Abstract
The storage temperature is important for maintaining the quality of raw fish meat. The characteristics of ordinary muscle (OM) and dark muscle (DM) differ. This study aimed to clarify the effects of storage temperature (refrigeration, ice storage, and super-chilled (SC) storage) on the bacterial flora and quality (biochemical changes, volatile organic compounds (VOCs), and off-flavor development) of both muscles of yellowtail (Seriola quinqueradiata). SC storage effectively extended the shelf life of the dorsal part of ordinary muscle (OM) and DM by reducing bacterial proliferation, VOC changes, and off-flavor formation. However, their effects on the inhibition of trimethylamine (TMA) accumulation and lipid oxidation are limited. (E,E)-2,4-octadienal and (E,E)-3,5-octadien-2-one were identified as candidate markers of OM quality deterioration, whereas 1-hexanol was identified as a potential marker for DM. Alcohols, esters, and ketones are potential spoilage indicators of yellowtail muscles (OM and DM). Pseudomonas was the dominant spoilage bacterium in OM and DM across all storage conditions, with Acinetobacter, Brochothrix, and Shewanella appearing in later storage stages. These findings highlight the importance of storage at lower temperatures and understanding the dynamics of spoilage-causing bacteria and changes in VOCs in raw fish meat (OM and DM) to prevent spoilage and maintain meat quality.
1. Introduction
Fish is a delicious and nutritious food. However, post-mortem fish easily deteriorates because of chemical reactions, endogenous enzymes, and spoilage-causing bacteria [1]. Among these, odor is a critical quality parameter that requires close attention. Temperature control for the quality preservation of fishery products is pivotal, with traditional refrigeration typically maintained at 4 °C to inhibit microbial growth [2,3]. Ice storage maintains freshness by inhibiting endogenous enzyme activity in food and by reducing microbial proliferation [4]. Super-chilled (SC) storage involves the partial freezing of water in food, typically cooling it to 1–2 °C below the initial freezing point. Storing food at SC temperatures offers three distinct advantages: maintaining freshness, retaining high quality, and suppressing the growth of harmful microbes [5,6]. Magnussen et al. reported that this method extends the shelf life of fresh cod by 2–3 days compared with traditional refrigeration, which provides a shelf life of 9–13 days [7].
Odor change is one of the most influential factors in degrading fish flesh quality. Some individuals avoid eating fish-based cuisine because of their unpleasant odor. Storage temperature plays an important role in the development of off-odors in seafood by affecting the growth of spoilage-causing bacteria, mainly gram-negative, and their metabolic activities [8]. In addition, volatile organic compounds (VOCs) derived from lipid oxidation are produced in fish muscle during storage, even in the absence of a bacterial contribution [9,10]. The progress of this lipid oxidation is affected by temperature. Therefore, to maintain the appropriate quality of fish meat, it is necessary to investigate the effect of storage temperature on changes in the volatile components of fish meat during storage.
Traditional bacterial colony counting methods may underestimate microbial diversity and spoilage-causing bacteria, owing to their inability to detect unculturable strains. Next-generation sequencing (NGS) enables the precise evaluation of bacterial taxa, including uncultivated microbes or those present in low abundance [11]. In some fish species, the predominant genera associated with flesh spoilage include Pseudomonas, Psychrobacter, Photobacterium, and Shewanella [12,13,14].
The yellowtail (Seriola quinqueradiata) is an important cultured fish species for domestic consumption and export in Japan. This fish species accounts for approximately 48% of the aquaculture production in Japan [15]. In Japanese cuisine, yellowtail ordinary muscle is eaten alone or with dark muscle (DM). DM has a higher concentration of unsaturated lipids and heme proteins, making it more prone to lipid oxidation. As a result, VOCs produced in DM differ from those in OM, leading to distinct odor profiles [9,10]. In our previous studies, VOC changes in the DM were found to be important for odor deterioration in yellowtail meat compared to OM. Nevertheless, there was no difference in the changes in the bacterial flora between OM and DM during refrigeration [16]. Pseudomonas were found to be predominant in yellowtail meat after spoilage in this study. Therefore, it is important to understand the changes in the VOCs and the bacterial flora of OM as well as DM of this species during storage. In addition, species other than Pseudomonas have been reported to be predominant in fish species other than yellowtail [12], and the predominant bacterial species in spoiled fish meat may differ depending on the storage temperature. Furthermore, many VOCs have been associated with spoilage in fish species [17]. However, the interactions between VOC formation and bacterial shifts during storage at different temperature conditions remain poorly understood. Additionally, limited research has examined how these changes differ between OM and DM, despite their distinct biochemical properties.
The DM and OM differed greatly in their characteristics (Table S1). Commercially valuable yellowtail is a typical red-fleshed fish with well-developed DM. We believe that using both muscle parts of this fish to determine the effects of storage temperature on bacterial flora, VOCs, and quality is necessary to provide insights for optimizing seafood preservation and supporting sustainable food supply chain management. Therefore, this study aimed to clarify how storage at different temperatures affects the VOC profiles (GC-MS analysis) and bacterial communities (NGS) of yellowtail OM and DM. Visible colony counts (VCCs), total volatile basic nitrogen (TVB-N), trimethylamine (TMA), and thiobarbituric acid reactive substances (TBARS) were used to evaluate spoilage progression. Sensory evaluations were conducted to assess the quality of yellowtail meat during storage.
2. Materials and Methods
2.1. Experimental Animals
Nine farmed yellowtails, each weighing 4.0 ± 0.1 kg, were purchased from a retailer in Hiroshima City. Fish were instantly slaughtered by medullary puncture at the market. They were transported to our laboratory on ice within 8 h. The killed fish were then filleted, skinned, and rinsed with an ice-cold 1% NaCl solution. The fillets were cut into 1.0 cm thick sections perpendicular to the lateral line. Each section was tightly wrapped in polyvinyl chloride (0.01 mm thick, KitcheNista, Chikusei, Japan) to prevent drying of the surface. For refrigeration, the samples were stored at 3 °C for 14 days. For ice storage, the fish were kept on ice for 21 days. For SC storage, the samples were immediately stored at −3 °C for 50 days in a portable low temperature refrigerator (SC-DF25, Twinbird, Tsubame, Japan) after being kept on ice until the fish muscle temperature reached 0 °C. The storage duration across different storage conditions was set at differences in microbial growth rates, spoilage progression, and biochemical degradation under each condition. After storage, all samples were separated into OM (dorsal part) and DM, minced in a food processor, and stored at −80 °C until all analyses—except for VCC.
