In Vitro Protective Effects of Resveratrol-Loaded Pluronic Micelles Against Hydrogen Peroxide-Induced Oxidative Damage in U87MG Glioblastoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formulation of Resveratrol and Rhodamine B-Loaded Micelles
2.3. Cell Line and Cultivation Condition
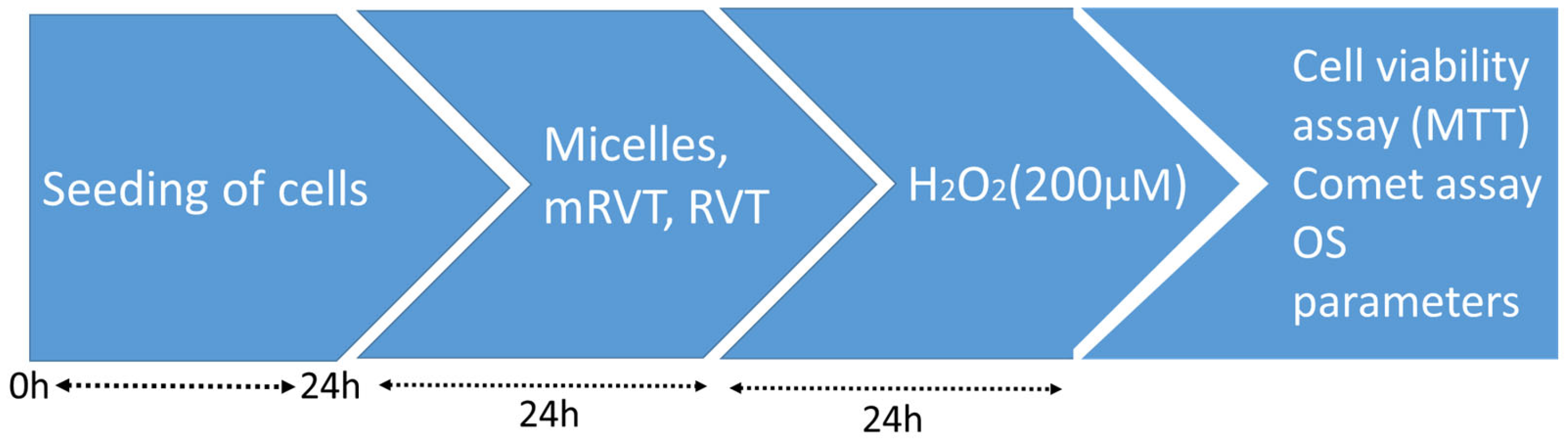
2.4. Cell Treatment
2.5. Cell Viability
2.6. Cells Homogenates Preparation
2.7. Oxidative Stress Parameters Measurement
2.7.1. Lipid Peroxidation Activity
2.7.2. Superoxide Dismutase Assay
2.7.3. Catalase Assay
2.8. AChE Activity in U87MG Glioblastoma Cell Homogenates
2.9. Single-Cell Gel Electrophoresis (The Comet Assay)
2.10. Nanoparticles Uptake by U87MG Cells
2.11. Statistical Analysis
3. Results
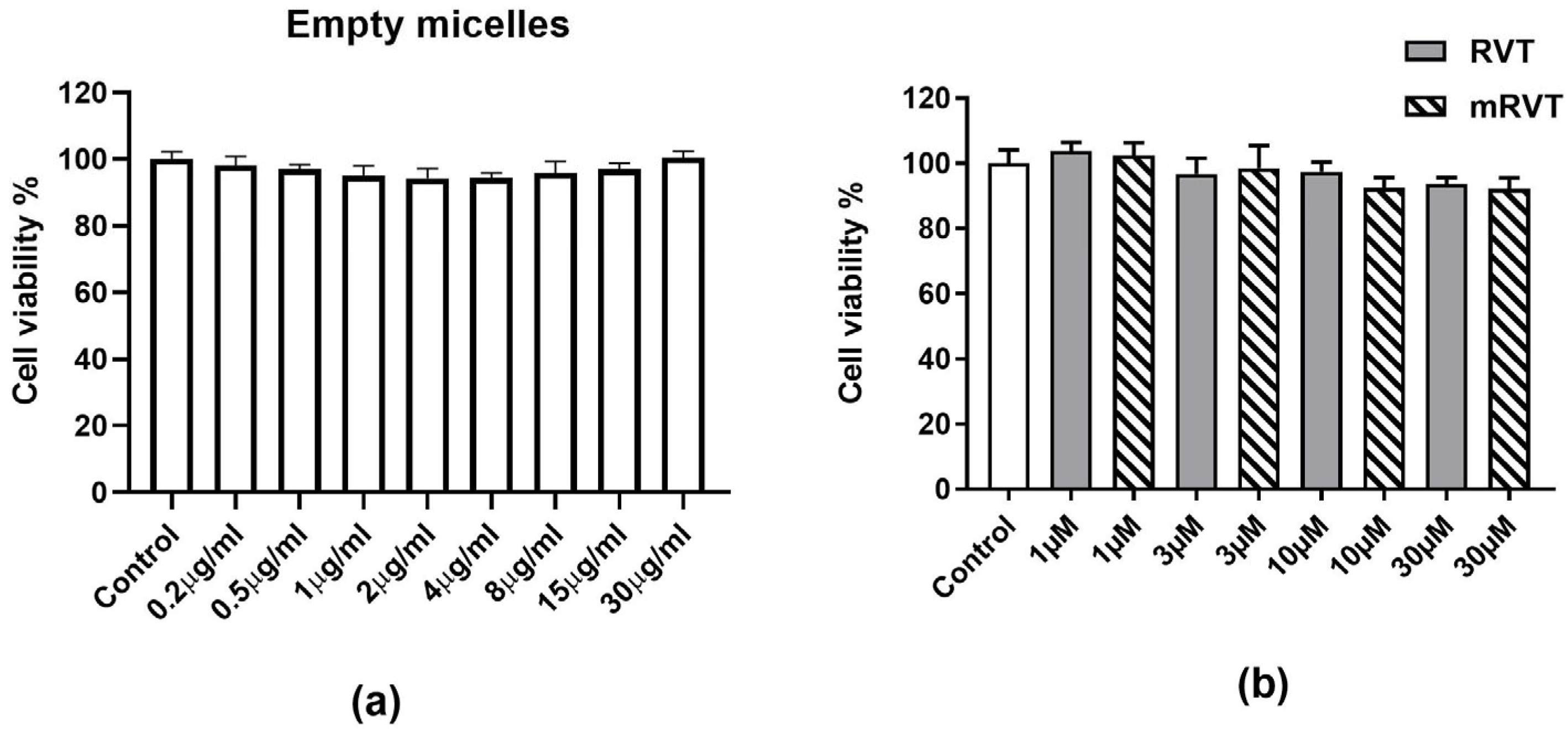
3.1. Effects of Empty Micelles, RVT, and mRVT Treatment on Cell Viability (MTT Assay)
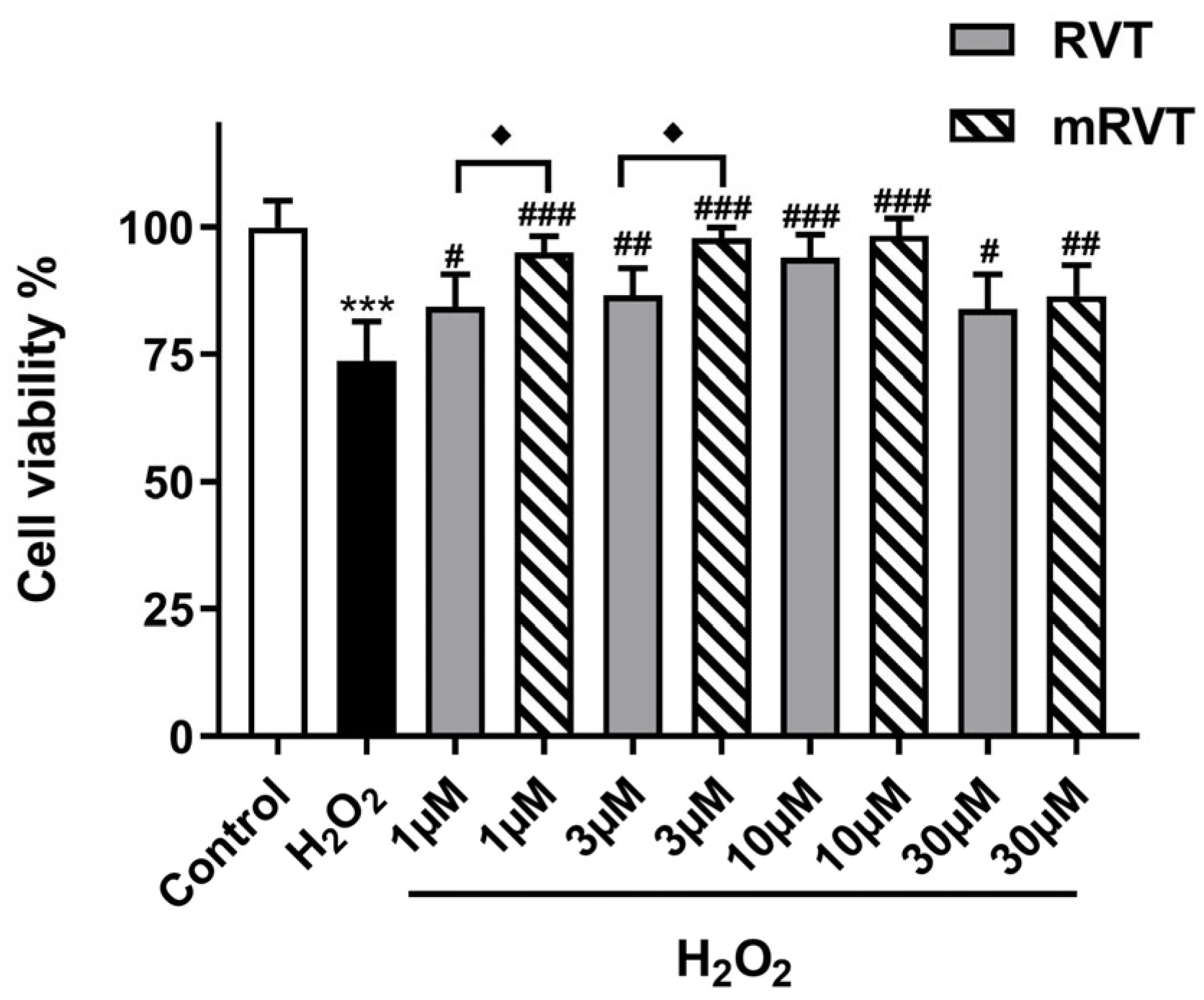
3.2. Cell Viability and Protection in the Model of H2O2-Induced Oxidative Damage
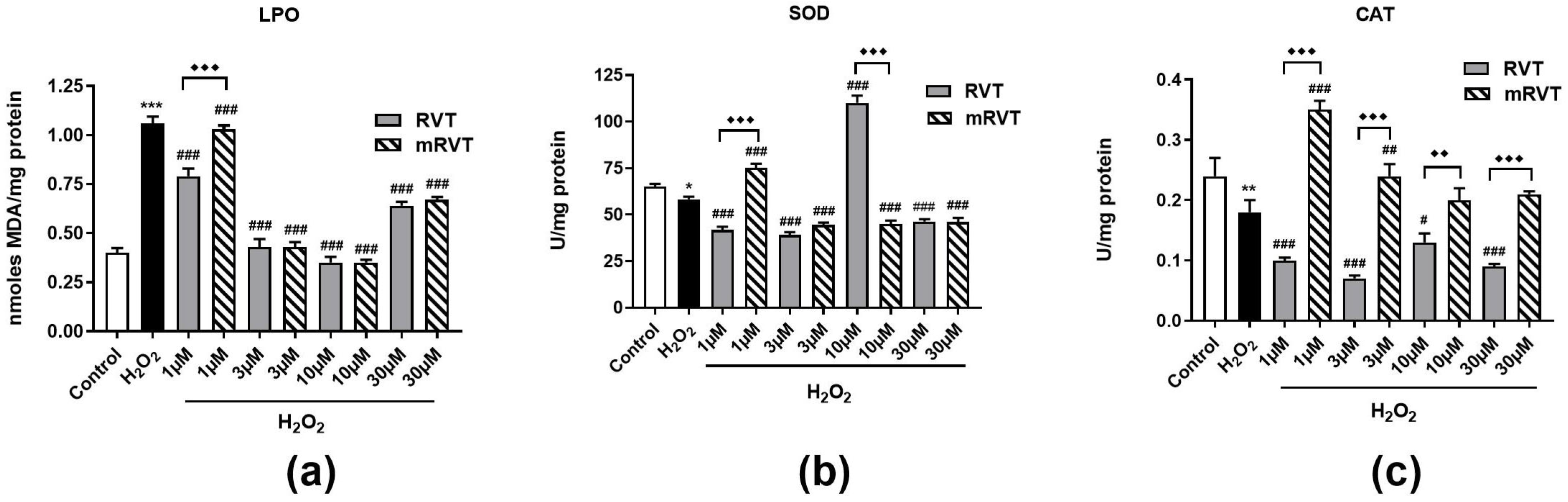
3.3. Effects of RVT and mRVT Treatment Against H2O2-Induced Oxidative Stress in U87MG Cells
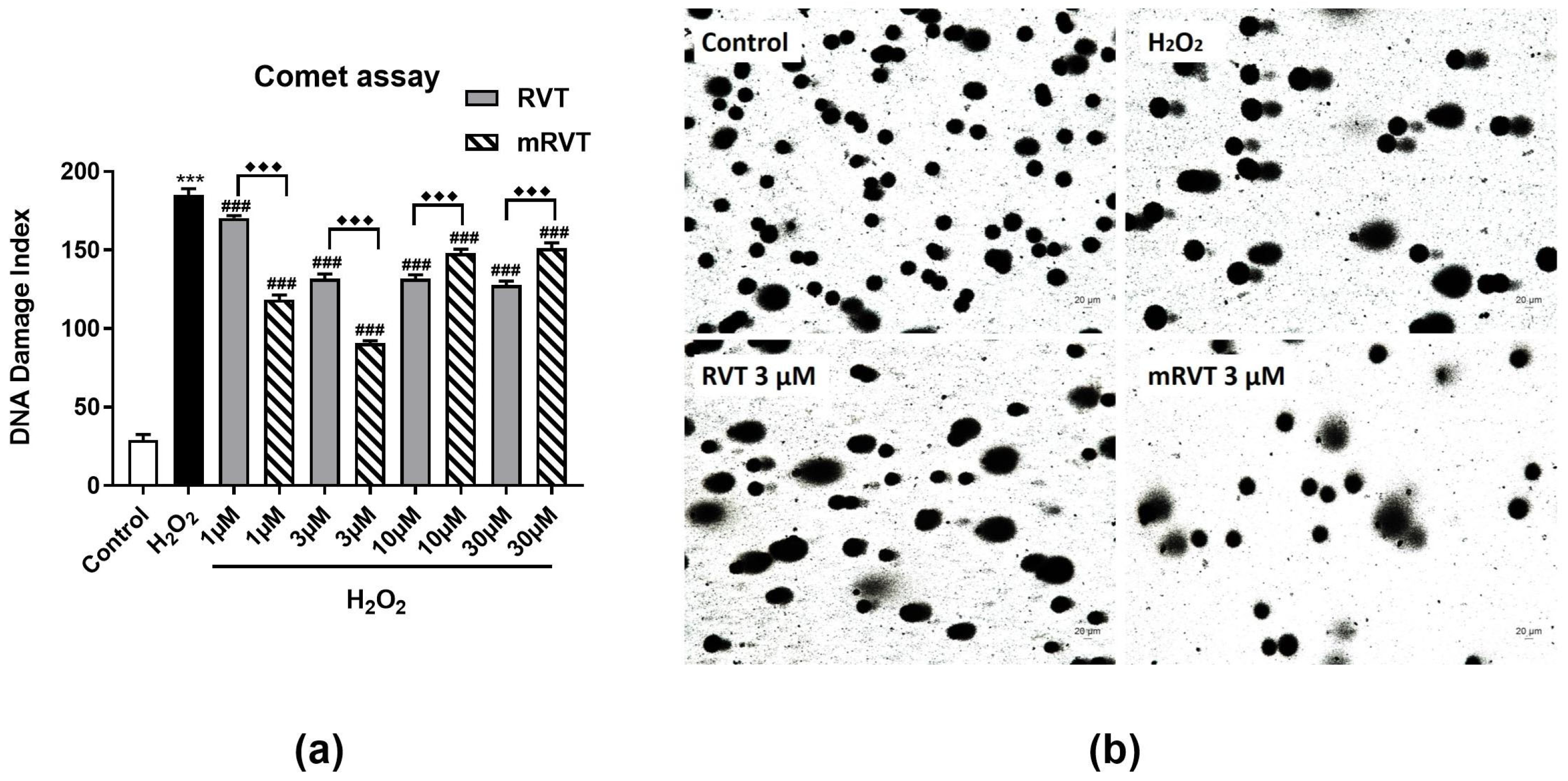
3.4. Effects of RVT and mRVT on DNA Damge
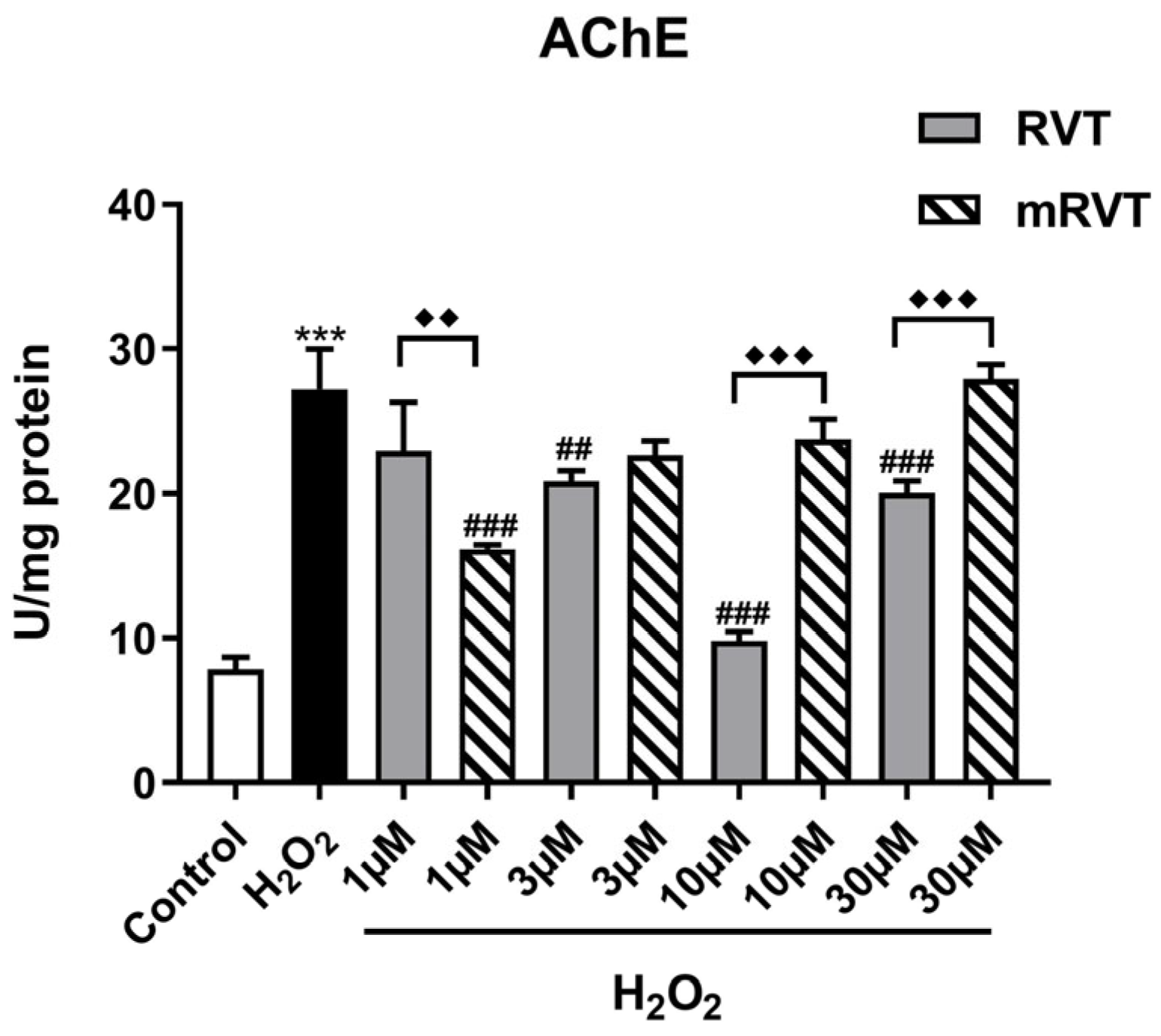
3.5. Effects of RVT and mRVT Treatments on H2O2-Induced Increase in Acetylcholinesterase Activity in U87MG Glioma Cells Homogenate
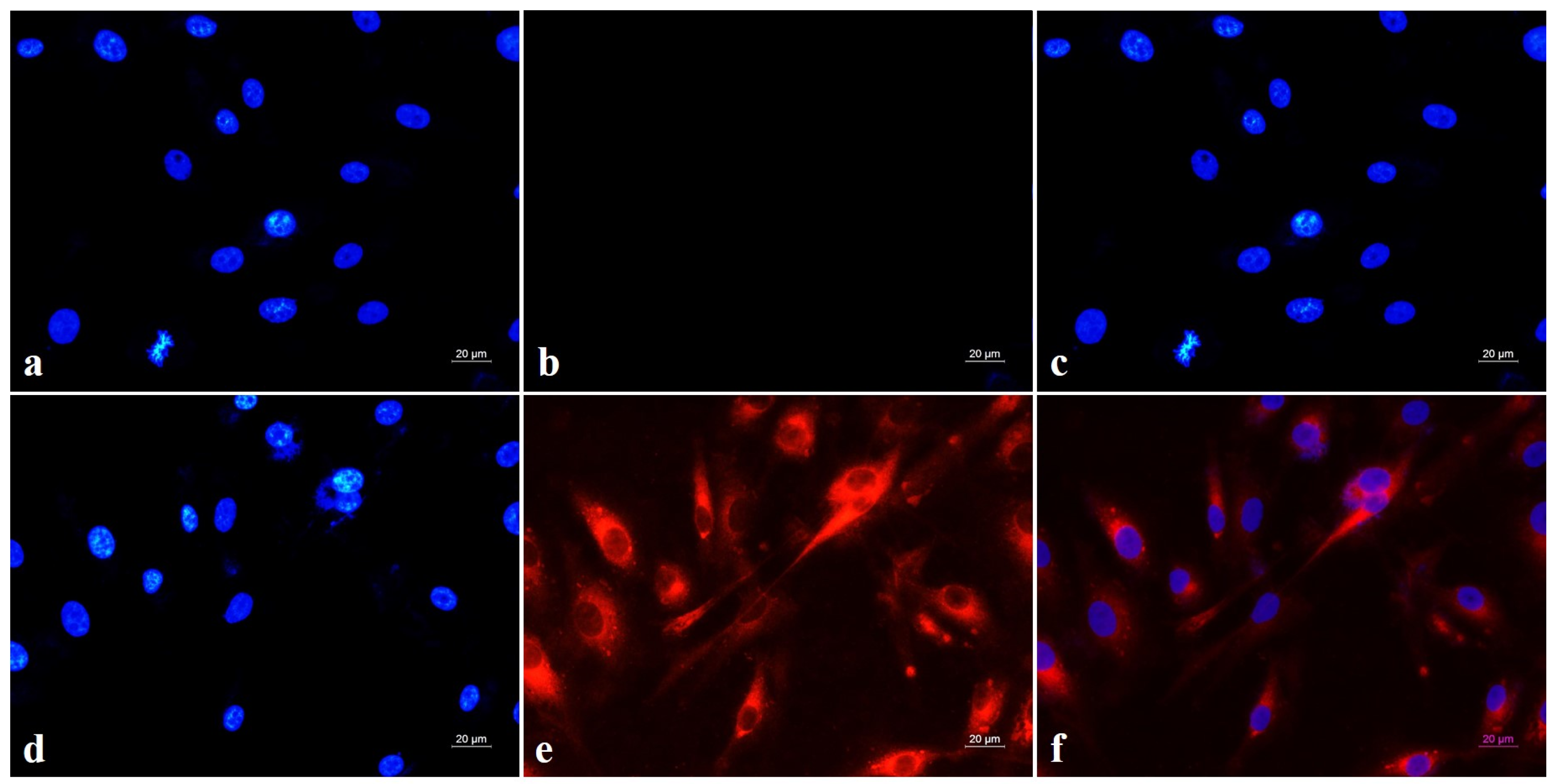
3.6. Intracellular Micellar Penetration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coyle, J.T.; Puttfarcken, P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993, 262, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Dringen, R. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 2000, 62, 649–671. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I.; Storey, K.B. Oxidative stress concept updated: Definitions, classifications, and regulatory pathways implicated. EXCLI J. 2021, 20, 956–967. [Google Scholar]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef]
- Scarian, E.; Viola, C.; Dragoni, F.; Di Gerlando, R.; Rizzo, B.; Diamanti, L.; Gagliardi, S.; Bordoni, M.; Pansarasa, O. New insights into oxidative stress and inflammatory response in neurodegenerative diseases. Int. J. Mol. Sci. 2024, 25, 2698. [Google Scholar] [CrossRef]
- Li, J.; Wuliji, O.; Li, W.; Jiang, Z.-G.; Ghanbari, H.A. Oxidative stress and neurodegenerative disorders. Int. J. Mol. Sci. 2013, 14, 24438–24475. [Google Scholar] [CrossRef]
