Numerical Modeling of Bowel Sound Propagation: Impact of Abdominal Tissue Properties
Abstract
1. Introduction
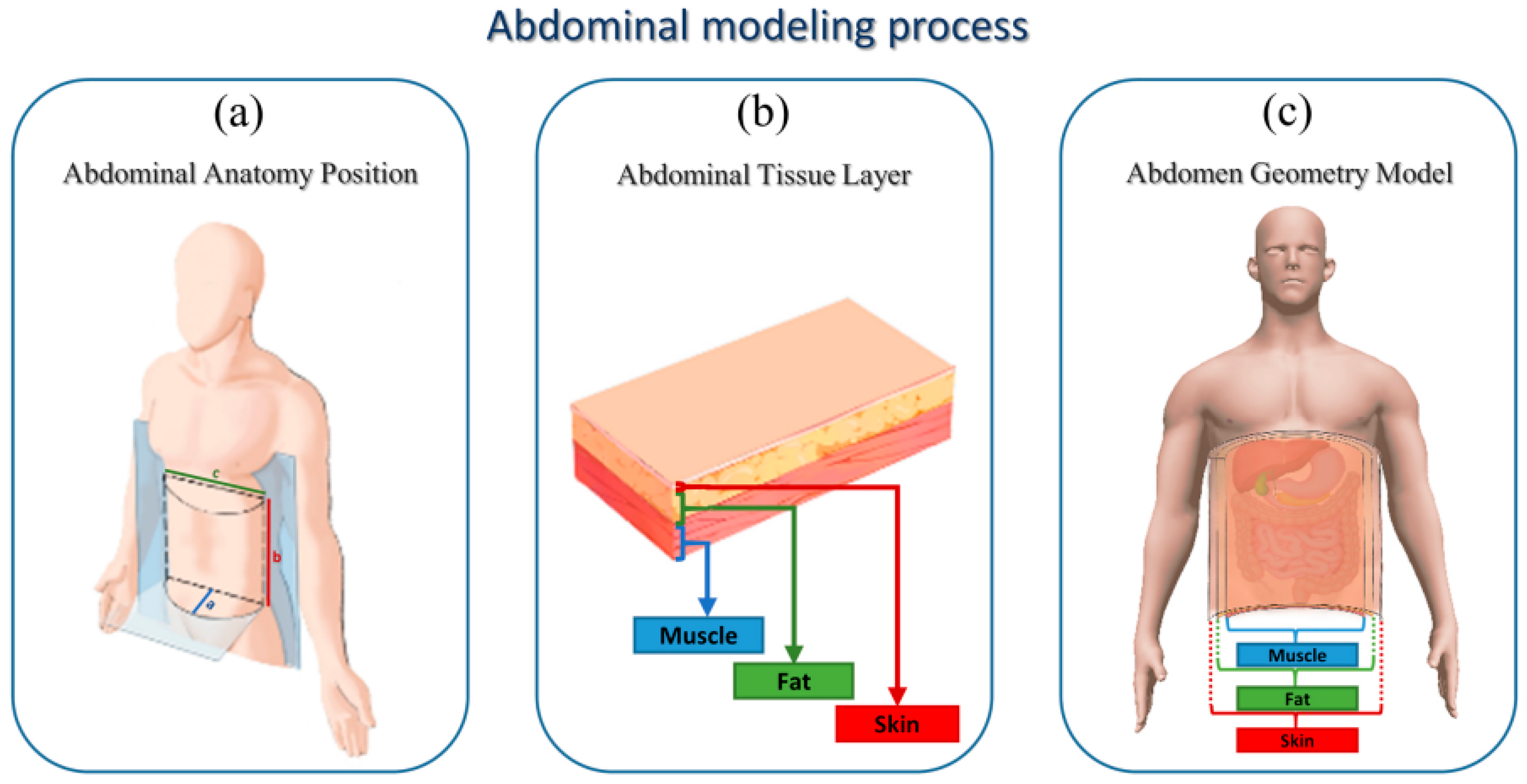
2. Theory and Model
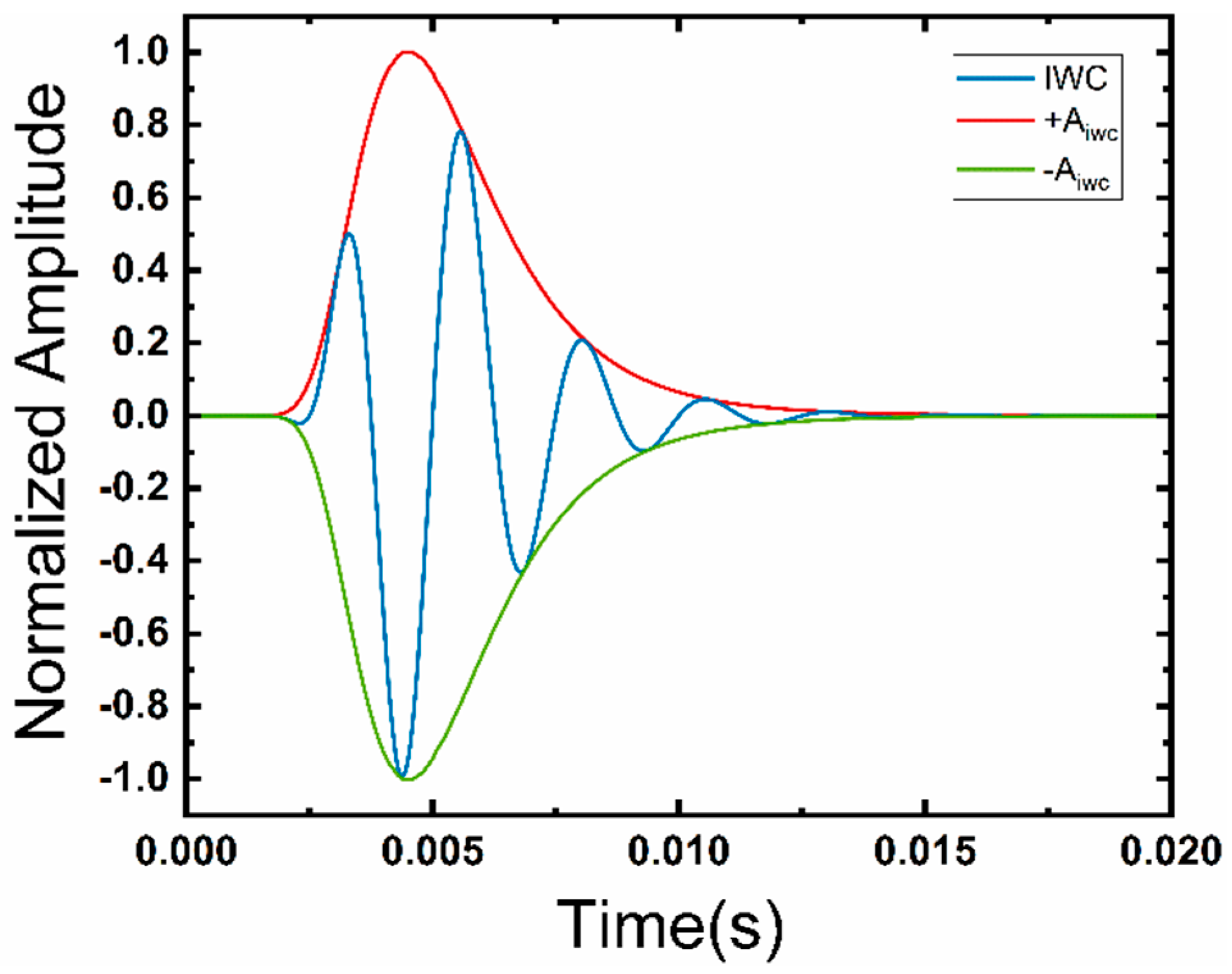
2.1. Generation of Bowel Sounds
2.2. Transmission of Bowel Sounds
2.2.1. Principle of Sound Propagation
2.2.2. Sound Propagation Models
3. Results and Discussion
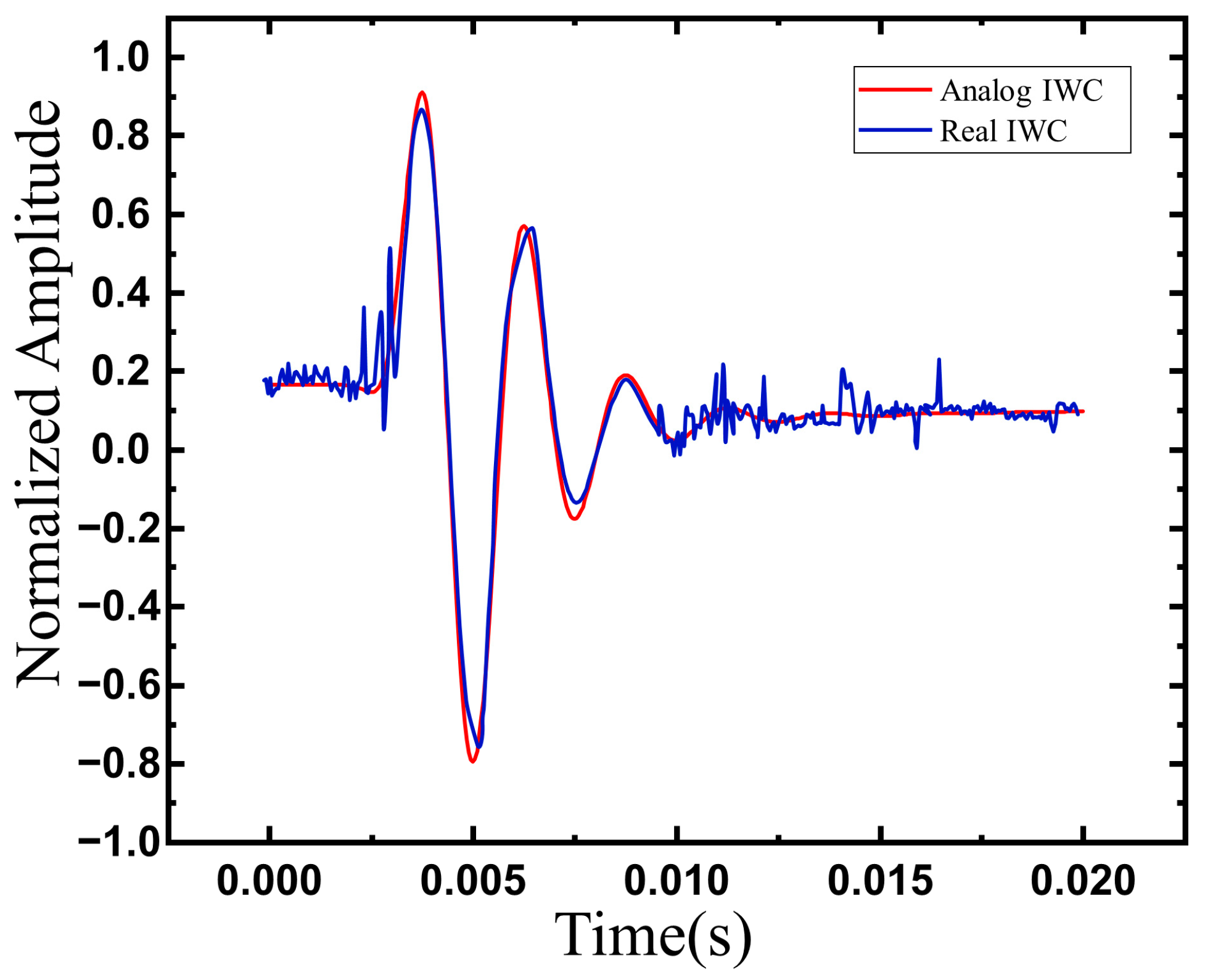
3.1. Model Validation
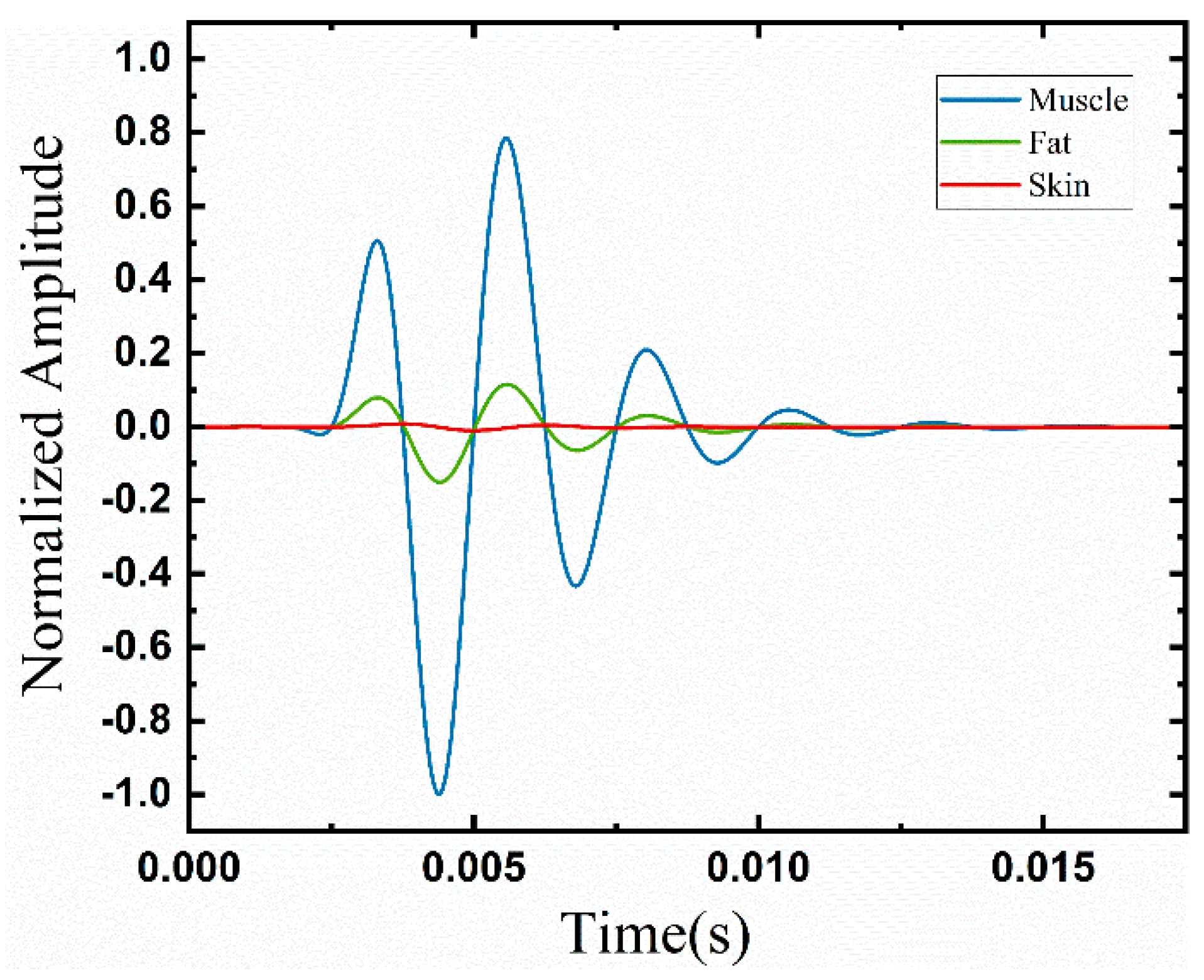
3.2. Tissue Attenuation
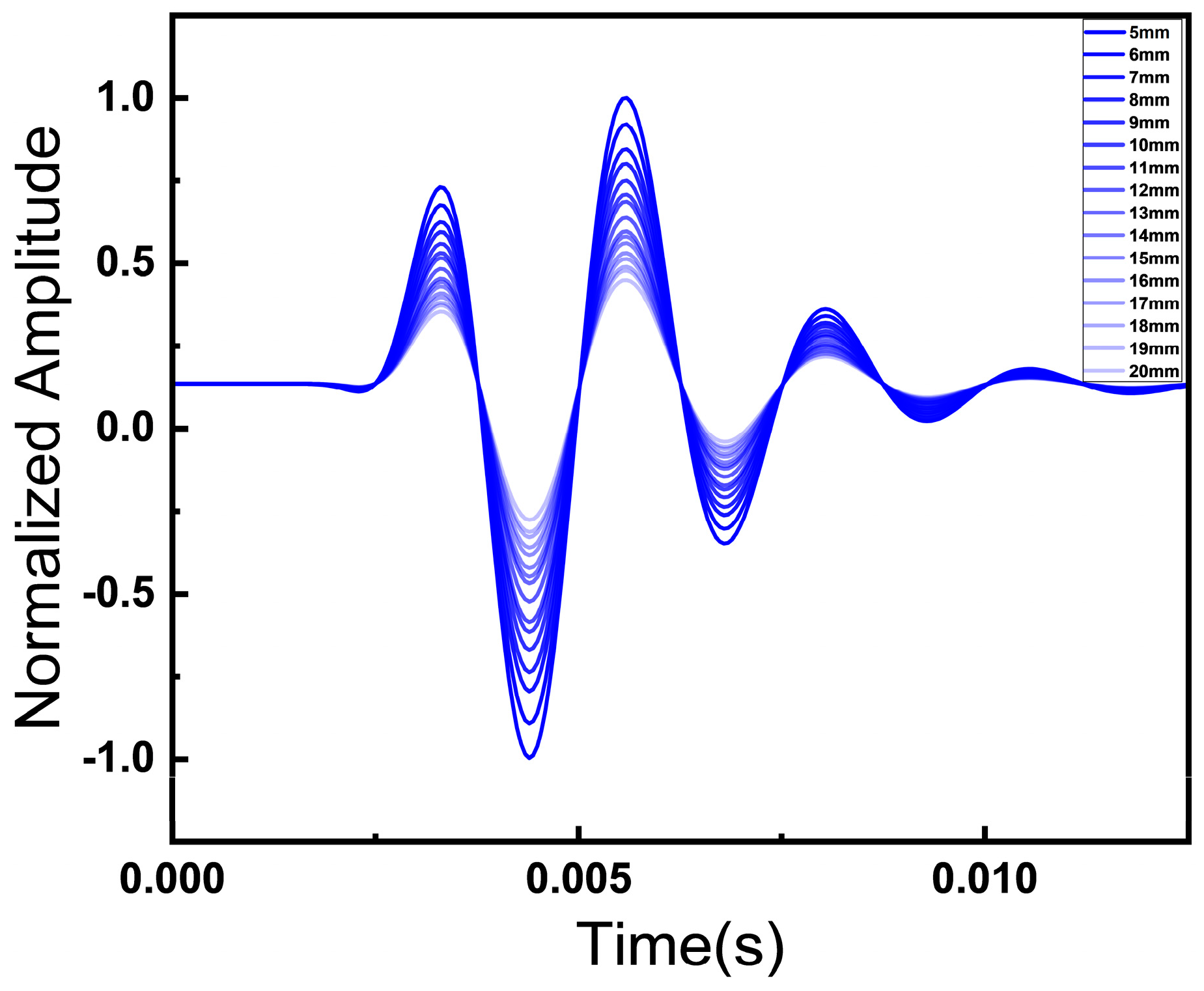
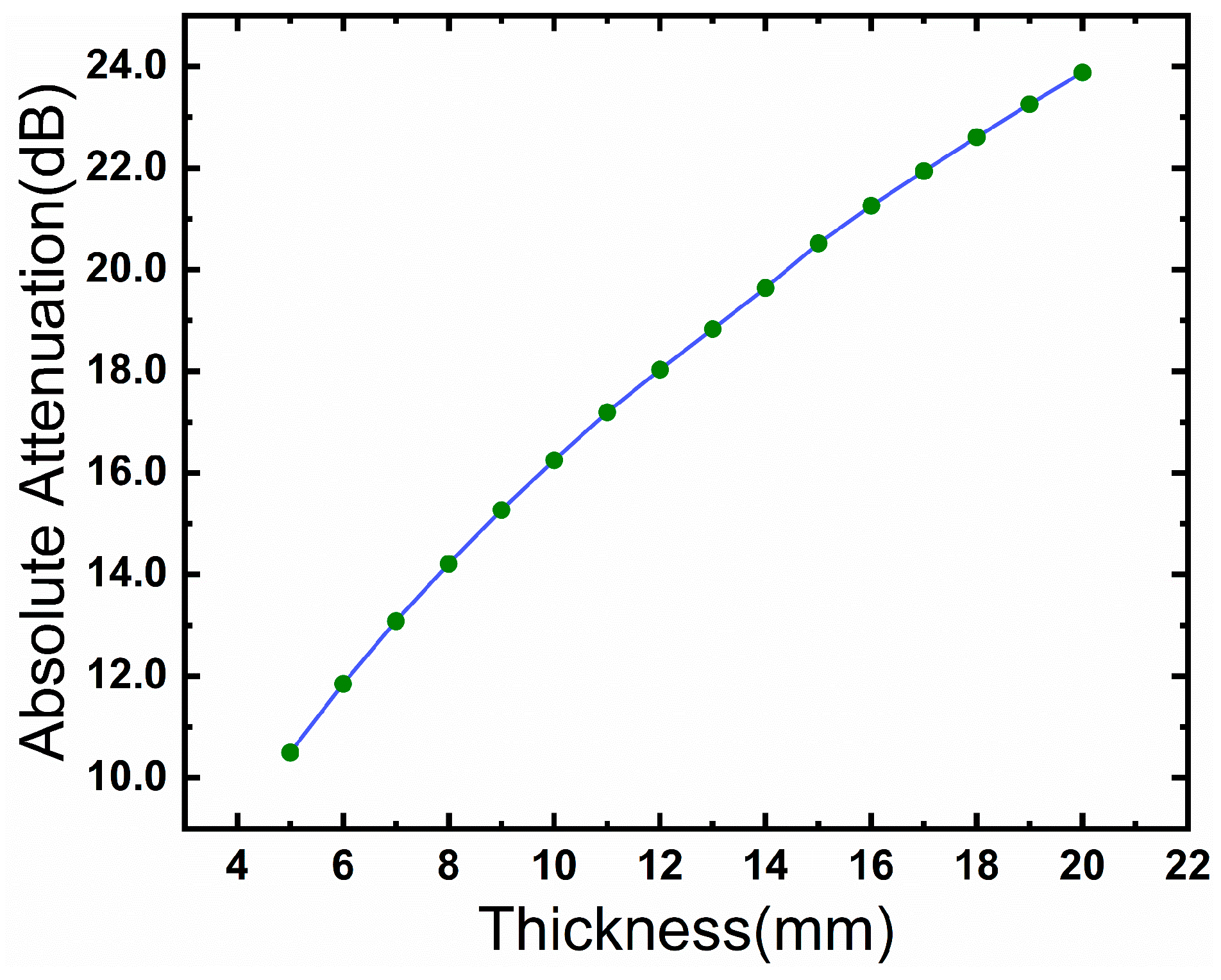
3.3. Fat Thickness Effects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dey, N.; Ashour, A.S.; Mohamed, W.S.; Nguyen, N.G. Acoustic Wave Technology. In Acoustic Sensors for Biomedical Applications; Dey, N., Ashour, A.S., Mohamed, W.S., Nguyen, N.G., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 21–31. ISBN 978-3-319-92225-6. [Google Scholar]
- Inderjeeth, A.-J.; Webberley, K.M.; Muir, J.; Marshall, B.J. The potential of computerised analysis of bowel sounds for diagnosis of gastrointestinal conditions: A systematic review. Syst. Rev. 2018, 7, 124. [Google Scholar] [CrossRef] [PubMed]