2.2. VCCs
Each sample (10 g) was homogenized in 90 mL of sterile peptone saline (0.1% polypeptone, 0.85% NaCl) for 2 min. The samples were then diluted in series using a saline solution. Plate count agar (PCA) was used to detect general viable bacteria [16]. Streptomycin-thallous acetate-actidione agar (STAA) was used for Brochothrix thermosphacta [18]. De Man, Rogosa, Sharpe agar (MRS, Merck KGaA, Darmstadt, Germany) was used for mesophilic LAB, and Violet Red Bile Glucose agar (VRBG, Merck KGaA) for Enterobacteriaceae. For these bacteria, 1 mL of the diluted solution was mixed with each medium. Aeromonas isolation agar (Sigma-Aldrich, St. Louis, MO, USA) with 5.0 mg/L of ampicillin (AIA) for Aeromonas spp., marine agar (Condalab, Madrid, Spain) for heterotrophic marine bacteria, cephaloridine fucidin cetrimide agar (Merck KGaA) with 1.0% (v/v) glycerol (CFC) for Pseudomonas species, and black colonies of iron agar (Condalab) with 5.0% NaCl for H2S-producing bacteria were used. A dilution (0.1 mL) of bacterial suspension was added to the medium. The incubation conditions were as follows: PCA at 30 °C for 72 h, STAA and VRBD at 25 °C for 48 h, MRS at 30 °C for 48 h, AIA and Marine agar at 20 °C for 72 h, CFC at 35 °C for 72 h, and iron agar at 25 °C for 96 h. After incubation, the colony-forming units in the samples were counted, resulting in VCC values (Log CFU/g). Microbial populations of 7 Log CFU/g have been consistently identified as the threshold for spoilage of fish products across various studies [19,20,21].
2.3. Determination of TVB-N, TMA, and TBARS Values
TVB-N was determined using the microdiffusion analysis method [22], with slight modifications. OM (1.0 g) and DM (0.5 g) were homogenized in 10 mL of 5% trichloroacetic acid (TCA). After centrifugation at 10,000× g for 20 min, TVB-N was measured as previously described [16].
TMA content was determined using the method described by Tanimoto et al. with slight modifications [16]. Briefly, TMA was extracted using TCA in the same manner as TVB-N. TMA was analyzed using GC-MS systems according to our previous study [23], employing an Rtx-Volatile Amine column (0.32 mm i.d. × 30 m, RESTEK, Center County, PA, USA).
TBARS was measured according to the method described by Kitabayashi et al. [24], with minor modifications on sample extraction. Briefly, OM (1.0 g) and DM (0.25 g) were used as samples.
2.4. Sensory Evaluation
Sensory evaluation was conducted with the approval of the Research Ethics Committee of the Prefectural University of Hiroshima (approval no. 20HH003-1). Forty-two students from the Prefectural University of Hiroshima, aged 23.5 ± 3.3 years, participated as trained panelists to compare the odor of OM and DM during storage using a scale method. Each sample (5 g) was placed in a vial covered with aluminum foil and left for 2 h at room temperature until sensory evaluation. To make an absolute evaluation of the putrid odor and odor intensity, a preliminary sensory evaluation experiment was conducted, and the following criteria were established: putrid odor was rated on a three-point scale; 1 (no odor), 2 (slight odor), and 3 (strong odor). The criteria for putrid odor were set at one point for the intensity of OM before storage and three points for its intensity on the 14th day of refrigeration. A five-point scale (1, very weak; 2, weak; 3, normal; 4, strong; 5, very strong) was used to rate odor intensity. As a criterion, the DM before storage was set at three points, and on the 14th day of refrigeration, it was set at five points.
2.5. DNA Extraction, PCR, and NGS
DNA extraction was performed as described in our previous study [23], with the following modifications: the bacterial pellet was resuspended in 500 μL of physiological saline (0.85% NaCl). Microbial DNA was extracted from the suspension using the Quick-DNA™ Fecal/Soil Microbe Miniprep Kit (Zymo Research Corporation, Irvine, CA, USA).
PCR and NGS of the V3-V4 region of the bacterial 16S rRNA gene were conducted as described in our previous study [25], except that the first PCR was performed for 25–40 cycles. The First PCR products were checked to ensure correct amplification by 2% agarose gel electrophoresis. A Fragment Analyzer (Advanced Analytical Technologies, Ankeny, IA, USA) with a dsDNA 915 Reagent Kit (Agilent, Santa Clara, CA, USA) was used to confirm the library quality from the products of the Second PCR.
2.6. Analysis of Sequencing Data
Metagenomic analysis was conducted using QIIME 2 software (2019.7, https://view.qiime2.Org (accessed on 24 February 2025), Table S2) according to our previous studies [23,25]. The DADA2 algorithm was employed for the quality control of the input sequences, and feature tables and data were generated. Briefly, for quality control of the input sequences, the quality score was set to not less than 30, the length of the sequences was filtered to at least 500 bp, and a feature table was constructed. The data were then subjected to denoising, and chimeric sequences were identified and removed. These were then used to construct phylogenetic trees. Alpha diversity metrics, including observed OTUs, Chao1, Good’s coverage, Shannon index, Simpson index, and beta diversity (weighted UniFrac distance) were determined. Principal coordinate analysis (PCoA) [26,27] of the weighted UniFrac distances was performed using the statistical software package R (Ver.4.0.4, https://www.r-project.org/ (accessed on 24 February 2025), Table S2).
For classification at the genus levels, the “Silva 132 99% OTUs from the 515F/806R region of sequences” database was used. Hierarchical clustering analysis (HCA) heatmaps [28] of the relative abundance data for microbial genera were generated using Ward’s method in MetaboAnalyst 5.0 (https://www.metaboanalyst.ca/, (accessed on 24 February 2025) Table S2). Data were log-transformed after correcting for the second-lowest relative abundance. Fifty genera with the highest total relative abundance were selected for the analysis.
2.7. Determination of VOCs
VOCs were extracted by solid-phase microextraction (SPME) according to our previous study [29]. A 1.2 g sample was homogenized with 6.0 mL of a saturated saline solution and centrifuged at 15,000× g for 10 min at 4 °C. Five milliliters of the supernatant and 5.0 µL of 0.010% cyclohexanol solution were placed in a vial, sealed, and maintained at 40 °C for 60 min. After incubation, the SPME fiber (65 µm polydimethylsiloxane/divinylbenzene, Merck KGaA) was exposed to the headspace at 40 °C for 30 min. The fibers were then inserted into the gas chromatograph inlet for analysis. VOCs were analyzed using a Shimadzu GCMS-QP2010 mass spectrometer (Shimadzu, Kyoto, Japan) equipped with a capillary column (Intert Cap Pure-WAX, 0.25 mm i.d. × 60 m, film thickness 0.25 µm, GL Science, Tokyo, Japan). The analysis, estimation, and semi-quantification of each volatile compound were performed as previously described [24].
2.8. Statistical Analysis
Analysis values are presented as mean. A two-tailed Dunnett’s test was used to test for significant differences between day 0 of storage and other storage days. Analysis of variance or Welch’s t-test was used to test for significant differences between OM and DM under the same storage conditions. These tests were performed using SPSS Statistics 17.0 (IBM Japan, Ltd., Tokyo, Japan, Table S2). The significance level was set at p < 0.05. Principal component analysis (PCA) [30,31] was performed for semi-quantitative VOC values using SIMCA 16 (Sartorius Stedim Biotech, Auvergne, France, Table S2).
