Abstract
Whole-body cryotherapy (WBC) is a widely used method that exposes the body to extremely low temperatures to induce physiological responses. While its analgesic, anti-inflammatory, and anti-edema effects are well-documented, the effects of WBC on the skin are not yet fully understood. The aim of this study was to evaluate the effects of a single session of WBC and a series of 10 and 20 treatments on selected biophysical properties of the skin in normal-weight and overweight or obese subjects. Twenty-three volunteers took part in the study—12 in the study group (SG, BMI > 27) and 11 in the control group (CG, BMI 18–25). Skin hydration, transepidermal water loss (TEWL), pH, and skin elasticity were measured each time before and after the 1st, 10th, and 20th WBC treatments. All assessments were carried out under standard conditions using specialized probes. The skin in response to the cryogenic stimulus differed according to BMI and anatomical location. In SG: skin firmness on the face (p < 0.001) and forearm (p < 0.001), hydration (p = 0.004), and pH (p = 0.005) on the forearm significantly improved, while TEWL increased after a series of treatments in both groups (p = 0.028). WBC appears to be a safe and effective method of modulating the biophysical properties of the skin, with effects varying by BMI and body region. However, the role of WBCs in exacerbating TEWL should continue to be observed, and in the future, it is also advisable to develop methods to offset this adverse effect.
1. Introduction
Whole-body cryotherapy (WBC) is defined as the technique of extreme cooling of the body (below −100 °C) for a short period (1–3 min) to induce a physiological response to cold [1]. The term cryostimulation refers to the use of cold exposure among healthy subjects (e.g., athletes); cryotherapy, on the other hand, refers to the use of cold in medical treatment including the management of injuries, inflammation, and other disorders [2].
Cryogenic temperatures have been successfully used in patients with rheumatoid arthritis [3], multiple sclerosis [4], fibromyalgia [5], and more recently, adjunctively in the treatment of obesity [6,7,8]. The effects of cryotherapy on improving mood and mental state are also indicated [9,10,11]. Additionally, growing evidence suggests the potential use of low temperatures in dermatology and cosmetology [12,13,14].
The skin is responsible for many functions including secretory, sensory, and defense [15]. It protects internal organs and tissues, receives signals from the environment, and prevents excessive water loss. In addition to its biological functions, it defines a person’s physical appearance, affecting his or her well-being and quality of life [16]. Skin characteristics such as hydration, pH, and TEWL ensure the integrity of the epidermal barrier. TEWL, defined as the amount of condensed water diffusing through a fixed area of the stratum corneum into the air in a given unit of time, is one of the most important parameters characterizing the skin barrier. Increased values may be associated with epidermal barrier dysfunction [17].
In cryotherapy, the skin is directly exposed to low temperatures. A decrease in peripheral temperature, primarily of the skin, triggers basic thermoregulatory responses aimed at maintaining a constant body temperature. There is a constriction of blood vessels in the skin bed, reducing blood flow and consequently minimizing heat transfer between the body’s core and outer layers [18,19].
As previous studies have indicated, the effects of low-temperature treatments on the human body are dependent on body composition [8,20]. These reactions are most likely related to the presence of adipose tissue acting as an insulator. On the other hand, however, it has also been pointed out that body composition has a strong influence on skin condition [21]. Individuals with a higher BMI have been reported to have higher tewametric scores, indicating reduced skin barrier capacity [22]. This may be related to the presence of chronic, subclinical inflammation that is part of obesity and metabolic syndrome. Its presence is associated with a higher incidence of certain dermatoses in obese individuals [23].
Single exposures to cold [13] and the use of a series of WBC treatments [24] and their effects on basic skin characteristics have already been the subject of scientific research. However, the number of publications addressing this topic is still small. A 2021 study [13] evaluated the effect of a single 3 min WBC treatment on skin features. A total of 77 young people (age 23.63 ± 1.36) participated. This study suggests that a single WBC treatment leads to an improvement in skin hydration and a decrease in acid–base reaction, but statistical significance was not reached. However, an unfavorable direction of change was indicated for skin barrier function as assessed by evaluating TEWL (transepidermal water loss), measured in men on the zygomatic bone. The study by Skrzek et al. [24] involved 22 middle-aged women (58.7 ± 7.54) who underwent 10 WBC treatments. Skin characteristics were measured before and after the first and last treatment. After the first treatment, there was an increase in hydration, which was statistically significant in only one of the eight measured locations on the body. An adverse effect on the hydration level of the stratum corneum was noted for the entire treatment series. Other changes in skin characteristics were not noted, while it was indicated that different parts of the skin react differently to low temperatures.
The aim of the present study was to evaluate the impact of a single cryogenic stimulus and a series of 20 WCB treatments on selected skin characteristics in overweight/obese and normal-weight subjects.
2. Materials and Methods
2.1. Study Group
A group of volunteers was recruited for the study, from which normal-weight and overweight or obese subjects were selected [25]. In each group, medical history was taken, and inclusion and exclusion criteria were applied. Study volunteers were grouped according to the indicated criteria in a study group (SG) with a BMI (body mass index) above 27 and a control group (CG) with a BMI within the normal range (18–25). Participants in the study group (SG), consisting of individuals with increased body fat, were matched to the control group (CG) based on age and gender. Ultimately, 23 subjects completed the project (Figure 1).
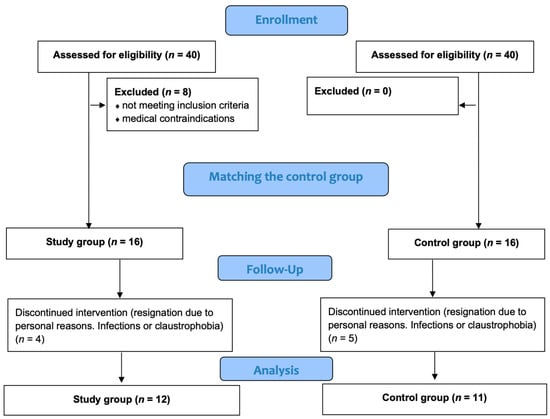
Figure 1.
Patient flow chart.