- Zhao, X.; Fang, J.; Li, S.; Gaur, U.; Xing, X.; Wang, H.; Zheng, W. Artemisinin attenuated hydrogen peroxide (H2O2)-induced oxidative injury in SH-SY5Y and hippocampal neurons via the activation of AMPK Pathway. Int. J. Mol. Sci. 2019, 20, 2680. [Google Scholar] [CrossRef]
- Dickens, M.G.; Franz, K.J. A prochelator activated by hydrogen peroxide prevents metal-induced amyloid-aggregation. Chem. Biochem. 2010, 11, 59–62. [Google Scholar] [CrossRef]
- Yamato, M.; Kudo, W.; Shiba, T.; Yamada, K.I.; Watanabe, T.; Utsumi, H. Determination of ROS associated with the degeneration of dopaminergic neurons during dopamine metabolism. Free Radic. Res. 2010, 44, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ferreiro, A.; Gil-Longo, J. Vascular pro-oxidant effects related to the autoxidation of dopamine. Free Radic. Res. 2009, 43, 295–303. [Google Scholar] [CrossRef]
- Arthur, P.G.; Lim, S.C.; Meloni, B.P.; Munns, S.E.; Chan, A.; Knuckey, N.W. The protective effect of hypoxic preconditioning on cortical neuronal cultures is associated with increases in the activity of several antioxidant enzymes. Brain Res. 2004, 1017, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.H.; Dar, K.B.; Anees, S.; Zargar, M.A.; Masood, A.; Sofi, M.A.; Ganie, S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, E.; Ivanova, D.; Zhelev, Z.; Bakalova, R.; Gulubova, M.; Aoki, I. Mitochondrial Dysfunction and Redox Imbalance as a Diagnostic Marker of “Free Radical Diseases”. Anticancer Res. 2017, 37, 5373–5381. [Google Scholar]
- Nguyen-Thi, P.T.; Vo, T.K.; Pham, T.H.T.; Nguyen, T.T.; Van Vo, G. Natural flavonoids as potential therapeutics in the management of Alzheimer’s disease: A review. 3 Biotech 2024, 14, 68. [Google Scholar] [CrossRef]
- Takaoka, M.J. Of the phenolic substances of white hellebore (Veratrum grandiflorum Loes. fil.). J. Fac. Sci. Hokkaido Imp. Univ. 1940, 3, 1–16. [Google Scholar] [CrossRef]
- Milardi, G.L.; Stringaro, A.; Colone, M.; Bonicontro, A.; Risuleo, G. The cell membrane is the main target of resveratrol as shown by interdisciplinary biomolecular/cellular and biophysical approaches. J. Membr. Biol. 2014, 247, 1–8. [Google Scholar] [CrossRef]
- Gambini, J.; Ingles, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef]
- Cristòfol, R.; Porquet, D.; Corpas, R.; Coto-Montes, A.; Serret, J.; Camins, A.; Pallàs, M.; Sanfeliu, C. Neurons from senescence-accelerated SAMP8 mice are protected against frailty by the sirtuin 1 promoting agents melatonin and resveratrol. J. Pineal Res. 2012, 52, 271–281. [Google Scholar] [CrossRef]