3. Results and Discussion
3.1. VCC
Table 1 shows the changes in VCCs in yellowtail muscle during storage at different temperatures. The counts of general viable bacteria, Enterobacteriaceae, Aeromonas spp., heterotrophic marine bacteria, and Pseudomonas spp. in OM and DM stored under all storage conditions increased significantly and reached 7 Log CFU/g, which could be prolonged as the storage temperature decreased. Although B. thermosphacta and H2S-producing bacteria in refrigerated or ice-stored OM and DM propagated significantly, their counts did not exceed the initial spoilage levels (7 Log CFU/g). However, there was no significant increase in the abundance of these bacteria at either muscle site during the SC storage. In the present study, microbial growth was assumed to follow first-order reaction. Therefore, at each temperature, the linear growth rate was calculated using the portion of storage days and the logarithm of VBC in a linear relationship, as shown in Table 2. Lactic acid bacteria that showed no obvious growth during storage were excluded. The growth rate (Log CFU/g/day) of each bacterium ranged from 0.42 to 0.77 in refrigerated storage, 0.19 to 0.35 in ice storage, and 0.02 to 0.16 in SC storage. This indicates a significant temperature-dependent inhibition of the growth of each bacterium during storage. Therefore, storage at lower temperatures, especially SC, can slow the propagation of mesophilic bacteria and various spoilage-causing bacteria in the yellowtail muscles. In greater amberjack fillets stored at −3 °C to 20 °C, bacterial growth was suppressed by storage at lower temperatures [32]. Additionally, SC storage effectively slows bacterial growth and extends the shelf life of fishery products [33]. These results are consistent with our findings.

Table 1.
Changes in visible cell counts of raw yellowtail muscles stored at different temperatures (log CFU/g).

Table 2.
Linear growth rate (k) of bacteria in raw yellowtail muscles stored at different temperatures (1/day).
3.2. TMA, TVB-N, and TBARS
Table 3 shows the changes in TVB-N, TMA, and TBARS levels in yellowtail muscle during storage at different temperatures. TVB-N in OM and DM increased significantly during storage under all storage conditions, except for OM in ice, whereas no large suppressive effect of decreasing temperature on the increase in TVB-N was observed. However, it did not exceed the spoilage threshold (acceptable limit) after storage (25–30 mg/100 g) [34,35,36,37]. No marked differences in TVB-N were observed between OM and DM when the same storage temperatures were compared. These findings differ from those of sea bass and greater amberjack, in which increases in TVB-N during storage were suppressed by storage at lower temperatures [32,38]. This discrepancy may be due to differences in fish species, origin, partial freezing conditions, and contaminating bacteria, or because the storage period was within the range not exceeding the spoilage threshold.

Table 3.
Changes in total volatile basic nitrogen (TVBN), trimethylamine (TMA), and thiobarbituric acid-reactive (TBARS) in raw yellowtail muscles stored at different temperatures.
The TMA levels significantly increased under all storage conditions. OM did not exceed the acceptable TMA limit of 50–100 µg/g [34,36]; however, DM exceeded these limits on days 6, 7, and 7 in refrigerated, ice, and SC storage, respectively. This indicates that the temperature difference between refrigeration and SC storage was insufficient to suppress TMA production in DM and is probably the result of enhanced enzyme activity with minimal reduction in TMAO reductase activity due to partial freezing during SC storage [39]. In other words, in DM, the increase in TMA levels during storage may be due to endogenous enzymes rather than bacterial proliferation. On the other hand, in OM, lower-temperature storage was found to have an inhibitory effect on the increase in TMA. Storage of rohu and mackerel at lower temperatures also inhibited TMA formation [38,40], supporting the results of the present study.
The TBARS levels in OM and DM also increased significantly during storage, with no notable differences between adjacent storage periods under different storage conditions, indicating that TBARS formation was not suppressed at lower temperatures. Banerjee and Maheswarappa noted that ice crystals formed during SC storage can promote autolysis as well as enzymatic and chemical reactions by damaging cell membranes and releasing oxidation-accelerating enzymes [41]. Thus, although SC storage inhibited spoilage by microorganisms, partial freezing did not suppress some biochemical changes. In pre-spoilage gilthead seabream fillets, storage at −1 °C was found to result in a greater increase in TBARS than storage at 3 °C [42]. In our previous study, storage in ice delayed the increase in TBARS in pre-spoilage yellowtail OM and DM [10]. The difference between these results and those of the present study may be due to the partial freezing mentioned above and the storage period (before and until spoilage).
3.3. Sensory Evaluation
Table 4 shows the results of sensory evaluation of OM and DM during storage at different temperatures. The average value of the putrid odor exceeded the two points (“weak putrid odor”) on day 6 for refrigerated, day 7 for ice storage, and day 17 for SC for both muscle parts. The odor intensity increased significantly during storage in both muscle groups. SC storage of OM suppressed the increase in odor intensity compared with other storage temperatures for OM, whereas lower storage temperatures for DM had a similar effect. These results suggest that SC storage can suppress odor changes in yellowtail muscles during storage, including spoilage, compared with refrigeration or ice storage. Storage causes greater odor deterioration in DM than in OM. Our previous study found that ice storage of yellowtail meat until it reached spoilage levels increased the odor intensity of DM, whereas it decreased that of OM [10]. This inconsistency with the results of this study on OM is thought to result from an increase in the number of components involved in the odor, owing to an increase in microorganisms.

Table 4.
Changes in sensory scores of raw yellowtail muscles stored at different temperatures.
3.4. NGS Analysis
Table 5 illustrates the changes in alpha diversity indices for OM and DM during storage at various temperatures. The Goods coverage index was 100.0%, indicating sufficient data to characterize the microbial communities. The alpha diversity indices for OM and DM decreased under all storage conditions except for the Simpson index of DM at certain temperatures. Similar decreases in alpha diversity have been reported for other fish species [32,43,44].

Table 5.
Changes in alpha diversity indices of raw yellowtail muscles stored at different temperatures.
Figure 1 illustrates the bacterial composition at the genus level, emphasizing the dominance of Pseudomonas during later stages of storage. On day 0, the most abundant genera were Staphylococcus (OM: 11.9%; DM: 15.4%) and Pseudomonas (OM: 9.8%; DM: 11.7%). By day 14 of refrigerated storage (Figure 1A), Pseudomonas was predominant, accounting for 88.9% in OM and 90.9% in DM. Under ice storage conditions on day 21 (Figure 1B), Pseudomonas remained the dominant genus (90.4% in OM and 91.7% in DM). On day 50 of SC storage (Figure 1C), Pseudomonas continued to dominate (91.8% in OM and 94.9% in DM). The increased abundance of Pseudomonas was consistently associated with spoilage progression under all storage conditions. The spoilage-causing bacteria Acinetobacter, Brochothrix, and Shewanella were present in the later stages of storage under all storage conditions, although in lower proportions than that of Pseudomonas. The relative abundance of these bacteria was lower in SC than in the other storage conditions.