The inclusion criteria considered in the process of qualifying participants for the project were BMI > 27 for the study group; BMI 18.5–25 for the control group; not taking winter baths, and no use of systematic cryotherapy at least 6 months prior; written consent for voluntary participation in the study; and consent of the qualifying physician. The exclusion criteria were chronic endocrine and dermatological diseases; skin conditions of fungal, bacterial, or viral origin; health problems (neurological or orthopedic) that prevent independent movement; taking anti-inflammatory medications and supplements containing vitamins and antioxidants; and lack of consent to participate in the study.
Participants read the study protocol and signed consent forms before starting the study. Participation in the project was completely voluntary. Each volunteer was informed that they could withdraw from the project at any stage, without providing a reason. The study protocol was approved by the Bioethics Committee of the Regional Medical Chamber in Kraków (177/KBL/OIL/2023 dated 25 September 2023). Interventional studies involving animals or humans and other studies that require ethical approval must specify the authority that provided approval and the corresponding ethical approval code.
2.2. Study Protocol
A medical consultation was conducted for each participant prior to the study. The study protocol consisted of a series of 20 sessions of systemic cryotherapy taking place daily (4 weeks excluding Saturdays and Sundays). Participants were allowed to miss one cryotherapy treatment; multiple absences resulted in exclusion from the study. Before each entry into the cryotherapy chamber, blood pressure was measured (blood pressure above 160/100 mmHg prevented participation in the treatment and further participation in the project).
Body composition was assessed before the first treatment in the cryochamber. Selected skin parameters were measured immediately before and after the 1st, 10th, and 20th WBC sessions at two locations: on the face near the zygomatic bone and on the inside of the forearm (Figure 2). It was assumed that the survey would be conducted on the dominant hand. In the room where the biophysical skin features were measured, constant conditions prevailed, as required by the manufacturer of the measurement probes, with humidity of 45% and air temperature of 21 °C monitored during the tests.
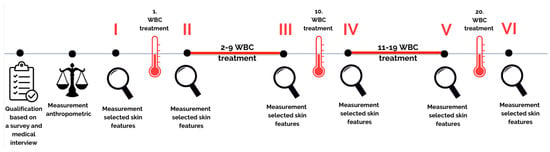
Figure 2.
Research flow chart.
2.3. Cyrotherapy
Preparation for the procedure followed standard protocols. Before entering the cryochamber, volunteers were asked to remove jewelry and glasses and to dress in appropriate attire: a cap, a mask covering the nose and mouth, a bathing suit/shorts, gloves, gaiters or long socks covering the tibias and knee joints, and clogs with thick soles. Each WBC procedure included a 30 s acclimatization in the cryochamber atrium (−60 °C), followed by a 3 min treatment in the cryochamber proper (−120 °C). Volunteers walked in a single file, changing direction every 30 s. They exited the cryochamber through its atrium. Immediately after exiting, participants underwent skin characteristics measurements and, in turn, were directed to the training room, where they performed aerobic exercises. Each treatment took place under the supervision of a specialist who monitored its progress through a window built into the cryochamber. Participants were informed that they could leave the cryochamber early if they experienced discomfort.
2.4. Anthropological Measurements
Body height was measured once using a Seca 216 growth meter (Hamburg, Germany), with an accuracy of 5 mm. Body weight was measured with a JAWON MEDICAL IOI-353-CE0197 scale (Jawon Medical Co., Ltd., Gyeongsan, Republic of Korea) using the bioimpedance method. In addition, BMI was calculated for each subject.
2.5. Skin Characteristic Measurements
Skin parameters were measured according to the protocol of the Skin Physiology Laboratory (CLNB) of the AKF in Krakow (Academy of Physical Culture in Krakow, certificate number PN-EN ISO 9001:2015: PW-08606-19). Measurements were performed on healthy, undamaged skin. The Multi Probe Adapter (MPA) System (Courage-Khazaka GmBH, Cologne, Germany) was used. The set included measuring probes from the same manufacturer: (1) Corneometer CM 825 (measurement error: ±3%); (2) Tewameter TM 300 (measurement error: water loss: ±0.5 g/h/m2 for RH ≥ 30%; ±1.0 g/hm2 for RH ≤ 30%); (3) Skin-pH-Meter PH 905 (error: ±0.1); and (4) Cutometer Dual MPA 580. Due to the storage of the pH measurement electrode in potassium chloride aqueous solution, the skin pH measurement was made last in the series of measurements so as not to disturb the measurement results of other parameters.
A 1-s measurement method was used for the corneometric and pH-metric measurements and a continuous (20 s) method for the tewametric measurements. The cutometer test included a suction phase (the skin was suctioned with a vacuum for 3 s) and a 3 s relaxation phase. All measurements were taken at a given location three times, and the arithmetic mean of the results was calculated.
2.6. Statistical Analysis
The results were analyzed using JASP 0.16.4 (University of Amsterdam, The Netherlands) and were presented using basic descriptive statistics. The type of distribution of variables was analyzed using the Shapiro–Wilk test. The initial characteristics of the study groups were compared using the Student’s t-test for independent groups. For variables with a normal distribution and with all other assumptions met, the ANOVA test for repeated measures with post hoc tests (Bonferroni test) was used. The effect size was calculated using the Omega squared coefficient (ω2) and interpreted as follows: 0.01–small, 0.06–medium, and 0.14–large. Effect size in post hoc tests was measured by Cohen’s coefficient (d) and interpreted as follows: 0.2–small, 0.5–medium, and 0.8–large. For variables with a non-normal distribution, the Friedman nonparametric test was used. The presence of correlation was checked using Spearman’s test (rho). The significance level (α) was set at p < 0.05.
3. Results
3.1. Group and Baseline Skin Characteristics
The study group (SG) consisted of six women and six men. The control group (CG) included eight women and three men. Detailed characteristics of each group are shown in Table 1. Biophysical characteristics of the skin measured before the study are shown in Table 2.

Table 1.
Characteristic of the group.

Table 2.
Baseline biophysical features of the participants’ skin on the face and forearm.
3.2. Study of the Impact of Single Exposure WBC
Initially, the effect of a single cold exposure on the measured skin characteristics was assessed. The effect of the 1st, 10th, and 20th treatments was checked. Different changes were indicated for SG and CG. In the normo-weight group, a significant increase was indicated in forearm hydration after the 1st treatment (p = 0.020; d = −0.835) and an increase in the R2 cutometer parameter on the forearm at the 20th treatment (p = 0.025; d = −0.796). In overweight or obese subjects: there was a significant decrease in TEWL on the forearm at the 10th treatment (p = 0.045; d = 0.652) and 20th treatment (p = 0.030; d = 0.721). There were no differences among the study groups after a single application of the cryogenic stimulus in the form of WBC for biophysical characteristics of facial skin (Table 3).