- Bai, Y.; Mao, Q.-Q.; Qin, J.; Zheng, X.Y.; Wang, Y.B.; Yang, K.; Shen, H.F.; Xie, L.P. Resveratrol induces apoptosis and cell cycle arrest of human T24 bladder cancer cells in vitro and inhibits tumor growth in vivo. Cancer Sci. 2010, 101, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Fremont, L. Minireview: Biological effects of resveratrol. Life Sci. 2020, 66, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Carrizzo, A.; Forte, M.; Damato, A.; Trimarco, V.; Salzano, F.; Bartolo, M.; Maciag, A.; Puca, A.A.; Vecchione, C. Antioxidant effects of resveratrol in cardiovascular, cerebral and metabolic diseases. Food Chem. Toxicol. 2013, 61, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Han, Q.; Wang, G.; Ma, W.-P.; Wang, J.; Wu, W.-X.; Guo, Y.; Liu, L.; Jiang, X.-Y.; Xie, X.-L.; et al. Resveratrol Protects Oxidative Stress-Induced Intestinal Epithelial Barrier Dysfunction by Upregulating Heme Oxygenase-1 Expression. Dig. Dis. Sci. 2016, 61, 2522–2534. [Google Scholar] [CrossRef]
- Jang, J.H.; Surh, Y.J. Protective effects of resveratrol on β-amyloid-induced oxidative PC12 cell death. Free Radic. Biol. Med. 2003, 34, 1100–1110. [Google Scholar] [CrossRef]
- Russo, A.; Palumbo, M.; Aliano, C.; Lempereur, L.; Scoto, G.; Renis, M. Red wine micronutrients as protective agents in Alzheimer-like induced insult. Life Sci. 2003, 72, 2369–2379. [Google Scholar] [CrossRef]
- Sharma, M.; Gupta, Y.K. Chronic treatment with trans resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats. Life Sci. 2002, 71, 2489–2498. [Google Scholar] [CrossRef]
- Savaskan, E.; Olivieri, G.; Meier, F.; Seifritz, E.; Wirz-Justice, A.; Muller-Spahn, F. Red wine ingredient resveratrol protects frombeta-amyloid neurotoxicity. Gerontology 2003, 49, 380–383. [Google Scholar] [CrossRef]
- Han, Y.S.; Zheng, W.H.; Bastianetto, S.; Chabot, J.G.; Quirion, R. Neuroprotective effects of resveratrol against beta-amyloid-induced neurotoxicity in rat hippo-campal neurons: Involvement of protein kinase C. Br. J. Pharmacol. 2004, 141, 997–1005. [Google Scholar] [CrossRef]
- Ginés, C.; Cuesta, S.; Kireev, R.; García, C.; Rancan, L.; Paredes, S.D.; Vara, E.; Tresguerres, J.A. Protective effect of resveratrol against inflammation, oxidative stress and apoptosis in pancreas of aged SAMP8 mice. Exp. Gerontol. 2017, 90, 61–70. [Google Scholar] [CrossRef]