- Liatsos, C.; Hadjileontiadis, L.J.; Mavrogiannis, C.; Patch, D.; Panas, S.M.; Burroughs, A.K. Bowel Sounds Analysis: A Novel Noninvasive Method for Diagnosis of Small-Volume Ascites. Dig. Dis. Sci. 2003, 48, 1630–1636. [Google Scholar] [CrossRef]
- Craine, B.L.; Silpa, M.L.; O’Toole, C.J. Two-Dimensional Positional Mapping of Gastrointestinal Sounds in Control and Functional Bowel Syndrome Patients. Dig. Dis. Sci. 2002, 47, 1290–1296. [Google Scholar] [CrossRef]
- Gu, Y.; Lim, H.J.; Moser, M.A.J. How Useful Are Bowel Sounds in Assessing the Abdomen? Dig. Surg. 2010, 27, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Elhardello, O.; Macfie, J. Bowel Sounds: Is it Time for Surgeons to Hang-up their Stethoscopes? World J. Surg. Surg. Res. Gen. Surg. 2018, 1, 1066. [Google Scholar]
- Namikawa, T.; Yamaguchi, S.; Fujisawa, K.; Ogawa, M.; Iwabu, J.; Munekage, M.; Uemura, S.; Maeda, H.; Kitagawa, H.; Kobayashi, M.; et al. Real-time bowel sound analysis using newly developed device in patients undergoing gastric surgery for gastric tumor. JGH Open 2021, 5, 454–458. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.-R.; Ma, Y.-L. Bowel Sounds and Monitoring Gastrointestinal Motility in Critically Ill Patients. Clin. Nurse Spec. 2012, 26, 29–34. [Google Scholar] [CrossRef]