Table 3.
Studying of the effect of single exposure on a cold exposure.
3.3. Study of the Impact of Multiple Exposures WBC
The next step investigated whether there was an adaptation of the skin to the cryogenic stimulus over time. A comparison of differences in measurements of skin biophysical characteristics before and after WBC treatment for the 1st, 10th, and 20th treatments was performed (Table 4). A difference was indicated for the effect on the tewametric score for the SG forearm (p = 0046; ω2 = 0.135). Further analysis of the results indicated a difference between the effect produced by the 10th and 20th treatments (p = 0.023; d = −1.023). The CG indicated a difference in skin firmness R0 (p = 0.009; ɷ2 = 0.205). A difference is noted between the effect induced by the 10th and 20th treatments (p = 0.032; d = 1.183), and between the effect induced by the 1st and 10th there was a difference at the level of statistical trend (p = 0.063; d = −1.152)

Table 4.
Studying the effect of adaptation on the effect of a single cold exposure.
3.4. Study of Impact of Series of WBC on Selected Skin Characteristics
To illustrate the effect of a series of treatments of WBC on selected skin characteristics, the results performed before the 1st (I), before the 10th (III), and before the 20th treatment (V) were compared among themselves.
The results of skin hydration measurements on the face and forearms are shown in Figure 3. Analysis of the results of the corneometric measurements on the face indicated significant differences in measurements only for the interaction measurement x group (p = 0.028; ω2 = 0.055).
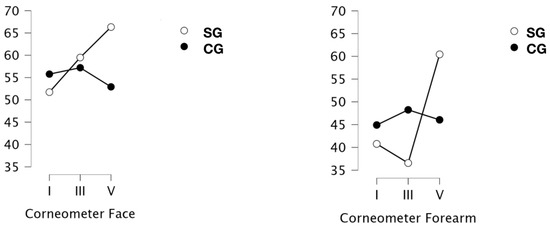
Figure 3.
Measurement of hydration [IU] of the skin of the face and forearms in a group of obese or overweight (SG) and normo-weight (CG) subjects taken before the project (I), after a series of 9 treatments (III) and 19 treatments (V) WBC.
For the forearm, significant differences in the results were indicated (p = 0.002; ɷ2 = 0.116). An interaction of score x group was noted (p < 0.001; ɷ2 = 0.139). Subsequent tests indicated that the results after a series of 4 weeks of treatments were significantly different from baseline (p = 0.012; d = −0.793) and also those after 2 weeks (p = 0.022; d = −0.826). When the type of group was included in the analysis, it was indicated that only the results obtained in the SG group were responsible for these differences. Thus, the results after the full 4-week series in the obese and overweight group were significantly different from the baseline results of this group (p = 0.004; d = −1500) and the results after the 9-treatment series (p = 0.002; d = −1820). In contrast, there were no differences between skin hydration at baseline and after 9 treatments.
The results of the tewametric measurements showing the magnitude of TEWL are shown in Figure 4. Significant differences were indicated for measurements on the face (p = 0.002; ω2 = 0.124), and a measurement x group interaction was also indicated (p < 0.001; ɷ2 = 0.287). Assessing the significance of the effect of the number of treatments on the state of the skin barrier assessed tewametrically, only a trend for the significance of difference I vs III was indicated. However, the results after 19 treatments were significantly different from the baseline (p = 0.003; d = −0.970). At the same time, the skin condition after 9 and 19 treatments did not differ. When group type was added to the analysis, it was indicated that baseline SC and CG scores differed significantly (p < 0.001; d = 1.665). In the obese and overweight group, significant differences were indicated after 9, when the lowest scores were observed, and after 19 treatments, where the highest TEWL values were indicated. For normal-weight subjects, baseline results differed from those determined after 9 treatments (p < 0.001; d = −2.064) and after 19 treatments (p = 0.003; d = −1.640). Both series lengths induced a significant increase in TEWL in this group.
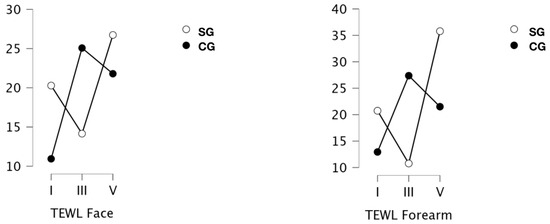
Figure 4.
Measurement of transepidermal water loss (TEWL) [g/m2/g] the skin of the face and forearms in a group of obese or overweight (SG) and normo-weight (CG) subjects taken before the project (I), after a series of 9 treatments (III) and 19 treatments (V) WBC.
Tewameter results on the skin of the forearms were significantly different (p = 0.006; ω2 = 0.132), and a measurement x group interaction was indicated (p < 0.001; ω2 = 0.225). It was indicated that the results after the 19th treatment were significantly higher than baseline (p = 0.028; d = −0.849) and those determined after nine treatments (p = 0.022; d = −0.928). Taking the group additionally into account, it was indicated that the baseline scores of participants in the two groups did not differ. The study group indicated significantly higher results after the full 4-week series of treatments compared to those obtained after 2 weeks (p < 0.001; d = −2.154).
The results of skin acid–base (pH) measurements are shown in Figure 5. Significant differences were indicated for measurements collected on facial skin only for the outcome x group interaction (p < 0.001; ω2 = 0.196). Post hoc tests indicated significantly higher indications for SG after 9 treatments (p = 0.042; d = −1.325) with a return decrease after another 2 weeks (III vs. V: p = 0.001; d = 1.709). It was also indicated that the SG group responded differently to a series of nine treatments, and in Measure III, SG results were significantly different from CG (p = 0.003; d = 1.831).
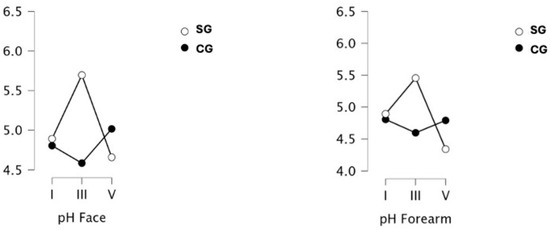
Figure 5.