- Fu, S.; Lv, R.; Wang, L.; Hou, H.; Liu, H.; Shao, S. Resveratrol, an antioxidant, protects spinal cord injury in rats by suppressing MAPK pathway. Saudi J. Biol. Sci. 2018, 25, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Francioso, A.; Mastromarino, P.; Masci, A.; d’Erme, M.; Mosca, L. Chemistry, stability and bioavailability of resveratrol. Med. Chem. 2014, 10, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Amri, A.; Chaumeil, J.C.; Sfar, S.; Charrueau, C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J. Control. Release 2012, 158, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Zupancic, S.; Lavric, Z.; Kristl, J. Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204. [Google Scholar] [CrossRef]
- Peterle, L.; Sanfilippo, S.; Borgia, F.; Li Pomi, F.; Vadalà, R.; Costa, R.; Cicero, N.; Gangemi, S. The Role of Nutraceuticals and Functional Foods in Skin Cancer: Mechanisms and Therapeutic Potential. Foods 2023, 12, 2629. [Google Scholar] [CrossRef]
- Oerlemans, C.; Bult, W.; Bos, M.; Storm, G.; Nijsen, J.F.; Hennink, W.E. Polymeric micelles in anticancer therapy: Targeting, imaging and triggered release. Pharm. Res. 2010, 27, 2569–2589. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Alakhov, V.Y. Pluronic® block copolymers in drug delivery: From micellar nanocontainers to biological response modifiers. Crit. Rev. Ther. Drug 2002, 19, 1–73. [Google Scholar] [CrossRef]
- Yoncheva, K.; Calleja, P.; Agueros, M.; Petrov, P.; Miladinova, I.; Tsvetanov, C.; Irache, J.M. Stabilized Micelles as Delivery Vehicles for Paclitaxel. Int. J. Pharm. 2012, 436, 258–264. [Google Scholar] [CrossRef]
- Taha, E.I.; Badran, M.M.; El-Anazi, M.H.; Bayomi, M.A.; El-Bagory, I.M. Role of Pluronic F127 Micelles in Enhancing Ocular Delivery of Ciprofloxacin. J. Mol. Liq. 2014, 199, 251–256. [Google Scholar] [CrossRef]
- Niu, J.; Yuan, M.; Chen, C.; Wang, L.; Tang, Z.; Fan, Y.; Liu, X.; Ma, Y.J.; Gan, Y. Berberine-Loaded Thiolated Pluronic F127 Polymeric Micelles for Improving Skin Permeation and Retention. Int. J. Nanomed. 2020, 15, 9987–10005. [Google Scholar] [CrossRef]
- Yordanov, Y.; Stefanova, D.; Spassova, I.; Kovacheva, D.; Tzankova, V.; Konstantinov, S.; Yoncheva, K. Formulation of Nanomicelles Loaded with Cannabidiol as a Platform for Neuroprotective Therapy. Pharmaceutics 2022, 14, 2625. [Google Scholar] [CrossRef] [PubMed]
- Hersh, A.M.; Alomari, S.; Tyler, B.M. Crossing the Blood-Brain Barrier: Advances in Nanoparticle Technology for Drug Delivery in Neuro-Oncology. Int. J. Mol. Sci. 2022, 23, 4153. [Google Scholar] [CrossRef] [PubMed]