- Ding, X.; Wu, Z.; Gao, M.; Chen, M.; Li, J.; Wu, T.; Lou, L. A High-Sensitivity Bowel Sound Electronic Monitor Based on Piezoelectric Micromachined Ultrasonic Transducers. Micromachines 2022, 13, 2221. [Google Scholar] [CrossRef]
- Wang, N.; Testa, A.; Marshall, B.J. Development of a bowel sound detector adapted to demonstrate the effect of food intake. Biomed. Eng. OnLine 2022, 21, 1. [Google Scholar] [CrossRef]
- Mansour, Z.; Uslar, V.; Weyhe, D.; Hollosi, D.; Strodthoff, N. SonicGuard Sensor—A Multichannel Acoustic Sensor for Long-Term Monitoring of Abdominal Sounds Examined through a Qualification Study. Sensors 2024, 24, 1843. [Google Scholar] [CrossRef]
- Du, X.; Allwood, G.; Webberley, K.M.; Osseiran, A.; Wan, W.; Volikova, A.; Marshall, B.J. A mathematical model of bowel sound generation. J. Acoust. Soc. Am. 2018, 144, EL485–EL491. [Google Scholar] [CrossRef]
- Ranta, R.; Louis-Dorr, V.; Heinrich, C.; Wolf, D.; Guillemin, F. Towards an acoustic map of abdominal activity. In Proceedings of the 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (IEEE Cat. No.03CH37439), Copenhagen, Denmark, 14–17 July 2025; IEEE: Cancun, Mexico, 2003; pp. 2769–2772. [Google Scholar]