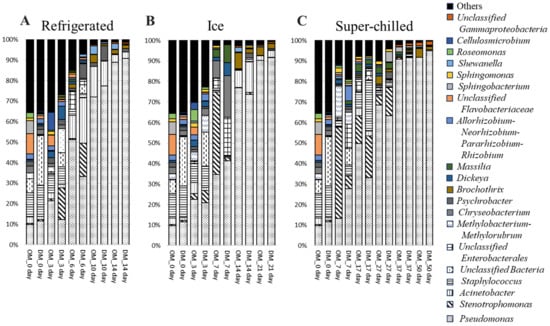
Figure 1.
Relative abundance (%) of bacterial genera in raw yellowtail muscles during storage at different temperatures based on the classification of partial 16S rDNA sequences. OM, dorsal part of ordinary muscle; DM, dark muscle. Storage time is indicated by # day.
In the VCC culture, the Aeromonas counts exceeded 7.5 Log CFU/g on the final sampling date under all conditions. Enterobacteriaceae displayed colony counts similar to those of Pseudomonas on all final sampling dates. These bacteria were not detected using NGS. These results align with the finding that traditional culture methods can detect spoilage bacteria that cannot be identified using NGS [45,46]. Similarly, Enterobacteriaceae, which have been identified as spoilage agents in freshwater fish [47], were observed in traditional cultures but were not detected by NGS. These discrepancies highlight the limitations of culture-dependent methods. In the VCC results, although H2S-producing bacteria multiplied significantly in refrigerated or iced OM and DM, these counts did not exceed the initial spoilage level (7 Log CFU/g) (Table 1) and the proportion did not increase significantly in the NGS results (Figure 1). Shewanella putrefaciens is considered the main spoilage bacterium in marine seafood stored at low temperatures. A study that used iced marine fish (cod, plaice, and flounder) found that the main H2S-producing organism was S. baltica [48]. Combined with the results for Pseudomonas, this reflects the competition between microflora and the dynamics of survival of the fittest in resource utilization. Shewanella is an important spoilage bacterium; however, its contribution to spoilage was not significant under SC conditions in the present study. The observations in the present study reinforce previous findings that culture-dependent and culture-independent methods capture complementary aspects of microbial communities, underscoring the need for approaches that accurately assess spoilage [49].
The PCoA results for the bacterial communities in OM and DM during storage at different temperatures are shown in Figure 2. The PCoA scores of the first three principal components (PCo1, PCo2, and PCo3) accounted for 40.5, 17.5, and 9.5% of the total variation, respectively. Across all storage conditions, the PCoA scores of the samples shifted rightward on the PC1 axis over the storage time, leading to the convergence of spoiled samples within a specific area. This suggests that the microbial communities in both muscle types evolved towards similar communities during storage, with the transition occurring more slowly at lower temperatures.
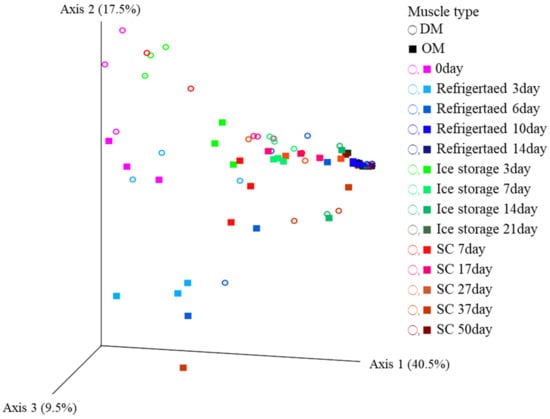
Figure 2.
Principal coordinate analysis based on the microbial community at the genus level in raw yellowtail muscles during storage at different temperatures. OM, dorsal part of ordinary muscle; DM, dark muscle. Analysis was performed using the weighted UniFrac method.
Figure 3 shows the distinct clustering patterns, emphasizing the dominance of Pseudomonas under all storage conditions. The samples were grouped into two main clusters. Cluster I consisted of samples stored for extended periods: OM refrigerated for 10 and 14 days and stored on ice for 14 and 21 days, and DM refrigerated for 10 and 14 days and stored on ice for 21 days. Cluster II included OM and DM samples from SC for 37 and 50 days, respectively. Pseudomonas (Cluster B) was the dominant genus in both clusters. Cluster I also contained relatively large populations of Acinetobacter, Brochothrix, and Shewanella (Cluster A). Cluster III included samples stored for shorter periods: days 0 (OM and DM), 3 (ice-stored OM and DM), and 3 (refrigerated DM). Cluster IV comprised samples from days 6 (refrigerated OM and DM), 27 (SC OM and DM), and 14 (ice-stored DM). Cluster V included samples stored for 7 days under various storage conditions (refrigerated OM and DM, stored on ice, and SC), 3 days (refrigerated OM), and 17 days (SC OM and DM). In Cluster III, the bacterial flora was more diverse, with Pseudomonas and other genera, such as Staphylococcus (Cluster C), Acinetobacter, and Psychrobacter (Cluster D), which were abundant. Regardless of the initial bacterial diversity, Pseudomonas consistently dominated at all storage temperatures as the storage progressed. However, spoilage-associated bacterial communities varied between refrigerated and ice-stored samples and between SC samples. These findings were consistent with the VCC data for B. thermosphacta, Pseudomonas spp., and H2S-producing bacteria (Shewanella spp.). When it was assumed that the shelf life was the shortest period of storage, in which both muscle sites belonged to clusters I and II, spoilage occurred after 10 (refrigeration), 14 (ice storage), and 37 (SC) days. By reaching 7 Log CFU/g of mesophilic bacteria, spoilage was observed at 14, 21, and 50 days for the refrigerated, ice-stored, and SC samples, respectively. The spoilage period of yellowtail meat estimated from the results of cluster analysis and VCC was slightly shorter in the cluster analysis than in the VCC analysis, suggesting that shelf life may be evaluated more accurately using NGS.
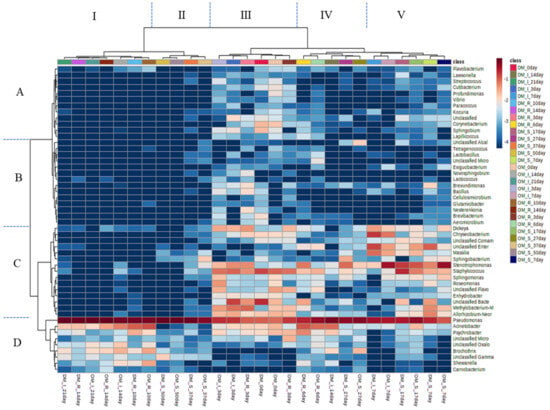
Figure 3.
Heat map visualization and hierarchical clustering based on the dataset of relative bacterial abundance at the genus level from raw yellowtail muscles during storage at different temperatures. OM, dorsal part of ordinary muscle; DM, dark muscle. The class indicates muscle type, storage method (R, refrigerated; I, ice; S, super-chilled), and storage time. Colors indicate the common logarithm of relative abundance.