- Lazarova, M.; Stefanova, M.; Tsvetanova, E.; Georgieva, A.; Tasheva, K.; Radeva, L.; Yoncheva, K. Resveratrol-Loaded Pluronic Micelles Ameliorate Scopolamine-Induced Cognitive Dysfunction Targeting Acetylcholinesterase Activity and Programmed Cell Death. Int. J. Mol. Sci. 2024, 25, 12777. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, J.V.; Featherstone, R.M. Anew and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1969, 7, 88–90. [Google Scholar] [CrossRef]
- Sulikovska, I.; Djeliova, V.; Kirazov, L.; Ivanov, I.; Dimitrova, M. Evaluation of different staining methods and image analyses of the results from a comet assay in human colorectal cancer cells treated with hydrogen peroxide. Acta Morphol. Anthropol. 2023, 30, 5–13. [Google Scholar] [CrossRef]
- Azqueta, A.; Langie, S.A.S.; Boutet-Robinet, E.; Duthie, S.; Ladeira, C.; Møller, P.; Collins, A.R.; Godschalk, R.W.L. DNA repair as a human biomonitoring tool: Comet assay approaches. Mutat. Res. Rev. Mutat. Res. 2019, 781, 71–87. [Google Scholar] [CrossRef]
- Noroozi, M.; Angerson, W.J.; Lean, M.E.J. Effects of flavonoids and Vitamin C on oxidative DNA damage to human lymphocytes. Am. J. Clin. Nutr. 1998, 67, 1210–1218. [Google Scholar] [CrossRef]
- Mayne, K.; White, J.A.; McMurran, C.E.; Rivera, F.J.; de la Fuente, A.G. Aging and neurodegenerative disease: Is the adaptive immune system a friend or foe? Front. Aging Neurosci. 2020, 12, 572090. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative stress and antioxidants in neurodegenerative disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- He, W.J.; Lv, C.H.; Chen, Z.; Shi, M.; Zeng, C.X.; Hou, D.X.; Qin, S. The regulatory effect of phytochemicals on chronic diseases by targeting Nrf2-ARE signaling pathway. Antioxidants 2023, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.; Gogia, N.; Singh, A. Exploring the efficacy of natural products in alleviating Alzheimer’s disease. Neural. Regen. Res. 2019, 14, 1321–1329. [Google Scholar] [PubMed]
- Venkatesan, R.; Ji, E.; Kim, S.Y. Phytochemicals that regulate neurodegenerative disease by targeting neurotrophins: A comprehensive review. BioMed Res. Int. 2015, 2015, 814068. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Jahan, S.; Alshahrani, S.; Alshehri, B.M.; Sameer, A.S.; Arafah, A.; Ahmad, A.; Rehman, M.U. Phytotherapeutic agents for neurodegenerative disorders: A neuropharmacological review. In Phytomedicine; Academic Press: Cambridge, MA, USA, 2021; pp. 581–620. [Google Scholar]
