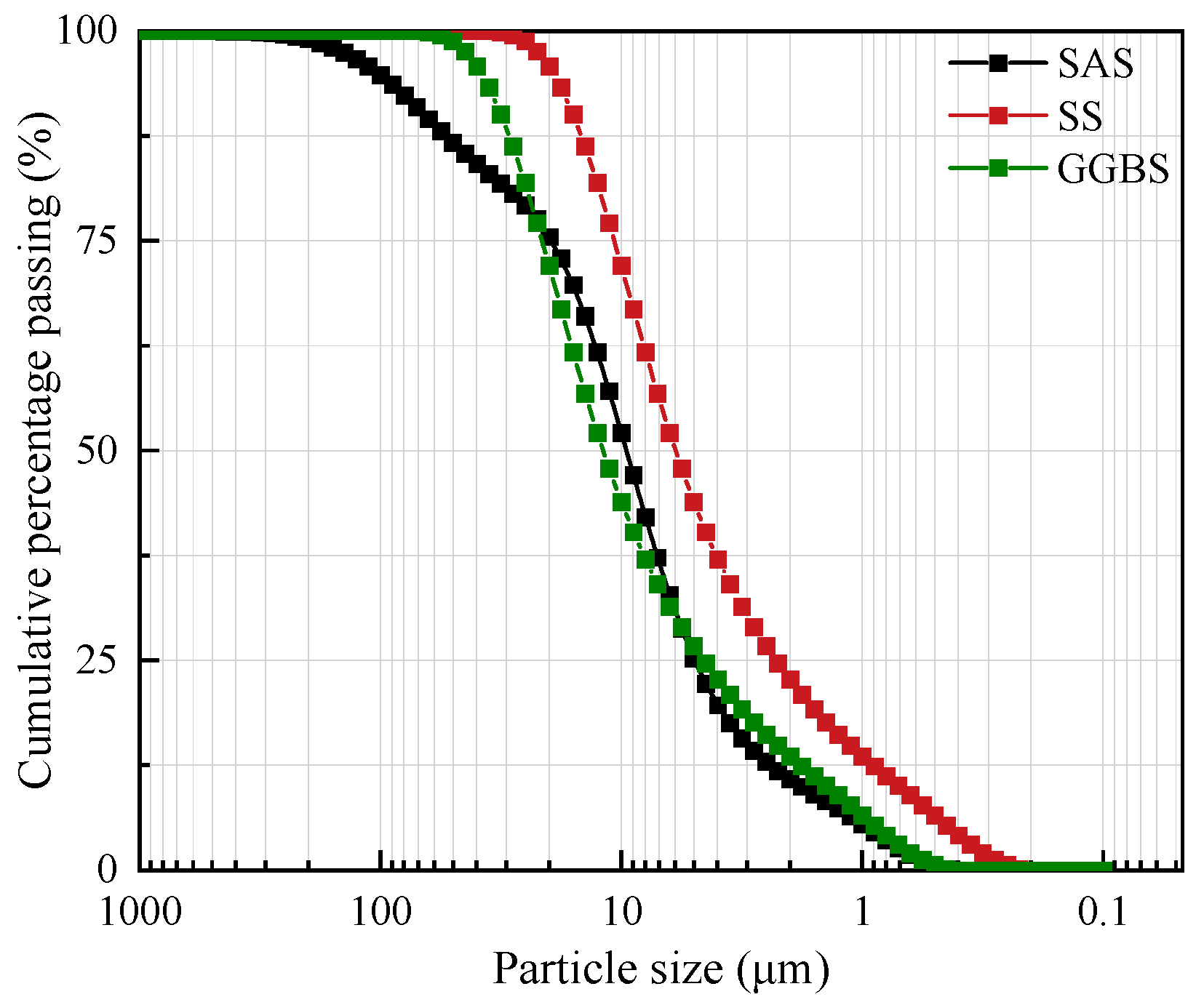





| Solid Waste Type | Chemical Composition and Content (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | CaO | Al2O3 | SO3 | Fe2O3 | MgO | K2O | Na2O | |
| SAS | 1.18 | 30.96 | 0.31 | 0.81 | 0.85 | 24.16 | 8.64 | 0.59 |
| SS | 14.84 | 48.64 | 3.72 | 0.64 | 27.55 | 1.21 | 0.56 | 0.04 |
| GGBS | 30.87 | 41.86 | 14.97 | 2.50 | 0.34 | 8.05 | 0.27 | 0.32 |
| Soil Classification Nomenclature | Clay/Silt/Sand | GS | wP (%) | wL (%) | IP | |
|---|---|---|---|---|---|---|
| Test Soil | Clay | - | 2.74 | 33.9 | 55.1 | 21.2 |
| Silt | - | 2.71 | 22.7 | 31.6 | 8.9 | |
| Sand | - | 2.67 | - | - | - | |
| Artificially prepared soil | CSS011 | 0∶50∶50 | 2.68 | 12.0 | 17.1 | 5.1 |
| CSS122 | 20∶40∶40 | 2.69 | 7.0 | 23.5 | 16.5 | |
| CSS111 | 33.3∶33.3∶33.3 | 2.70 | 16.2 | 24.7 | 8.5 | |
| CSS100 | 100∶0∶0 | 2.74 | 33.9 | 55.1 | 21.2 | |
| Soil Type | Binder | Mixing Ratio of Binder (%) | Curing Age (d) | Freeze–Thaw Cycle Times (Cycles) | Water Admixture (%) |
| CSS011 CSS122 CSS111 CSS100 | SSGM P.O 42.5 | 15 | 28 | 0, 2, ,4, 6, 8, 10 | 1.2wL |
| Soil Type | a | b | R2 |
|---|---|---|---|
| CSS011 | 76.4 | 0.85 | 0.9828 |
| CSS122 | 289.6 | 0.85 | 0.9949 |
| CSS111 | 229.9 | 0.82 | 0.9991 |
| CSS100 | 359.9 | 0.94 | 0.9710 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, R.; Yang, J.; Wu, Y. Study on the Influence of Clay Content on the Freeze–Thaw Characteristics and Mechanisms of Solidified Low-Liquid-Limit Clay. Appl. Sci. 2025, 15, 3005. https://doi.org/10.3390/app15063005
Lu R, Yang J, Wu Y. Study on the Influence of Clay Content on the Freeze–Thaw Characteristics and Mechanisms of Solidified Low-Liquid-Limit Clay. Applied Sciences. 2025; 15(6):3005. https://doi.org/10.3390/app15063005
Chicago/Turabian StyleLu, Ruifan, Junjie Yang, and Yalei Wu. 2025. "Study on the Influence of Clay Content on the Freeze–Thaw Characteristics and Mechanisms of Solidified Low-Liquid-Limit Clay" Applied Sciences 15, no. 6: 3005. https://doi.org/10.3390/app15063005
APA StyleLu, R., Yang, J., & Wu, Y. (2025). Study on the Influence of Clay Content on the Freeze–Thaw Characteristics and Mechanisms of Solidified Low-Liquid-Limit Clay. Applied Sciences, 15(6), 3005. https://doi.org/10.3390/app15063005