The predominance of Pseudomonas aligns with previous findings that associate this genus with the spoilage of refrigerated muscle products, such as crayfish and beef, owing to its ability to thrive at low temperatures and generate volatile metabolites responsible for sensory deterioration [45,50]. Anagnostopoulos et al. reported that Psychrobacter and Pseudomonas dominated the bacterial communities in red seabream after 12 days of ice storage [51], whereas Parlapani et al. observed similar trends in thawed common cuttlefish stored at 2 °C in Greece [52]. Jingbin et al. reported the dominance of Pseudomonas in partially frozen European crucian carp after storage [53]. In the present study, Pseudomonas emerged as the predominant spoilage bacterium across various temperatures and muscle types, which is consistent with previous findings. This hardy gram-negative bacterium thrives in various storage environments for aquatic products, leveraging its ability to metabolize lipids and proteins [54].
In addition to Pseudomonas, other bacteria identified after spoilage in this study included Acinetobacter, Brochothrix, and Shewanella. Similar results have been reported for other seafood products [55,56]. In the present study, Acinetobacter increased only during refrigeration and ice storage, suggesting that it contributes to spoilage, albeit to a lesser extent than Pseudomonas. This also indicates that this bacterium has a lower proliferation rate at low temperatures than that of Pseudomonas. Brochothrix has been reported to be present in foods of animal origin including meat, seafood, and dairy products [57]. Brochothrix was detected at low proportions, notably during SC storage, and was not dominant. The reduced growth of Brochothrix relative to Pseudomonas may reflect competitive exclusion dynamics under cold storage conditions, where psychrotrophic bacteria, such as Pseudomonas, outcompete other spoilage bacteria by utilizing available nutrients more efficiently [58]. In contrast, Shewanella was found to be a minor component of thawed hake and plaice fillets stored at 0 or 10 °C [59]. Thus, storage temperature plays a critical role in determining bacterial growth rate and community composition.
3.5. Changes in VOCs
Table S3 shows the changes in VOCs in yellowtail muscle stored at different temperatures. In total, 145 compounds were identified in this study. The VOCs were classified into thirty-nine aldehydes, twenty-four alcohols, ten alkanes, five aromatic compounds, eleven acids, one alkyl halide, three esters, five furans, twenty-two ketones, one terpene, two sulfides, and twenty-two unidentified compounds (unknowns). Most of the detected aldehydes, alcohols, and ketones were consistent with previous findings and were derived from the degradation of lipids in fish meat [16,60]. Figure 4 shows the PCA results for VOCs in the yellowtail muscle during storage at different temperatures. The scores for PC1 and PC2 accounted for 32.5% and 22.5% of the total variation, respectively. In DM, the samples at all storage temperatures moved in the positive direction of the PC2 score with storage, with longer storage periods moving to the left at each storage temperature. For OM, the samples at all storage temperatures moved in the negative direction of the PC1 score and in the positive direction of the PC2 score during storage. The storage periods of the samples with similar scores for both OM and DM were as follows: SC storage > ice storage > refrigeration. These results suggest VOC alterations during storage for both muscle parts at all storage temperatures, and these changes can be suppressed at lower temperatures, that is, SC. These results agree with the above-mentioned results for the VCCs.
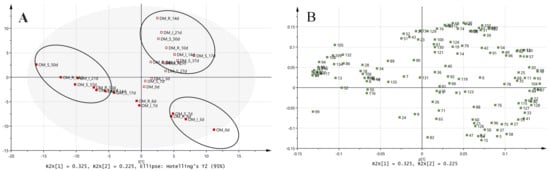
Figure 4.
Score scatter plot (A) and loading plot (B) of principal component analysis (PC1 and PC2) based on volatiles in raw yellowtail muscles during storage at different temperatures. The data were preprocessed using an auto-scale. (A) OM, dorsal part of ordinary muscle. DM, dark muscle. The muscle type (OM and DM), storage method (R, refrigerated; I, ice; S, super-chilled), and storage time (#d) are indicated. (B) The numbers in the loading plot indicate the peak numbers in Supplementary Table S1.
The loading plots showed that in OM, VOCs such as (E,E)-2,4-octadienal and (E,E)-2,4-heptadienal with high PC1 factor loadings and VOCs such as (E,E)-3,5-octadien-2-one and (E,E)-2,6-nonadienal with low PC1 factor loadings significantly decreased or increased during storage, respectively. In DM, VOCs, such as unknown compounds with KI (1742) and 1-hexanol with high PC2 factor loadings, increased significantly during storage at all temperatures. In DM, a few VOCs increased significantly during the storage period at all temperatures. Although many VOCs in DM increased during the early stages of storage, they plateaued or decreased during the later stages of storage. This is believed to be the result of lipid oxidation, which leads to an increase in the concentration of VOCs and their subsequent degradation. In DM and OM at all storage temperatures, esters such as ethyl acetate were not detected early in storage but were detected later in storage, with some exceptions. Esters are produced by esterification of alcohols and carboxylic acids in meat via microbial esterase activity [17]. Pseudomonas and Brochothrix are spoil-causing bacteria that are capable of producing esters [17]. Therefore, it is highly probable that esters are produced during bacterial growth. Under all storage conditions, 3-methylbutanol in OM was undetectable before storage and was detected in late storage. The VOC in DM significantly increased during late storage. 2-Methylbutanol in OM was undetectable before storage but was detected during late storage under all storage conditions. Proteins, especially structural proteins, can be hydrolyzed into peptides and amino acids by microbial proteases; the peptides can be transferred into bacterial cells and subsequently degraded into amino acids. These amino acids undergo transamination and decarboxylation to produce alpha-keto acids. The alpha-keto acids are enzymatically reduced to their respective alcohols via aldehydes [61]. 3-Methyl-1-butanol is produced by leucine catabolism [17]. Spoilage-causing bacteria such as Pseudomonas, Shewanella, and Brochothrix can produce 2-methyl-1-butanol and 3-methyl-1-butanol [17]. 2,3-Butanedione and 3-hydroxy-2-butanone in DM and OM, with the exception of some samples, significantly increased during the late storage period at all storage temperatures. Thus, it is suggested that the above-mentioned esters and alcohols, 2,3-butanedione and 3-hydroxy-2-butanone, are possible signs of spoilage during the storage of yellowtail muscle (DM and OM). 3-Hydroxy-2-butanone and its oxidized form, 2, 3-butanedione, are produced by pyruvate fermentation under anaerobic conditions [62]. 3-Hydroxy-2-butanone can originate from microbial degradation of aspartate [17]. Pseudomonas and Brochothrix are spoil-causing bacteria capable of producing these VOCs [17]. On the other hand, because the change in the behavior of volatile components during storage differs depending on the muscle type and storage conditions, there are no common markers of quality deterioration of the yellowtail muscles. (E,E)-2,4-octadienal and (E,E)-3,5-octadien-2-one were identified as potential quality deterioration markers for OM, whereas 1-hexanol was a candidate for DM. Aldehydes are generated during the hydrolysis of triglycerides and fatty acid metabolism such as the beta-oxidation of unsaturated fatty acids or the autoxidation of lipids [17]. Aldehydes can also be generated from amino acids via transamination [17]. Some methyl ketones are produced from lipolytic activity but also from several other pathways, including alkane degradation by unique alpha-oxidation by bacteria and the dehydrogenation of secondary alcohols by bacteria. (E,E)-2,4-octadienal and (E,E)-3,5-octadien-2-one are produced in fish oil by the action of macroalgal lipoxygenase with high 13-lipoxygenase activity [63]. (E,E)-3,5-octadien-2-one formed by the n-3 fatty acid oxidation is a VOC of rotten yellowtail [64]. In our previous study, we reported that (E,E)-3,5-octadien-2-one is a characteristic component of red sea bream spoilage [23]. However, it is unclear whether these compounds are produced by spoilage-causing bacteria, and further investigation is required. 1-Hexanol is produced by the reduction of hexanal, which is produced by lipid oxidation [17]. Pseudomonas and Shewanella generate this VOC. These underscore the importance of understanding the dynamics of spoilage-causing bacteria and changes in VOCs in raw fish meat (OM and DM) to maintain meat quality.