- Chen, B.; Zhao, J.; Zhang, R.; Zhang, L.; Zhang, Q.; Yang, H.; An, J. Neuroprotective effects of natural compounds on neurotoxininduced oxidative stress and cell apoptosis. Nutr. Neurosci. 2022, 25, 1078–1099. [Google Scholar] [CrossRef]
- Tie, F.; Fu, Y.; Hu, N.; Wang, H. Silibinin protects against H2O2-induced oxidative damage in SH-SY5Y cells by improving mitochondrial function. Antioxidants 2022, 11, 1101. [Google Scholar] [CrossRef]
- Wang, C.M.; Yang, C.Q.; Cheng, B.H.; Chen, J.; Bai, B. Orexin-A protects SH-SY5Y cells against H2O2-induced oxidative damage via the PI3K/MEK1/2/ERK1/2 signaling pathway. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418785739. [Google Scholar] [CrossRef]
- Jang, M.H.; Piao, X.L.; Young, K.H.; Cho, E.J. Resveratrol oligomers from vitis amurensis attenuate -amyloid-induced oxidative stress in PC12 cells. Biol. Pharm. Bull. 2007, 30, 1130–1134. [Google Scholar] [CrossRef]
- Loh, K.P.; Huang, S.H.; Silva, R.D.; Tan, B.K.H.; Zhu, Y.Z. Oxidative stress: Apoptosis in neuronal injury. Curr. Alzheimer Res. 2006, 3, 327–337. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Concept and some practical aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Konno, T.; Melo, E.P.; Chambers, J.E.; Avezov, E. Intracellular sources of ROS/H2O2 in health and neurodegeneration: Spotlight on endoplasmic reticulum. Cells 2021, 10, 233. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992, 59, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Surh, Y.J. Possible role of NF-κB in Bcl-XL protection against hydrogen peroxide-induced PC12 cell death. Redox Rep. 2014, 9, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Aruoma, O.I. DNA damage by oxygen-derived species. Itsmechanism and measurement in mammalian systems. FEBS Lett. 1991, 28, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, H.; Sun, Y.; Lin, X.; Chen, B.; Tan, C.; Cao, G.; Wang, Z. Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur. J. Pharmacol. 2007, 564, 18–25. [Google Scholar] [CrossRef]
- Asada, K. Ascorbate peroxidase-a hydrogen peroxide-scavenging enzyme in plants. Physiol. Plant. 1992, 85, 235–241. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Yoshikawa, A.; Saito, Y.; Maruyama, K. Lignan compounds and 4,4′-dihydroxybiphenyl protect C2C12 cells against damage from oxidative stress. Biochem. Biophys. Res. Commun. 2006, 344, 394–399. [Google Scholar] [CrossRef]
- El Gamal, A.A.; AlSaid, M.S.; Raish, M.; Al-Sohaibani, M.; Al-Massarani, S.M.; Ahmad, A.; Rafatullah, S. Beetroot (Beta vulgaris L.) extract ameliorates gentamicin-induced nephrotoxicity associated oxidative stress, inflammation, and apoptosis in rodent model. Mediat. Inflamm. 2014, 2014, 983952. [Google Scholar] [CrossRef]