Measurement of acid–base reaction (pH) of the skin of the face and forearms in a group of obese or overweight (SG) and normo-weight (CG) subjects taken before the project (I), after a series of 9 treatments (III) and 19 treatments (V) WBC.
As with skin pH on the face, on the forearm significant differences occurred only for the outcome × group interaction (p = 0.007; ω2 = 0.133). The directions of change for both groups closely resembled what was observed on facial skin. pH in the SG increased significantly in Measure III (difference vs. CG p = 0.011; d = 1.354) and decreased in Measure V (difference vs. III p = 0.005; d = 1.757).
Cutometric assessment of skin firmness is possible by monitoring the R0 index. Its changes in SG and CG are shown in Figure 6. Significant differences in this index assessed on the face were indicated (p = 0.001; ω2 = 0.137), and interaction with the group was also indicated (p = 0.006; ω2 = 0.096). Significantly lower scores were observed for measurement V compared to baseline (I) measurement (p < 0.001; d = 0.995), and significant differences between the SG and CG were also indicated (p < 0.001; d = 1.865). A closer analysis including these two factors (measurement and group) indicated significant differences between SG and CG in all measurements: baseline difference (p < 0.001; d = 2.611); after nine treatments (p = 0.005; d = 2.061) except the last one. Results after 20 treatments were similar in both groups. Something that was not observed for participants with normal BMI values was significant decreases in R0 after 19 treatments (p < 0.001; d = 1.839) compared to baseline. This decrease ultimately allowed, after a 4-week series of treatments, for results similar to those observed in the control group throughout the project.
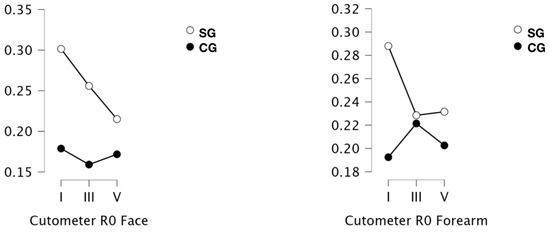
Figure 6.
Measurement of cutometric measurement (firmness assessment, R0) of the skin of the face and forearms in a group of obese or overweight (SG) and normo-weight (CG) subjects taken before the project (I), after a series of 9 treatments (III) and 19 treatments (V) WBC.
For skin on the forearms, significant differences were indicated only after accounting for the group (interaction: p = 0.004; ω2 = 0.160). The variable itself was also a differentiating factor: group (p < 0.001; ω2 = 0.268). In the group with higher BMI values, significantly higher scores were observed in Measure I compared to CG (p < 0.001; d = 2.324). In the overweight and obese group, a significant decrease in R0 was observed after 19 runs (p < 0.001; d = 1.374), after 9 runs the decrease was within the statistical trend (p = 0.072; d = 1.448).
The results of cutometric measurements, this time showing visco-elasticity of the skin (R2), are shown in Figure 7. The results for the face and forearm did not differ between the groups at any of the measurement points.
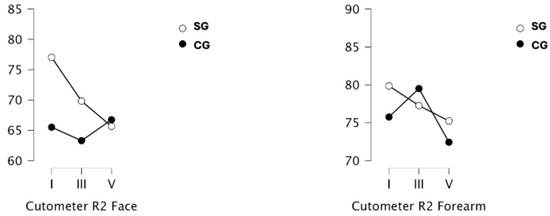
Figure 7.
Measurement of cutometric measurement (visco-elasticity evaluation, R2) of the skin of the face and forearms in a group of obese or overweight (SG) and normo-weight (CG) subjects taken before the project (I), after a series of 9 treatments (III) and 19 treatments (V) WBC.
3.5. Correlations
The results of the correlation analysis are shown in Table 5. The results of skin parameters measured on the forearm correlated with those assessed on the face for hydration (p < 0.001; Spearman’s rho = 0.749), TEWL (p = 0.031; Spearman’s rho = 0.453), pH (p < 0.001; Spearman’s rho = 0.868) and the R0 parameter (<0.001; Spearman’s rho = 0.893). Also indicated was the presence of a correlation between the R0 value and hydration (p = 0.022; Spearman’s rho = −0.474) of the skin on the forearm. A correlation was noted between TEWL and R0 on the forearm (0.054; Spearman’s rho = 0.409) and facial TEWL with: R0 (p < 0.001; Spearman’s rho = 0.653); R2 (p = 0.017; Spearman’s rho = 0.492) and BMI (p < 0.001; Spearman’s rho = 0.758). A correlation with the R0 parameter was also indicated for BMI, both for the face (p > 0.001; Spearman’s rho = 0.675) and forearm (p < 0.001; Spearman’s rho = 0.714).

Table 5.
Spearman’s Correlations.
4. Discussion
The literature highlights the significance of cryotherapy in cosmetology, emphasizing the strong effect of cryogenic temperatures on the condition of human skin [12,26]. In the present study, skin elasticity and stratum corneum hydration in the SG significantly improved after a series of WBC treatments. The effect of cryogenic temperatures on the skin is multidirectional. It is known that WBC has an inhibitory effect on the amount of ROS and stimulates antioxidant production [27,28]. However, other mechanisms regarding the effect on the skin are not yet fully understood. It has been suggested that stimulation of circulation will increase the supply of oxygen to the cells and nutrients, which consequently improves the appearance of the skin [1,29]. The present results primarily indicate that the effect of cryochamber treatments on the skin is dependent on BMI, with those with elevated values of this index responding with positive changes in a number of the biophysical measurements used in this project.
In the present project, it was chosen to evaluate skin features on only one limb. The literature data indicate that skin features may differ between the dominant and non-dominant hand. Higher TEWL was indicated on the dominant hand in the study of Treffel et al., [30], while Enright and Nikolis [31], in skin topography imaging, indicated higher SEW values associated with winkles depth on the dominant hand. This indicates that the dominant hand may be more prone to skin aging. Although other studies have not confirmed a relationship between skin features and the dominant and non-dominant hand [32,33], randomizing the choice of limb seemed an inappropriate choice.
The values of stratum corneum hydration, pH and skin elasticity differed significantly depending on the site of measurement. Obese subjects showed higher hydration and firmness values and lower pH values on the face. In normal-weight subjects, significantly higher pH values were recorded on the face. Piotrowska et al. [13] and Skrzek et al. [24] also reported higher hydration values on the face compared to the extremities. However, the pH results in the analyzed studies were inconsistent. Piotrowska et al. [13] reported higher pH on facial skin, while Skrzek et al. [24] noted no pH differences in the measured areas. The directions of changes in the results of pH measurements obtained in the present study are inconclusive, but it is worth mentioning that in all the mentioned studies, pH values were within the range of physiological values.