- Williams, C.J.B. Physical Examination of the Abdomen in Health and Disease:—Acoustic Signs. BMJ 1852, s2–4, 1–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Praticò, F.G.; Fedele, R.; Briante, P.G. On the Dependence of Acoustic Pore Shape Factors on Porous Asphalt Volumetrics. Sustainability 2021, 13, 11541. [Google Scholar] [CrossRef]
- Mast, T.D.; Hinkelman, L.M.; Orr, M.J.; Waag, R.C. The effect of abdominal wall morphology on ultrasonic pulse distortion. Part II. Simulations. J. Acoust. Soc. Am. 1998, 104, 3651–3664. [Google Scholar] [CrossRef]
- Awal, R.; Jusoh, M.; Yahya, M.S.; Latiff, N.A.A.; Rahman, S.A.; Nazri, A.; Zakaria, H.A.; Saat, S. ACOUSTIC Wave Propagation in High Scale Impedance Mismatch Mediums. Chem. Biotechnol. Eng. 2021, 22, 1–9. [Google Scholar] [CrossRef]
- Qaleh Jooq, M.K.; Mir, A.; Mirzakuchaki, S.; Farmani, A. Semi-analytical modeling of high performance nano-scale complementary logic gates utilizing ballistic carbon nanotube transistors. Phys. E Low-Dimens. Syst. Nanostruct. 2018, 104, 286–296. [Google Scholar] [CrossRef]
- COMSOL Multiphysics®; Version 6.2.; COMSOL AB: Stockholm, Sweden, 2023; Available online: www.comsol.com (accessed on 1 January 2024).
- Kim, D.-M.; Park, S.-K.; Park, S.-G. A Study on the Performance Evaluation Criteria and Methods of Abdominal Ultrasound Devices Based on International Standards. Safety 2021, 7, 31. [Google Scholar] [CrossRef]