- Gopalan, M.; Jadhav, A.S. Protective effect beetroot Beta vulgaris extract against H2O2 Induced Oxidative stress in U87MG gliomacells. South Asian J. Exp. Biol. 2021, 11, 266–274. [Google Scholar] [CrossRef]
- Lu, X.; Ji, C.; Xu, H.; Li, X.; Ding, H.; Ye, M.; Zhu, Z.; Ding, D.; Jiang, X.; Ding, X.; et al. Resveratrol-loaded polymeric micelles protect cells from Abeta-induced oxidative stress. Int. J. Pharm. 2009, 375, 89–96. [Google Scholar] [CrossRef]
- Raj, A.; Dey, S.; Rao, S.; Madhunapantula, V.; Manjula, S.N. Neuroprotective And Cognitive Enhancing Effect Of Methanolic Morus Alba Leaf Fraction In U87mg Cell Lines And Experimental Rat Model. Asian J. Pharm. Clin. Res. 2019, 12, 238–243. [Google Scholar]
- Martemucci, G.; Portincasa, P.; Di Ciaula, A.; Mariano, M.; Centonze, V.; D’Alessandro, A.G. Oxidative stress, aging, antioxidant supplementation and their impact on human health: An overview. Mech. Ageing Dev. 2022, 206, 111707. [Google Scholar] [CrossRef]
- Quincozes-Santos, A.; Andreazza, A.C.; Nardin, P.; Funchal, C.; Gonçalves, C.A.; Gottfried, C. Resveratrol attenuates oxidative-induced DNA damage in C6 Glioma cells. Neurotoxicology 2007, 28, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Hirrlinger, J.; Resch, A.; Gutterer, J.M.; Dringen, R. Oligodendroglial cells in culture effectively dispose of exogenous hydrogen peroxide: Comparison with cultured neurones, astroglial and microglial cells. J. Neurochem. 2002, 82, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Ballard, C.; Greig, N.; Guillozet-Bongaarts, A.; Enz, A.; Darvesh, S. Cholinesterases: Roles in the brain during health and disease. Curr. Alzheimer Res. 2005, 2, 307–318. [Google Scholar] [CrossRef]
- Francis, P.T.; Palmer, A.M.; Snape, M.; Wilcock, G.K. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry 1999, 66, 137–147. [Google Scholar] [CrossRef]
- Foudah, A.I.; Devi, S.; Alam, A.; Salkini, M.A.; Ross, S.A. Anticholinergic Effect of Resveratrol with Vitamin E on Scopolamine-Induced Alzheimer’s Disease in Rats: Mechanistic Approach to Prevent Inflammation. Front. Pharmacol. 2023, 14, 1115721. [Google Scholar] [CrossRef]
- Deng, Z.; Li, Y.; Liu, H.; Xiao, S.; Li, L.; Tian, J.; Cheng, C.; Zhang, G.; Zhang, F. The role of sirtuin 1 and its activator, resveratrol in osteoarthritis. Biosci. Rep. 2019, 39, BSR20190189. [Google Scholar] [CrossRef]