Previous studies have shown that skin on different areas of the body responds differently to WBC. Consistent with previous observations, also in the present study, the skin on the forearm responded more strongly to the cryogenic stimulus than the skin on the face [13,24,34].
A cross-sectional study [35] found that obesity affects the degree of hydration of the stratum corneum on the forearm. In the present study, no differences were found in baseline hydration parameters between the groups, nor was there a relationship between BMI and the degree of hydration. After the 19th treatment, however, SG skin had higher values of hydration of the stratum corneum than before the treatment series, with no differences noted in CG.
An improvement in hydration was observed after the first treatment on forearm skin in CG, and the noted increase reached statistical significance. An improvement in hydration was also indicated in an earlier study [13], but statistical significance was not achieved. In that study [13], the skin of the volunteers was in the range corresponding to dry, while in the present study, the skin of the volunteers was determined to be properly moisturized, which may affect the final outcome of the study and suggest that for dry skin, the cryogenic stimulus alone will not be sufficient.
The only adverse change noted in both groups during the treatment series was increased TEWL, which may be due to an increased vascular reflex in response to cold. Piotrowska et al. [13] also reported an increase in TEWL after a single WBC treatment. SG was initially characterized by higher TEWL values than CG, which is consistent with previous observations [36,37]. Guida et. et al. [38] reported significantly lower TEWL values in obese subjects compared to the normal-weight group, but no correlation between TEWL and BMI was indicated. In the present study, such a correlation was noted, as was Yew et al. [22]. The authors noted that as BMI increased by 1 km/m2, TEWL increased (0.221%). Obese patients have larger skin folds and thicker layers of subcutaneous fat. For proper thermoregulation, this group, compared to normal fat subjects, will sweat more, hence water evaporation from the skin surface will be higher [39].
The normal pH of the skin is in the range of 4.1–4.8, which ensures the integrity and integrity of the stratum corneum and the regulation of epidermal barrier homeostasis [40]. Acidic skin pH also protects the commensal skin microflora, which acts as the first line of defense against pathogens [41]. As a result of a number of factors (e.g., diabetes, skin aging, and skin diseases), microbial dysbiosis can occur, increasing the risk of infection [42]. Ma et al. [36] did not indicate changes in the skin microbiota among individuals with varying degrees of body fatness. The present study did not indicate differences in baseline pH measurements between the groups, which is consistent with the results of Yew et al. [43], who also showed no differences in pH measurements in subjects with normal and high BMI. However, the study by Ibuki et al. [44] indicated that skin pH was slightly higher in the group with higher BMI. Initially, the pH in the SG increased significantly, reaching values on the borderline of physiological skin pH, with a subsequent significant decrease. CG reacted inversely to the treatments, but pH values were within the range of physiological values throughout. In other work on cryotherapy [24,45], the pH values also remained in a constant acidic environment, but in contrast, no significant differences were noted during the treatment series. Therefore, it can be concluded that cryotherapy does not negatively affect the pH of the skin, and thus it can be assumed that it does not have a negative effect on the skin’s microbiome and hydrolipid barrier controlled by its well-being. However, confirmation of this hypothesis requires further research.
Measurement of pH is assessed by the presence of water present on the skin surface. The highest pH values were indicated in the most hydrated areas [46]. Increased TEWL would therefore indicate higher pH values. It is worth noting that an inverse relationship was noted in the study participants. As TEWL increased, pH values decreased, and vice versa. Both characteristics are key indicators of proper hydrolipid barrier function. It is possible that the skin, in response to increased water loss, triggers defensive reactions to maintain the integrity of the epidermal barrier, thus decreasing the pH value.
The SG group was characterized by higher R0 values in the cutometric measurement, indicating lower skin firmness than observed in subjects with normal BMI values. It is worth noting that the average age of the two groups was similar to each other, and therefore the influence of age on this skin parameter can be excluded. The influence of obesity on the rate of aging of the body including the skin has been increasingly noted in the literature [47,48,49]. Liu et al. [47] pointed to strong correlations between obesity and facial skin aging. In a study on an animal model [50], it was observed that the collagen content of the skin was similar in a group of obese and lean mice, but the surface area of the obese skin was 60% larger which translates into a weaker mechanical strength of this tissue.
The skin of the obese subjects in the present study responded differently to the cryogenic stimulus than the skin of subjects with normal BMI values. Before the 10th treatment, the SG’s skin on the forearm had lower corneometric values than baseline and higher tewametric values, followed by an increase in these values. The forearm skin of normal-weight subjects reacted inversely. There was an initial increase in these values and a decrease between the 10th and 20th treatments. The pH value initially increased in the SG, with a subsequent decrease in values. Again, the CG’s skin reacted inversely.
Walker et al. [51] noted no differences in epidermal thickness among obese (BMI 35–50) and normal-weight (BMI 18–27) subjects. The present study did not examine epidermal thickness. There is an important element that is worth considering in future studies using high-frequency ultrasound imaging.
Obesity causes structural and functional changes in microvessels, including endothelial dysfunction. However, studies of obese individuals have noted significant alterations in the expression of genes encoding proteins that alter skin oxygenation and may contribute to poorer wound and ulcer healing [51]. Control of vascular tone and secretion of paracrine factors is impaired, affecting organ function and contributing to an altered release of adipokines and inflammatory cytokines [52]. As mentioned earlier, cryotherapy results in enhanced microvascular reflex. Systemic microvascular dysfunction in obese individuals may therefore also affect the skin and the effectiveness of treatments. Accordingly, it can be concluded that the skin of obese people, with a baseline higher degree of vascular dysfunction, responds more strongly to the cryogenic stimulus.
Individuals exposed to a series of repeated, intermittent periods of cold exposure should exhibit a number of physiological adaptations that alter the strength and quality of the body’s response to the initial episodes of cold exposure [19]. The present study, however, indicates that the vast majority of skin parameters studied do not show adaptations to the cryogenic stimulus, and each treatment, regardless of whether it is the first or last in a series, produces an identical effect. Adaptation differences were observed for TEWL on facial skin in the group of subjects with higher BMI (the last treatment induced a stronger effect than the first treatment). Similarly, different responses to the first and last treatments in the series were observed in skin hydration and for the R0 parameter in SG. In each case, these changes were on the skin on the forearms, which, as described earlier, responds more strongly than the skin on the face.