4. Conclusions
In the present study, the effects of storage temperature on changes in the bacterial flora and quality of different muscle parts of yellowtail fish were investigated. SC storage effectively extended the shelf life of OM and DM by slowing bacterial growth and off-flavor development. However, storage at lower temperatures had limited inhibitory effects on the biochemical changes. Therefore, although SC storage is an effective method for maintaining fish meat quality for the fishing industry and consumers, further measures that can control these changes are needed. An analysis of VOCs indicated dynamic changes in their profiles, with these trends becoming less pronounced at lower temperatures. Notably, the patterns of change differed between OM and DM. Several VOCs were identified at both muscle sites as candidate markers of quality deterioration and signs of spoilage. These are expected to be new quality indicators for fish meat. In the bacterial flora analysis, Pseudomonas was the dominant spoilage bacterium under all storage conditions, regardless of the muscle type. Additionally, spoilage-associated genera such as Acinetobacter, Brochothrix, and Shewanella were present in certain proportions in the later stages of storage, with some exceptions. The metabolic activity of spoilage bacteria may significantly influence VOC concentrations. Further research on the correlation between specific spoilage VOCs and microbial activity is recommended to better understand the mechanisms of fish spoilage and odor production. Such insights could guide the development of more effective preservation strategies to support the sustainability and economic viability of the fishing industry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15062996/s1, Table S1: Biochemical and physiological properties of ordinary muscle (OM) and dark muscle (DM); Table S2: Application program and software used in this study; Table S3a: Changes in volatile organic compounds (VOCs) in raw yellowtail muscles stored at 3 °C (ng/g).; Table S3b: Changes in volatile organic compounds (VOCs) in raw yellowtail muscles stored at 0 °C (ng/g); Table S3c: Changes in volatile organic compounds (VOCs) in raw yellowtail muscles stored at −3 °C (ng/g).
Author Contributions
Conceptualization, S.T., A.M., A.F. and Y.J.; methodology, S.T., A.M., A.F. and G.O.; software, Y.J., S.I., G.O. and S.T.; validation, S.T. and A.F.; formal analysis, Y.J. and S.I.; investigation, Y.J., S.I., G.O. and S.T.; resources, S.T.; data curation, Y.J. and S.I.; writing—original draft preparation, Y.J., S.T. and S.I.; writing—review and editing, Y.J., S.T., S.I., A.M., A.F. and G.O.; visualization, Y.J. and S.I.; supervision, S.T.; project administration, S.T.; funding acquisition, S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by JSPS KAKENHI (grant number: JP20K02346).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Research Ethics Committee of Prefectural University of Hiroshima (protocol code 20HH003-1 and date (5 July 2023) of approval).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Materials. Further inquiries can be directed at the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DM | Dark muscle |
| SC | Super-chilled |
| VOC | Volatile organic compound |
| OM | Ordinary muscle |
| TMA | Trimethylamine |
| NGS | Next-generation sequencing |
| VCC | Visible colony count |
| TVB-N | Total volatile basic nitrogen |
| TBARS | Thiobarbituric acid reactive substances |
| CFU | Colony-forming unit |
| TCA | Trichloroacetic acid |
| HCA | Hierarchical clustering analysis |
| PCoA | Principal coordinate analysis |
| PCA | Principal component analysis |
References
- Yu, D.; Wu, L.; Regenstein, J.M.; Jiang, Q.; Yang, F.; Xu, Y.; Xia, W. Recent advances in quality retention of non-frozen fish and fishery products: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1747–1759. [Google Scholar] [CrossRef] [PubMed]
- Sandria, G.; Chen, F.C.; Chambers, E.; Coppings, R.; Chambers, D.H. A comprehensive evaluation of temperatures within home refrigerators. J. Food Sci. 2007, 4, 275–283. [Google Scholar]
- Evans, J.A. Effects of food and beverage storage, distribution, display, and consumer handling on shelf life. J. Food Sci. 2011, 7, 107–140. [Google Scholar] [CrossRef]
- Ge, L.; Xu, Y.; Xia, W.; Jiang, Q.; Jiang, X. Differential role of endogenous cathepsin and microorganism in texture softening of ice-stored grass carp (Ctenopharyngodon idella) fillets. J. Sci. Food. Agri. 2016, 96, 3233–3239. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; You, Y.; Yong, K.H.; Li, Y.; Jun, S. Impact of supercooling storage on physical and chemical properties of yellowfin tuna (Thunnus albacares). J. Food Eng. 2023, 373, 111818. [Google Scholar] [CrossRef]
- Kaale, L.D.; Eikevik, T.M.; Rustad, T.; Kolsaker, K. Superchilling of food: A review. J. Food Eng. 2011, 107, 141–146. [Google Scholar] [CrossRef]
- Magnussen, O.M.; Haugland, A.; Hemmingsen, A.K.T.; Johansen, S.; Nordtvedt, T.S. Advances in superchilling of food–process characteristics and product quality. Trends Food Sci. Technol. 2008, 19, 418–424. [Google Scholar] [CrossRef]
- Wu, T.; Wang, M.; Wang, P.; Tian, H.; Zhan, P. Advances in the formation and control methods of undesirable flavors in fish. Foods 2022, 11, 2504. [Google Scholar] [CrossRef]
- Tanimoto, S.; Shimoda, M. Changes in volatile compounds of dark and ordinary muscles of yellowtail (Seriola quinqueradiata) during short-term cold storage. J. Aquat. Food Prod. Technol. 2016, 25, 185–196. [Google Scholar] [CrossRef]
- Tanimoto, S.; Kikutani, H.; Kitabayashi, K.; Ohkita, T.; Arita, R.; Nishimura, S.; Takemoto, R.; Mabuchi, R.; Shimoda, M. Qualitative changes in each part of yellowtail (Seriola quinqueradiata) flesh during cold storage. Fish Sci. 2018, 84, 135–148. [Google Scholar] [CrossRef]
- Mayo, B.; Rachid, C.T.C.C.; Alegría, Á.; Leite, A.M.O.; Peixoto, R.S.; Delgado, S. Impact of next generation sequencing techniques in food microbiology. Curr. Genom. 2014, 15, 293–309. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulos, D.A.; Parlapani, F.F.; Boziaris, I.S. The evolution of knowledge on seafood spoilage microbiota from the 20th to the 21st century: Have we finished or just begun? Trends Food Sci. Technol. 2022, 120, 236–247. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Mallouchos, A.; Haroutounian, S.A.; Boziaris, I.S. Volatile organic compounds of microbial and non-microbial origin produced on model fish substrate uninoculated and inoculated with gilt-head sea bream spoilage bacteria. LWT-Food Sci. Technol. 2017, 78, 54–62. [Google Scholar] [CrossRef]
- Parlapani, F.F. Microbial diversity of seafood. Curr. Opin. Food Sci. 2021, 37, 45–51. [Google Scholar] [CrossRef]
- Production Statistics of The Fishery and Aquaculture Industries for 2020, Ministry of Agriculture, Forestry and Fisheries, Japan. Available online: https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00500216&tstat=000001015174&cycle=7&year=20220&month=0&tclass1=000001015175&tclass2=000001214760 (accessed on 24 February 2025).