- Athar, M.; Back, J.H.; Tang, X.; Kim, A. Resveratrol: A reviewof preclinical studies for human cancer prevention. Toxicol. Appl. Pharmacol. 2007, 224, 274–283. [Google Scholar] [CrossRef]
- Szende, B.; Tyihбk, E.; Kirбly-Vйghely, Z. Dose-dependent effect of resveratrol on proliferation and apoptosis in endothelial and tumor cell cultures. Exp. Mol. Med. 2000, 32, 88–92. [Google Scholar] [CrossRef]
- Sotomatsu, A.; Nakano, M.; Hirai, S. Phospholipid peroxidation induced by the catechol-Fe31(Cu21) complex: A possible mechanism of nigrostriatal cell damage. Arch. Biochem. Biophys. 1990, 283, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.; Burdon, R. Free radical–Lipid interactions and their pathological consequences. Prog. Lipid Res. 1993, 32, 71–110. [Google Scholar] [CrossRef] [PubMed]
- Galati, G.; Sabzevari, O.; Wilson, J.X.; O’Brien, P.J. Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicology 2002, 177, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Minotti, G.; Aust, S.D. The role of iron in oxygen radical mediated lipid peroxidation. Chem. Biol. Interact. 1989, 71, 1–19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulikovska, I.; Tsvetanova, E.; Georgieva, A.; Djeliova, V.; Radeva, L.; Yoncheva, K.; Lazarova, M. In Vitro Protective Effects of Resveratrol-Loaded Pluronic Micelles Against Hydrogen Peroxide-Induced Oxidative Damage in U87MG Glioblastoma Cells. Appl. Sci. 2025, 15, 2995. https://doi.org/10.3390/app15062995
Sulikovska I, Tsvetanova E, Georgieva A, Djeliova V, Radeva L, Yoncheva K, Lazarova M. In Vitro Protective Effects of Resveratrol-Loaded Pluronic Micelles Against Hydrogen Peroxide-Induced Oxidative Damage in U87MG Glioblastoma Cells. Applied Sciences. 2025; 15(6):2995. https://doi.org/10.3390/app15062995
Chicago/Turabian StyleSulikovska, Inna, Elina Tsvetanova, Almira Georgieva, Vera Djeliova, Lyubomira Radeva, Krassimira Yoncheva, and Maria Lazarova. 2025. "In Vitro Protective Effects of Resveratrol-Loaded Pluronic Micelles Against Hydrogen Peroxide-Induced Oxidative Damage in U87MG Glioblastoma Cells" Applied Sciences 15, no. 6: 2995. https://doi.org/10.3390/app15062995
APA StyleSulikovska, I., Tsvetanova, E., Georgieva, A., Djeliova, V., Radeva, L., Yoncheva, K., & Lazarova, M. (2025). In Vitro Protective Effects of Resveratrol-Loaded Pluronic Micelles Against Hydrogen Peroxide-Induced Oxidative Damage in U87MG Glioblastoma Cells. Applied Sciences, 15(6), 2995. https://doi.org/10.3390/app15062995