The present study confirms the positive effects of 20 WBC treatments on the skin, particularly in obese individuals. Other works have also indicated the positive effects of WBC treatments in obese individuals on weight and fat loss [6,53,54], improvement of lipid profile [55], and anti-inflammatory effects of this form of physical treatment [6,20,56]. Thus, cryogenic treatments should be considered as a safe form of health support for people struggling with overweight and obesity.
Study Limitations
Study participants were matched in terms of study group characteristics, but the groups were small in size. Further studies conducted with a larger number of volunteers would provide a more complete picture of the effects of a series of systemic cryotherapy treatments on skin conditions.
5. Conclusions
Whole-body cryotherapy appears to be a safe treatment for the skin. Baseline measurements of skin characteristics, including TEWL and skin firmness, showed variability among study groups. Positive effects of cryotherapy among obese subjects were indicated for forearm skin hydration and pH, and skin firmness in both locations. The upper extremities appear to be more sensitive to cold exposure than the facial region. The effect of the cryogenic stimulus will depend on the number of treatments, BMI, and the anatomical location of the assessed skin areas.
Author Contributions
Conceptualization, A.P. and A.D.; methodology, A.D., A.P., E.Z., M.Z. and A.S.; software, O.C.-L. and A.D.; validation, A.D., A.P. and O.C.-L.; formal analysis, A.D. and A.P.; investigation, A.D.; resources, A.D., O.C.-L.; A.S., E.Z., D.K., M.Z. and A.P.; data curation, A.D., A.S. and D.K.; writing—original draft preparation, A.D. and A.P.; writing—review and editing, A.D. and O.C.-L.; A.S., E.Z., D.K., M.Z. and A.P.; visualization, A.D., O.C.-L., A.S., E.Z., D.K., M.Z. and A.P.; supervision, A.D., A.P., E.Z. and M.Z.; project administration, A.D.; funding acquisition, A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Academy of Physical Culture in Krakow, grant numbers 165/MN/INP/2023 and 317/BS/INP/2023. The acquisition of the measurement apparatus utilized in this project was facilitated by funds secured through a grant from the Ministry of Science and Higher Education (Poland) under the designation “Regional Initiative for Perfection” for the years 2019–2022, project number 022/RID/2018/19.
Institutional Review Board Statement
The study was conducted in accordance with the principles outlined in the 7th revision of the Declaration of Helsinki and received approval from the bioethical committee of the Regional Medical Chamber in Krakow (no: 177/KBL/OIL/2023, dated 25 September 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to the bioethics committee decision.
Acknowledgments
The authors would like to express their sincere thanks to Wanda Pilch for her support and mentoring.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Sieron, A.; Cieslar, G.; Stanek, A. Cryotherapy: Theoretical Bases, Biological Effects, Clinical; α-Medica Press: Bielsko-Biała, Poland, 2010. [Google Scholar]
- Legrand, F.D.; Dugué, B.; Costello, J.; Bleakley, C.; Miller, E.; Broatch, J.R.; Polidori, G.; Lubkowska, A.; Louis, J.; Lombardi, G.; et al. Evaluating Safety Risks of Whole-Body Cryotherapy/Cryostimulation (WBC): A Scoping Review from an International Consortium. Eur. J. Med. Res. 2023, 28, 387. [Google Scholar] [CrossRef] [PubMed]
- Sadura-Sieklucka, T.; Solłtysiuk, B.; Karlicka, A.; Sokolłowska, B.; Kontny, E.; Ksiezopolska-Orlłowska, K. Effects of Whole Body Cryotherapy in Patients with Rheumatoid Arthritis Considering Immune Parameters. Reumatologia 2019, 57, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, A.; Maciak, K.; Miller, E.D.; Starosta, M.; Saluk, J. Targeting Vascular Impairment, Neuroinflammation, and Oxidative Stress Dynamics with Whole-Body Cryotherapy in Multiple Sclerosis Treatment. Int. J. Mol. Sci. 2024, 25, 3858. [Google Scholar] [CrossRef] [PubMed]
- Klemm, P.; Becker, J.; Aykara, I.; Asendorf, T.; Dischereit, G.; Neumann, E.; Müller-Ladner, U.; Lange, U. Serial Whole-Body Cryotherapy in Fibromyalgia Is Effective and Alters Cytokine Profiles. Adv. Rheumatol. 2021, 61, 3. [Google Scholar] [CrossRef]
- Wiecek, M.; Szymura, J.; Sproull, J.; Szygula, Z. Whole-Body Cryotherapy Is an Effective Method of Reducing Abdominal Obesity in Menopausal Women with Metabolic Syndrome. J. Clin. Med. 2020, 9, 2797. [Google Scholar] [CrossRef]
- Rymaszewska, J.E.; Stańczykiewicz, B.; Lion, K.; Misiak, B. The Impact of Whole-Body Cryotherapy on Lipid Profile: A Systematic Review and Meta-Analysis. Complement. Ther. Med. 2020, 55, 102568. [Google Scholar] [CrossRef]
- Fontana, J.M.; Bozgeyik, S.; Gobbi, M.; Piterà, P.; Giusti, E.M.; Dugué, B.; Lombardi, G.; Capodaglio, P. Whole-Body Cryostimulation in Obesity. A Scoping Review. J. Therm. Biol. 2022, 106, 103250. [Google Scholar] [CrossRef]
- Bouzigon, R.; Grappe, F.; Ravier, G.; Dugue, B. Whole-and Partial-Body Cryostimulation/Cryotherapy: Current Technologies and Practical Applications. J. Therm. Biol. 2016, 61, 67–81. [Google Scholar] [CrossRef]