- Tanimoto, S.; Hirata, Y.; Ishizu, S.; Wang, R.; Furuta, A.; Mabuchi, R.; Okada, G. Changes in the quality and microflora of yellowtail (Seriola quinqueradiata) muscles during cold storage. Foods 2024, 13, 1086. [Google Scholar] [CrossRef]
- Casaburi, A.; Piombino, P.; Nychas, G.; Villani, F.; Ercolini, D. Bacterial populations and the volatilome associated to meat spoilage. Food Microbiol. 2015, 45, 83–102. [Google Scholar] [CrossRef]
- Gardner, G.A. Streptomycin-thallous acetate-actidione (STAA) agar, a medium for the selective enumeration of Brochothrix thermosphacta. Int. J. Food Microbiol. 1985, 2, 69–70. [Google Scholar] [CrossRef]
- Shewan, J.M.; Hobbs, G.; Hodgkiss, W. The Pseudomonas and Achromobacter groups of bacteria in the spoilage of marine white fish. J. Appl. Microbiol. 1960, 23, 463–468. [Google Scholar] [CrossRef]
- Petruzzi, L.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Microbial spoilage of foods: Fundamentals. Food Microbiol. 2017, 1–21. [Google Scholar] [CrossRef]
- Nangulohi, M.N.; Shikongo-Nambabi, N.; Shoolongela, A.; Schneider, M. Control of bacterial contamination during marine fish processing. J. Biol. Life Sci. 2011, 3, 1–10. [Google Scholar] [CrossRef][Green Version]
- Conway, E.J. Microdiffusion analysis and volumetric error. Nature 1948, 161, 583. [Google Scholar] [CrossRef]
- Wang, R.; Ishizu, S.; Kondo, M.; Furuta, A.; Okada, G.; Tanimoto, S. Changes in quality and bacterial flora of red sea bream (Pagrus major) flesh during ice storage. Food Sci. Technol. Res. 2024, 30, 599–611. [Google Scholar] [CrossRef]
- Kitabayashi, K.; Tanimoto, S.; Kikutani, H.; Ohkita, T.; Mabuchi, R.; Shimoda, M. Effect of nitrogen gas packaging on odor development in yellowtail (Seriola quinqueradiata) muscle during ice storage. Fish. Sci. 2019, 85, 247–257. [Google Scholar] [CrossRef]
- Wang, R.; Hirabayashi, M.; Furuta, A.; Okazaki, T.; Tanimoto, S. Changes in extractive components and bacterial flora in live mussels (Mytilus galloprovincialis) during storage at different temperatures. J. Food Sci. 2023, 88, 1654–1671. [Google Scholar] [CrossRef]
- Regueira-Iglesias, A.; Balsa-Castro, C.; Blanco-Pintos, T.; Tomás, I. Critical review of 16S rRNA gene sequencing workflow in microbiome studies: From primer selection to advanced data analysis. Mol Oral Microbiol. 2023, 38, 347–399. [Google Scholar] [CrossRef]
- Calle, M.L. Statistical analysis of metagenomics data. Genomics Inform. 2019, 17, e6. [Google Scholar] [CrossRef]
- Zhang, Z.; Murtagh, F.; Van Poucke, S.; Lin, S.; Lan, P. Hierarchical cluster analysis in clinical research with heterogeneous study population: Highlighting its visualization with R. Ann. Transl. Med. 2017, 5, 75. [Google Scholar] [CrossRef]
- Mukojima, K.; Yoshii, M.; Nakashio, A.; Mabuchi, R.; Furuta, A.; Tanimoto, S. Effect of vacuum packing on the odor of yellowtail (Seriola quinqueradiata) flesh stored after heating. Fish. Sci. 2023, 89, 709–721. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Phil. Trans. R. Soc. A 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Mohammad, N.; Behrooz, A.B.; Ioannis, K.K. Volatilomic with chemometrics: A toward authentication approach. Eur. Food Res. Technol. 2023, 249, 2215–2226. [Google Scholar] [CrossRef]
- Zhong, H.; Wei, S.; Kang, M.; Sun, Q.; Xia, Q.; Wang, Z.; Han, Z.; Liu, Y.; Liu, M.; Liu, S. Effects of different storage condtions on microbial community and quality changes of greater amberjack (Seriola dumerili) fillets. LWT 2023, 179, 114640. [Google Scholar] [CrossRef]
- Park, D.H.; Lee, S.; Byeon, Y.M.; Kim, E.J.; Choi, M.J. Effect of supercooling storage applied with stepwise algorithm for fishes (salmon and olive flounder) and its freshness during extended storage. Food Biosci. 2022, 49, 101950. [Google Scholar] [CrossRef]
- Koseki, S.; Kitakami, S.; Kato, N.; Arai, K. Changes in stiffness and freshness (k value) of seafood after death. J. Sch. Mar. Sci. Technol. Tokai Univ. 2006, 4, 31–46. [Google Scholar]
- Sharifian, S.; Zakipour, E.; Mortazavi, M.S.; Arshadi, A. Quality assessment of tiger tooth croaker (Otolithes ruber) during ice storage. Int. J. Food Prop. 2011, 14, 309–318. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Vatsa, S.; Srivastav, P.P.; Pathak, S.S. A comprehensive review on freshness of fish and assessment: Analytical methods and recent innovations. Food Res. Int. 2020, 133, 109157. [Google Scholar] [CrossRef]
- European Commission. Commission Decision of 8 March 1995 fixing the total volatile basic nitrogen (TVB-N) limit values for certain categories of fishery products and specifying the analysis methods to be used. Off. J. Eur. Communities 1995, 97, 84–87. [Google Scholar]
- Prabhakar, P.K.; Srivastav, P.P.; Pathak, S.S. Kinetics of Total Volatile Basic Nitrogen and Trimethylamine Formation in Stored Rohu (Labeo rohita) Fish. J. Aquat. Food Prod. Technol. 2019, 28, 452–464. [Google Scholar] [CrossRef]
- Qiu, H.; Guo, X.; Deng, X.; Guo, X.; Mao, X.; Xu, C.; Zhang, J. The influence of endogenous cathepsin in different subcellular fractions on the quality deterioration of northern pike (Esox lucius) fillets during refrigeration and partial freezing storage. Food Sci. Biotechnol. 2020, 29, 1331–1341. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, S.G.; Lin, H.M. Changes in the physicochemical and volatile flavor characteristics of Scomberomorus ni-phnius during chilled and frozen storage. Food Sci. Technol. Res. 2012, 18, 747–754. [Google Scholar] [CrossRef][Green Version]
- Banerjee, R.; Maheswarappa, N.B. Superchilling of muscle foods: Potential alternative for chilling and freezing. Crit. Rev. Food Sci. Nutr. 2019, 59, 1256–1263. [Google Scholar] [CrossRef]