- Tran, Q.D.; Jang, G.-W.; Kwon, H.-S.; Cho, W.-H.; Cho, S.-H.; Cho, Y.-H.; Seo, H.-S. Shape optimization of acoustic lenses for underwater imaging. J. Mech. Sci. Technol. 2016, 30, 4633–4644. [Google Scholar] [CrossRef]
- Čertík, O. Theoretical Physics Reference. Available online: https://ondrejcertik.com/projects/contributions/tfr/ (accessed on 5 March 2025).
- Lancerotto, L.; Stecco, C.; Macchi, V.; Porzionato, A.; Stecco, A.; De Caro, R. Layers of the abdominal wall: Anatomical investigation of subcutaneous tissue and superficial fascia. Surg. Radiol. Anat. 2011, 33, 835–842. [Google Scholar] [CrossRef]
- Lawson, T.L.; Berland, L.L.; Foley, W.D. Coronal upper abdominal anatomy: Technique and gastrointestinal applications. Gastrointest. Radiol. 1981, 6, 115–128. [Google Scholar] [CrossRef]
- Arpaia, P.; Cuneo, D.; Grassini, S.; Mancino, F.; Minucci, S.; Moccaldi, N.; Sannino, I. A finite element model of abdominal human tissue for improving the accuracy in insulin absorption assessment: A feasibility study. Meas. Sens. 2021, 18, 100218. [Google Scholar] [CrossRef]
- Nadimi, E.S.; Blanes-Vidal, V.; Harslund, J.L.F.; Ramezani, M.H.; Kjeldsen, J.; Johansen, P.M.; Thiel, D.; Tarokh, V. In vivo and in situ measurement and modelling of intra-body effective complex permittivity. Healthc. Technol. Lett. 2015, 2, 135–140. [Google Scholar] [CrossRef]