- Schaal, K.; Le Meur, Y.; Louis, J.; Filliard, J.R.; Hellard, P.; Casazza, G.; Hausswirth, C. Whole-Body Cryostimulation Limits Overreaching in Elite Synchronized Swimmers. Med. Sci. Sports Exerc. 2015, 47, 1416–1425. [Google Scholar] [CrossRef]
- Rymaszewska, J.; Ramsey, D. Whole Body Cryotherapy as a Novel Adjuvant Therapy for Depression and Anxiety. Arch. Psychiatry Psychother. 2008, 2, 49–57. [Google Scholar]
- Dzidek, A.; Piotrowska, A. The Use of Cryotherapy in Cosmetology and the Influence of Cryogenic Temperatures on Selected Skin Parameters—A Review of the Literature. Cosmetics 2022, 9, 100. [Google Scholar] [CrossRef]
- Piotrowska, A.; Aszklar, K.; Dzidek, A.; Ptaszek, B.; Czerwińska-Ledwig, O.; Pilch, W. The Impact of a Single Whole Body Cryostimulation Treatment on Selected Skin Properties of Healthy Young Subjects. Cryobiology 2021, 100, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Rho, N.K. Revisiting the Role of Local Cryotherapy for Acne Treatment: A Review and Update. J. Clin. Med. 2023, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Sybilski, A. Skin—The Most Important Organ of Our Body. Let’s Take Care of It! Pediatr. Med. Rodz. 2012, 8, 375–379. [Google Scholar]
- Harris-Tryon, T.; Grice, E. Microbiota and Maintenance of Skin Barrier Function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef]
- Alexander, H.; Brown, S.; Danby, S.; Flohr, C. Research Techniques Made Simple: Transepidermal Water Loss Measurement as a Research Tool. J. Investig. Dermatol. 2018, 138, 2295–2300.e1. [Google Scholar] [CrossRef]
- Lubkowska, A. Therapeutic Application of Cryotherapy in Chronic Diseases-Clinical Practice. Fam. Med. Prim. Care Rev. 2013, 15, 233–239. [Google Scholar]
- Castellani, J.W.; Young, A.J. Human Physiological Responses to Cold Exposure: Acute Responses and Acclimatization to Prolonged Exposure. Auton. Neurosci. 2016, 196, 63–74. [Google Scholar] [CrossRef]
- Pilch, W.; Piotrowska, A.; Wyrostek, J.; Czerwińska-Ledwig, O.; Ziemann, E.; Antosiewicz, J.; Zasada, M.; Kulesa-Mrowiecka, M.; Żychowska, M. Different Changes in Adipokines, Lipid Profile, and TNF-Alpha Levels between 10 and 20 Whole Body Cryostimulation Sessions in Individuals with I and II Degrees of Obesity. Biomedicines 2022, 10, 269. [Google Scholar] [CrossRef]
- Hirt, P.A.; Castillo, D.E.; Yosipovitch, G.; Keri, J.E. Skin Changes in the Obese Patient. J. Am. Acad. Dermatol. 2019, 81, 1037–1057. [Google Scholar] [CrossRef]
- Yew, Y.W.; Mina, T.; Ng, H.K.; Lam, B.C.C.; Riboli, E.; Lee, E.S.; Lee, J.; Ngeow, J.; Elliott, P.; Thng, S.T.G.; et al. Investigating Causal Relationships between Obesity and Skin Barrier Function in a Multi-Ethnic Asian General Population Cohort. Int. J. Obes. 2023, 47, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Yang, Y.; Liao, Y.; Shi, Y.; Zhang, L. Emerging Roles of Adipose Tissue in the Pathogenesis of Psoriasis and Atopic Dermatitis in Obesity. JID Innov. 2022, 2, 100064. [Google Scholar] [CrossRef]
- Skrzek, A.; Ciszek, A.; Nowicka, D.; Dębiec-Bąk, A. Evaluation of Changes in Selected Skin Parameters under the Influence of Extremely Low Temperature. Cryobiology 2019, 86, 19–24. [Google Scholar] [CrossRef]
- Weir, C.; Jan, A. BMI Classificatioon Percentile and Cut Off Points; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- O’Connor, M.; Wang, J.V.; Saedi, N. Whole-and Partial-Body Cryotherapy in Aesthetic Dermatology: Evaluating a Trendy Treatment. J. Cosmet. Dermatol. 2019, 18, 1435–1437. [Google Scholar] [CrossRef]
- Wojciak, G.; Szymura, J.; Szygula, Z.; Gradek, J.; Wiecek, M. The Effect of Repeated Whole-Body Cryotherapy on SIRT1 and SIRT3 Concentrations and Oxidative Status in Older and Young Men Performing Different Levels of Physical Activity. Antioxidants 2021, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Mrowicka, M.; Malinowska, K.; Mrowicki, J.; Saluk-Juszczak, J.; Kȩdziora, J. Effects of Whole-Body Cryotherapy on a Total Antioxidative Status and Activities of Antioxidative Enzymes in Blood of Depressive Multiple Sclerosis Patients. World J. Biol. Psychiatry 2011, 12, 223–227. [Google Scholar] [CrossRef]
- Herrera, E.; Sandoval, M.C.; Camargo, D.M.; Salvini, T.F. Motor and Sensory Nerve Conduction Are Affected Differently by Ice Pack, Ice Massage, and Cold Water Immersion. Phys. Ther. 2010, 90, 581–591. [Google Scholar] [CrossRef]
- Treffel, P.; Panisset, F.; Faivre, B.; Agache, P. Hydration, transepidermal water loss, pH and skin surface parameters: Correlations and variations between dominant and non-dominant forearms. Br. J. Dermatol. 1994, 130, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Enright, K.M.; Nikolis, A. In vivo determination of the skin surface topography and biophysical properties of human hands: Effects of sex and hand dominance. Ski. Res. Technol. 2020, 26, 277–283. [Google Scholar] [CrossRef]
- Piotrowska, A.; Czerwińska-Ledwig, O.; Kotarba, P. Selected hand skin characteristics of laboratory diagnosticians. Med. Pr. Work. Health Saf. 2020, 71, 725–734. [Google Scholar] [CrossRef]
- Szymoniak-Lipska, M.; Dańczak-Pazdrowska, A.; Lipski, A.; Korecka, K.; Żaba, R.; Polańska, A. Transepidermal water loss (TEWL) and transonychial water loss (TOWL) measurements in healthy nail apparatus. Ski. Res. Technol. 2024, 30, e13851. [Google Scholar] [CrossRef]