- Tsironi, T.N.; Petros, S.; Taoukis, P.S. Effect of storage temperature and osmotic pre-treatment with alternative solutes on the shelf-life of gilthead seabream (Sparus arata) fillets. Aquacult. Fish. 2017, 2, 39–47. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; Magdy, M. Metabarcoding profiling of microbial diversity associated with trout fish farming. Sci. Rep. 2021, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Clols-Fuentes, J.; Nguinkal, J.A.; Unger, P.; Kreikemeyer, B.; Palm, H.W. Bacterial community in african catfish (Clarias gariepinus) recirculating aquaculture systems under different stocking densities. Front. Mar. Sci. 2023, 10, 234. [Google Scholar] [CrossRef]
- Xia, J.; Jiang, N.; Zhang, B.; Sun, R.; Zhu, Y.; Xu, W.; Ma, Y. Bacterial changes in boiled crayfish between different storage periods and characterizations of the specific spoilage bacteria. Foods 2023, 12, 3006. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Georgalaki, M.; Anastasiou, R.; Alexandropoulou, A.-M.; Manolopoulou, E.; Zoumpopoulou, G.; Tsakalidou, E. Study of the microbiome of the cretan sour cream staka using amplicon sequencing and shotgun metagenomics and isolation of novel strains with an important antimicrobial potential. Foods 2024, 13, 1129. [Google Scholar] [CrossRef]
- Lindberg, A.M.; Ljungh, Å.; Ahrne, S.; Löfdahl, S.; Molin, G. Enterobacteriaceae found in high numbers in fish, minced meat and pasteurised milk or cream and the presence of toxin encoding genes. Int. J. Food Microbiol. 1998, 39, 11–17. [Google Scholar] [CrossRef]
- Vogel, B.F.; Venkateswaran, K.; Satomi, M.; Gram, L. Identification of Shewanella baltica as the most important h2s-producing species during iced storage of danish marine fish. Appl. Environ. Microbiol. 2005, 71, 6689–6697. [Google Scholar] [CrossRef]
- Chaillou, S.; Chaulot-Talmon, A.; Caekebeke, H.; Cardinal, M.; Christieans, S.; Denis, C.; Desmonts, M.; Dousset, X.; Feurer, C.; Hamon, E.; et al. Origin and ecological selection of core and food-specific bacterial communities associated with meat and seafood spoilage. ISME J. 2014, 9, 1105–1118. [Google Scholar] [CrossRef]
- Ercolini, D.; Russo, F.; Nasi, A.; Ferranti, P.; Villani, F. Mesophilic and psychrotrophic bacteria from meat and their spoilage potential in vitro and in beef. Appl. Environ. Microbiol. 2009, 75, 1990–2001. [Google Scholar] [CrossRef]
- Anagnostopoulos, D.A.; Parlapani, F.F.; Mallouchos, A.; Angelidou, A.; Syropoulou, F.; Minos, G.; Boziaris, I.S. Volatile organic compounds and 16s metabarcoding in ice-stored red seabream (Pagrus major). Foods 2022, 11, 666. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Michailidou, S.; Anagnostopoulos, D.A.; Sakellariou, A.; Pasentsis, K.; Psomopoulos, F.; Argiriou, A.; Haroutounian, S.A.; Boziaris, I.S. Microbial spoilage investigation of thawed common cuttlefish (Sepia officinalis) stored at 2 °C using next generation sequencing and volatilome analysis. Food Microbiol. 2018, 76, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Jingbin, Z.; Sijia, S.; Dongping, L.; Yongkang, L. Microbial communities and biogenic amines of crucian carp (Carassius auratus) fillets during partial freezing and chilled storage. Int. J. Food Prop. 2017, 20, 1053–1064. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Mullaeva, S.A.; Sazonova, O.I.; Petrikov, K.V.; Vetrova, A.A. Current research on simultaneous oxidation of aliphatic and aromatic hydrocarbons by bacteria of genus pseudomonas. Folia Microbiol. 2022, 67, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Parlapani, F.F.; Meziti, A.; Kormas, K.A.; Boziaris, I.S. Indigenous and spoilage microbiota of farmed sea bream stored in ice identified by phenotypic and 16s RNA gene analysis. Food Microbiol. 2013, 33, 85–89. [Google Scholar] [CrossRef]
- Tahiluddin, A.B.; Maribao, I.; Amlani, M.; Sarri, J.H. A review on spoilage microorganisms in fresh and processed aquatic food products. Food Bull. 2022, 10, 245–257. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Jones, D. The Genus Brochothrix. In The Prokaryotes: A Handbook on the Biology of Bacteria; Dworkin, M., Ed.; Springer: Berlin, Germany, 2006; Volume 3, pp. 861–872. [Google Scholar] [CrossRef]
- Wong, J.X.; Ramli, S.; Son, R. A review: Characteristics and prevalence of psychrotolerant food spoilage bacteria in chill-stored meat, milk and fish. Food Res. 2023, 7, 215–226. [Google Scholar] [CrossRef]
- Zotta, T.; Parente, E.; Ianniello, R.G.; De Filippis, F.; Ricciardi, A. dynamics of bacterial communities and interaction networks in thawed fish fillets during chilled storage in air. Int. J. Food Microbiol. 2019, 293, 102–113. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Role of lipids in food flavor generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef]
- Zhuang, S.; Hong, H.; Zhang, L.; Luo, Y. Spoilage-related microbiota in fish and crustaceans during storage: Research progress and future trends. Compr. Rev. Food Sci. Food Saf. 2021, 20, 252–288. [Google Scholar] [CrossRef]
- Audrain, B.; Farag, M.A.; Ryu, C.M.; Ghigo, J.M. Role of bacterial volatile compounds in bacterial biology. FEMS Microbiol. Rev. 2015, 39, 222–233. [Google Scholar] [CrossRef]
- Hu, S.P.; Pan, B.S. Modification of fish oil aroma using a macroalgal lipoxygenase. J Amer. Oil Chem. Soc. 2000, 77, 343–348. [Google Scholar] [CrossRef]
- Lin, H.; Xue, C.H.; Li, Z.J.; Lou, W.F.; Chen, X.B. Studies on the volatile compounds of spoiled yellowtail (Seriola aureovittata) during storage. Period. Ocean Univ. China 1995, 25, 474–480. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).