- Ahmed, H.M. Ultrasonic Estimation of Soft Tissue Visco Elastic Properties. arXiv 2022, arXiv:2210.02446. [Google Scholar]
- Mast, T.D.; Hinkelman, L.M.; Orr, M.J.; Sparrow, V.W.; Waag, R.C. Simulation of ultrasonic pulse propagation through the abdominal wall. J. Acoust. Soc. Am. 1997, 102, 1177–1190. [Google Scholar] [CrossRef] [PubMed]
- Goss, S.A.; Johnston, R.L.; Dunn, F. Comprehensive compilation of empirical ultrasonic properties of mammalian tissues. J. Acoust. Soc. Am. 1978, 64, 423–457. [Google Scholar] [CrossRef]
- Woodard, H.Q. The composition of body tissues. Br. J. Radiol. 1986, 59, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.L. A review of finite-element methods for time-harmonic acoustics. J. Acoust. Soc. Am. 2006, 119, 1315–1330. [Google Scholar] [CrossRef]
- Chen, Z.; Montlouis, W. Bowel Movement Signal Modeling and Parameters Extraction. In Proceedings of the 2020 Asia-Pacific Signal and Information Processing Association Annual Summit and Conference (APSIPA ASC), Auckland, New Zealand, 7–10 December 2020. [Google Scholar]