- Cholewka, A.; Stanek, A.; Sieroń, A.; Drzazga, Z. Thermography Study of Skin Response Due to Whole-Body Cryotherapy. Ski. Res. Technol. 2012, 18, 180–187. [Google Scholar] [CrossRef]
- de Farias Pires, T.; Azambuja, A.P.; Vançan Russo Horimoto, A.R.; Nakamura, M.S.; de Oliveira Alvim, R.; Krieger, J.E.; Pereira, A.C. A Population-Based Study of the Stratum Corneum Moisture. Clin. Cosmet. Investig. Dermatol. 2016, 9, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, H.; Jia, Q.; Bai, T.; Yang, S.; Wang, M.; Li, Y.; Shao, L. Facial Physiological Characteristics and Skin Microbiomes Changes Are Associated with Body Mass Index (BMI). Clin. Cosmet. Investig. Dermatol. 2024, 17, 513–528. [Google Scholar] [CrossRef]
- Yang, B.; Lai, Q.; Chen, A.; Ye, L.; Wang, X.; Lai, Y.; Liu, D.; Man, M.Q. Body Mass Index z Scores Correlate with Epidermal Function in Chinese Children. Diabetes Metab. Syndr. Obes. 2023, 16, 3393–3401. [Google Scholar] [CrossRef]
- Guida, B.; Nino, M.; Perrino, N.R.; Laccetti, R.; Trio, R.; Labella, S.; Balato, N. The Impact of Obesity on Skin Disease and Epidermal Permeability Barrier Status. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 191–195. [Google Scholar] [CrossRef]
- Yosipovitch, G.; DeVore, A.; Dawn, A. Obesity and the Skin: Skin Physiology and Skin Manifestations of Obesity. J. Am. Acad. Dermatol. 2007, 56, 901–916. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E. PH in Nature, Humans and Skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef]
- Hachem, J.P.; Crumrine, D.; Fluhr, J.; Brown, B.E.; Feingold, K.R.; Elias, P.M. PH Directly Regulates Epidermal Permeability Barrier Homeostasis, and Stratum Corneum Integrity/Cohesion. J. Investig. Dermatol. 2003, 121, 345–353. [Google Scholar] [CrossRef]
- Smythe, P.; Wilkinson, H.N. The Skin Microbiome: Current Landscape and Future Opportunities. Int. J. Mol. Sci. 2023, 24, 3950. [Google Scholar] [CrossRef]
- Yew, Y.; Thing, T.; Chambers, J.; Chian, L. Relationship of Adiposity and Skin Epidermal Barrier Status: Results from an Asian General Population Cohort Study. J. Am. Acad. Dermatol. 2019, 81, AB117. [Google Scholar]
- Ibuki, A.; Kuriyama, S.; Toyosaki, Y.; Aiba, M.; Hidaka, M.; Horie, Y.; Fujimoto, C.; Isami, F.; Shibata, E.; Terauchi, Y.; et al. Aging-like Physiological Changes in the Skin of Japanese Obese Diabetic Patients. SAGE Open Med. 2018, 6, 1–6. [Google Scholar] [CrossRef]
- Misiorek, A.; Szyszkowska Kępińska, M. Evaluation of the Influence of Whole-Body Cryotherapy on Selected Skin Parameters in Healthy Individuals: Pilot Study. Cryobiology 2021, 100, 77–80. [Google Scholar] [CrossRef]
- Du Plessis, J.L.; Stefaniak, A.B.; Wilhelm, K.P. Measurement of Skin Surface PH. Curr. Probl. Dermatol. 2018, 54, 19–25. [Google Scholar] [CrossRef]
- Liu, M.; Feng, J. Association between Adiposity and Facial Aging: Results from a Mendelian Randomization Study. Eur. J. Med. Res. 2023, 28, 350. [Google Scholar] [CrossRef]
- Santos, A.L.; Sinha, S. Obesity and Aging: Molecular Mechanisms and Therapeutic Approaches. Ageing Res. Rev. 2021, 67, 101268. [Google Scholar] [CrossRef]
- Kruglikov, I.L.; Scherer, P.E. Skin Aging: Are Adipocytes the next Target? Aging 2016, 8, 1457–1469. [Google Scholar] [CrossRef]
- Enser, M.; Avery, N.C. Mechanical and Chemical Properties of the Skin and Its Collagen from Lean and Obese-Hyperglycaemic (Ob/Ob) Mice. Diabetologia 1984, 27, 44–49. [Google Scholar] [CrossRef]
- Walker, J.M.; Garcet, S.; Aleman, J.O.; Mason, C.E.; Danko, D.; Zuffa, S.; Swann, J.R.; Krueger, J.; Breslow, J.L.; Holt, P.R. Obesity and Ethnicity Alter Gene Expression in Skin. Sci. Rep. 2020, 10, 14079. [Google Scholar] [CrossRef] [PubMed]
- Sorop, O.; Olver, D.; Van De Wouw, J.; Heinonen, I.; Van Duin, R.W.; Duncker, D.J.; Merkus, D. The Microcirculation: A Key Player in Obesity-Associated Cardiovascular Disease. Cardiovasc. Res. 2017, 113, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Pilch, W.; Wyrostek, J.; Major, P.; Zuziak, R.; Piotrowska, A.; Czerwińska-Ledwig, O.; Grzybkowska, A.; Zasada, M.; Ziemann, E.; Żychowska, M. The Effect of Whole-Body Cryostimulation on Body Composition and Leukocyte Expression of HSPA1A, HSPB1, and CRP in Obese Men. Cryobiology 2020, 94, 100–106. [Google Scholar] [CrossRef]
- Loap, S.; Lathe, R. Mechanism Underlying Tissue Cryotherapy to Combat Obesity/Overweight: Triggering Thermogenesis. J. Obes. 2018, 2018, 5789647. [Google Scholar] [CrossRef]
- Lubkowska, A.; Dudzińska, W.; Bryczkowska, I.; Dołęgowska, B. Body Composition, Lipid Profile, Adipokine Concentration, and Antioxidant Capacity Changes during Interventions to Treat Overweight with Exercise Programme and Whole-Body Cryostimulation. Oxid. Med. Cell. Longev. 2015, 2015, 803197. [Google Scholar] [CrossRef]
- Ziemann, E.; Olek, R.A.; Grzywacz, T.; Antosiewicz, J.; Kujach, S.; Łuszczyk, M.; Smaruj, M.; Śledziewska, E.; Laskowski, R. Whole-Body Cryostimulation as an Effective Method of Reducing Low-Grade Inflammation in Obese Men. J. Physiol. Sci. 2013, 63, 333–343. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).