- Koch, T. Predicting the Intramuscular Fat Content in Porcine M. longissimus via Ultrasound Spectral Analysis with Consideration of Structural and Compositional Traits. Ph.D. Thesis, Georg-August-University Göttingen, Göttingen, Germany, 2011. [Google Scholar]
- Vasisht, D.; Zhang, G.; Abari, O.; Lu, H.-M.; Flanz, J.; Katabi, D. In-body backscatter communication and localization. In Proceedings of the 2018 Conference of the ACM Special Interest Group on Data Communication, Budapest, Hungary, 20–25 August 2018; ACM: Budapest Hungary, 2018; pp. 132–146. [Google Scholar]
- Lewandowski, Z.; Dychała, E.; Pisula-Lewandowska, A.; Danel, D.P. Comparison of Skinfold Thickness Measured by Caliper and Ultrasound Scanner in Normative Weight Women. Int J Env. Res Public Health 2022, 19, 16230. [Google Scholar] [CrossRef]
- Pérez-Chirinos Buxadé, C.; Solà-Perez, T.; Castizo-Olier, J.; Carrasco-Marginet, M.; Roy, A.; Marfell-Jones, M.; Irurtia, A. Assessing subcutaneous adipose tissue by simple and portable field instruments: Skinfolds versus A-mode ultrasound measurements. PLoS ONE 2018, 13, e0205226. [Google Scholar] [CrossRef]
- Masui, A.; Tsuji, H.; Tamura, K.; Tarumi, N.; Sugiura, T.; Iwasaka, T.; Inada, M. Effect of Body Characteristics on the Variables of Signal-averaged Electrocardiograms in Healthy Subjects. Chest 1994, 105, 1357–1359. [Google Scholar] [CrossRef][Green Version]
- Liao, Y.-Y.; Yang, K.-C.; Lee, M.-J.; Huang, K.-C.; Chen, J.-D.; Yeh, C.-K. Multifeature analysis of an ultrasound quantitative diagnostic index for classifying nonalcoholic fatty liver disease. Sci. Rep. 2016, 6, 35083. [Google Scholar] [CrossRef]
- Duchnowski, P.; Śmigielski, W. Usefulness of myocardial damage biomarkers in predicting cardiogenic shock in patients undergoing heart valve surgery. Pol. Heart J. 2024, 82, 423–426. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, X.; Xu, Y.; Zou, Y. Numerical Modeling of Bowel Sound Propagation: Impact of Abdominal Tissue Properties. Appl. Sci. 2025, 15, 2929. https://doi.org/10.3390/app15062929
Deng X, Xu Y, Zou Y. Numerical Modeling of Bowel Sound Propagation: Impact of Abdominal Tissue Properties. Applied Sciences. 2025; 15(6):2929. https://doi.org/10.3390/app15062929
Chicago/Turabian StyleDeng, Xingyu, Yazhen Xu, and Yuanwen Zou. 2025. "Numerical Modeling of Bowel Sound Propagation: Impact of Abdominal Tissue Properties" Applied Sciences 15, no. 6: 2929. https://doi.org/10.3390/app15062929
APA StyleDeng, X., Xu, Y., & Zou, Y. (2025). Numerical Modeling of Bowel Sound Propagation: Impact of Abdominal Tissue Properties. Applied Sciences, 15(6), 2929. https://doi.org/10.3390/app15062929






